Abstract
AIM
To systematically review the literature on epidemiology, disease burden, and treatment outcomes for Crohn’s disease (CD) patients with complex perianal fistulas.
METHODS
PubMed, Embase, and Cochrane were searched for relevant articles (published 2000-November 2016) and congress abstracts (published 2011-November 2016).
RESULTS
Of 535 records reviewed, 62 relevant sources were identified (mostly small observational studies). The cumulative incidence of complex perianal fistulas in CD from two referral-centre studies was 12%-14% (follow-up time, 12 years in one study; not reported in the second study). Complex perianal fistulas result in greatly diminished quality of life; up to 59% of patients are at risk of faecal incontinence. Treatments include combinations of medical and surgical interventions and expanded allogeneic adipose-derived stem cells. High proportions of patients experience lack of or inadequate response to treatment (failure and relapse rates, respectively: medical, 12%-73% and 0%-41%; surgical: 0%-100% and 11%-20%; combined medical/surgical: 0%-80% and 0%-50%; stem cells: 29%-47% and not reported). Few studies (1 of infliximab; 3 of surgical interventions) have been conducted in treatment-refractory patients, a population with high unmet needs. Limited data exist on the clinical value of anti-tumour necrosis factor-α dose escalation in patients with complex perianal fistulas in CD.
CONCLUSION
Complex perianal fistulas in CD pose substantial clinical and humanistic burden. There is a need for effective treatments, especially for patients refractory to anti-tumour necrosis factor-α agents, as evidenced by high failure and relapse rates.
Keywords: Burden, Complex perianal fistulas, Crohn’s disease, Epidemiology, Outcomes, Systematic literature review, Treatment
Core tip: Complex perianal fistulas in Crohn’s disease (CD) impose considerable burden. Rates of failure and relapse are generally high with currently available treatments. Effective treatment options for complex perianal fistulas in patients with CD, especially those who are refractory to anti-tumour necrosis factor-α agents, are needed.
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory disease of the gastrointestinal tract characterised by transmural inflammation that can disrupt the mucosal integrity of the intestine and anal canal, leading to complications such as abscesses and fistulas (i.e., tracts between intestinal segments and other organs or adjacent tissue or skin)[1]. It is estimated that 26% of patients with CD experience perianal fistulas in the two decades after diagnosis[2]. An estimated 70% to 80% of perianal fistulas are complex perianal fistulas (CPF)[3], defined by the American Gastroenterological Association as those involving the upper part of the sphincter complex (i.e., high intersphincteric, high transsphincteric, suprasphincteric, or extrasphincteric origin of the fistula tract); have multiple external openings (tracts); are associated with pain or fluctuation suggesting a perianal abscess; and/or are associated with a rectovaginal fistula or anorectal stricture[4]. The available literature indicates that CPF can result in a high disease burden, greatly diminished health-related quality of life (HRQOL), and increased health care resource use and costs[5].
Treatment approaches for CPF in patients with CD include combinations of medical and surgical options, with the aims of achieving healing of the fistula, treating and preventing further septic complications, resolving fistula discharge, and improving patients’ HRQOL[6]. Currently, anti-tumour necrosis factor alpha (TNF-α) agents, particularly infliximab, are generally recommended as the first-line medical therapy for CPF in patients with CD[5,6]. Adjuvant use of antibiotics at induction and thiopurines during maintenance are additional options[5]. Relapse is common after stopping medical treatment, and it is generally estimated that only one-third of patients achieve remission, often defined as closure of external openings and a lack of drainage (although definitions of remission vary across studies)[3,7].
To our knowledge, the literature on epidemiology, burden, and management of CPF in patients with CD has not been systematically reviewed. The objective of this study was to conduct a systematic review of the literature on the epidemiology and disease burden of CPF in Europe and treatment outcomes for CPF in patients with CD globally.
