Abstract
Colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women, worldwide. In the early stages of the disease, biomarkers predicting early relapse would improve survival rates. In metastatic patients, the use of predictive biomarkers could potentially result in more personalized treatments and better outcomes. The CXC family of chemokines (CXCL1 to 17) are small (8 to 10 kDa) secreted proteins that attract neutrophils and lymphocytes. These chemokines signal through chemokine receptors (CXCR) 1 to 8. Several studies have reported that these chemokines and receptors have a role in either the promotion or inhibition of cancer, depending on their capacity to suppress or stimulate the action of the immune system, respectively. In general terms, activation of the CXCR1/CXCR2 pathway or the CXCR4/CXCR7 pathway is associated with tumor aggressiveness and poor prognosis; therefore, the specific inhibition of these receptors is a possible therapeutic strategy. On the other hand, the lesser known CXCR3 and CXCR5 axes are generally considered to be tumor suppressor signaling pathways, and their stimulation has been suggested as a way to fight cancer. These pathways have been studied in tumor tissues (using immunohistochemistry or measuring mRNA levels) or serum [using enzyme-linked immuno sorbent assay (ELISA) or multiplexing techniques], among other sample types. Common variants in genes encoding for the CXC chemokines have also been investigated as possible biomarkers of the disease. This review summarizes the most recent findings on the role of CXC chemokines and their receptors in CRC and discusses their possible value as prognostic or predictive biomarkers as well as the possibility of targeting them as a therapeutic strategy.
Keywords: Biomarkers, Treatment, Chemotherapy, Oxaliplatin, Irinotecan, Immunotherapy, Colorectal cancer, CXC chemokines, Immune system, Bevacizumab
Core tip: The contribution of the immune system to the development and progression of cancer is now fully acknowledged. The specific action of the immune system depends on the type of immune cells that are recruited to the tumor sites. Chemokines from the CXC subfamily are released by tumor cells and cells within the tumor microenvironment, whereupon they attract cells with anti-tumor (e.g., CD4+ and CD8+ lymphocytes) or pro-tumor activity (e.g., myeloid-derived suppressor cells). Chemokines have been proposed as prognostic factors, as biomarkers of response to therapy and as drug targets. The present review addresses the most recent findings in the field.
INTRODUCTION
Colorectal cancer (CRC) is the third most common type of cancer worldwide, representing approximately 10% of all diagnosed cancers, and it is the most frequent cause of cancer-related deaths[1]. In Europe, the prognosis of localized disease is quite good; the 5-year overall survival rates are near 90%; however, in patients with regional or distant disease, these rates drop to 70% and down to 20%, respectively (source: Europacolon, http://www.europacolon.com/colorectalcancer.php?Action=Colorectalcancer)[2] . One of the main reasons behind this poor survival rate is that, in almost all cases, tumors become resistant to therapy. Therefore, it is necessary to find useful and reliable predictive biomarkers that allow physicians to assign effective drugs specific to each case. In general, the current first-line treatment for metastatic CRC (mCRC) is based upon combinations of chemotherapy [fluoropyrimidines, oxaliplatin (OXA) and/or irinotecan], consisting of doublets (FOLFOX or FOLFIRI) or triplets (FOLFOXIRI) in specific cases, and rational molecularly targeting agents [anti-epidermal growth factor receptor (anti-EGFR), cetuximab or panitumumab; anti-vascular endothelial growth factor (anti-VEGF), bevacizumab][3]. Mutations in the RAS and BRAF family of oncogenes are indicative of the inefficacy of anti-EGFR drugs and are the only predictive markers available currently[4,5]. The recent classification of CRC into four molecular subtypes has shed light on the biology of this tumor. However, the clinical usefulness and application of this classification is still under discussion[6,7].
It has been proposed that serum levels of certain chemokines may be used as predictive markers for the response to chemotherapy. The CXC family of chemokines and their receptors are crucial for inflammation and antitumor immunity, which are key factors in CRC progression. These small proteins are secreted not only by tumor cells but also by leukocytes, fibroblasts, endothelial cells and epithelial cells. They modulate tumor behavior by regulating angiogenesis, activating tumor-specific immune responses and directly stimulating tumor proliferation in an autocrine or paracrine fashion. The CXC family of chemokines and receptors have been associated with metastasis and resistance to treatment. Several studies have reported the expression of CXC chemokines and/or their receptors in tumors (in epithelial tumor cells, fibroblasts or infiltrating leukocytes) or in plasma/serum samples from CRC patients, and this expression has been associated with patient outcomes. Furthermore, the CXC genes are highly polymorphic, and certain genetic variants have been associated with prognosis and response to treatment. The aim of the present work is to review the literature and discuss the possible use of the CXC family of chemokines as predictive or prognostic markers in CRC (Table 1). We will focus on the main pathways that are activated after the appropriate chemokines bind to the receptors CXCR1, CXCR2, CXCR4, CXCR7, CXCR3, CXCR5, CXCR6 and CXCR8.
Table 1.
CXC chemokines as biomarkers
| Biomarker | Cancer type | Expression | Where is it measured? | Treatment | Predictive value | Ref. |
| CXCL1/2 | Breast cancer | High | Paraffin embedded tissue | Chemoresistance 5FU | Poor | [17] |
| CXCL1 | CRC | High | Tumor and cell lines | OXA + CURCUMIN | Poor | [18] |
| CXCL1/8 CXCR2 | CRC, Prostate cancer | High | Cell lines and in vivo | OXA + SCH-527123 | Poor | [22,24] |
| CXCL8 | CRC | Low | Serum | Chemotherapy + Bevacizumab | Good | [25,26] |
| CXCL8 | Breast cancer | Polymorphism, TT genotype | Peripheral blood (DNA) | FOLFOX+ Bevacizumab vs FOLFOX alone | Good | [26] |
| CXCR1 | CRC | Polymorphism | Whole blood (DNA) | OXA based + Bevacizumab | Good | [27] |
| CXCL7/8 CXCR2 | CRC | High | mRNA levels tumors | Surgery + ady vs Ady alone | Poor | [28] |
| CXCR4 | CRC | Colocalization-Lgr5 | CRC cell lines and tumors | Poor | [35] | |
| CXCR4 | CRC | Colocalization-CD133 | CRC cell lines and in vivo | 5FU; 5FU and OXA | Poor | [36,38] |
| CXCR4-microbiota | CRC | High | in vivo and in vitro | Lipopolysaccharides | Poor | [39] |
| CXCL12-visfatin | CRC | Interaction | CRC cells | Poor | [40] | |
| CXCR4 | CRC | High | Paraffin embedded tissue | Patients that underwent hepatectomy | Poor | [41] |
| CXCR4 | CRC | High | CRC cells | OXA or 5FU therapies including anti-VEGF | Poor | [46] |
| CXCL10-KRAS mut | CRC CMS2 and CMS3 | Low | TCGA, tumors | Poor | [52] | |
| CXCL10 | CRC | High | CRC cells, tumors, in vivo | Good | [53] | |
| CXCL10 | CRC | Expression | Post-surgical localized CRC | Good | [53,56] | |
| CXCR3 | CRC | High | Protein levels in primary tumors | Poor | [57] | |
| CXCL10/11 | CRC | High | Serum | Poor | [58] | |
| CXCL9/10 | CRC | High | in vivo | Anthracyclines + STAT KO | Good | [60] |
| CXCL9/10/11 | Rectal cancer | High | mRNA levels tumors | 5FU + Radiotherapy | Good | [64,65] |
| CXCL4 | CRC | High | in vivo | 5FU | Poor | [66] |
| CXCL13 CXCR5 | CRC | High | Plasma levels and paraffin embedded tissue | Poor | [69,70] | |
| CXCL13-microbiota | CRC | High | Tumors | Good | [71] | |
| CXCR5+ CD8+ T cell | CRC | Presence | Tumors | Good | [73] | |
| CXCL13 | CRC | Low | mRNA levels tumors | Good | [56,74] | |
| CXCL16 | CRC | High | Serum | Poor | [81] | |
| CXCL17 | CRC | High | mRNA levels cell lines and tumors | Poor | [87] |
CRC: Colorectal cancer; OXA: Oxaliplatin; VEGF: Vascular endothelial growth factor; 5FU: 5-fluorouracil; KO: Knock out.
