Abstract
Purpose
Renal denervation (RD) has been demonstrated to be an effective approach to reduce blood pressure for those with resistant hypertension. Yet, we aimed to explore the effect and possible mechanism of RD on blood-pressure response to hemorrhagic shock in spontaneously hypertensive rats.
Methods
A total of 48 male spontaneously hypertensive rats were randomized to three groups: study group, sham-operation group and control group. RD was achieved by cutting off renal nerves and swabbing phenol on it. Ten weeks after RD, 8 rats in each group were sacrificed to collect the kidney and heart tissues. The remaining rats were subjected to an operation to induce hemorrhagic shock which would lead to 40% loss of total blood volume, and observed for 120 min. The serum concentration of norepinephrine was measured before and three weeks after RD.
Results
The blood-pressure and norepinephrine levels were reduced significantly after RD (p < 0.05). Systolic blood pressure and diastolic blood pressure of the surgery group were higher than those in the sham and control groups at 15, 30 and 45 min after hemorrhagic shock (p < 0.05), while no significant difference was observed at 60, 90 and 120 min (p > 0.05). Additionally, the beta-1 adrenergic receptor (β1-AR) in the study group was significantly higher than those in the other two groups (p < 0.05) after hemorrhagic shock.
Conclusion
This study demonstrated that RD could to some extent improve blood-pressure response to hemorrhagic shock in an established model of severe hemorrhagic shock in spontaneously hypertensive rats. The mechanism might be associated with up-regulation of β1-AR.
Keywords: Renal denervation, Hemorrhage, Beta-1 adrenergic receptor, Spontaneously hypertensive rats
Introduction
Hypertension is one of the most important modifiable risk factors for cardiovascular mortality and morbidity.1 It is estimated that approximately 9–18% hypertension patients are consistent with the diagnostic criteria of resistant hypertension (RHTN).2, 3 RHTN is diagnosed when blood pressure (BP) remains above the target level despite therapy with three or more antihypertensive agents of different types at maximum tolerable doses with one being a diuretic.4 RHTN has been characterized as a multifactorial phenomenon due to multiple biological mechanisms. However, the hyperactivity of the sympathetic nervous system, composed of efferent sympathetic nerves and afferent sensory nerves, plays a crucial role in the pathogenesis and progression of RHTN. Elevated renal sympathetic activity enhances renin and norepinephrine (NE) release, leading to peripheral arterial vasoconstriction, and subsequent increase in arterial BP. Recently,catheter-based ablation of afferent and efferent sympathetic nerves surrounding the renal arteries has been featured as a safe and effective approach for patients with RHTN.5, 6, 7, 8 In animal models of hypertension, renal denervation (RD), especially phenol-based renal nerve ablation, has demonstrated an anti-hypertensive effect.9, 10, 11
Sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis could be activated by stimuli of various stress, and both of them were involved as the major components of the stress response. Activation of SNS and HPA axis results in the secretion of stress hormones, including glucocorticoids from the adrenal cortex and catecholamines from the adrenal medulla and sympathetic nerve termini. The catecholamines NE and epinephrine mediate the early stress response via the SNS, but they also have a key role in homeostatic BP control through the activation of adrenergic receptors located on the heart and the blood vessels. Yann Vuignier et al showed that the reactivity of the SNS is blunted after RD in their study.12 In symplicity HTN-2 one patient was given drugs to correct hypotension,13 but the specific mechanism is unknown. Independently of its effect on BP, RD could affect the stress response, which are directly related to renal SNS activity. So far, few clinical studies have evaluated the impact of RD on the stress response. Therefore, we suspected that whether RD could affect stress tolerance and even the mortality. Furthermore, hemorrhage is a common trauma stress.
Beta-1 adrenergic receptor (β1-AR) is the predominant subtype of β-AR in the heart, and leads to most of the actions of adrenergic stimulation in cardiac muscle cells.14, 15 The activation of β1-AR can increase cardiac rate and contractility in response to NE and epinephrine during stress. Evidence from both animal- and patient-based studies has proved that β1-AR may play an important role in the development and clinical course of progressive cardiac dilatation and heart failure.16 Moreover, lots of pathological mechanisms within acute myocardial infarction and chronic heart failure can regulate the β1-AR expression.17, 18
Spontaneously hypertensive rat (SHR) represents an experimental model of hypertension and many of their characteristics mimic those found in human essential hypertension. Because RD is applied to the patients with resistant hypertension, it seems to be more helpful and important to reveal the precise effects of RD on BP in SHR, rather than other ordinary rats. There is still lack of studies focusing on the effect of RD on BP response to hemorrhagic shock in SHR. Based on the existing knowledge, the objectives of the study were to assess the effect of RD on BP response in SHR when subjected to hemorrhagic shock. Besides, the role of RD in regulation of the expression of β1-AR during the pathylogical process of hemorrhagic shock would also be explored. This study focused on the BP response to hemorrhagic shock after RD, not the effect of this procedure on BP.