MATERIALS AND METHODS
A systematic search of electronic medical literature databases and relevant conferences (i.e., Crohn’s and Colitis Foundation of America’s clinical and research conference/Advances in Inflammatory Bowel Diseases, Digestive Disease Week, European Crohn’s and Colitis Organisation, American College of Gastroenterology, United European Gastroenterology Week, and International Society for Pharmacoepidemiology; 2011 forward) was conducted in November 2016. The MEDLINE, MEDLINE In-Process, Embase, and Cochrane Library (including Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Database of Abstracts of Reviews of Effects) databases were searched (2000 forward for full articles; 2011 forward for conference abstracts) using combinations of free text and Medical Subject Headings terms. The population of interest was adult (≥ 18 years) patients with CD and/or CPF undergoing medical or surgical therapy. CPF were generally defined as fistulas with intersphincteric, transsphincteric, suprasphincteric, extrasphincteric, or horseshoe tracts, although definitions of CPF varied between studies. Rectovaginal fistulas were included if they were included by authors as part of their definition of CPF; however, we did not specifically focus on rectovaginal fistulas. Studies were included if they reported the epidemiology, burden, costs, treatment patterns, and/or treatment outcomes. Studies not distinguishing between simple perianal fistulas and CPF were excluded if outcomes were not reported separately for the CPF population. Comments, case reports, and studies in animals were excluded. For a full list of the predefined inclusion and exclusion criteria see Supplementary Table 1. Supplementary Table 2 presents the MEDLINE search strategy. Additional studies were identified via the bibliographies of relevant systematic literature reviews and conference abstracts. Searches of professional association websites, and the National Guidelines Clearinghouse and Turning Research into Practice databases were also conducted to capture clinical guidelines.
Articles were selected by two independent researchers via a two-step screening process. Titles and abstracts of identified articles were first reviewed for relevance according to the inclusion and exclusion criteria. Full-text articles selected were then reviewed for relevance using the same criteria. Any disagreements regarding the inclusion of an article were resolved by consensus, using a third reviewer to reach a final decision. For information about epidemiology, HRQOL and economic burden, and treatment guidelines and patterns for CPF in patients with CD, we focused on European studies. Because clinical studies are not prone to change from country to country, we also included clinical studies conducted in any country if they reported on relevant treatment outcomes. For the purposes of this review, we classified surgical procedures as major or minor, as outlined in Supplementary Table 3.
RESULTS
Literature search results
The search of the medical literature databases yielded 535 records, from which 116 sources were selected after abstract review, and 33 sources were deemed eligible for inclusion after full-text review. An additional 29 sources were identified from the search of conference abstracts and the grey literature, for a total of 62 articles and abstracts (Figure 1).
Figure 1.
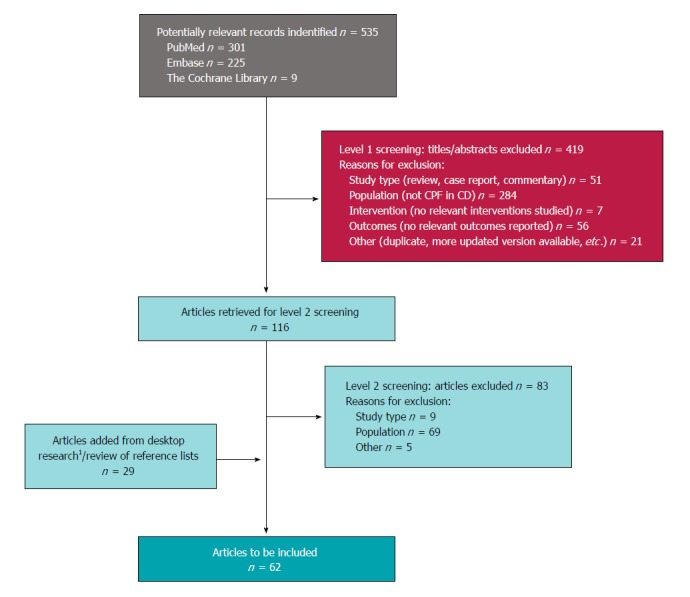
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram. 1Includes 12 studies identified via search of congress abstracts, and not published elsewhere. CD: Crohn’s disease; CPF: Complex perianal fistula; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Epidemiology of complex perianal fistulas in CD (n = 2 studies)
The cumulative incidence of CPF in patients with CD reported in two separate retrospective Spanish studies (including 2391 and 350 distinct CD patients) was 12% and 14% (follow-up: Mean 12 years per patient and total 15 years within institution, respectively)[8,9] (Figure 2). In the larger study, the authors reported an annual incidence of CPF of 0.7 per 100 patient-years[8]. No epidemiologic studies presenting data on the proportion of CPF among CD patients with perianal fistulas were identified. However, in clinical studies, CPF accounted for 52% to 88% of the total number of perianal fistulas among patients with CD[10-16] (Table 1).
Figure 2.