LITERATURE SEARCH
We established the work of Verbeke et al[8] as reference from which we searched the literature. We used PubMed as the primary source to find all of the articles published on this topic. The following keywords were employed in the search: chemokine/s, resistance, CRC, chemotherapy, 5 fluorouracil, OXA, cetuximab, bevacizumab, CXCL1, CXCL2, CXCL3, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8, CXCL9, CXCL10, CXCL11, CXCL12, CXCL13, CXCL16, CXCL17, CXCR1, CXCR2, CXCR4, CXCR2 inhibitor, CXCR4 inhibitor, reparixin, cancer, repertaxin, and plerixafor. In addition to PubMed, we also performed a brief Google search for “Chemokine CRC”.
THE CXC FAMILY OF CHEMOKINES
CXC chemokines are small proteins; CXC refers to the location of the two cysteine residues near the N-terminal, with the X representing any amino acid (cysteine-containing motif)[9]. These chemokines can be sub-classified based on the presence or absence of the tripeptide motif (Glu-Leu-Arg at the NH2 terminus) as ELR+ or ELR-, respectively. The ELR+ chemokines are considered to be angiogenic, whereas the ELR- chemokines are considered to be angiostatic[10-12]. Nevertheless, contradictory results have been published, which we will discuss in the present review.
CXC chemokines are named according to their original function, and they also follow the standard nomenclature CXCLx, where the L stands for “ligand” and the x corresponds to a number (Table 2). To exert their function, chemokines bind to their cognate CXCR which, in the majority of cases, is a 7-transmembrane G protein-coupled receptor[13]. After binding, the receptors change their conformation and activate the coupled G protein, initiating the corresponding signaling pathway. Only a few of the CXCRs are atypical receptors, which are not coupled to a G protein but to beta-arrestins; these receptors include the atypical chemokine receptor 3 (ACKR3) also known as CXCR7[14]. Certain chemokines can bind to more than one receptor and vice versa, as is the case for the promiscuous CXCR2[15] (Table 2). After a given CXCL binds and activates the corresponding CXCR, the receptor is usually internalized through clathrin-mediated endocytosis for further degradation or recycling to the plasma membrane[16] (Figure 1).
Table 2.
The CXC chemokines and their receptors
| CXC ligand | Chemokine name | Receptor | ELR domain |
| CXCL1 | Growth-regulated oncogene (Gro-α) | CXCR2 | ELR+ |
| CXCL2 | Growth-regulated oncogene (Gro-β) | CXCR2 | ELR+ |
| CXCL3 | Growth-regulated oncogene (Gro-γ) | CXCR2 | ELR+ |
| CXCL4 | Platelet factor-4 (PF-4) | CXCR3 | ELR- |
| CXCL5 | Epithelial cell-derived neutrophil-activating peptide-78 (ENA-78) | CXCR2 | ELR+ |
| CXCL6 | Granulocyte chemotactic protein-2 (GCP-2) | CXCR1/2 | ELR+ |
| CXCL7 | Neutrophil-activating peptide-2 (NAP-2) | CXCR2 | ELR+ |
| CXCL8 | Interleukin-8 (IL-8) | CXCR1/2 | ELR+ |
| CXCL9 | Monokine induced by interferon-γ (Mig) | CXCR3 | ELR- |
| CXCL10 | Inducible protein-10 (IP-10) | CXCR3 | ELR- |
| CXCL11 | Interferon-inducible T cell alpha chemoattractant (I-TAC) | CXCR3/7 | ELR- |
| CXCL12 | Stromal cell-derived factor-1 (SDF-1) | CXCR4/7 | ELR- |
| CXCL13 | B cell-attracting chemokine-1 (BCA-1) | CXCR5 | ELR- |
| CXCL16 | Scavenger receptor that binds phosphatidylserine and oxidized lipoprotein (SR-PSOX) | CXCR6 | ELR- |
| CXCL17 | VEGF-correlated chemokine-1 (VCC-1) | CXCR8 | ELR+ |
VEGF: Vascular endothelial growth factor.
Figure 1.
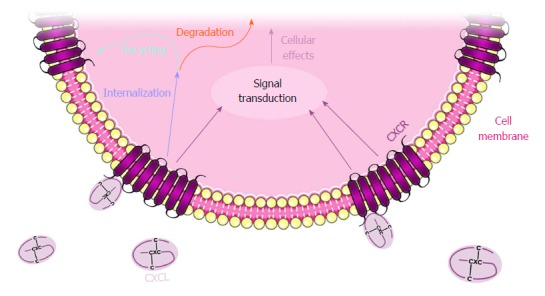
CXCL and CXCR regulatory mechanisms. Figure 1 illustrates CXCL binding to the CXCR located at the cellular membrane, internalizing and transducing the signal into the nucleus. CXCR internalization is usually followed by degradation or recycling to the plasma membrane.
Originally, the role of these chemokines and their receptors was associated with inflammatory processes wherein leukocytes accumulate in injured or infected tissues. Given that inflammation is a hallmark of cancer, it is not surprising that once in the nucleus, the CXC chemokines exert their activity through various pathways, such as tumor necrosis factor alpha (TNF-α)/nuclear factor κB (NF-κB) and protein kinase B (PKB/AKT)[17,18]. These pathways are related to survival, proliferation, angiogenesis, pathological processes such as tumor growth and metastasis[19,20], and even responses to anticancer treatments (Figure 2).
Figure 2.