Materials and methods
Animals
The experiment scheme and the use of rat were approved by Animal Use and Management Ethics Committee of Zhejiang University of Traditional Chinese Medicine (Hangzhou, China). The implementation process and experimental design were undertaken in accordance with animal welfare guidelines provided by the CPCSEA and World Medical Association Declaration of Helsinki on Ethical Principles. Forty-eight SHRs were randomly assigned to three groups: the study group, sham-operation group and control group. Before and three weeks after RD, the plasma was collected, centrifuged and stored at −80 °C for measurement of NE activity. At week 10 after surgery, 8 animals from each group were sacrificed with a large dose of chloral hydrate for the separation of the kidney and heart tissue, and the other 8 rats were used for hemorrhagic shock operation. All the kidneys were frozen for measurement to evaluate the completion of RD, and heart tissues were collected for measuring the β1-AR expression. NE in blood and kidney were used as measures of sympathetic activity (Fig. 1).
Fig. 1.
Experimental flow chart. SHR: Spontaneously hypertensive rat; BP: blood pressure; NE: norepinephrine; RD: renal denervation, β1-AR: beta-1 adrenergic receptor.
Materials
The male SHRs (n = 48) weighing 240–260 g were purchased from Beijing Vitalriver Co., Ltd (License number SCXK [JING] 2012-0001). Animals were housed in the experimental animal center of the said university until they were aged 12 weeks. Rat NE ELISA kit (Wuhan Sino-American Biotechnology Co., Ltd., Hubei, China), rat NE ELISA kit (Beijing Boosen Biological Technology Co., Ltd., Beijing, China), and the multi-channel physiological recorder (MedLab-U/8c, Nanjing Mei Yi Technology Co., Ltd., Nanjing, China) were used in the present study.
Renal denervation procedure
The RD procedure was performed as previous description.19, 20 The rats were anesthetized intraperitoneally at room temperature with chloral hydrate at 10% chloral hydrate (3 ml/kg). After anesthesia and sterilization, a midline incision was made in the abdomen to expose the ureters and the arteries, veins and nerves in the sheath. Under a microscope (magnification, 25×), the renal nerve was stripped and painted with 10% phenol in 90% ethanol for 3 min to ensure the destruction of any remaining nerves. In the sham-operation group, the sympathetic nerve was treated with normal saline. The wound was subsequently closed. Within 3 days following RDN, intraperitoneal injection of 16 U of penicillin was administered once daily in case of infection.
Hemorrhagic shock operation21, 22
After 10-week follow-up observation, rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 ml/kg). Polyethylene catheters (PE 50 tubing, outside diameter is 0.965 mm, and inside diameter is 0.58 mm; experimental animal center of Committee of Zhejiang University of Traditional Chinese Medicine, Hangzhou, China) containing saline and heparin were then introduced into the left femoral artery for blood withdrawal. The left femoral artery was also connected to the multi-channel physiological recorder for monitoring systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate. After 15 min tranquillization, hemorrhagic shock was induced by removing 40% of the total blood volume (22.4 ml/kg). The operation was divided into 3 stages to achieve a uniform hemorrhagic shock state: the first stage of 20% (T0 – T20, 30 min), the second stage of 10% (T20 – T30, 15 min), and the third stage of 10% (T30 – T40, 15 min). Hemodynamic indexes were compared at each time point before and at 15, 30, 45, 60, 90 and 120 min after shock induction. The plasma and heart tissue of each rat were collected at the last time point, and the number of dead rats was recorded.
Measurement of blood pressure
BP of the tail artery was measured non-invasively as previous description.19, 20 Briefly, the resting rat was fixed on a plastic restrainer at room temperature until the temperature was increased to 39 °C. The proximal tail was attached to the computer via a 4-channel dynamic signal acquisition system. The mean BP was obtained from three consecutive readings at 3 min intervals.
Measurement of the norepinephrine concentration
The NE concentration in serum of the kidney was detected by ELISA. In brief, chromogenic reagent A (50 μl) and B (50 μl) were consecutively added to each well, to which stop solution (50 μl) was added until vortexing, and then incubated in the dark at 37 °C for 15 min. The optical density (OD) was measured at 450 nm by spectrophotometer, and the standard curve was delineated to calculate the NE concentration.