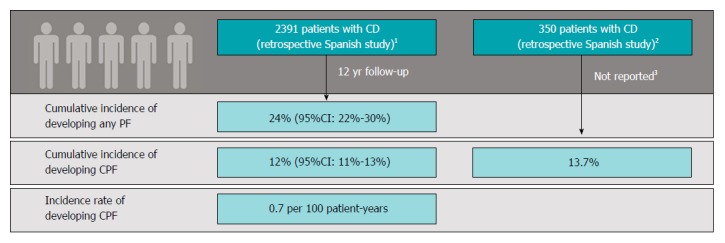
Epidemiology of complex perianal fistula in Crohn’s disease. 1Chaparro et al[8] published in 2011. Definition of complex perianal fistulas (CPF): A fistula meeting any of the following criteria: High location (high intersphincteric, high transsphincteric, extrasphincteric, or suprasphincteric), multiple external openings, perianal abscess, anal stenosis, or proctitis. Retrospective multicentre study conducted in 11 hospitals in Madrid, Spain (study period was not reported); 2Morete et al[9] published in 2013. Definition of CPF not given. Retrospective single-centre study (Ferrol, Spain) with 15 yr of follow-up; 3Analysis of patients followed up at reporting institution over 15 yr; mean per-patient follow-up not noted. Note: Both studies were presented as conference abstracts only and were not published in a peer-reviewed journal. Thus, data are limited and difficult to assess for robustness. CD: Crohn’s disease; CI: Confidence interval; CPF: Complex perianal fistula; PF: Perianal fistula.
Table 1.
Epidemiology of complex perianal fistula in Crohn’s disease from non-epidemiologic studies
| Ref. | Country, study type | Definition of complex perianal fistula | Sample Size | Mean (SD) age | Sex, % female | Mean CD duration (SD) | Mean fistulising CD duration (SD) | Disease location, % | Proportion of CPF among total fistulas, % |
| Haennig et al[10] 2015 | France, retrospective | According to AGA | 81 | 31 (13) yr; median: 26.9 yr | 52% | NR | Newly referred patients | Perineum: 69% | 88% |
| Rectum: 42% | |||||||||
| Ileum: 7% | |||||||||
| Colon: 40% | |||||||||
| Ileum-colon: 52% | |||||||||
| Active proctitis: 80% | |||||||||
| Alessandroni et al[11] 2013 | Italy, retrospective | Not provided (rectovaginal and rectourethral fistulas were excluded) | 210 | Median, 34 (range 9-74) yr1 | 47%1 | NR | NR | Ileal disease1: 27%, colonic1: 26%, ileocolonic involvement1: 47% | 86% |
| Riss et al[12] 2013 | Austria, Retrospective | Transsphincteric, extrasphincteric, suprasphincteric, and rectovaginal fistulas were classified as complex | 69 | Median, 46.5 (range 18-64) yr | 68% | Median, 202.2 mo (range 29-406.5 mo) | NR | NR | 84% |
| Molendijk et al[13] 2014 | Holland, Retrospective | High intersphincteric, transsphincteric, extrasphincteric, or suprasphincteric were classified as complex | 232 | Median, 29.4 (9.1-77.3) yr | 58% | NR; median age at diagnosis: 22.8 yr (4.0-68.7) | Newly diagnosed patients | Upper GI: 5% | 78% |
| Small bowel: 7% | |||||||||
| Ileocecal: 15% | |||||||||
| Large bowel: 38% | |||||||||
| Small + large bowel: 29% | |||||||||
| Whole GI tract: 1% | |||||||||
| Isolated perianal disease: 6% | |||||||||
| Rectal involvement: 41% | |||||||||
| Lahat et al[14] 2012 | Israel, Retrospective | According to AGA | 52 | 10 yr (9.2; range, 1-37) | 5.3 (6.5; range, 1-29) | Terminal | 75%2 | ||
| Ileum: 35% | |||||||||
| Colon: 27% | |||||||||
| Ileocolon: 39% | |||||||||
| Bell et al[15] 2003 | United Kingdom, Retrospective | Transsphincteric, translevator, supralevator and extrasphincteric perianal fistulas were classified as complex | 110 perianal fistulas3 | Median, 35 (range, 20-91) yr | 53% | Median, 8 (range, 0-32) yr | Median, 3 yr (range, 0-32 yr) | Ileocolonic or colonic: 85%, | 72% of fistulas |
| Rectal involvement: 65% | |||||||||
| Mueller et al[16] 2007 | Germany, Prospective | Complex fistula was defined as rectovaginal or fistula with three or more perianal openings | 88 | Median, 23 (range 8-51) yr4 | 52% | NR | NR | Isolated small intestinal disease4: 4%, isolated colonic disease4: 11%, small intestinal and colonic disease4: 85% | 52% |
Baseline patient characteristics were only reported for 229 patients of whom 19 patients with rectovaginal or rectourethral fistulas were then excluded from the study;
74% of patients with ileocolonic or colonic disease had complex fistula, compared with 72% of patients with ileal disease only (P = NS);
NR how many patients had perianal fistulas or CPF; a total of 87 patients with CD and active fistulas were enrolled; 34 patients (39%) had a single fistula, 24 (28%) had two fistulas and 29 (33%) had three or more fistulas during the course of their disease, giving a total number of fistulas of 169 fistulas, of which 110 were perianal fistulas (79 complex perianal and 31 simple perianal fistulas);
Reported for all 97 patients with perianal disease. Baseline characteristics not reported separately for 88 patients with CD and perianal fistulas. AGA: American Gastroenterological Association; CD: Crohn’s disease; CPF: Complex perianal fistula; GI: Gastrointestinal; NR: Not reported; NS: Not significant.
