Abstract
A mussel-inspired polydopamine (PDA), resulting from the oxidative polymerization of dopamine, was reported to be an attractive substrate for advancing biomaterial applications. Thus, this study determined the osteoconductive/osteoinductive properties of titanium (Ti) surfaces coated with PDA and the facilitation of the PDA layer to immobilize bone morphogenetic protein-2 (BMP-2) on Ti substrates. The surface chemistry of PDA or PDA/BMP-2-coated Ti was confirmed by contact angle measurement, scanning electron microscopy (SEM), immunofluorescence staining, atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS). We verified the osteogenic potential of periodontal ligament stem cells (PDLSCs) cultured on the PDA or PDA/BMP-2-Ti surfaces. The osteogenic differentiation of the PDLSCs was assessed by measuring alkaline phosphatase (ALP) activity, intracellular calcium levels, as well as by evaluating osteocalcin (OCN), osterix (OSX), and runt-related transcription factor 2 (RUNX2) protein levels. The PDLSCs cultured on PDA/BMP-2-Ti showed the highest osteogenic activity compared with those on the control Ti and PDA-coated Ti surfaces. Moreover, PDLSCs on PDA and PDA/BMP-2-Ti expressed increased levels of integrin β1 and actin molecules compared to cells on control Ti. Blocking integrin β1 significantly decreased the osteogenic activity of PDLSCs on PDA/BMP-2 surfaces. This study suggests that the PDA coating can efficiently encourage the immobilization of BMP-2 on Ti surfaces and that this modified Ti substrate highly enhanced the osteogenic differentiation of PDLSCs by integrin-mediated cell-matrix adhesion mechanisms.
Electronic supplementary material
The online version of this article (10.1007/s12079-018-0468-0) contains supplementary material, which is available to authorized users.
Keywords: Titanium, Polydopamine, BMP-2, PDLSCs, Osteogenic differentiation
Introduction
Titanium (Ti) substrates are considered basic biomaterials with satisfactory biocompatibility and high mechanical strength in orthopedic or dental implantology. The properties of implant surfaces are critical factors for the successful osseointegration between Ti implants and bone tissue and have demanded the development of additive and subtractive surface modification techniques (Cochran et al. 1998; Oates et al. 2007).
Implant surfaces with improved osteoconductive or osteoinductive features can strengthen cell adhesion on biomaterial substrates, achieve more rapid bone healing, and enhance bone-to-implant contact (Chou et al. 1995). Cell attachment to implant materials are the first events in cell and biomaterial interactions that significantly control the integration of Ti substrates and surrounding bone tissue. Ultimately, these processes play an important role in the success or failure of biomaterial implantation (Liu et al. 2007; Baxter et al. 2002). Thus, the crucial role of biocompatible implant materials is to offer a suitable extracellular microenvironment that enhances cell adhesion, proliferation, and differentiation to encourage osseointegration and faster bone formation (Abrams et al. 2000).
Many studies on implant modification have been directed towards the surface immobilization of bioactive molecules to enhance the osseointegration of Ti implants. However, many of these methods have obstacles such as complicated chemical reactions or a time-consuming procedure. In a recent study, a mussel-inspired polydopamine (PDA), resulting from the oxidative polymerization of dopamine, was reported to be an attractive substrate for advancing biomaterial applications (Lee et al. 2006). The PDA coating is a simple and accessible technique for the conjugation of various bioactive substances and peptides on implant surfaces (Chien et al. 2012; Ku et al. 2010). Thus, multiple approaches to overcoming physical adsorption using PDA have been developed. Previous studies showing cellular activity on the PDA-coated substrates suggested that PDA can enhance the adherence, proliferation, and differentiation of specific cell types without cellular toxicity (Ku et al. 2010; Lynge et al. 2011; Bhang et al. 2013).
Although implant geometry and hydrophilicity are improved by certain modification techniques, different types of cells respond to specific surface properties (Kononen et al. 1992; Oakley and Brunette 1995). A previous study demonstrated the osteoregenerative potential of periodontal ligament stem cells (PDLSCs) in bone defects from peri-implantitis (Park et al. 2015). Our previous report was the first to verify the increased osteogenic activity of PDLSCs when cultured on PDA film, indicating that the cell-bioadhesive substrate interaction can contribute to cellular processes rather than simple mechanical binding operations (Lee et al. 2014).
The present study investigated the osteoconductive and osteoinductive properties of Ti surfaces through a simple PDA coating method. Moreover, BMP-2 was immobilized on Ti substrates in a one-step process using a mixture of BMP-2 and a PDA solution for effective osteoinduction. The adhesion conditions and the osteogenic differentiation of human PDLSCs were also determined to evaluate the efficacy of the modified surfaces.
Materials and methods
Materials
Fetal bovine serum (FBS) was purchased from Gibco-BRL (Gaithersburg, MD, USA). OCN, OSX, integrin β1, F-actin, β-actin, and goat anti-mouse and goat anti-rabbit antibodies were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). BMP-2 antibody blocking peptide was obtained by LSBio (LifeSpan Biosciences, Inc., WA, USA). Unless otherwise specified, the chemicals and laboratory supplies were from Sigma Chemical Company (St. Louis, MO, USA) and Falcon Labware (Becton-Dickinson, Franklin Lakes, NJ, USA), respectively.
Periodontal ligament stem cell culture
Periodontal ligaments were obtained from extracted human molars donated by the Kyung Hee University Department of Oral and Maxillofacial Surgery. All subjects involved in this study were informed about its purpose and procedures, and the study was approved by the Review Board of Kyung Hee University. Written informed consent was obtained from all donors or guardians on the behalf of minor participants.
Periodontal ligaments were collected from the middle thirds of roots and cultured in alpha-minimal essential medium (α-MEM; Invitrogen, Carlsbad, CA, USA) containing 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL; Sigma Chemical Company) according to a previously described method (Seo et al. 2004; Kim et al. 2013). After two passages, the cells were subjected to magnetic isolation using antibodies to detect the STRO-1 antigen (mesenchymal stem cell marker; Millipore, Billerica, MA, USA) and magnetic beads (Miltenyi Biotec, Germany). The resulting STRO-1(+) cell population was cultured in α-MEM plus 10% FBS at 37 °C in a humidified gas mixture of 5% CO2/95% air. All experiments were carried out with passage 4–7 cells.