Measurement of beta-1 adrenergic receptor expression in the myocardium
The β1-AR expression in the myocardium was measured by immunohistochemistry. In brief, the paraffin-embedded heart tissue was sliced into sections and placed onto clean glass slides. The primary antibody (Anti-beta1 adrenergic receptor antibody, ab3442, ABCAM, Massachusetts, USA) at a dilution of 1:300 was added to each glass slide, and the slides were incubated at 4 °C overnight. The slides were then treated with secondary antibodies for 30 min at room temperature. Finally, the sections were visualized by applying diaminobenzidine chromogen (DAB; Zhongshan Biotech, Beijing, China), counterstained with methyl green, dehydrated, mounted, and cover-slipped. Three noncontiguous microscopic areas were randomly selected and photographed with a digital camera (×400; DM3000; Leica, Germany). Three selected areas from each slide was captured and stored as high-resolution image files (2047×1532 pixels). Computer-aided image analysis software (Image-Pro Plus 6.0, Media Cybernetics) was introduced to discriminate the immunostained area and calculate the integrated optical density (IOD).
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, USA) was employed for statistical analysis. Quantitative data were expressed as means ± standard deviation (SD). Normal distribution was tested before comparisons. The difference during the different periods of the same group was compared by an paired sample t-test. Multiple comparisons of means among groups were analyzed by one-way analysis of variance (ANOVA) test, while between-group comparisons of means were examined by Least Significant Difference (LSD) t-test or Student–Newman–Keuls test where appropriate. Differences were considered statistically significant at p < 0.05.
Results
Effect of renal denervation on animal survive
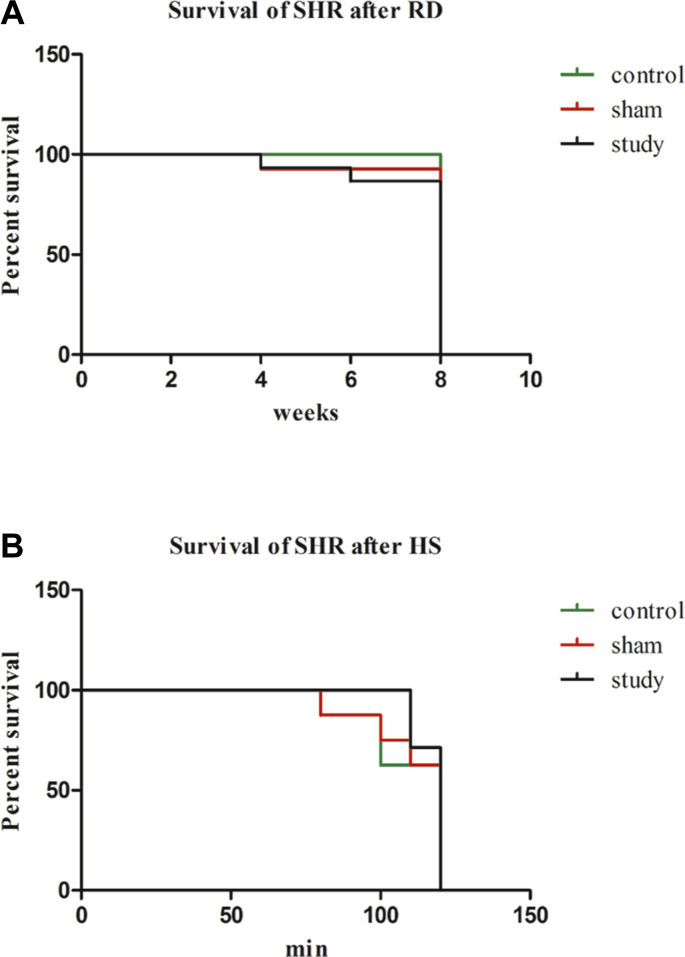
Three rats died after RD and sham operation because of infection, overdose of anesthesia or intestinal paralysis. Eight rats in the control group, 7 rats in the sham-operation group, and 7 rats in the study group were euthanized randomly with an overdose of anesthetic (Fig. 2).
Fig. 2.
The survival situation of experimental animals during different periods. (A) Survival of SHR after RD: (B) Survival of SHR after HS. SHR: Spontaneously hypertensive rat; RD: renal denervation; HS: hemorrhagic shock.
Effect of renal denervation on systolic blood pressure and diastolic blood pressure
As shown in Table 1, there were no significant differences in SBP and DBP before RD among the three groups (p > 0.05). After RD, SBP and DBP in the study group were reduced significantly at weeks 2, 4, 6 and 8, as compared with the other two groups (p < 0.05), confirming RDN's BP-lowering efficacy. In addition, no significant differences in SBP and DBP were observed between the sham operation and control groups after RD (p > 0.05) (Table 1).
Table 1.