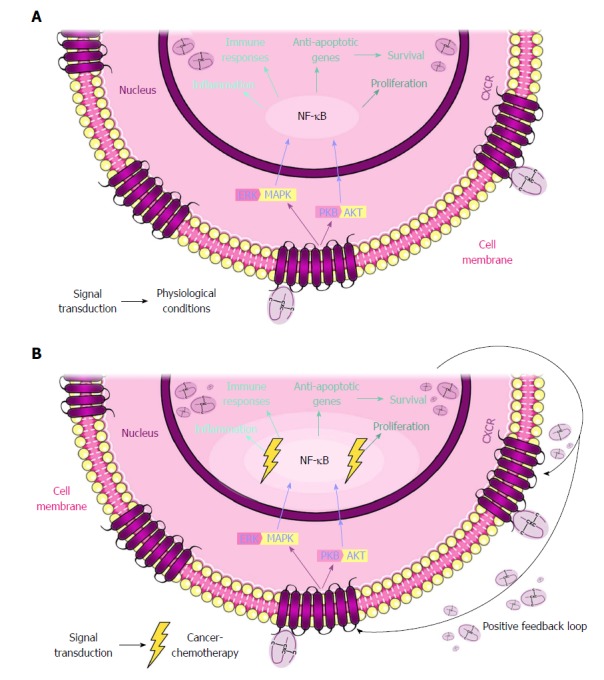
CXCL and CXCR network in physiological and cancer conditions. A: The binding of CXCL to CXCR triggers the activation of signaling pathways, such as PKB/AKT and ERK/MAPK. Both pathways share NF-κB as a downstream factor which, in the nucleus, promotes the transcription of the CXCLs. These pathways maintain the physiologic conditions inside the cell. B: In a cancer cell exposed to chemotherapy, the situation is different. The chemotherapeutic treatment promotes the hyperactivation of these pathways. As a result, the CXCLs are also more abundant, promoting an autocrine response and, further, a positive feedback loop. This response results in pathological processes, such as tumor growth and metastasis, and in resistance to anticancer treatments. PKB: Protein kinase B; ERK: Extracellular signal-regulated kinases; MAPK: Mitogen-activated protein kinase; NF-κB: Nuclear factor κB.
AXIS 1: CXCR1 AND CXCR2
The CXCR1/CXCR2 pathway is the most widely studied pathway in cancer. CXCL1, 2, 3 and 8 are proangiogenic and chemoattractive chemokines that all bind to the receptor CXCR2, for which CXCL1 has the highest affinity. All four chemokines have been found to be upregulated in CRC[21]. One of the most relevant studies demonstrating the role of CXCL1 and 2 in treatment resistance and metastasis was performed by the group of Joan Massagué. This group showed that CXCL1 and CXCL2 are overexpressed in breast cancer cells that are primed for survival in metastatic sites. These two chemokines attract CD11b+Gr1+myeloid cells which, in turn, produce more chemokines and enhance cancer cell survival. Regarding chemoresistance, this group found that chemotherapeutic agents, such as 5 Fluorouracil (5FU), doxorubicin hydrochloride, cyclophosphamide monohydrate and paclitaxel trigger a stromal reaction, leading to TNF-α production. TNF-α increases CXCL1/2 expression via NF-κB, which results in a feedback loop that promotes cancer cell survival[17]. NF-κB is a transcription factor that can be activated downstream of the PKB/AKT and mitogen-activated protein kinase (MAPK) signaling pathways. Increased activity of NF-κB has been associated with the high expression of genes related to cancer cell survival, proliferation, angiogenesis, and also the development of resistance to anticancer drugs (Figure 2). As CXCL1 and 2 (and other chemokines that bind to CXCR1 and 2) can activate this pathway, it is not surprising that these chemokines play a role in chemoresistance and may also serve as predictive biomarkers in CRC.
For instance, we reported that CRC cells with acquired resistance to OXA exhibited high intracellular and secreted levels of CXCL1, 2 and 8, which was a consequence of the upregulated activation of NF-κB in the resistant cells. Both the inhibition of NF-κB with curcumin and the silencing of genes encoding for these chemokines led to a partial reversion of the resistance phenotype. The combination of curcumin and OXA was especially synergistic in resistant cells. Moreover, in experiments with tissue explants derived from hepatic CRC metastasis, we determined that higher levels of CXCL1 led to higher sensitivity of explants to the combination of curcumin + OXA. Thus, the tumor or serum levels of CXCL1 may serve as a useful biomarker for OXA resistance and may be indicative of curcumin + OXA sensitivity[18]. In another study, Ning et al[22] reported that CXCL8 mediated resistance to OXA in CRC cell lines through its binding to CXCR2 and AKT/MAPK/NF-κB signaling. The gene silencing of CXCR2 sensitized cells to the platinum drug. Treatment of CRC cell lines with a CXCR2 antagonist (SCH-527123) resulted in decreased migration and invasion, and increased apoptosis, acting synergistically with OXA. Further work performed in vivo demonstrated similar results[23]. These results, together with those reported by Wilson et al[24] in prostate cancer cells, suggest that the levels of at least CXCL1 and 8 (and also CXCR2) can identify CRC patients who are unlikely to respond to OXA, who may be putative candidates for treatment with CXCR2 inhibitors in combination with this platinum agent.
The role of the CXCR1/CXCR2 pathway in the response and resistance to chemotherapy is not limited to OXA. Due to the role of these chemokines and receptors in promoting angiogenesis, it has been speculated that they can influence the response and resistance to antiangiogenic drugs. Two works reported that low serum levels of CXCL8 in mCRC patients treated with chemotherapy plus bevacizumab had better outcomes. First, in a short communication from Abajo et al[25], low levels of CXCL8 measured before treatment with bevacizumab, irinotecan and gemcitabine, were associated with better response rates in a small cohort of patients. This group also reported a general decrease in the levels of CXCL8 in post-treatment samples[25]. In another study, Di Salvatore et al[26] analyzed the serum levels of CXCL8 in 120 RAS mutant patients treated with first-line 5FU + Leucovorin + OXA (known as FOLFOX) + bevacizumab. They found that low levels of CXCL8 were associated with longer progression-free (PFS) and overall survival (OS). The same group also studied a polymorphism (c-251T>A) in the CXCL8 gene and reported that the TT genotype was associated with longer PFS and OS in the aforementioned patients but not in another cohort that was treated only with FOLFOX. Interestingly, patients carrying the TT genotype had significantly lower serum levels of CXCL8 compared with patients with the AT or AA genotype[26]. Another polymorphism (rs2234671) in the CXCR1 gene was found to be associated with the overall response rate in 132 mCRC patients treated with OXA-based chemotherapy plus bevacizumab. However, the authors did not provide a biological explanation underlying these results[27]. Two methods of measuring chemokines and their receptors include the immunohistochemical staining of proteins in tumor tissues and the qPCR evaluation of mRNA levels. Using these techniques, Desurmont et al[28] determined that high levels of CXCR2 and its ligands, CXCL7 and 8, were associated with worse PFS and OS in CRC patients who underwent neoadjuvant chemotherapy (different schedules) followed by metastatic hepatectomy, but not in those who were treated with surgery alone. CXCR2 and CXCL7 mRNA levels were higher in metastasis from chemotherapy-treated patients compared to metastasis from patients who underwent surgery alone. These data suggest not only the prognostic value of CXCR2 (and CXCL7) but also its involvement in disease aggressiveness. Therefore, targeting the CXCR1/2-mediated signaling pathway has been suggested as a possible therapeutic approach for treating CRC and other tumors.