Preparation of PDA-coated Ti discs and immobilization of BMP-2
The PDA solution was prepared by dissolving 1 mg of L-DOPA in 1 ml of 10 mM Tris buffer base (pH 8.5 Sigma-Aldrich). Pristine Ti discs were treated with the PDA solution overnight at room temperature and washed three times with sterile phosphate-buffered saline (PBS). The obtained substrates were then dried in a vacuum oven. Immobilization of BMP-2 was performed by dissolving 50 and 100 ng in 1 ml of sterile PBS. The substrates were treated with the BMP-2 solution overnight at room temperature and washed three times with sterile PBS.
Surface characterization of Ti
Ti discs, 15 mm in diameter and 1 mm in thickness, prepared to fit a 24-well tissue culture plate, were supplied by the Department of Oral and Maxillofacial Surgery of Kyung Hee University. The water contact angle was determined using a telescopic goniometer (Phoenix 300; SEO, South Korea). Each Ti image was examined by scanning electron microscopy (SEM; S-4700, Hitachi). The samples were washed with PBS twice and dried in a vacuum oven. They were then freeze-dried overnight and observed by SEM. Photographs were taken at 10 kV using a 10 k magnification. The BMP-2 modified-Ti surfaces were detected by immunofluorescence staining. The Ti surfaces were also analyzed using an atomic force microscope (AFM; XE-100; PSIA Inc., Suwon, Korea) in noncontact mode. The AFM observations were measured at ambient temperature under a 0.5 Hz scan rate. Digital NC-AFM images were acquired using the XEI 4.1.1 program. The chemical composition of the Ti surfaces was analyzed by X-ray photoelectron spectroscopy (XPS). The XPS measurements of the scanned areas were approximately 100 × 100 μm, the measurement time was 30 min, and the pass energy was 187.85 eV.
Alkaline phosphatase (ALP) activity
ALP activity was measured as previously described (Kim et al. 2013). Briefly, the cells were washed twice with PBS and lysed in 50 mM Tris-HCl buffer (pH 7.0) containing 1% (v/v) Triton X-100 and 1 mM phenylmethylsulfonyl fluoride (PMSF). Total protein was then quantified using the Bradford procedure (Bradford 1976). The entire cell lysate was assayed by adding 200 μL p-nitrophenylphosphate (Sigma Chemical Company) as a substrate for 30 min at 37 °C. The reaction was stopped by adding 3 M NaOH and the absorbance was read on a spectrophotometer at 405 nm. The enzyme activity was expressed as mM/100 μg protein.
Intracellular calcium assay
The cells were washed three times with PBS and lysed in 50 mM Tris-HCl buffer (pH 7.0) containing 1% (v/v) Triton X-100 and 1 mM PMSF without EDTA. The protein content was then quantified according to the Bradford method (Bradford 1976). The intracellular calcium content was measured using a calcium assay kit according to the manufacturer’s instructions (BioAssay Systems, Hayward, CA, USA.), and the absorbance was read on a spectrophotometer at 602 nm. The calcium content level was expressed as mg/100 mg of protein.
RNA isolation and real-time reverse-transcriptase polymerase chain reaction
This process was performed as described in our previous study (Kim et al. 2013). Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen) following the manufacturer’s protocol. Real-time quantification of RNA targets was then performed using a Rotor-Gene 2000 real-time thermal cycling system (Corbett Research, Australia) and a QuantiTect® SYBR® Green reverse-transcriptase polymerase chain reaction (RT-PCR) kit (Qiagen, CA, USA). The reaction mix (20 μL) contained 200 ng total RNA, 0.5 μM of each primer, and appropriate amounts of enzymes and fluorescent dyes, as recommended by the supplier. The Rotor-Gene 2000 cycler was programmed as follows: 30 min at 50°C for reverse transcription, 15 min at 95°C for DNA polymerase activation, 15 s at 95°C for denaturing, and 45 cycles of 15 s at 94°C, 30 s at 55°C, and 30 s at 72°C. Data were collected during the extension step (30 s at 72°C). The PCR reaction was followed by melting curve analysis to verify the specificity and identity of the RT-PCR products, which can distinguish specific PCR products from non-specific PCR products resulting from primer dimer formation. The temperature of the PCR products was increased from 65°C to 99°C at a rate of 1°C/5 s, and the resulting data were analyzed using the software provided by the manufacturer. The primers used were 5′- GTCTCACTGCCTCTCACTTG-3′ (sense) and 5′-CACACATCTCCTCCCTTCTG-3′ (antisense) for RUNX2; 5′-GCAGACCTGACATCCAGTACC-3′ (sense) and 5′- GATGGCCTTGTATGCACCTTC-3′ (antisense) for OPN; 5′- ATGAGAGCCCTCACACTCTCG-3′ (sense) and 5′- GTCAGCCAACTCGTCACAGTCC-3′ (antisense) for OCN; and 5′- GCTCTCCAGAACATCATCC-3′ (sense) and 5′-TGCTTCACCACCTTCTTG-3′ (antisense) for GAPDH.
Western blot analysis
Western blot analysis was conducted as previously reported (Kim et al. 2013). Protein extract samples (20 μg) were separated by 8–10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes. The blots were washed with TBST [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20], blocked with 5% skim milk for 1 h, and incubated with the appropriate primary antibodies (anti-OCN, anti-OSX, anti-integrin β1, or anti-β-actin; Santa Cruz Biotechnology) at the dilutions recommended by the supplier. The membranes were then washed and the primary antibodies were detected using goat anti-rabbit immunoglobulin G (IgG) or goat anti-mouse IgG conjugated to horseradish peroxidase. The blots were developed with enhanced chemiluminescence (Santa Cruz Biotechnology) and exposed to X-ray film (Eastman-Kodak, Rochester, NY, USA).
Immunofluorescence staining
The cells were fixed and treated with mouse anti-OCN, anti-OSX, anti-RUNX2, anti-integrin β1, or anti-F-actin antibody (1:100; Santa Cruz Biotechnology) for 1 h at room temperature. Fluorescein isothiocyanate–conjugated anti-mouse IgG (1:100) was then added for 1 h at room temperature as previously reported (Kim et al. 2013). Images were obtained using a fluorescence microscope (Fluoview 300; Olympus).
siRNA transfection
The cells were transfected for 24 h with a Stealth RNAi™ small interfering RNA (siRNA) specific to integrin β1 [5'- UAGAAAUGUUGGAACACUUUCGUCC-3' (sense) and 5'- GGACGAAAGUGUUCCAACAUUUCUA-3' (antisense), 200 pmol/L; Invitrogen] or a negative control siRNA (scrambled), using Lipofectamine® 2000 according to the manufacturer’s instructions as previously described (Kim et al. 2013).