SBP and DBP of each group during different periods before HS(mmHg), mean ± SD.
| Items | control |
sham |
study |
F | p | ||
|---|---|---|---|---|---|---|---|
| n = 16 | n = 15 | n = 14 | |||||
| preoperation | SBP | 223.46 ± 10.31 | 220.20 ± 9.00 | 222.43 ± 11.79 | 0.394 | 0.788 | 0.567 |
| DBP | 169.13 ± 16.93 | 178.99 ± 14.87 | 172.39 ± 13.57 | 2.310 | 0.151 | 0.572 | |
| 2 weeks | SBP | 221.44.±11.06 | 218.60 ± 10.06 | 202.07 ± 11.08‡ | 13.800 | <0.001∗ | <0.001† |
| DBP | 173.42 ± 10.58 | 171.72 ± 19.68 | 157.70 ± 18.91§ | 19.508 | <0.001∗ | <0.001† | |
| 4 weeks | SBP | 223.94 ± 8.02 | 218.33 ± 7.98 | 204.29 ± 12.34 | 16.478 | <0.001∗ | <0.001† |
| DBP | 182.77 ± 11.68 | 175.36 ± 6.75 | 148.96 ± 13.44 | 38.391 | <0.001∗ | <0.001† | |
| 6 weeks | SBP | 221.94 ± 10.27 | 220.60 ± 7.64 | 200.86 ± 12.18 | 19.595 | <0.001∗ | <0.001† |
| DBP | 176.62 ± 14.75 | 175.07 ± 12.48 | 140.11 ± 19.65 | 48.697 | <0.001∗ | <0.001† | |
| 8 weeks | SBP | 224.62 ± 12.46 | 220.60 ± 8.82 | 201.50 ± 12.73 | 16.924 | <0.001∗ | <0.001† |
| DBP | 176.01 ± 8.67 | 183.85 ± 15.46 | 138.56 ± 11.12 | 56.774 | <0.001∗ | <0.001† | |
Notes: ∗p < 0.05, compared with control, †p < 0.05, compared with sham-operation, which were both detected by Least Significant Difference t-test; ‡t = 23.975, p < 0.001, compared with same group before operation, §t = 13.104, p < 0.001, compared same group before operation, which were both detected by an paired sample t-test. SBP: systolic blood pressure; DBP: diastolic blood pressure; HS: hemorrhagic shock.
Effect of renal denervation on the serum norepinephrine concentration
There were no significant differences in the serum NE concentrations among the study, sham operation and control groups before RD (174.38 ± 12.25 ng/L vs. 169.17 ± 14.02 ng/L, p = 0.266 and 175.06 ± 10.9 ng/L, p = 0.882) (Fig. 3A). Three weeks after RD, the NE concentration in the study group, compared with the sham operation and control groups, was reduced significantly (77.66 ± 11.06 ng/L vs. 171.72 ± 11.47 ng/L, p < 0.001 and 177.17 ± 9.87 ng/L, p < 0.001) (Fig. 3A). In addition, there were no significant differences in the NE concentration between the sham operation and control groups (p > 0.05) (Fig. 3A). At the last time point after hemorrhagic shock, the serum NE concentration in the study group (n = 7) was significantly lower than those in the sham operation (n = 8) and control (n = 8) groups (119.25 ± 10.39 ng/L vs. 223.00 ± 8.99 ng/L, p < 0.001 and 224.14 ± 8.85 ng/L, p < 0.001) (Fig. 4A).
Fig. 3.
Effects of renal denervation (RD) on norepinephrine (NE) concentrations after RD. (A) NE concentrations in serum before and three weeks after RD. △: p < 0.05, vs control; □: p < 0.05, vs sham operation; *: p < 0.05, vs same group before operation. (B) NE concentrations in kidney ten weeks after RD. △: p < 0.05, vs control; □: p < 0.05, vs sham operation.
Fig. 4.
Effects of renal denervation (RD) on norepinephrine (NE) concentrations after hemorrhagic shock (HS). (A) NE concentrations in serum after HS. △: p < 0.05, vs control; □: p < 0.05, vs sham operation. (B) NE concentrations in kidney after HS. △: p < 0.05, vs control; □: p < 0.05, vs sham operation.
Effect of renal denervation on the concentration in the kidney tissue
After RD, the NE concentration in the kidney of the study group (n = 7) was significantly lower than those in the sham operation (n = 7) and control (n = 8) groups (80.37 ± 6.48 ng/L vs. 147.27 ± 5.81 ng/L, p < 0.001 and 150.26 ± 7.00 ng/L, p < 0.001) (Fig. 3B), thus indicating that the RD was effective. There were no significant differences in the NE concentration between the sham operation and control groups (p > 0.05) (Fig. 3B). After hemorrhagic shock, the NE concentration in the kidney of the study group (n = 7) was significantly lower than those in the sham operation group (n = 8) and the control (n = 8) group (101.08 ± 9.58 ng/L vs. 187.69 ± 10.50 ng/L, p < 0.001 and 181.20 ± 9.49 ng/L, p < 0.001) (Fig. 4B).