Health-related quality of life burden of CD patients with perianal fistulas (n = 1 study)
A postal HRQOL questionnaire was administered to 69 perianal CD patients (84% with CPF) with no current stoma, identified retrospectively from a single surgical department in Austria, who underwent surgical treatment[12]. (Surgical treatments were fistulotomy, loose seton drainage, advancement flap, or stoma.) HRQOL was statistically significantly reduced compared with age- and sex-matched healthy controls[12]. Median SF-12 physical health score was 47.9 (range, 25.5-57.2) for patients compared with 54.3 (range, 34.6-61.8) for controls (P = 0.03). Patients’ Inflammatory Bowel Disease Questionnaire (IBDQ) score was 157 (range, 60-199.5) compared with 188.5 (range, 125-206.5) for controls (P < 0.0001). Faecal incontinence, defined as the involuntary leakage of solid stool, liquid stool, or gas at the time of follow-up, was observed in 59% of patients (incontinence of solid stool: 30%, liquid stool: 54%, gas: 52%) and had a negative impact on HRQOL (IBDQ; P = 0.0006 vs healthy age-matched controls without faecal incontinence)[12].
Treatment of complex perianal fistulas in CD patients treatment goals (n = 1 study)
The short-term goals in the treatment of perianal CD, including CPF, are abscess drainage (to manage or prevent sepsis) and reduction of symptoms[17]. The long-term goals are resolving fistula discharge, improvement in HRQOL, fistula healing, preserving continence, and avoiding proctectomy with stoma[17].
Treatment options, choices, and guidelines (n = 6 studies)
Treatment options for CPF include a combination of medical (e.g., antibiotics, immunosuppressants, and anti-TNF-α drugs) and surgical interventions (e.g., plugs, glue, fistulotomy, fistulectomy, placement of setons, stoma, ligation of the intersphincteric fistula tract, advancement flap, colectomy, proctectomy), as well as stem cell therapy. Figure 3 presents real-world treatment patterns as identified in two retrospective studies - one conducted in Spain and one in the Netherlands[13,18].
Figure 3.
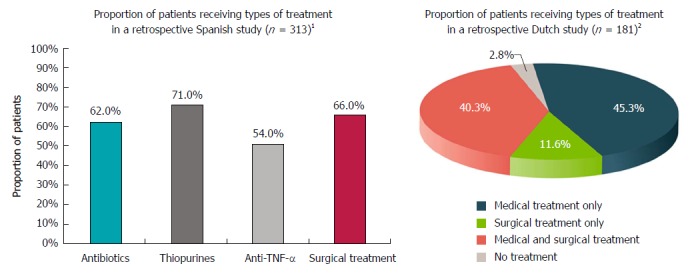
Treatment choices. 1Retrospective multicentre study (study period or median follow-up time were not reported) enrolling patients with Crohn’s disease (CD) and PFs; 80% had complex perianal fistulas (CPF). The graph presents treatment selection for patients with any fistula. Patients could have received multiple treatments (proportion of patients who received combination therapy was not reported). The most common surgical intervention was fistulotomy (37%), followed by placement of setons (32%)[18]. 2Retrospective single-centre study enrolling patients with CD and any perianal fistula [n = 232 patients, of which 181 (78%) had CPF]; patient identification: 1980-2000, follow-up through January 1, 2010; median follow-up was 10.0 yr (range, 0.5-37.5 yr). The graph presents treatment choices for 181 patients with CD and CPF. The most common type of surgery (alone or in combination with medical treatment) was faecal diversion (stoma; 63.6% of 94 patients with CPF who underwent surgery; 33.1% of all 181 patients with CPF), followed by colectomy (55.3% of patients with CPF who underwent surgery; 28.7% of all patients with CPF), fistulectomy (42.6% of patients with CPF who underwent surgery; 22.1% of all patients with CPF), and rectum amputation (proctectomy; 25.5% of patients with CPF who underwent surgery; 13.3% of all patients with CPF)[13].