As the CXCR1/2-mediated signaling pathway is associated with inflammation, several studies have described how the inhibition of CXCR1 or CXCR2 could potentially be used for preventive purposes[29-31]. Notably, the study by Jamieson et al[31] showed that CXCR2 acts as a potent pro-tumorigenic CXCR which directs the recruitment of tumor-promoting leukocytes into tissues (including the intestines) during tumor-inducing and tumor-driven inflammation. Thus, the deletion or inhibition of this receptor led to the profound suppression of inflammation-driven and spontaneous tumorigenesis in a mouse model of colon cancer, suggesting that CXCR2 antagonists may have therapeutic and prophylactic potential in the treatment of cancer[31]. Additionally, in the metastatic setting, it has been shown that treatment with inhibitors of this pathway may be a good strategy[23,32-34]. Interestingly, the use of two different inhibitors against CXCR1 and 2 reduced the development of metastasis in mouse models but had no effect on primary tumors. This effect was due, in part, to the capacity of the inhibitors to abrogate neovascularization[15].
AXIS 2: CXCR4 AND CXCR7
The CXCR4/CXCR7 pathway is activated after the binding of CXCL12 to CXCR4 or 7. Strikingly, although CXCL12 is an ELR- chemokine, the activity of this pathway has been primarily associated with stemness, the development of metastasis and poor prognosis. CXCR4 has been shown to co-localize with CRC stem cell markers, such as Lgr5, CD133 and CD44, and this co-localization was associated with epithelial-mesenchymal transition (EMT) processes and resistance to therapy[35-38]. Similar to CXCR1/2, the NF-κB transcription factor appears to have a role in the induction of EMT. In this case, it has been shown that lipopolysaccharides can promote the migratory capacity of CRC in vivo and in vitro by inducing CXCR4 expression and EMT through NF-κB signaling[39]. Additionally, the link between obesity and CRC has been attributed to the interaction between the adipokine visfatin and CXCL12[40]. CXCR4 expression appears to be increased in tumors compared with normal tissues and in metastasis compared with primary tumors. This increase is associated with poor prognosis, as shown in the work by Yopp et al[41]. This group assessed CXCR4 (among other factors) expression by immunohistochemical staining in 75 patients who underwent partial hepatectomy with curative intention and found that CXCR4 positivity was negatively associated with disease-specific and recurrence-free survival[41]. This finding suggests the possibility of targeting CXCR4 for therapeutic purposes. A lentiviral vector carrying siRNA targeting CXCR4 was used in CRC cell lines and a mouse model; the result was a reduction in cell proliferation, migration and invasion and the formation of hepatic metastasis[42]. Other researchers are attempting to exploit the membrane localization of CXCR4 to direct nanoparticles that recognize this receptor, facilitating their entry into the cells to deliver antineoplastic drugs[43]. Specific inhibitors, such as plerixafor, have been identified as a possible therapy for CRC and are under investigation in several clinical trials involving solid and non-solid tumors. Regardless of the strategy, it is important to take into account the biology of CXCR4 and 7. Two studies reported inconsistent regulation and effects between these two receptors. Romain et al[44] reported elevated levels of both receptors in late stage carcinomas compared to normal mucosa, colon polyps or early carcinomas. However, this group demonstrated that while CXCR4 expression was strongly induced by hypoxia-inducible factor 1 alpha (HIF-1α), this was not the case of CXCR7. Moreover, the reduction of CXCR4 expression was associated with a decrease in AKT and extracellular signal-regulated kinases (ERK) activation which, was not observed for CXCR7. The authors conclude that CXCR4 is a more promising target compared to CXCR7 in CRC[44]. Similarly, Heckmann and colleagues showed different (and partly antidromic) patterns of expression between CXCR7 and CXCR4 after stimulation with CXCL12 in colon cancer cells. CXCR4 overexpression was associated with increased levels of microRNA-217 and -218 in CXCR4-overexpressing cells, but this was not the case for the overexpression of CXCR7. Additionally, CXCR4 cells were more sensitive to 5FU compared with CXCR7 cells[45]. Thus, the clinical application of CXCR4 antagonists, such as plerixafor, should be studied with caution. Finally, the relationship between this pathway and the resistance to treatment of CRC has also been studied. High levels of CXCR4 were found to be associated with resistance to all chemotherapeutic agents used (5FU, irinotecan and OXA), including anti-VEGF therapies. In SW1116 colon cancer cells with acquired resistance to OXA or 5FU, the expression of and signaling through CXCR4 was found to be associated with the chemoresistant phenotype. Specifically, the authors demonstrated that the ERK1/2/MAPK and phosphatidylinositol-3-kinases (PI3K)/AKT pathway was important in CXCR4-mediated chemoresistance[46]. The effect of CXCR4 on resistance to OXA can be exploited therapeutically by using endostar, a modified endostatin. When endostar and OXA are administered together, they have a synergistic effect, primarily due to the reduction in CXCR4 levels[47]. In two different orthotopic mouse models, treatment with anti-VEGFR2 therapy resulted in the upregulation of both CXCL12 and CXCR4. CXCR4-expressing immunosuppressive innate immune cells (Ly6low monocytes) were recruited to the tumor site upon treatment with anti-VEGFR2, which was abrogated after CXCR4 blockade[48].
AXIS 3: CXCR3
The chemokines CXCL4, CXCL9, CXCL10 and CXCL11 are considered to exert an angiostatic effect through their binding to CXCR3, although CXCL11 can also bind to CXCR7[49]. CXCR3 is found on peripheral blood activated T cells in vitro and on a significant fraction of circulating CD4+ and CD8+ T cells, B cells and natural killer (NK) cells, but not on monocytes or neutrophils[50]. These chemokines are upregulated by interferon gamma (IFN-γ) and, therefore, they have important roles in inflammatory diseases, such as ulcerative colitis[50] and active pulmonary tuberculosis[51]. However, the infiltration of activated CD4+ and CD8+ T cells in tumors is considered to be a good prognostic factor; consequently, most of the research on the expression of these chemokines or their receptors in the context of CRC is focused in that direction. An interesting study by Lal et al[52] suggests that KRAS mutations are associated with reduced levels of CXCL10 and with the suppression of cytotoxic T cells, neutrophils and the IFN-γ pathway, especially in CRC consensus molecular subtype (CMS) 2 and CMS3. Other studies also reported the potential of these chemokines as good prognostic factors, primarily because of their association with the high infiltration of CD8+ T and CD4+ T helper 1 (Th1) effector cells[53-55]. Mice injected with colonic tumor cells overexpressing CXCL10 were protected against metastasis development[53]. Two independent studies demonstrated the post-surgical positive prognostic value of CXCL10 expression in localized colon cancer[53,56]. Specifically, in the study by Agesen et al[57], CXCL10 levels were part of a 13-gene expression classifier called ColoGuideEX for the prognosis prediction in stage II CRC. Interestingly, some authors have reported the opposite results. For instance, a high level of CXCR3 protein expression in primary CRC tumors was shown to be indicative of a poor prognosis[57]. In another study, the authors investigated the molecular factors underlying the poor prognosis of CRC tumor neuroendocrine differentiation and found that neuroendocrine-like cells secreted high levels of CXCL10 and CXCL11 which, in turn, promoted the recruitment of tumor-associated macrophages, thereby promoting the proliferation and invasion of CRC cells and leading to a poor prognosis[58].