Statistical analysis
All data are expressed as means ± standard deviation. One-way analysis of variance was used for multiple comparisons (Duncan’s multiple range test). Analyses were performed using the SPSS software (ver. 10.0; SPSS Inc., Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Results
Surface characteristics of PDA-coated Ti substrates
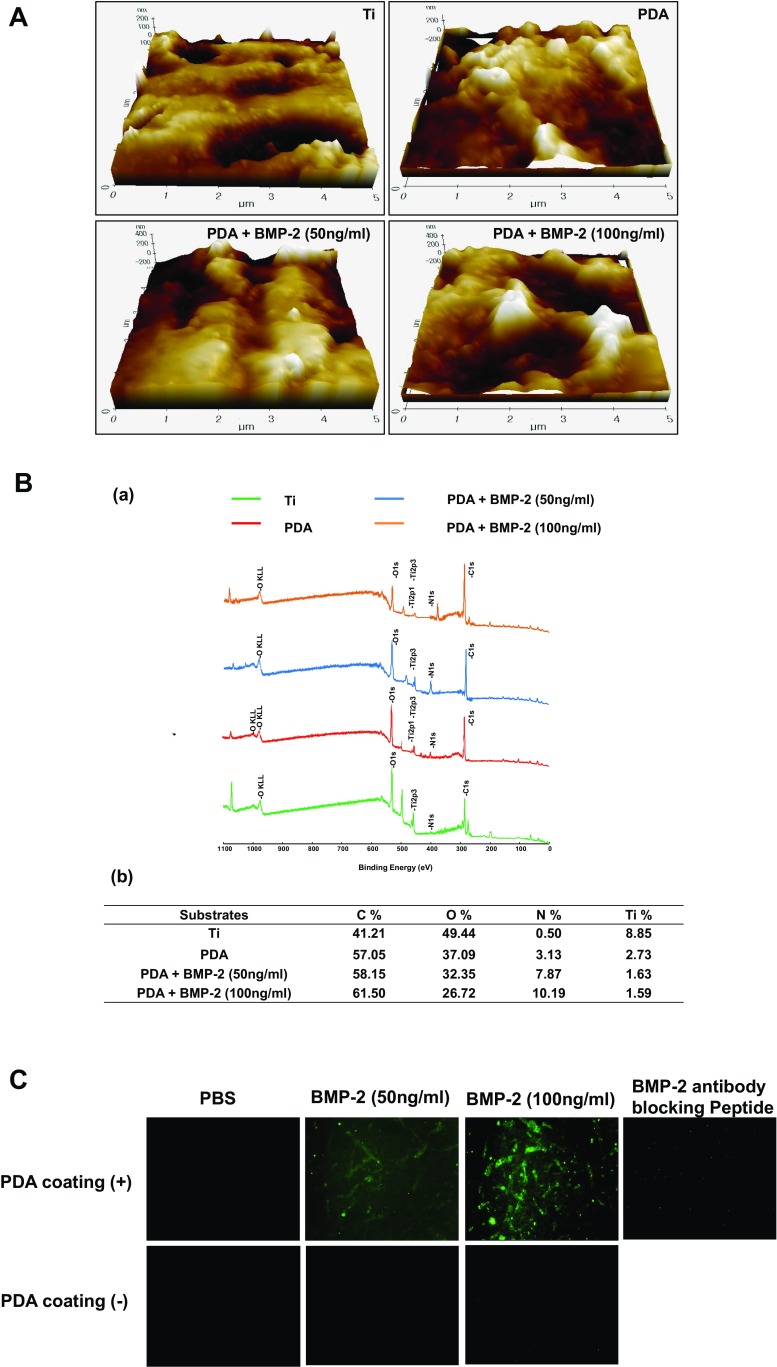
In order to coat Ti with PDA, the Ti substrates were submerged in a dopamine solution (1 mg/mL in 10 mM Tris buffer, pH 8.5) with shaking for 24 h at room temperature. PDA-coated Ti substrates were then coated with BMP-2 (50 or 100 ng/ml) and the water contact angle of the Ti surfaces was assessed to verify the change in hydrophilicity of the modified surfaces. The pristine Ti (PT) exhibited a water contact angle of 33° and the PDA-coating caused the Ti surfaces to become more hydrophilic, as indicated by the decrease in contact angle (10° in the PDA-coated surface). Alternatively, the immobilization of BMP-2 did not significantly change the contact angle on PDA-coated surfaces (Fig. 1a). We next analyzed the surface morphology and roughness of the modified Ti substrates by SEM and AFM. The polymerized PDA particles were observed on the PDA-coated Ti surface by SEM (Fig. 1b). An increase in the surface roughness and thickness, measured by AFM, indicated that the PDA and BMP-2 layers were coated onto the Ti surfaces (Fig. 2a). Finally, the PDA-coated Ti surfaces were analyzed by XPS to verify the PDA coating and immobilization of BMP-2. The XPS spectra demonstrated that the atomic chemical composition of the PT surfaces was changed by the PDA coating and the BMP-2 immobilization. The modified Ti surfaces displayed the N1 s peaks originating from nitrogen components of the PDA layer. The N1 s peaks were increased in the BMP-2-immobilized surfaces, indicating that the PDA material can attend BMP-2 adhesion onto Ti surfaces (Fig. 2b-a). Quantification of the atomic constitution by XPS indicated that PT surfaces barely displayed a nitrogen component (0.5%), whereas the PDA-layered Ti demonstrated 3.13% nitrogen; following 50 or 100 ng/ml of BMP-2 immobilization, this content was further elevated to 7.87% or 10.19%, respectively (Fig. 2b-b). These results indicated that PDA and BMP-2 were successfully layered onto the Ti surfaces. The immobilization of BMP-2 was also analyzed fluorescently by visualizing the BMP-2 attachment on PT or PDA-coated surfaces. PDA coating significantly encouraged BMP-2 immobilization on the Ti substrates indicating that PDA coating increases protein affinity onto Ti surfaces more practically than physical adsorption (Fig. 2c). When BMP-2 antibody was blocked, immunofluorescence staining disappeared indicating the specificity of BMP-2 antibody binding affinity used in this study.