Global, European, Italian, and German treatment guidelines vary with respect to treatment of CPF in patients with CD; however, drainage of sepsis is generally recommended as first-line intervention before initiating immunosuppressive treatment[17,19-21]. Recommended first-line medical treatments include anti-TNF-α agents, particularly infliximab (the only anti-TNF-α agent approved for fistulas in patients with CD), sometimes in combination with antibiotics and/or thiopurines; plugs are recommended as first-line surgical therapy[17,19,20]. For maintenance therapy following induction, guidelines generally recommend azathioprine/6-mercaptopurine, often combined with infliximab or adalimumab, seton drainage, or a combination of drainage and medical therapy[19,20]. Upon failure of anti-TNF-α agents, treatment options include azathioprine/6-mercaptopurine, methotrexate, or tacrolimus, with antibiotics as adjunctive treatment[17,20]. Ustekinumab is now also available for treatment of CD after failure of anti-TNF-α agents but has not been specifically studied in CD patients with CPF[22,23]. Proctectomy or diverting stoma is the last resort for severe, therapy-refractory disease[17,19,20].
Clinical treatment outcomes (n = 45 studies)
Clinical treatment outcomes (e.g., response, relapse, and maintenance of healing) were described in 20 prospective studies and 24 retrospective studies (design was unclear in one study). Supplementary Table 4 presents information on each study, including study design, definitions of CPF, and study endpoints (e.g., response and relapse). All included studies presented information on the treatment of patients with CPF and CD. [Three studies - Alessandroni et al[11] (2013), Arberas-Diez et al[18] (2013), and Haennig et al[10] (2015) - reported results for CD patients with any fistula, but the majority (≥ 80%) of the population had CPF]. Therefore, studies of infliximab and other treatment options were not included if they enrolled patients with any perianal fistulising CD but relevant outcomes were not reported separately for patients with CPF. Treatment options investigated in the included studies were anti-TNF-α agents (infliximab, adalimumab, or unspecified anti-TNF-α agent) (n = 10), major and minor surgery (as defined in Supplementary Table 3) with (n = 15) or without (n = 12) concurrent medical treatment, stem cell therapy (n = 4), and “usual care” (defined as a standard medical care delivered at a study author’s institution, consisting of medical and/or surgical approaches) (n = 4) (Figure 4; Supplementary Table 4). One study investigating "usual care" reported response rates for patients treated with antibiotics and thiopurines without concurrent surgical or anti-TNF-α therapies[18].
Figure 4.
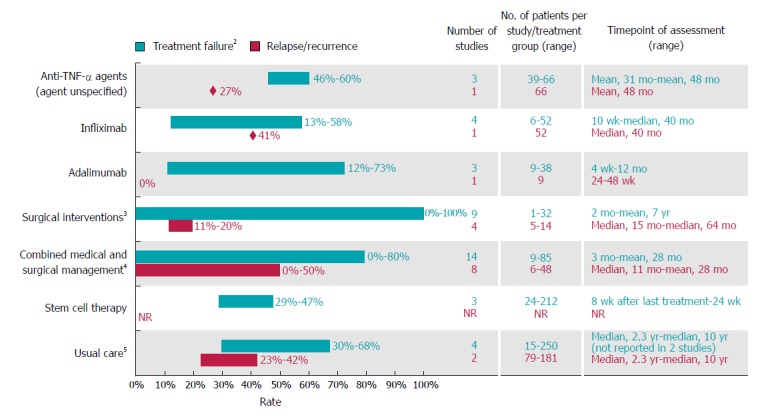
Rates of treatment failure and relapse or recurrence among Crohn’s disease patients with complex perianal fistula1. 1For studies with mixed populations (i.e., patients with any fistula and those with complex fistula), only results for patients with Crohn’s disease and complex perianal fistulas were considered; 2Defined as lack of or inadequate response to therapy (i.e., lack of complete response or lack of healing response); 3Most studies (10 of 12) reported outcomes for surgical procedures that were considered major procedures in this review, ligation of intersphincteric fistula tract, advancement flap repair, mucosal advancement flap with injection of platelet-rich plasma into the fistula tract, myocutaneous flaps and proctocolectomy with permanent ileostomy, gracilis muscle transposition, over-the-scope-clip proctology, fistula tract transposition or standard surgical management (including both major and minor procedures)[34-36,42,48-50,53,54,66]. One study reported outcomes with permanent seton (minor procedure; other minor procedures reported in studies mentioned above included biologic fistula plug, and fibrin glue)[52]. One study did not specify the type of surgery[11]; 4Most (12 of 15) studies assessed surgical procedures that are considered minor procedures (seton drainage (most frequently), abscess drainage, fistulotomy, fibrin glue)[10,29,38-41,43,67-71]. Two studies assessed surgical procedures that are considered major procedures (mucosal advancement flap, resection, stoma, proctectomy)[37,51]. Surgery type was not defined in one study[72]; 5Defined as usual care used at each centre [standard medical care (excluding anti-tumour necrosis factors) and surgery in two studies, and standard medical care (including anti-tumour necrosis factor alpha agents) and surgery in two studies]. TNF-α: Tumour necrosis factor alpha.