Taken together, these findings appear to indicate that targeting CXCR3 or its ligands would not be an effective therapeutic strategy against CRC. However, some authors have speculated otherwise about the possibility of enhancing this pathway indirectly. It is worth commenting on three different papers that address three different strategies. First, Brackett et al[59] used a Toll-like receptor 5 (TLR5) agonist called entolimod in a murine mCRC model. This group found that entolimod induced the expression of CXCL9 and CXCL10 in tumors which, in turn, led to the recruitment of CXCR3+ NK cells to the liver, thereby activating dendritic cells (DCs) and stimulating the CD8+ T cell response. Consequently, the drug exerted an anti-metastatic effect and a tumor-specific and durable immune memory[59]. Second, Yang et al[60] studied the effect of signal transducer and activator transcription 3 (STAT3) inhibition in combination with immunogenic chemotherapy (anthracyclines). This group showed that, when injected in mice, STAT3 KO cells developed tumors with higher infiltration of DCs and cytotoxic T cells after treatment with anthracyclines. These tumors also displayed higher levels of CXCL9 and 10. Consequently, STAT3 inhibition improved the outcome of chemotherapy-treated mice by synergizing with immunogenic chemotherapy[60]. Another interesting approach involved the depletion of regulatory T cells (Treg) in mice bearing CRC tumors. This depletion was associated with an increase in T cell infiltration and proliferation and an increase in CXCL9 and 10, which led to the accumulation of CXCR3+ T cells and the increased mRNA expression of IFN-γ, which suggests Treg cell targeting as a possible anti-tumor immunotherapy[61]. It is important to note that some studies have indicated that CXCR3 targeting may be a good strategy against CRC cancer[62,63].
Finally, high mRNA levels of CXCL9 and 11[64] as well as CXCL10[64,65] appear to be associated with the response to neoadjuvant chemotherapy (5FU) plus radiotherapy in patients with locally advanced rectal cancer; on the other hand, CXCL4 was shown to be upregulated after treatment with 5FU, which was associated with accelerated growth in vivo[66]. These studies suggest the possibility of using members of this pathway as predictive biomarkers.
AXIS 4: MISCELLANY
Finally, it is worth commenting on the lesser known chemokines CXCL13, 16 and 17. Similar to that of the previously mentioned chemokines, the CXCL13-CXCR5 axis is involved in regulating lymphocyte migration and promoting inflammation[67]. However, the role of this axis in promoting or inhibiting tumor development or progression is not clear, as the current articles report contradictory results. Zhu et al[68] reported that this pathway promotes the growth, migration and invasion of colon cancer cells, likely through the PI3K/AKT pathway. Accordingly, high plasma levels of CXCL13 and positive immunohistochemical staining of CXCL13 and CXCR5 in tumors from CRC patients were associated with clinical and pathological characteristics typically related with a worse prognosis[69,70]. In contrast, high levels of CXCL13 in colorectal tumors have also been shown to be associated with the infiltration of follicular Th and B cells and, consequently, with a better prognosis. Cremonesi et al[71] attributed the increase in CXCL13 levels to the presence of gut microbiota, whereas in the work of Bindea et al[72], genomic instability affected CXCL13 expression, which led to alterations in T and B cell infiltration and clinical outcomes. In addition, it appears that a significant CD8+ T cell subset in colorectal tumors that are CXCR5+ potentially contribute to anti-tumor activity[73]. Not surprisingly, low levels of CXCL13 have been reported as a negative prognostic factor in CRC patients[56,74]. These contradictory results may be affected by several factors, including tumor stage, treatment, molecular subtype and microsatellite instability.
CXCR6 is expressed on the surface of CD4+ T cells, CD8+ T cells, NK cells and plasma cells and is responsible for inducing the chemotactic migration of these cells to inflamed tissues. The ligand of CXCR6, CXCL16, has two forms, the trans-membrane (TM) form and the soluble(s) form. TM-CXCL16 is expressed on macrophages, DCs, monocytes, and B cells where it functions as a cell adhesion molecule for cells that express CXCR6[75]. TM-CXCL16 is also a novel scavenger receptor that binds to phosphatidylserine and oxidized lipoprotein. In the membrane, this chemokine is cleaved by the disintegrin-like metalloproteinases ADAM10 and ADAM17, producing fragments that are released outside the cells[76]. sCXCL16 functions as a chemotactic factor for CXCR6-expressing Th1, T cells and NK cells. Interestingly, soluble CXCL16 can promote angiogenesis in human umbilical vein endothelial cells[77]. Again, given its capacity to promote tumor immunity, many of the published works describe CXCL16 as a positive prognostic factor[78-80]. On the other hand, Matsushita et al[81] reported that CRC patients had higher levels of CXCL16 in the serum compared to healthy controls, and these levels increased with tumor stage and were correlated with poor survival. In patients with localized disease, higher serum levels of CXCL16 were associated with a higher probability of metachronous liver recurrence and worse survival. In vitro, treatment with recombinant CXCL16 promoted cell growth, migration, invasion and EMT[81]. Thus, the exact role of CXCL16 in CRC warrants further investigation.
Finally, CXCL17 is a 119-amino acid CXC chemokine that binds specifically to CXCR8[82,83]. This chemokine is expressed in breast and colon cancer and acts as a chemoattractant for monocytes, macrophages and mature and immature DCs, thereby playing an important role in angiogenesis[84-86]. CXCL17 expression is co-regulated with VEGF expression[82,84] and can attract neutrophils to tumor sites and promote tumorigenesis through angiogenesis in mouse models[86]. Very little is known about the role of CXCL17 in CRC. Ohlsson et al[87] reported high mRNA levels of CXCL17 in several CRC cell lines as well as very high mRNA and protein levels of CXCL17 in primary CRC tumors compared to normal colon tissue. CXCL17-positive cells measured by immunofluorescence and immunohistochemistry were significantly more abundant in colon cancer tissues (80%) compared with the controls (normal colon samples from proximal or distal resection margin). Interestingly, CXCL17 was more abundant in tumor epithelial cells (17.2%) in comparison with tumor stromal cells (2.7%), and CXCL17 mRNA levels were correlated with the myeloid cell marker CD86, which is primarily expressed in antigen presenting cells (APCs), suggesting that this chemokine may contribute to the infiltration of APCs into the tumor[87]. Taken together, these findings indicate that the high expression of CXCL17 in CRC may be an indicator of poor prognosis in colon cancer, although further studies are warranted to elucidate the exact role of this chemokine in this malignancy.
In summary, very little information is available on the role of these less-known chemokines in CRC development or response to treatment.