Effect of renal denervation on systolic blood pressure and diastolic blood pressure after hemorrhagic shock
Before hemorrhagic shock, the SBP and DBP in the study group were significantly lower than those in the other two groups (p < 0.05). At 15, 30 and 45 min after hemorrhagic shock, the SBP and DBP of the study group were significantly higher than those of the other two groups (p < 0.05), but no significant differences in the SBP and DBP were observed at 60, 90 and 120 min after hemorrhagic shock among the three groups (p > 0.05) (Table 2, Fig. 5).
Table 2.
SBP and DBP of each group during different periods after HS(mmHg), mean ± SD.
| Items | control n = 8 |
sham n = 8 |
study n = 7 |
F | p | ||
|---|---|---|---|---|---|---|---|
| preoperation | SBP | 141.25 ± 9.08 | 143.00 ± 12.06 | 111.14 ± 5.05 | 26.805 | <0.001∗ | <0.001† |
| DBP | 113.25 ± 7.92 | 109.75 ± 8.96 | 97.14 ± 7.84 | 7.865 | 0.001∗ | 0.008† | |
| 15 min | SBP | 62.38 ± 9.78 | 62.88 ± 7.02 | 79.86 ± 5.67 | 11.982 | <0.001∗ | <0.001† |
| DBP | 57.75 ± 8.05 | 52.63 ± 3.78 | 72.71 ± 8.00 | 17.089 | <0.001∗ | <0.001† | |
| 30 min | SBP | 61.13 ± 9.39 | 62.63 ± 7.67 | 80.00 ± 4.66 | 13.883 | <0.001∗ | <0.001† |
| DBP | 53.88 ± 5.84 | 50.50 ± 5.90 | 65.57 ± 4.43 | 15.282 | 0.001∗ | <0.001† | |
| 45 min | SBP | 61.13 ± 11.08 | 63.00 ± 9.64 | 80.29 ± 14.00 | 6.007 | 0.005∗ | 0.009† |
| DBP | 50.88 ± 9.60 | 49.25 ± 7.27 | 63.86 ± 3.13 | 8.733 | 0.003∗ | 0.001† | |
| 60 min | SBP | 58.75 ± 16.20 | 62.00 ± 13.71 | 70.86 ± 9.32 | 1.571 | 0.100 | 0.221 |
| DBP | 49.63 ± 11.14 | 46.13 ± 8.92 | 53.00 ± 5.03 | 1.122 | 0.471 | 0.150 | |
| 90 min | SBP | 53.29 ± 14.51 | 57.57 ± 10.78 | 63.14 ± 12.55 | 1.059 | 0.164 | 0.423 |
| DBP | 51.71 ± 7.78 | 41.71 ± 12.12 | 42.86 ± 13.56 | 1.608 | 0.164 | 0.854 | |
| 120 min | SBP | 52.00 ± 15.36 | 56.80 ± 11.45 | 64.60 ± 4.67 | 1.559 | 0.106 | 0.300 |
| DBP | 52.50 ± 6.00 | 50.50 ± 4.20 | 47.40 ± 8.36 | 0.514 | 0.332 | 0.589 | |
Notes: ∗p < 0.05, compared with control; †p < 0.05, compared with sham-operation, which were both detected by Least Significant Difference t-test. SBP: systolic blood pressure; DBP: diastolic blood pressure; HS: hemorrhagic shock.
Fig. 5.
Effects of renal denervation (RD) on systolic blood pressure (SBP) and diastolic blood pressure (DBP) after hemorrhagic shock (HS). (A) △: p < 0.05, vs control; □: p < 0.05, vs sham operation. (B) △: p < 0.05, vs control; □: p < 0.05, vs sham operation.
Effect of renal denervation on beta-1 adrenergic receptor expression before and after hemorrhagic shock
Different shades of brown yellow granules were observed in the immunohistochemically stained sections. The β1-AR expression in the study group was significantly higher than those in the other two groups (p < 0.05) after hemorrhagic shock, although the differences were not statistically significant before hemorrhagic shock (p > 0.05) (Table 3, Fig. 6).
Table 3.
The IOD of β1-AR in myocardium, mean ± SD.
| Items | n | IOD | F | p | |
|---|---|---|---|---|---|
| RD | control | 8 | 22169 ± 2882.391 | 0.015 | 0.780 |
| sham | 7 | 23043 ± 3972.917 | 0.839 | ||
| study | 7 | 22668 ± 3354.595 | |||
| RD + HS | control | 8 | 27534 ± 6913.565 | 20.192 | <0.001∗ |
| sham | 8 | 28538 ± 6252.929 | <0.001† | ||
| study | 7 | 51632 ± 1107.896‡ | |||
Notes: ∗p < 0.05, compared with RD + HS-control, †p < 0.05, compared with RD + HS-sham-operation, which were both detected by Least Significant Difference t-test. ‡t = −6.653, p < 0.001, compared with RD-study, which were detected by an paired sample t-test. IOD: integrated optical density; β1-AR: beta-1 adrenergic receptor; RD: renal denervation; HS: hemorrhagic shock.