Fig. 1.
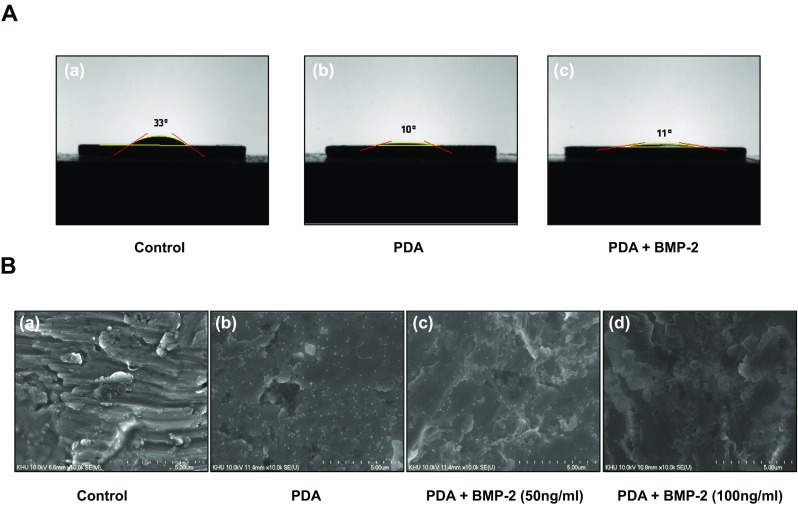
Contact angle and SEM images of surface-modified Ti discs. a The water contact angles of (a) Ti, (b) PDA-coated, and (c) PDA/BMP-2-coated surfaces were assessed. b Topographical features of (a) Ti, (b) PDA, (c) PDA/BMP-2 (50 ng/ml), and (d) PDA/BMP-2 (100 ng/ml)-coated substrates were examined by scanning electron microscopy at ×10 k magnification
Fig. 2.
Surface characterization of modified Ti substrates. a AFM images of Ti, PDA, PDA/BMP-2 (50 ng/ml), and PDA/BMP-2 (100 ng/ml)-Ti substrates. The scale bar indicates 5 μm. b XPS peaks of Ti, PDA, PDA/BMP-2 (50 ng/ml), and PDA/BMP-2 (100 ng/ml)-Ti substrates (a), quantification of atomic chemical composition of each Ti surface (b). c Fluorescent visualization of labeled FITC-BMP-2 antibody immobilized onto pristine Ti (PT) or PDA-coated Ti substrates. The specific binding affinity of BMP-2 antibody was also assessed using blocking peptide
Effect of PDA-BMP-2 coated Ti surfaces on osteogenic differentiation of PDLSCs
To determine whether the PDA-mediated BMP-2 peptide immobilization on a Ti surface can regulate the osteogenic differentiation of PDLSCs, the cells were cultured on PT, PDA-layered Ti, or PDA-BMP-2-coated Ti surfaces. The ALP activity and intracellular calcium concentration ([Ca2+]i) of PDLSCs, as markers of osteogenic differentiation, were assessed on days 4 and 7. The PDA coating alone on the Ti surfaces increased the ALP activity and [Ca2+]i of PDLSCs compared with control PT, and the PDA-BMP-2 immobilization significantly facilitated the osteogenic differentiation of PDLSCs (Fig. 3a, b).
Fig. 3.
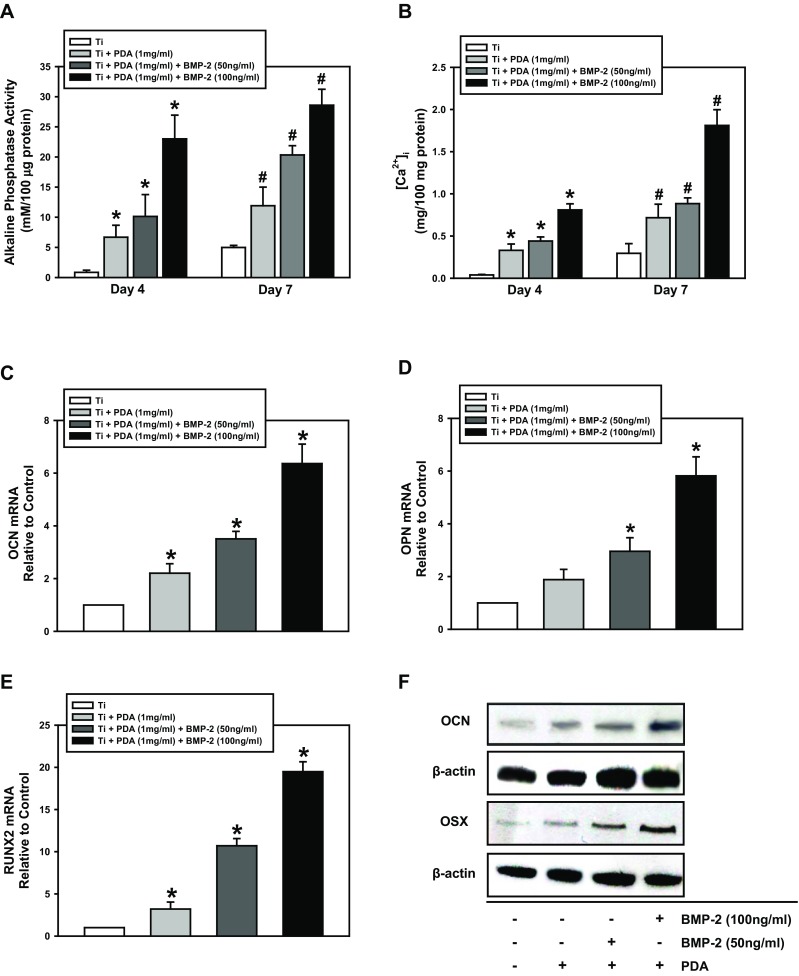
Osteogenic differentiation of PDLSCs cultured on PDA-modified surfaces. a ALP activity and (b) intracellular calcium levels were determined after 4 and 7 days of osteogenic induction. (c) Cells were cultured on Ti, PDA, PDA/BMP-2 (50 ng/ml), and PDA/BMP-2 (100 ng/ml)-Ti substrates for 4 or 7 days, and (a) ALP activity, (b) intracellular calcium levels, (c - e) real time RT-PCR, and (f) Western blot for osteogenic markers were then assessed as described in the “Materials and Methods” section. The values reported are the means ± S.D. of five independent experiments. *P < 0.05 vs. 4 day-control value, and #P < 0.05 vs. 7-day control value
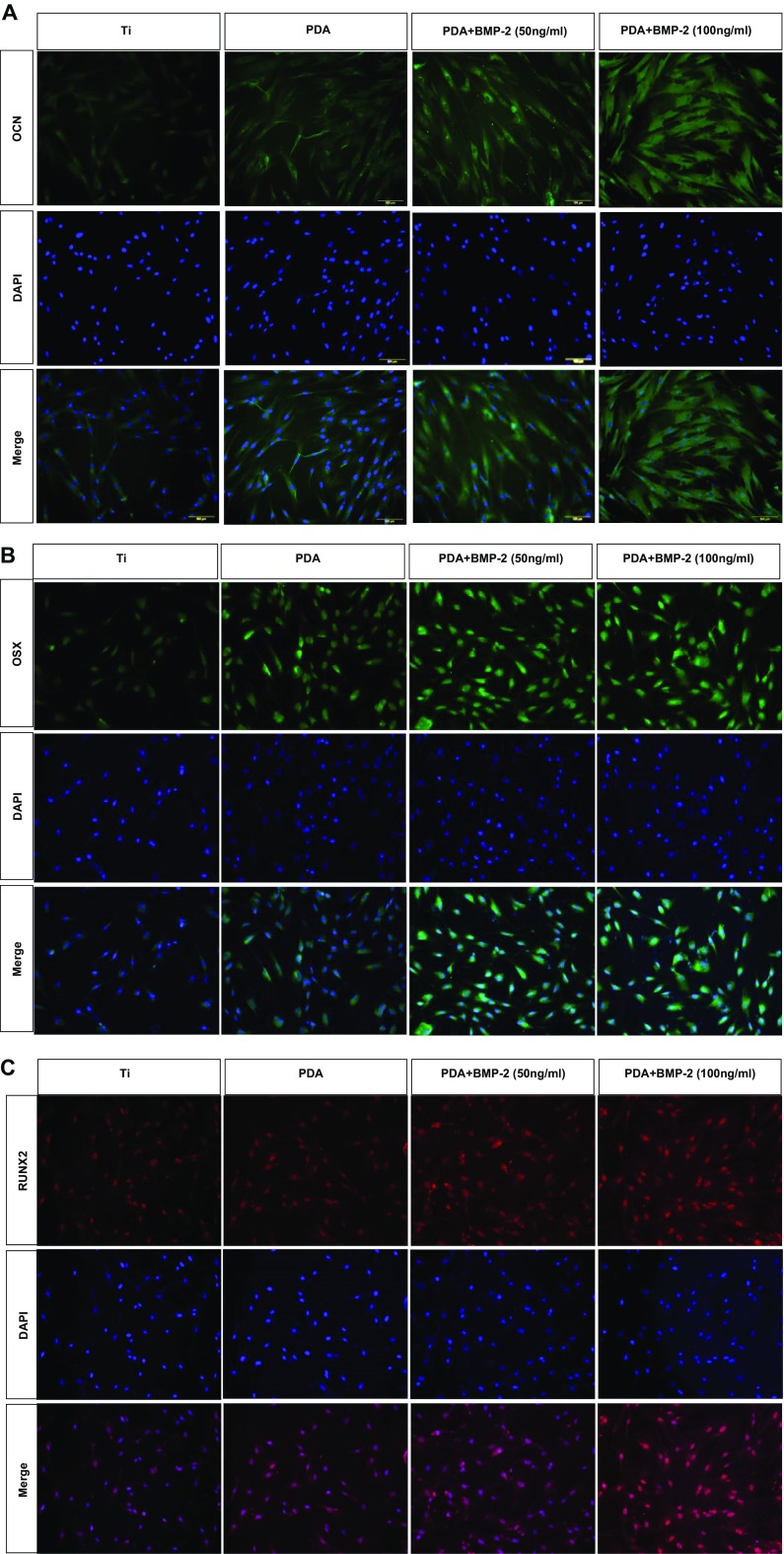