CONCLUSION
The importance of the immune system in the surveillance, prognosis and treatment of cancer is undeniable. The recent development of immunotherapies and the rapidness of their application to the clinics for several tumor types demonstrate the possibilities of exploiting the immune system as a target for drug development. Moreover, the recent publication of the international validation of the consensus Immunoscore[88] as a reliable tool to classify colon cancer and to prognosticate its outcome also reveals the utility of using immune factors [in this case, total tumor-infiltrating T cell (CD3+) and cytotoxic T cell counts (CD8+)] as biomarkers. Therefore, other immune system-related factors thought to be important in cancer development and/or progression may also serve as prognostic and/or predictive biomarkers. Specially, those soluble factors that are released from tumor cells and are involved in the attraction of tumor-promoting or -killing cells are interesting candidates. In the present review, we have described the potential utility of chemokines from the CXC family as biomarkers of response and prognosis in CRC. Moreover, as these chemokines exert their function through binding to membrane receptors, these receptors also appear to be excellent drug targets. Some challenges still need to be overcome. First, some researchers have doubts about the systemic value of a factor that exerts its function locally. Second, due to certain contradictory reports, a deeper knowledge of the exact roles of each chemokine and its receptor(s) is urgently needed. Nevertheless, we are convinced that in the following years, these small secreted proteins will have a large role in predictive and personalized cancer medicine.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no potential conflicts of interest.
Peer-review started: July 5, 2018
First decision: August 25, 2018
Article in press: October 16, 2018
P- Reviewer: Kim SM, Mastoraki A, Nakayama Y, Popp C S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
Contributor Information
Sara Cabrero-de las Heras, Program Against Cancer Therapeutic Resistance, Catalan Institute of Oncology, Germans Trias i Pujol health research institute, Badalona, Barcelona 08916, Catalunya, Spain; Program of Predictive and Personalized Cancer Medicine, Germans Trias i Pujol health research institute, Badalona, Barcelona 08916, Catalunya, Spain.
Eva Martínez-Balibrea, Program Against Cancer Therapeutic Resistance, Catalan Institute of Oncology, Germans Trias i Pujol health research institute, Badalona, Barcelona 08916, Catalunya, Spain; Program of Predictive and Personalized Cancer Medicine, Germans Trias i Pujol health research institute, Badalona, Barcelona 08916, Catalunya, Spain. embalibrea@iconcologia.net.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Welch HG, Robertson DJ. Colorectal Cancer on the Decline--Why Screening Can’t Explain It All. N Engl J Med. 2016;374:1605–1607. doi: 10.1056/NEJMp1600448. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 4.Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, Falcone A, Loupakis F. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat Rev Clin Oncol. 2015;12:607–619. doi: 10.1038/nrclinonc.2015.129. [DOI] [PubMed] [Google Scholar]
- 5.Dienstmann R, Salazar R, Tabernero J. Overcoming Resistance to Anti-EGFR Therapy in Colorectal Cancer. Am Soc Clin Oncol Educ Book. 2015:e149–e156. doi: 10.14694/EdBook_AM.2015.35.e149. [DOI] [PubMed] [Google Scholar]
- 6.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 8.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117–129. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112:139–149. doi: 10.1016/j.pharmthera.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachelerie F, Graham GJ, Locati M, Mantovani A, Murphy PM, Nibbs R, Rot A, Sozzani S, Thelen M. New nomenclature for atypical chemokine receptors. Nat Immunol. 2014;15:207–208. doi: 10.1038/ni.2812. [DOI] [PubMed] [Google Scholar]
- 15.Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011;300:180–188. doi: 10.1016/j.canlet.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchese A. Endocytic trafficking of chemokine receptors. Curr Opin Cell Biol. 2014;27:72–77. doi: 10.1016/j.ceb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz de Porras V, Bystrup S, Martínez-Cardús A, Pluvinet R, Sumoy L, Howells L, James MI, Iwuji C, Manzano JL, Layos L, et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep. 2016;6:24675. doi: 10.1038/srep24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24:18–27. doi: 10.3109/08941939.2010.521232. [DOI] [PubMed] [Google Scholar]
- 20.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandapalli OR, Ehrmann F, Ehemann V, Gaida M, Macher-Goeppinger S, Wente M, Schirmacher P, Brand K. Down-regulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine. 2012;57:46–53. doi: 10.1016/j.cyto.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli Venkata KC, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11:1353–1364. doi: 10.1158/1535-7163.MCT-11-0915. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O’Sullivan JM, Wilson RH, Johnston PG, Waugh DJ. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther. 2008;327:746–759. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- 25.Abajo A, Boni V, Lopez I, Gonzalez-Huarriz M, Bitarte N, Rodriguez J, Zarate R, Bandres E, Garcia-Foncillas J. Identification of predictive circulating biomarkers of bevacizumab-containing regimen efficacy in pre-treated metastatic colorectal cancer patients. Br J Cancer. 2012;107:287–290. doi: 10.1038/bjc.2012.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Salvatore M, Pietrantonio F, Orlandi A, Del Re M, Berenato R, Rossi E, Caporale M, Guarino D, Martinetti A, Basso M, et al. IL-8 and eNOS polymorphisms predict bevacizumab-based first line treatment outcomes in RAS mutant metastatic colorectal cancer patients. Oncotarget. 2017;8:16887–16898. doi: 10.18632/oncotarget.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerger A, El-Khoueiry A, Zhang W, Yang D, Singh H, Bohanes P, Ning Y, Winder T, Labonte MJ, Wilson PM, et al. Pharmacogenetic angiogenesis profiling for first-line Bevacizumab plus oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2011;17:5783–5792. doi: 10.1158/1078-0432.CCR-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desurmont T, Skrypek N, Duhamel A, Jonckheere N, Millet G, Leteurtre E, Gosset P, Duchene B, Ramdane N, Hebbar M, et al. Overexpression of chemokine receptor CXCR2 and ligand CXCL7 in liver metastases from colon cancer is correlated to shorter disease-free and overall survival. Cancer Sci. 2015;106:262–269. doi: 10.1111/cas.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Triner D, Xue X, Schwartz AJ, Jung I, Colacino JA, Shah YM. Epithelial Hypoxia-Inducible Factor 2α Facilitates the Progression of Colon Tumors through Recruiting Neutrophils. Mol Cell Biol. 2017;37 doi: 10.1128/MCB.00481-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YS, Choi D, Kim NY, Yang S, Jung E, Hong M, Yang D, Lenz HJ, Hong YK. CXCR2 inhibition enhances sulindac-mediated suppression of colon cancer development. Int J Cancer. 2014;135:232–237. doi: 10.1002/ijc.28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, Uchida K, Mohri Y, Inoue Y, Kusunoki M. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48:2244–2251. doi: 10.1016/j.ejca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Kikuchi H, Ohta M, Kawabata T, Hiramatsu Y, Kondo K, Baba M, Kamiya K, Tanaka T, Kitagawa M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–9762. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- 34.Lin SC, Hsiao KY, Chang N, Hou PC, Tsai SJ. Loss of dual-specificity phosphatase-2 promotes angiogenesis and metastasis via up-regulation of interleukin-8 in colon cancer. J Pathol. 2017;241:638–648. doi: 10.1002/path.4868. [DOI] [PubMed] [Google Scholar]