Fig. 6.
Effects of renal denervation (RD) on β1-adrenergic receptor (β1-AR) expression in myocardium before and after hemorrhagic shock (HS). (A) RD-control; (B) RD-sham operation; (C) RD-study; (D) RD + HS-control; (E) RD + HS-sham operation; (F) RD + HS-study.
Discussion
Hemorrhage is a major cause of morbidity and mortality in both humans and animals.23 In response to hypovolemia, a major manifestation at the early stage of hemorrhagic shock, the neurohumoral mechanisms which including SNS and renin-angiotensin-aldosterone system (RAAS) would be activated to play a compensated role by increasing heart rate and peripheral vascular resistance. Besides, high-concentration catecholamines including NE are released into systemic circulation in hemorrhagic shock,24 and NE is the predominant one. Moreover, a large amount of NE is produced by sympathetic postganglionic fibers. Although animals respond to hemorrhage with several reactive types, the major pathway is the reflex activation of the sympathetic nerves to maintain arterial pressure and organ perfusion,25 and lack of renal sympathetic activation may affect restoration of BP in the short term following blood loss. The results of this study showed that SBP and DBP in the study group were significantly higher than those in the other two groups at 15, 30 and 45 min after hemorrhagic shock, indicating that from initiation time to 45 min during hemorrhagic shock, the BP response in the study group was better than those in the other two groups, while the serum and kidney NE concentrations were lower. Many findings had demonstrated the relative importance of renal nerves in the physiological response to hemorrhage. But Machino et al26 demonstrated that the RAAS was inhibited in SHR after RD. To this day, the mechanism contributing to this response when the two important compensatory mechanisms of SNS and RAAS are blocked remains to be answered.
Three subtypes of β-AR (β1-AR, β2-AR and β3-AR) have been identified in the heart. β1-AR and β2-AR subtypes are expressed as a ratio of 70:30 in normal.27, 28 In response to NE and epinephrine, stimulation of β1-AR has positive inotropic, chronotropic and lusitropic effects in the myocardium.29 In addition, inotropic treatment with the β1 adrenoceptor agonist dobutamine has been used to increase cardiac output in hemorrhagic shock. Dynamic regulation of β-AR subtype expression and functional desensitization are considered to be adaptive or protective mechanisms in the heart.30 For example, β1-AR is the predominant adult isoform (β1 vs. β2 59% vs. 41%), whereas β2-AR is more highly expressed in neonates (β1 vs. β2 36% vs. 64%) in rats.31 In addition, NE has higher affinity in combining with β1-AR than the other subtypes of adrenergic receptors. Moreover, in certain pathophysiological conditions, β-AR expression is subtype-selective in the heart. For instance, β2-AR is up-regulated in the transplanted human heart,32 but down-regulated in chronic heart failure.18 Acute hypovolemia in hemorrhagic shock would result in acute myocardial infarction, as observed in the present immunohistochemistry of some myocardium tissues from SHR. Acute myocardial infarction is a type of acute coronary syndrome that can decrease the cardiac output as a result of impaired cardiac pump function. To compensate for the decreased cardiac output, one of the reactions in the infarcted myocardium is to up-regulate β1-AR expression17 rather than any other types of β-AR. The results of our study confirmed the above illustration. Watanabe et al33 reported that in Dahl salt sensitive hypertensive rats, RD significantly prolonged survival, and increased tyrosine hydroxylase and β1-adrenergic receptors in the left ventricular myocardium. In the present study the β1-AR expression in the study group was significantly higher than those in the other two groups (p < 0.05) after hemorrhagic shock. So we hypothesized that up-regulation of β1-AR may increase the cardiac contractility, frequency and rate and improve BP response against hemorrhagic shock following RD. This study does not provide explanation of this issue. It was found in our study that there was no significant difference in SBP and DBP at 60, 90 and 120 min after hemorrhagic shock between the three groups. To our knowledge, hemodynamic and sympathetic responses to acute hemorrhage represent two distinct phases: in the initial phase, the sympathetic nerve activity and heart rate are increased to maintain the BP, recovery of the circulation volume can prevent the situation to be exacerbation in this phase. When hemorrhage continues to evolve into the next phase, decreases in renal sympathetic nerve activity and heart rate follow. We speculated that it may evolve into the irreversible period from 60 min after hemorrhagic shock in the present study. As BP was progressively decreased, the seriously insufficient perfusion of systemic microcirculation could cause cardiovascular collapse and death.