To investigate the effect of PDA-modified Ti on PDLSC behavior further, we analyzed the mRNA expression of osteogenic target genes (OCN, OPN, and RUNX2) using real-time RT-PCR. PDA coating or PDA-mediated BMP-2 immobilization increased the mRNA expression of each osteogenic gene compared with the control PT. Moreover, the expression of all genes showed the highest level in cells on PDA-coated surfaces with BMP-2 deposition (Fig. 3c - e). Analysis of osteogenic marker proteins, using Western blotting, supported the results of the mRNA evaluation. The protein levels of the osteogenic markers (OCN and OSX) on day 7 of osteogenic induction were increased in PDLSCs cultured on the PDA coating alone or on PDA-BMP-2-Ti substrates and were significantly augmented by BMP-2 immobilization, indicating that BMP-2 fixation via PDA coating can promote osteogenic differentiation of PDLSCs (Fig. 3f). Immunofluorescence staining of OCN, OSX, and RUNX2 indicated that PDA-layered Ti surfaces enhance PDLSC differentiation into the osteogenic lineage. The pattern of protein expression levels in response to each surface was consistent with the Western blotting data (Fig. 4).
Fig. 4.
Immunofluorescence analysis of (a) OCN, (b) OSX, and (c) RUNX2 proteins. The nuclei were stained with DAPI (blue staining). A representative result from three independent experiments is shown. (magnification ×200)
Involvement of integrin in PDLSC adhesion onto PDA-layered Ti surfaces
To understand whether the PDA coating itself can directly interact with cells, cell-matrix adhesions were next investigated by confirmation of an adhesion receptor and integrin, and actin cytoskeletal organization. Western blotting revealed that PDA coating, or PDA-mediated BMP-2 immobilization, increased the protein levels of integrin β1 (Fig. 5a). These results were supported by immunofluorescence analysis to verify the function of PDA layering in intensifying cell adhesion (Fig. 5b). Immunofluorescence staining of F-actin, one of the major microfilaments of the cytoskeleton, was performed to identify the main integrin that mediates cell adhesion on PDA (Fig. 5c). These results indicated that PDA performs similarly to extracellular matrix (ECM) proteins to organize cell-matrix adhesions. We eventually investigated the influence of integrin β1 on the osteogenic differentiation of PDLSCs on the PDA/BMP-2 immobilized Ti surface. The knockdown efficiency of integrin β1-specific siRNA was first validated. Figure 6a demonstrates that the transfection of integrin β1 siRNA effectively blocked protein levels of PDLSCs on the PDA/BMP-2 Ti surface. The knockdown of integrin β1 significantly decreased ALP activity and the [Ca2+]i of PDLSCs (Fig. 6b, c). These results suggest that integrin β1 is involved not only in adhesion events but also in the osteogenic differentiation of PDLSCs on PDA-layered Ti substrates.