- 35.Wu W, Cao J, Ji Z, Wang J, Jiang T, Ding H. Co-expression of Lgr5 and CXCR4 characterizes cancer stem-like cells of colorectal cancer. Oncotarget. 2016;7:81144–81155. doi: 10.18632/oncotarget.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu P, Zhao N, Sheng D, Hou J, Hao C, Yang X, Zhu B, Zhang S, Han Z, Wei L, et al. Inhibition of Growth and Metastasis of Colon Cancer by Delivering 5-Fluorouracil-loaded Pluronic P85 Copolymer Micelles. Sci Rep. 2016;6:20896. doi: 10.1038/srep20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler MJ, Lowthers EL, Richard CL, Hajducek DM, Spagnuolo PA, Blay J. Chemotherapeutic agents attenuate CXCL12-mediated migration of colon cancer cells by selecting for CXCR4-negative cells and increasing peptidase CD26. BMC Cancer. 2015;15:882. doi: 10.1186/s12885-015-1702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margolin DA, Silinsky J, Grimes C, Spencer N, Aycock M, Green H, Cordova J, Davis NK, Driscoll T, Li L. Lymph node stromal cells enhance drug-resistant colon cancer cell tumor formation through SDF-1α/CXCR4 paracrine signaling. Neoplasia. 2011;13:874–886. doi: 10.1593/neo.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu WT, Jing YY, Yan F, Han ZP, Lai FB, Zeng JX, Yu GF, Fan QM, Li R, Zhao QD, et al. LPS-induced CXCR4-dependent migratory properties and a mesenchymal-like phenotype of colorectal cancer cells. Cell Adh Migr. 2017;11:13–23. doi: 10.1080/19336918.2015.1134404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang WS, Chen CN, Sze CI, Teng CC. Visfatin induces stromal cell-derived factor-1 expression by β1 integrin signaling in colorectal cancer cells. J Cell Physiol. 2013;228:1017–1024. doi: 10.1002/jcp.24248. [DOI] [PubMed] [Google Scholar]
- 41.Yopp AC, Shia J, Butte JM, Allen PJ, Fong Y, Jarnagin WR, DeMatteo RP, D’Angelica MI. CXCR4 expression predicts patient outcome and recurrence patterns after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2012;19 Suppl 3:S339–S346. doi: 10.1245/s10434-011-1774-4. [DOI] [PubMed] [Google Scholar]
- 42.Wang TB, Hu BG, Liu DW, Shi HP, Dong WG. The influence of lentivirus-mediated CXCR4 RNA interference on hepatic metastasis of colorectal cancer. Int J Oncol. 2014;44:1861–1869. doi: 10.3892/ijo.2014.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unzueta U, Céspedes MV, Ferrer-Miralles N, Casanova I, Cedano J, Corchero JL, Domingo-Espín J, Villaverde A, Mangues R, Vázquez E. Intracellular CXCR4+ cell targeting with T22-empowered protein-only nanoparticles. Int J Nanomedicine. 2012;7:4533–4544. doi: 10.2147/IJN.S34450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romain B, Hachet-Haas M, Rohr S, Brigand C, Galzi JL, Gaub MP, Pencreach E, Guenot D. Hypoxia differentially regulated CXCR4 and CXCR7 signaling in colon cancer. Mol Cancer. 2014;13:58. doi: 10.1186/1476-4598-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heckmann D, Maier P, Laufs S, Li L, Sleeman JP, Trunk MJ, Leupold JH, Wenz F, Zeller WJ, Fruehauf S, et al. The disparate twins: a comparative study of CXCR4 and CXCR7 in SDF-1α-induced gene expression, invasion and chemosensitivity of colon cancer. Clin Cancer Res. 2014;20:604–616. doi: 10.1158/1078-0432.CCR-13-0582. [DOI] [PubMed] [Google Scholar]
- 46.Huang WS, Hsieh MC, Huang CY, Kuo YH, Tung SY, Shen CH, Hsieh YY, Teng CC, Lee KF, Chen TC, et al. The Association of CXC Receptor 4 Mediated Signaling Pathway with Oxaliplatin-Resistant Human Colorectal Cancer Cells. PLoS One. 2016;11:e0159927. doi: 10.1371/journal.pone.0159927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin F, Ji H, Jia C, Brockmeier U, Hermann DM, Metzen E, Zhu Y, Chi B. Synergistic antitumor effects of endostar in combination with oxaliplatin via inhibition of HIF and CXCR4 in the colorectal cell line SW1116. PLoS One. 2012;7:e47161. doi: 10.1371/journal.pone.0047161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung K, Heishi T, Incio J, Huang Y, Beech EY, Pinter M, Ho WW, Kawaguchi K, Rahbari NN, Chung E, et al. Targeting CXCR4-dependent immunosuppressive Ly6Clow monocytes improves antiangiogenic therapy in colorectal cancer. Proc Natl Acad Sci USA. 2017;114:10455–10460. doi: 10.1073/pnas.1710754114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 52.Lal N, White BS, Goussous G, Pickles O, Mason MJ, Beggs AD, Taniere P, Willcox BE, Guinney J, Middleton GW. KRAS Mutation and Consensus Molecular Subtypes 2 and 3 Are Independently Associated with Reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin Cancer Res. 2018;24:224–233. doi: 10.1158/1078-0432.CCR-17-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kistner L, Doll D, Holtorf A, Nitsche U, Janssen KP. Interferon-inducible CXC-chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget. 2017;8:89998–90012. doi: 10.18632/oncotarget.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zumwalt TJ, Arnold M, Goel A, Boland CR. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget. 2015;6:2981–2991. doi: 10.18632/oncotarget.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Z, Huang X, Han X, Li Z, Zhu Q, Yan J, Yu S, Jin Z, Wang Z, Zheng Q, et al. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed Pharmacother. 2016;78:8–13. doi: 10.1016/j.biopha.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 56.Agesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, Lothe RA. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61:1560–1567. doi: 10.1136/gutjnl-2011-301179. [DOI] [PubMed] [Google Scholar]