Rates of treatment failure: Lack of response or inadequate response
Across studies and treatment options reviewed, treatment failures (calculated based on lack of or inadequate response, as presented in the included studies) and/or relapses were common (Figure 4).
Rates of remission
Three studies (3 retrospective reviews of prospectively collected data reported by the same first author, perhaps with overlapping populations, and examining treatment with some combination of an anti-TNF-α [agent not specified] plus azathioprine and/or antibiotics[24-26]) reported clinical remission rate and radiological remission rate, one retrospective study (examining fibrin glue with or without adult stem cells) included Magnetic resonance imaging (MRI) results as part of their definition of remission[27], and two studies (one prospective trial of adalimumab and one retrospective study examining infliximab plus other medications such as 6-mercatopurine, azathioprine, methotrexate, and/or antibiotics) provided rates for both clinical and endoscopic ultrasound-confirmed remission[28,29]. Overall, only a small proportion of patients with CD and CPF treated with anti-TNF-α (agent not specified) achieved radiological healing. Specifically, among 39 patients with mean follow-up of 31 mo, 54% were in clinical remission, but only 10% with radiological closure[24]; among 51 patients with mean follow-up time of 42 mo, 53% were in clinical remission, but only 14% with radiological closure[25]; and among 66 patients with mean follow-up of 48.50 mo, 40% were in clinical remission, but only 24% had radiological closure[26]. The rate of combined clinical and radiological remission was comparable to the rate of clinical remission for patients who received darvadstrocel (Cx601) stem cell therapy (50% vs 53% of 107 patients receiving Cx601 plus standard of care followed up for 24 wk compared with 34% vs 41% of 105 patients receiving placebo plus standard of care[3]).
Anti-TNF-α treatment response rates
Ten studies reported outcomes after anti-TNF-α treatment (4 for infliximab [infusions or local injections], 3 for adalimumab, and 3 for unspecified anti-TNF-α treatment; 5 were retrospective, 4 were prospective, and 1 had an unclear study design; follow-up ranged from 4 wk to a mean of 48 mo across studies; see Supplementary Table 4 for details). In studies evaluating infliximab induction treatment, complete response was not consistently achieved (Figure 4). In a retrospective United Kingdom study, 42.3% of 52 CD patients with CPF had complete response (defined as complete closure of the fistula with no further drainage) and 44.2% had a partial response (defined as reduction in number, size, or drainage of fistulas) to infliximab after a median follow-up of 41 mo[30]. A prospective Italian study found that 3 of 6 CD patients with CPF experienced clinical closure of the CPF at the end of the induction period (i.e., the week 10 visit)[31]. Moreover, relapse is common. In the aforementioned UK study, the relapse rate at the median follow-up time of 36.5 mo was 41% among patients with initial complete response and 47.8% among those with initial partial response[30]. In another prospective Italian cohort study, the initial rate of persistent closure at 12 mo among 12 CD patients with CPF was 87.5% but was 62.5% at the end of the follow-up (median, 35 mo)[32].
The proportion of adalimumab-treated patients that achieved complete cessation of drainage varied. The complete response rate after adalimumab treatment was 88% at 48 wk among a subset of nine patients enrolled in a randomised prospective study conducted in the United States (US) who underwent assessments by endoscopic ultrasound every 12 wk and was 73% among 11 control patients who received standard of care without endoscopic ultrasound[28]. Complete response was 28% among 38 CD patients with CPF at 12 mo in a retrospective multicentre study conducted in Spain[33]. In a retrospective assessment of data gathered prospectively in Turkey, clinical response after an anti-TNF-α-based triple treatment (unspecified anti-TNF-α agent, azathioprine, and antibiotic) among 66 CD patients with CPF after a mean follow-up of 48.50 mo was 40%[26].