Fig. 5.
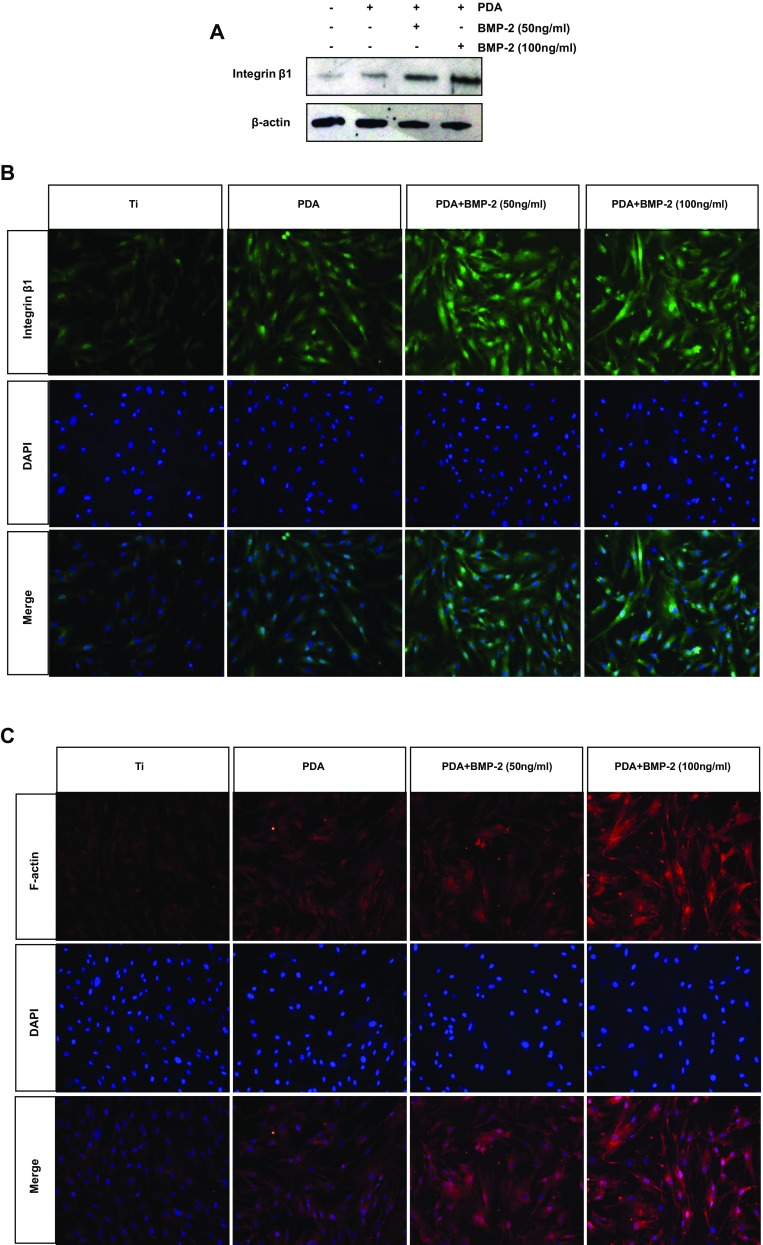
Evaluation of integrin β1 and F-actin proteins in PDLSCs. a Cells were cultured on each Ti substrate for 7 days, and then the protein levels of integrin β1 were determined by western blot analysis. The fluorescent visualization of (b) integrin β1 and (c) F-actin were assessed (magnification 200×). The nuclei were stained with DAPI (blue staining)
Fig. 6.
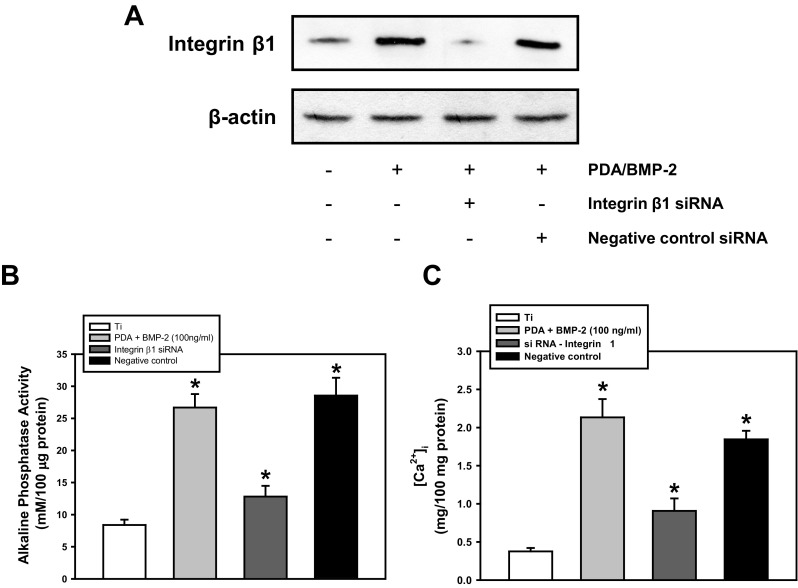
Effect of integrin β1 knockdown on osteogenic activity PDLSCs. a Cells were transfected with integrin β1-specific siRNA, and (a) the protein levels of integrin β1, (b) ALP activity, and (c) intracellular calcium levels were assessed after 4 days of osteogenic induction. A representative result from five independent experiments is shown. * P < 0.05 vs. control value
Discussion
The present study investigated whether the PDA-mediated surface modification allowed the development of osteoinductive Ti substrates that could promote the osteogenic potential of PDLSCs. Modification of the implant surface chemistry is another strategy to improve the efficiency of osseointegration. Successful integration between implant substrates and surrounding bone tissue requires promising cell adhesion and subsequent cellular events including proliferation/differentiation on the biomaterial surface (Liu et al. 2007; Baxter et al. 2002). Thus, various surface modification techniques have been developed to enhance the physical adsorption and chemical immobilization of bioactive molecules onto artificial materials (Xu et al. 2010; Martins et al. 2009).