- 57.Wu Z, Han X, Yan J, Pan Y, Gong J, Di J, Cheng Z, Jin Z, Wang Z, Zheng Q, et al. The prognostic significance of chemokine receptor CXCR3 expression in colorectal carcinoma. Biomed Pharmacother. 2012;66:373–377. doi: 10.1016/j.biopha.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Zeng YJ, Lai W, Wu H, Liu L, Xu HY, Wang J, Chu ZH. Neuroendocrine-like cells -derived CXCL10 and CXCL11 induce the infiltration of tumor-associated macrophage leading to the poor prognosis of colorectal cancer. Oncotarget. 2016;7:27394–27407. doi: 10.18632/oncotarget.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brackett CM, Kojouharov B, Veith J, Greene KF, Burdelya LG, Gollnick SO, Abrams SI, Gudkov AV. Toll-like receptor-5 agonist, entolimod, suppresses metastasis and induces immunity by stimulating an NK-dendritic-CD8+ T-cell axis. Proc Natl Acad Sci USA. 2016;113:E874–E883. doi: 10.1073/pnas.1521359113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H, Yamazaki T, Pietrocola F, Zhou H, Zitvogel L, Ma Y, Kroemer G. STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells. Cancer Res. 2015;75:3812–3822. doi: 10.1158/0008-5472.CAN-15-1122. [DOI] [PubMed] [Google Scholar]
- 61.Akeus P, Langenes V, Kristensen J, von Mentzer A, Sparwasser T, Raghavan S, Quiding-Järbrink M. Treg-cell depletion promotes chemokine production and accumulation of CXCR3(+) conventional T cells in intestinal tumors. Eur J Immunol. 2015;45:1654–1666. doi: 10.1002/eji.201445058. [DOI] [PubMed] [Google Scholar]
- 62.Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer. 2013;132:276–287. doi: 10.1002/ijc.27670. [DOI] [PubMed] [Google Scholar]
- 63.Rupertus K, Sinistra J, Scheuer C, Nickels RM, Schilling MK, Menger MD, Kollmar O. Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer. Clin Exp Metastasis. 2014;31:447–459. doi: 10.1007/s10585-014-9639-4. [DOI] [PubMed] [Google Scholar]
- 64.Agostini M, Janssen KP, Kim IJ, D’Angelo E, Pizzini S, Zangrando A, Zanon C, Pastrello C, Maretto I, Digito M, et al. An integrative approach for the identification of prognostic and predictive biomarkers in rectal cancer. Oncotarget. 2015;6:32561–32574. doi: 10.18632/oncotarget.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li C, Wang Z, Liu F, Zhu J, Yang L, Cai G, Zhang Z, Huang W, Cai S, Xu Y. CXCL10 mRNA expression predicts response to neoadjuvant chemoradiotherapy in rectal cancer patients. Tumour Biol. 2014;35:9683–9691. doi: 10.1007/s13277-014-2234-0. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Gao J, Wang X, Deng S, Ye H, Guan W, Wu M, Zhu S, Yu Y, Han W. CXCL4 mediates tumor regrowth after chemotherapy by suppression of antitumor immunity. Cancer Biol Ther. 2015;16:1775–1783. doi: 10.1080/15384047.2015.1095404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Res. 2006;66:9576–9582. doi: 10.1158/0008-5472.CAN-06-1507. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 2015;400:287–295. doi: 10.1007/s11010-014-2285-y. [DOI] [PubMed] [Google Scholar]
- 69.Olsen RS, Nijm J, Andersson RE, Dimberg J, Wågsäter D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017;23:6212–6219. doi: 10.3748/wjg.v23.i34.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, Wu HR. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014;18:1916–1924. [PubMed] [Google Scholar]
- 71.Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, Trella E, Galati-Fournier V, Oertli D, Däster SR, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67:1984–1994. doi: 10.1136/gutjnl-2016-313498. [DOI] [PubMed] [Google Scholar]
- 72.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Xing J, Zhang C, Yang X, Wang S, Wang Z, Li X, Yu E. CXCR5+CD8+ T cells infiltrate the colorectal tumors and nearby lymph nodes, and are associated with enhanced IgG response in B cells. Exp Cell Res. 2017;356:57–63. doi: 10.1016/j.yexcr.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 74.Rachidi SM, Qin T, Sun S, Zheng WJ, Li Z. Molecular profiling of multiple human cancers defines an inflammatory cancer-associated molecular pattern and uncovers KPNA2 as a uniform poor prognostic cancer marker. PLoS One. 2013;8:e57911. doi: 10.1371/journal.pone.0057911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 76.Schramme A, Abdel-Bakky MS, Kämpfer-Kolb N, Pfeilschifter J, Gutwein P. The role of CXCL16 and its processing metalloproteinases ADAM10 and ADAM17 in the proliferation and migration of human mesangial cells. Biochem Biophys Res Commun. 2008;370:311–316. doi: 10.1016/j.bbrc.2008.03.088. [DOI] [PubMed] [Google Scholar]
- 77.Yu X, Zhao R, Lin S, Bai X, Zhang L, Yuan S, Sun L. CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1α in human umbilical vein endothelial cells. Oncol Rep. 2016;35:1557–1565. doi: 10.3892/or.2015.4520. [DOI] [PubMed] [Google Scholar]
- 78.Chung S, Dwabe S, Elshimali Y, Sukhija H, Aroh C, Vadgama JV. Identification of Novel Biomarkers for Metastatic Colorectal Cancer Using Angiogenesis-Antibody Array and Intracellular Signaling Array. PLoS One. 2015;10:e0134948. doi: 10.1371/journal.pone.0134948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kee JY, Ito A, Hojo S, Hashimoto I, Igarashi Y, Tsuneyama K, Tsukada K, Irimura T, Shibahara N, Takasaki I, et al. CXCL16 suppresses liver metastasis of colorectal cancer by promoting TNF-α-induced apoptosis by tumor-associated macrophages. BMC Cancer. 2014;14:949. doi: 10.1186/1471-2407-14-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kee JY, Ito A, Hojo S, Hashimoto I, Igarashi Y, Tsukada K, Irimura T, Shibahara N, Nakayama T, Yoshie O, et al. Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncol Rep. 2013;29:975–982. doi: 10.3892/or.2012.2185. [DOI] [PubMed] [Google Scholar]
- 81.Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, Inoue Y, Kusunoki M. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Ann Surg Oncol. 2012;19 Suppl 3:S518–S527. doi: 10.1245/s10434-011-1993-8. [DOI] [PubMed] [Google Scholar]
- 82.Lee WY, Wang CJ, Lin TY, Hsiao CL, Luo CW. CXCL17, an orphan chemokine, acts as a novel angiogenic and anti-inflammatory factor. Am J Physiol Endocrinol Metab. 2013;304:E32–E40. doi: 10.1152/ajpendo.00083.2012. [DOI] [PubMed] [Google Scholar]
- 83.Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol. 2015;194:29–33. doi: 10.4049/jimmunol.1401704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weinstein EJ, Head R, Griggs DW, Sun D, Evans RJ, Swearingen ML, Westlin MM, Mazzarella R. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. 2006;350:74–81. doi: 10.1016/j.bbrc.2006.08.194. [DOI] [PubMed] [Google Scholar]
- 85.Mu X, Chen Y, Wang S, Huang X, Pan H, Li M. Overexpression of VCC-1 gene in human hepatocellular carcinoma cells promotes cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai) 2009;41:631–637. doi: 10.1093/abbs/gmp051. [DOI] [PubMed] [Google Scholar]
- 86.Matsui A, Yokoo H, Negishi Y, Endo-Takahashi Y, Chun NA, Kadouchi I, Suzuki R, Maruyama K, Aramaki Y, Semba K, et al. CXCL17 expression by tumor cells recruits CD11b+Gr1 high F4/80- cells and promotes tumor progression. PLoS One. 2012;7:e44080. doi: 10.1371/journal.pone.0044080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohlsson L, Hammarström ML, Lindmark G, Hammarström S, Sitohy B. Ectopic expression of the chemokine CXCL17 in colon cancer cells. Br J Cancer. 2016;114:697–703. doi: 10.1038/bjc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]