Surgical treatment response rates
Twelve studies reported outcomes after surgical interventions without combined medical treatment (8 retrospective and 4 prospective; duration of follow-up across studies ranged from 2 mo to 7 years). Ten studies focused on major surgical procedures, one focused on minor surgical procedures, and one did not report the type of procedure (see Figure 4 and Supplementary Table 4 for details of each procedure). Across all 12 studies, response rates with surgical procedures ranged from 31% to 100%. In a retrospective Canadian study, the healing rate among patients who underwent seton drain placement was 31.3% (10 of 32 patients; assessment at 12 wk), 33.3% (1 of 3 patients) among patients who underwent advancement flaps, and 75% (3 of 4 patients) among patients who had fistula plugs inserted[34]. In an Austrian retrospective cohort study, all five patients who underwent myocutaneous flaps and proctocolectomy with permanent ileostomy experienced healing at 3 mo, although one patient relapsed at 6 mo[35]. Similarly, in a retrospective Brazilian study, at a median follow-up of 15.2 mo, 1 of 5 patients who underwent fistula tract transposition experienced a recurrence associated with a new tract formation[36].
Combined medical and surgical response rates
Fifteen studies reported outcomes after combined medical and surgical treatments (10 prospective and 5 retrospective studies; follow-up ranged from 3 mo to 5 years across studies; 2 studies reported outcomes for major surgical procedures combined with medical treatment, 12 for minor surgical procedures combined with medical treatment, and 1 for unspecified surgery type combined with medical treatment; see Figure 4 and Supplementary Table 4 for details). Overall, response rates ranged from 20% to 100%. Response rates to combined infliximab and seton drainage therapy ranged from 43% to 100%[10,29,37-42]. (Haennig et al[10] (2015) included 71 patients with CD with CPF of 81 patients total). In a retrospective observational study, relapse was reported in 41% of 71 CD patients (with CPF) who underwent combined infliximab and seton drainage therapy[10].
The results of three comparative studies suggested that combined medical and surgical treatment may have clinical outcome benefits over medical (drug) or surgical treatment alone. In a retrospective United States study of 20 CD patients with CPF, outcomes were significantly better in patients who had an examination and seton placement under anaesthesia prior to infliximab compared with patients receiving infliximab alone in terms of initial response (within 3 mo of third infusion; 100% vs 71.5%, P = 0.026), recurrence rate (50% vs 100%, P = 0.036), and mean time to recurrence (13 mo vs 2.1 mo, P = 0.001)[41]. Similarly, in a retrospective Korean study of 85 CD patients with CPF[43], patients receiving combined medical (antibiotics and/or immunomodulators; no anti-TNF-α) and fistulotomy and/or seton drainage showed significantly better response rates at 3 mo (68%) than patients receiving medical treatment alone (40.9%) or surgical treatment alone (38.5%) (P = 0.03). In a prospective Italian study of 35 CD patients with CPF, although the rates of response and relapse were not significantly different among patients who received infliximab only, surgery (i.e., draining seton) only, or infliximab combined with surgery, patients in the latter group showed significantly shorter mean (standard deviation [SD]) time to healing of fistulas [3.1 (0.8) mo] than those who received surgery alone [mean (SD), 4.2 (1.3) mo; P = 0.041] and significantly longer mean time to relapse than those who received infliximab only [mean (SD), 2.6 (0.7) mo; P = 0.012] or surgery only [mean (SD), 3.6 (0.5) mo; P = 0.016][38].
Response rates after allogeneic adipose-derived stem cell treatment
Expanded allogeneic adipose-derived stem cells (eASCs) are a novel treatment option for patients with CD and CPF who do not respond to conventional and/or biological treatments[44] or in whom systemic immunosuppression needs to be avoided. Across studies of eASCs, response (defined as ≥ 50% reduction in the number of draining fistulas) has been achieved in 66% to 71% of patients and combined clinical and radiological remission (defined as absence of abscess ≥ 2 cm in diameter) has been achieved in 30% to 50% of patients[3,45,46]. In a phase 3 trial of darvadstrocel, a suspension of eASCs, in 212 CD patients with CPF, significantly more patients treated with darvadstrocel achieved combined remission 24 wk after receiving a single injection compared with control patients (50% vs 34%, P = 0.024); numerical improvements in response and clinical remission rates were also observed[3]. Moreover, the median time to clinical remission was shorter with darvadstrocel than with control (6.7 wk vs 14.6 wk; hazard ratio, 0.57; 95%CI: 0.41-0.79), as was the median time to response (6.3 wk vs 11.7 wk; hazard ratio, 0.59; 95%CI, 0.43-0.81)[3].