The adherence of numerous biomolecules and cells has been reported with the PDA coating, which is constructed by dopamine polymerization (Bhang et al. 2013; Lai et al. 2011). Previous studies have shown that the PDA layer can be considered a biocompatible microenvironment that modulates cellular activity (Liu et al. 2013; Rim et al. 2012). Our recent study demonstrated that the osteogenic potential of PDLSCs was significantly enhanced when cells were grown on PDA film, and that the PDA substrate can induce cellular responses depending on the adhesive mechanism (Lee et al. 2014). Thus, PDA acts as a biologically active molecule not only for attaching cells to a substrate but also for regulating cellular functions. The PDA-mediated functionalization also allows the stable and efficient immobilization of BMP-2 peptides, an osteoinductive factor (Yamaguchi et al. 2000) that has proven to be strong on Ti surfaces, whereas many of the surface conjugation methods for bioactive molecules encounter obstacles such as complicated chemical reactions and time-consuming adsorption processes. Recent research has reported that PDA layering consistently yields much more advanced peptide immobilization on the polymer scaffolds compared with physical adsorption, indicating that the PDA catechol functional groups are associated with binding and the configuration of peptides on substrate surfaces (Ko et al. 2013). Subsequently, surface modification by the PDA coating and PDA-mediated BMP-2 adsorption changed the surface properties, including the hydrophilicity, morphology, and roughness of the Ti substrates, in which the surface wettability and roughness were significantly increased by the PDA coating or PDA-BMP-2. Interestingly, this reformation of the Ti surfaces by PDA promoted the osteogenic differentiation of PDLSCs compared with non-modified, pristine Ti, and the immobilization of BMP-2 further increased PDLSC-osteogenesis, which provides a surface geometry that can regulate stem cell behavior. In accord with the present study, previous studies reported increases in stem cell proliferation and differentiation on PDA- or PDA/peptide-layered substrates (Ko et al. 2013; Luo et al. 2013; Lee et al. 2012). Thus, the modification of Ti surfaces by PDA and PDA/BMP-2 can clearly improve cellular responses and induce new bone tissue formation.
The present study ultimately demonstrated that the PDA and PDA/BMP-2 layers encouraged cell-matrix adhesion mechanisms, which provided linkages between the cells and the biomaterial substrates. A previous study showed that the surface topography can influence the intracellular cytoskeleton and adhesion organization and thus regulate the cell adhesive behavior on the ECM (Chou et al. 1995). Integrins represent a major class of cell adhesion receptors that play important roles in embryonic development, tissue repair, and stem cell regulation (Humphries et al. 2006). It has also been reported that the principal types of integrins can affect cell-biomaterial interactions (Keselowsky et al. 2005). This study verified that the PDA coating highly modulated integrin β1 in the PDLSCs cultured on PDA-coated Ti during osteogenic induction, suggesting that PDA-induced cell adhesion is mainly mediated by integrin β1. Immunofluorescence analysis of the actin cytoskeleton further confirmed the adhesion function of the PDA coating. Even though additional detailed studies are required, these findings indicate that the PDA layering performs similarly to ECM materials to facilitate cell-matrix organization.
Additionally, this study showed that PDA-bound BMP-2 significantly increased the integrin β1 and actin proteins. Blockage of integrin β1 significantly decreased the osteogenic activity of PDLSCs on PDA/BMP-2 surfaces. Several studies have reported cross-talk between integrins and BMP signaling pathways without integrins and BMP receptor interactions (Margadant and Sonnenberg 2010; Ivaska et al. 2011). Similar to the present finding but with different physical material properties, matrix-bound BMP-2 induces β3 integrin-dependent myoblast spreading and recruits adhesion dynamics (Fourel et al. 2016). Whether these BMP actions rely on the cooperation of integrin receptors or on the recruitment of additional pathways remains to be elucidated in the current experimental conditions.
This study suggests that the PDA coating can efficiently encourage the immobilization of BMP-2 on Ti surfaces and that this modified Ti substrate highly enhanced the osteogenic differentiation of PDLSCs by integrin-mediated cell-matrix adhesion mechanisms. This bioadhesive material can be used as a medical substance for bone regeneration in the field of dental and orthopedic implants.
Electronic supplementary material
Osteogenic differentiation of human osteoblast cell line (hFOB 1.19) cultured on PDA-modified surfaces. (A) ALP activity, (B) intracellular calcium levels, and (C) protein levels of osteogenic markers, OCN and OSX were determined after 4 and 7 days of osteogenic induction. The values reported are the means ± S.D. of four independent experiments. *P < 0.05 vs. 4 day-control value, and #P < 0.05 vs. 7-day control value. (PDF 78 kb)
Effect of BMP-2 neutralization on PDLSCs osteogenesis. Cells were cultured on Ti, PDA, PDA/BMP-2 (100 ng/ml), and PDA/BMP-2 (100 ng/ml) plus neutralizing antibody-Ti substrates for 4 or 7 days, and (A) ALP activity, (B) intracellular calcium levels, (C) real time RT-PCR for OCN, OPN, RUNX2 were then assessed. The values reported are the means ± S.D. of five independent experiments. *P < 0.05 vs. each control value, and #P < 0.05 vs. PDA/BMP-2 (100 ng/ml) value. (PDF 11 kb)
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2015R1A2A2A01006490).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Abrams GA, Goodman SL, Nealey PE, Franco M, Murphy CJ. Nanoscaletopography of the basement membrane underlying the corneal epithelium of therhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004410050004. [DOI] [PubMed] [Google Scholar]
- Baxter LC, Frauchiger V, Textor M, ap Gwynn I, Richards RG. Fibroblast and osteoblast adhesion and morphology on calcium phosphate surfaces. Eur Cell Mater. 2002;4:1–17. doi: 10.22203/eCM.v004a01. [DOI] [PubMed] [Google Scholar]