This study is the first to focus on BP and β1-AR change after RDN, in response to a common trauma stress induced by hemorrhagic shock. To this day, the mechanism of RD in improving BP response to hemorrhagic shock is still unclear. One of the possible mechanisms, except β1-AR accounting for the better compensation initiated by RD, may be regeneration of renal sympathetic nerves. There is considerable evidence that efferent renal sympathetic nerves reinnervate the kidney after RD in animals and humans. Therefore, the long-term reduction in arterial pressure has been related with lack of afferent renal sensory reinnervation.34 However, other available data shows that renal reinnervation will take months to year(s) in humans versus weeks in animals. In normotensive rats, reinnervation of the afferent renal sensory nerves and the efferent renal sympathetic nerves occurs over the same time course, both being complete at 9–12 weeks after RD.35 Similarly, report of Booth et al36 showed the reinnervation of renal afferent and efferent nerves at 5.5 months after catheter-based radiofrequency renal denervation in sheep and by 11 months the functional afferent and efferent responses to electric stimulation were normal.
Several limitations need to be recognized in our study. First, the sample size is limited, which limits the statistical effectiveness and further observation of β1-AR changes in heart at the early stage of hemorrhagic shock. Further studies with larger sample sizes are warranted to more definitively determine the relation between β1-AR and BP. In addition, the experiment only involved changes in macro indicators including NE and BP, and failed to further explain the possible mechanisms from molecular biology. As in all experimental animal studies, the relevance of the findings to the clinical situation is unclear, but as most human hemorrhagic shock is hyperdynamic in nature it is likely that this model has clinical relevance. It also indirectly indicates the relative safety of RD.
In conclusion, this study demonstrated that RD could to some extent improve BP response to hemorrhagic shock in an established model of severe hemorrhagic shock in SHR. The mechanism might be associated with up-regulation of β1-AR. This study would offer a theoretical basis for the intensive study on safety and efficacy of clinical renal arteries ablation in the treatment of hypertension. Also this may provide a valuable time for the rescue of patients with hemorrhagic shock after RD, which can prolong the therapeutic time window of the shock compensatory period and improve their prognosis.
Acknowledgements
The authors would like to thank Fang Mingsun and other teachers from the experimental animal center of Zhejiang Medical University for their invaluable assistance during the experiment.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjtee.2018.09.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zambon A., Arfè A., Corrao G. Relationships of different types of event to cardiovascular death in trials of antihypertensive treatment: an aid to definition of total cardiovascular disease risk in hypertension. J Hypertens. 2014;32:495–508. doi: 10.1097/HJH.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 2.Epstein M. Resistant hypertension: prevalence and evolving concept. J Clin Hypertens. 2007;9:2–6. doi: 10.1111/j.1524-6175.2007.06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorostidi M., Banegas J.R., De la Sierra A. Ambulatory blood pressure monitoring in daily clinical practice-the Spanish ABPM Registry experience. Eur J Clin Invest. 2016;46:92–98. doi: 10.1111/eci.12565. [DOI] [PubMed] [Google Scholar]
- 4.Kumbhani D.J., Steg P.G., Cannon C.P. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34:1204–1214. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 5.Manolis A.J., Poulimenos L.E., Kallistratos M.S. Sympathetic overactivity in hypertension and cardiovascular disease. Curr Vasc Pharmacol. 2014;12:4–15. doi: 10.2174/15701611113119990140. [DOI] [PubMed] [Google Scholar]
- 6.Dibona G.F. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 7.Doroszko A., Janus A., Szahidewicz-Krupska E. Resistant Hypertension. Adv Clin Exp Med. 2016;25:173–183. doi: 10.17219/acem/58998. [DOI] [PubMed] [Google Scholar]
- 8.Krum H., Schlaich M., Whitbourn R. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 9.Azizi M., Sapoval M., Gosse P. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 10.Mahfoud F., Bohm M., Azizi M. Proceedings from the European clinical consensus conference for renal denervation:considerations on future clinical trial design. Eur Heart J. 2015;36:2219–2227. doi: 10.1093/eurheartj/ehv192. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Wu N., Yan W. The effects of renal denervation on resistant hypertension patients: a meta-analysis. Blood Press Monit. 2016;21:206–214. doi: 10.1097/MBP.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 12.Vuignier Y., Grouzmann E., Mullerb O. Blood pressure and renal responses to orthostatic stress before and after radiofrequency renal denervation in patients with resistant hypertension. Front Cardiovasc Med. 2018;5:42. doi: 10.3389/fcvm.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Investigators HTN-2 Symplicity, Esler M.D., Krum H. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 14.Fu Q., Xiang Y.K. Trafficking of β-adrenergic receptors: implications in intracellular receptor signaling. Prog Mol Biol Transl Sci. 2015;132:151–188. doi: 10.1016/bs.pmbts.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodde O.E., Bruck H., Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. J Pharmacol Sci. 2006;100:323–337. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaev V.O., Boivin V., Störk S. A novel fluorescence method for the rapid detection of functional ß1-adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol. 2007;50:423–431. doi: 10.1016/j.jacc.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Ihl-Vahl R., Marquetant R., Bremerich J. Regulation of beta-adrenergic receptors in acute myocardial ischemia: subtype-selective increase of mRNA specific for beta 1-adrenergic receptors. J Mol Cell Cardiol. 1995;27:437–452. doi: 10.1016/s0022-2828(08)80040-5. [DOI] [PubMed] [Google Scholar]
- 18.Brodde O.E. Pathophysiology of the beta-adrenoceptor system in chronic heart failure: consequences for treatment with agonists, partial agonists or antagonists? Eur Heart J. 1991;12:54–62. doi: 10.1093/eurheartj/12.suppl_f.54. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W., Guo Y., Tan L. Impact of renal denervation on renalase expression in adult rats with spontaneous hypertension. Exp Ther Med. 2012;4:493–496. doi: 10.3892/etm.2012.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pires N.M., Igreja B., Moura E. Blood pressure decrease in spontaneously hypertensive rats folowing renal denervation or dopamine β-hydroxylase inhibition with etamicastat. Hypertens Res. 2015;38:605–612. doi: 10.1038/hr.2015.50. [DOI] [PubMed] [Google Scholar]
- 21.Taguchi K., Maruyama T., Iwao Y. Pharmacokinetics of single and repeated injection of hemoglobin-vesicles in hemorrhagic shock rat model. J Control Release. 2009;136:232–239. doi: 10.1016/j.jconrel.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi K., Ogaki S., Watanabe H. Fluid resuscitation with hemoglobin vesicles prevents escherichia coli growth via complement activation in a hemorrhagic shock rat model. J Pharmacol Exp Ther. 2011;337:201–208. doi: 10.1124/jpet.110.177832. [DOI] [PubMed] [Google Scholar]
- 23.Hall K.E., Holowaychuk M.K., Sharp C.R. Multicenter prospective evaluation of dogs with trauma. J Am Vet Med Assoc. 2014;244:300–308. doi: 10.2460/javma.244.3.300. [DOI] [PubMed] [Google Scholar]
- 24.Nakai M., Kawamura M., Kunieda T. Intrapulmonary bronchial circulation during hemorrhage. Heart Ves. 1991;6:90–95. doi: 10.1007/BF02058754. [DOI] [PubMed] [Google Scholar]
- 25.Malpas S.C., Evans R.G., Head G.A. Lukoshkova contribution of renal nerves to renal blood flow variability during hemorrhage. Am J Physiol. 1998;274:R1283–R1294. doi: 10.1152/ajpregu.1998.274.5.R1283. [DOI] [PubMed] [Google Scholar]
- 26.Machino T., Murakoshi N., Sato A. Anti-hypertensive effect ofradiofrequency renal denervation in spontaneously hypertensive rats. Life Sci. 2014;110:86–92. doi: 10.1016/j.lfs.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Port J.D., Bristow M.R. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- 28.Steinfath M., Lavicky J., Schmitz W. Regional distribution of beta 1- and beta 2-adrenoceptors in the failing and nonfailing human heart. Eur J Clin Pharmacol. 1992;42:607–611. doi: 10.1007/BF00265923. [DOI] [PubMed] [Google Scholar]
- 29.Femminella G.D., Barrese V., Ferrara N. Tailoring therapy for heart failure: the pharmacogenomics of adrenergic receptor signaling. Pharmgenomics Pers Med. 2014;7:267–273. doi: 10.2147/PGPM.S49799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Wang B., Cui H. let-7e replacement yields potent anti-arrhythmic efficacy via targeting beta 1-adrenergic receptor in rat heart. J Cell Mol Med. 2014;18:1334–1343. doi: 10.1111/jcmm.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morisco C., Zebrowski D.C., Vatner D.E. Beta-adrenergic cardiac hypertrophy is mediated primarily by the b1-subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–573. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 32.Farrukh H.M., White M., Port J.D. Up-regulation of beta 2-adrenergic receptors in previously transplanted, denervated nonfailing human hearts. J Am Coll Cardiol. 1993;22:1902–1908. doi: 10.1016/0735-1097(93)90777-x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H., Iwanaga Y., Miyaji Y. Renal denervation mitigates cardiac remodeling and renal damage in Dahl rats: a comparison with β-receptor blockade. Hypertens Res. 2016;39:217–226. doi: 10.1038/hr.2015.133. [DOI] [PubMed] [Google Scholar]
- 34.Mulder Jan, Hökfelt Tomas, Mark M. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R675–R682. doi: 10.1152/ajpregu.00599.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodionova K., Fiedler C., Guenther F. Complex reinnervation pattern after unilateral renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R806–R818. doi: 10.1152/ajpregu.00227.2014. [DOI] [PubMed] [Google Scholar]
- 36.Booth L.C., Nishi E.E., Yao S.T. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 Months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 2015;65:393–400. doi: 10.1161/HYPERTENSIONAHA.114.04176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.