- Bhang SH, Kwon SH, Lee S, Kim GC, Han AM, Kwon YH, Kim BS. Enhanced neuronal differentiation of pheochromocytoma 12 cells on polydopamine-modified surface. Biochem Biophys Res Commun. 2013;430:1294–1300. doi: 10.1016/j.bbrc.2012.11.123. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chien HW, Kuo WH, Wang MJ, Tsai SW, Tsai WB. Tunable micropatterned substrates based on poly(dopamine) deposition via microcontact printing. Langmuir. 2012;28:5775–5782. doi: 10.1021/la300147p. [DOI] [PubMed] [Google Scholar]
- Chou L, Firth JD, Uitto VJ, Brunette DM. Substratum surface topography alters cell shape and regulates fibronectin mRNA level, mRNA stability, secretion and assembly in human fibroblasts. J Cell Sci. 1995;108:1563–1573. doi: 10.1242/jcs.108.4.1563. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface. J Biomed Mater Res. 1998;40:1–11. doi: 10.1002/(SICI)1097-4636(199804)40:1<1::AID-JBM1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Fourel L, Valat A, Faurobert E, Guillot R, Bourrin-Reynard I, Ren K, Lafanechère L, Planus E, Picart C, Albiges-Rizo C. β3 integrin-mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. J Cell Biol. 2016;212:693–706. doi: 10.1083/jcb.201508018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska Johanna, Heino Jyrki. Cooperation Between Integrins and Growth Factor Receptors in Signaling and Endocytosis. Annual Review of Cell and Developmental Biology. 2011;27(1):291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee JK, Park YD, Kang KL, Lee JC, Heo JS. Hesperetin alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of periodontal ligament stem cells. PLoS One. 2013;8:e67504. doi: 10.1371/journal.pone.0067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E, Yang K, Shin J, Cho SW. Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules. 2013;14:3202–3213. doi: 10.1021/bm4008343. [DOI] [PubMed] [Google Scholar]
- Kononen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J Biomed Mater Res. 1992;26:1325–1341. doi: 10.1002/jbm.820261006. [DOI] [PubMed] [Google Scholar]
- Ku SH, Ryu J, Hong SK, Lee H, Park CB. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials. 2010;31:2535–2541. doi: 10.1016/j.biomaterials.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Lai M, Cai K, Zhao L, Chen X, Hou Y, Yang Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules. 2011;12:1097–1105. doi: 10.1021/bm1014365. [DOI] [PubMed] [Google Scholar]
- Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YB, Shin YM, Lee JH, Jun I, Kang JK, Park JC, Shin H. Polydopamine-mediated immobilization of multiple bioactive molecules for the development of functional vascular graft materials. Biomaterials. 2012;33:8343–8352. doi: 10.1016/j.biomaterials.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yi JK, An SY, Heo JS. Increased osteogenic differentiation of periodontal ligament stem cells on Polydopamine film occurs via activation of integrin and PI3K signaling pathways. Cell Physiol Biochem. 2014;34:1824–1834. doi: 10.1159/000366381. [DOI] [PubMed] [Google Scholar]
- Liu X, Lim JK, Donahue HJ, Dhurjati R, Mastro AM, Vogler EA. Influence of substratumsurface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: phenotypic and genotypic responses observed in vitro. Biomaterials. 2007;28:4535–4550. doi: 10.1016/j.biomaterials.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YT, Lee TM, Lui TS. Enhanced osteoblastic cell response on zirconia by bio-inspired surface modification. Colloids Surf B Biointerfaces. 2013;106:37–45. doi: 10.1016/j.colsurfb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Luo R, Tang L, Zhong S, Yang Z, Wang J, Weng Y, Tu Q, Jiang C, Huang N. In vitro investigation of enhanced hemocompatibility and endothelial cell proliferation associated with quinone-rich polydopamine coating. ACS Appl Mater Interfaces. 2013;5:1704–1714. doi: 10.1021/am3027635. [DOI] [PubMed] [Google Scholar]
- Lynge ME, Ogaki R, Laursen AO, Lovmand J, Sutherland DS, Städler B. Polydopamine/liposome coatings and their interaction with myoblast cells. ACS Appl Mater Interfaces. 2011;3:2142–2147. doi: 10.1021/am200358p. [DOI] [PubMed] [Google Scholar]
- Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A, Pinho ED, Faria S, Pashkuleva I, Marques AP, Reis RL, Neves NM. Surface modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological performance. Small. 2009;5:1195–1206. doi: 10.1002/smll.200801648. [DOI] [PubMed] [Google Scholar]
- Oakley C, Brunette DM. Response of single, pairs, and clusters of epithelial cells to substratum topography. Biochem Cell Biol. 1995;73:473–489. doi: 10.1139/o95-053. [DOI] [PubMed] [Google Scholar]
- Oates TW, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J, Toutenburg H, Cochran DL. Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. Int J Oral Maxillofac Implants. 2007;22:755–760. [PubMed] [Google Scholar]
- Park SY, Kim KH, Gwak EH, Rhee SH, Lee JC, Shin SY, Koo KT, Lee YM, Seol YJ. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J Biomed Mater Res A. 2015;103:38–47. doi: 10.1002/jbm.a.35145. [DOI] [PubMed] [Google Scholar]
- Rim NG, Kim SJ, Shin YM, Jun I, Lim DW, Park JH, Shin H. Mussel-inspired surface modification of poly (L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surf B Biointerfaces. 2012;91:189–197. doi: 10.1016/j.colsurfb.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Xu FJ, Wang ZH, Yang WT. Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules. Biomaterials. 2010;31:3139–3147. doi: 10.1016/j.biomaterials.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Osteogenic differentiation of human osteoblast cell line (hFOB 1.19) cultured on PDA-modified surfaces. (A) ALP activity, (B) intracellular calcium levels, and (C) protein levels of osteogenic markers, OCN and OSX were determined after 4 and 7 days of osteogenic induction. The values reported are the means ± S.D. of four independent experiments. *P < 0.05 vs. 4 day-control value, and #P < 0.05 vs. 7-day control value. (PDF 78 kb)
Effect of BMP-2 neutralization on PDLSCs osteogenesis. Cells were cultured on Ti, PDA, PDA/BMP-2 (100 ng/ml), and PDA/BMP-2 (100 ng/ml) plus neutralizing antibody-Ti substrates for 4 or 7 days, and (A) ALP activity, (B) intracellular calcium levels, (C) real time RT-PCR for OCN, OPN, RUNX2 were then assessed. The values reported are the means ± S.D. of five independent experiments. *P < 0.05 vs. each control value, and #P < 0.05 vs. PDA/BMP-2 (100 ng/ml) value. (PDF 11 kb)