“Usual care” response rates
Four studies presenting outcomes after usual care were identified. Results by specific treatment were not consistently available in these studies. Usual care varied among countries and included both medical interventions (usual medical care included anti-TNF-α agents in the Netherlands[13] and Spain[18] but not did not include anti-TNF-α agents in the United Kingdom[15] or Ukraine[47]) and surgical interventions (see Supplementary Table 4). Overall, complete response after usual care was achieved in 32% to 77% of patients, with higher response rates generally reported when “usual care” included anti-TNF-α therapy. This wide range in response rates is likely due to variations in the treatment options considered usual care in the studies. In a retrospective Dutch study including 181 patients with CD and CPF, although the initial remission rate was 64.6%, at a median time of follow-up of 10 years, remission was maintained in only 37% of patients[13]. In a retrospective Spanish study, complete response rates appeared to be more favourable with an anti-TNF-α agent combined with thiopurines (77% of patients) than with anti-TNF-α monotherapy (59%); however, outcomes were reported only for patients with any type of fistula [approximately 250 of 313 patients (80%) had complex fistulas][18]. Corresponding complete response rates for patients with complex fistulas treated with only thiopurines, antibiotics, or surgical procedures were 32%, 37%, and 50%, respectively. Treatment outcomes for surgery combined with an anti-TNF-α agent were not reported[18]. A retrospective UK study reported that the overall healing rate for CPF treated with standard care was 70% (55/79 fistulas); median time to healing of CPF was 42.8 mo (range, 1.8-397 mo), with a median of 6 treatment episodes (range, 1-23 episodes); the majority of episodes leading to healing consisted of surgical treatment[15]. In a Ukrainian study, 11 of 15 patients with complex fistulas underwent transanal advancement flap, and 4 of 15 patients underwent non-cutting seton procedure (both procedures were followed by medical treatment); of these, 5 of 11 (45%) and 2 of 4 (50%) healed, respectively[47].
Outcomes in treatment-refractory patients
Although most patients in the studies identified received previous or concurrent medical and surgical therapies, only four studies were conducted specifically in patients refractory to medical and/or surgical therapies. In a prospective study of perifistular injections of infliximab in 12 CD patients with CPF refractory to medical therapy with immunomodulators and/or intravenous infliximab, persistent closure (i.e., 12 mo after treatment) occurred in 62.5% of patients (5/8) at the end of follow-up (median, 35 mo)[32]. Three retrospective studies of patients refractory to some combination of medical (e.g., anti-TNF-α agents, azathioprine, steroids) and surgical interventions evaluated specific surgical interventions (myocutaneous flaps and proctocolectomy with permanent ileostomy, gracilis muscle transposition, or over-the-scope-clip proctology), with healing rates ranging from 64% of patients to 100% of patients[35,48,49].
Outcomes after anti-TNF-α dose escalation or switching
There is limited evidence on anti-TNF-α dose escalation in this population. One small retrospective study showed that adalimumab dose escalation to 40 mg weekly (from 160 mg + 80 mg or 80 mg + 40 mg induction) in patients who had no response (n = 3 patients) or inadequate response (n = 6 patients) to adalimumab led to remission in only 3 of 9 patients[33]. No studies reporting outcomes after switching between different anti-TNF-α agents for the treatment of CPF were identified.
Predictors of treatment outcomes
Evidence of correlation between patient characteristics and CPF treatment response is limited and inconsistent. One retrospective study of 210 CD patients with perianal fistulas found that the risk of poor outcomes after treatment of CPF in patients with CD was related significantly to disease location, rectal involvement, and need for more than one surgical procedure[11]. (Alessandroni et al[11] (2013) included 210 patients with perianal fistulas [181 (86%) with CPF]. Another retrospective study reported no correlation between fistula response and disease location, duration, number of fistulas, or treatment modality[24].
Other outcomes
Few studies (n = 11) reported patients’ continence after surgical procedures, and in most of these studies no negative effect on continence was observed[27,34,36,46,47,49-54]. In addition, the need for colostomy or ileostomy after treatment failure generally was not consistently reported across studies. In a Dutch study, 33.1% of 181 patients with CD and CPF and 15.7% of 51 patients with simple fistulas underwent faecal diversion (stoma; not reported if transient or permanent) during a median follow-up of 10.0 years[13]. Among 210 patients with CD and any perianal fistulas (86% with CPF), the cumulative probability of defunctioning stoma or proctectomy during 72 mo of follow-up was 0.3. This probability was not influenced by patients’ type of fistula, age at diagnosis, or sex and not significantly reduced by immunosuppressive or biological therapy; however, the risk was significantly related to disease localisation, rectal involvement, and the need for more than one surgical procedure[11].
DISCUSSION
This systematic literature review revealed that published data on the epidemiology and burden of CPF in patients with CD are scarce. The cumulative incidence of CPF in patients with CD ranged from 12% to 14% (follow-up: mean 12 years per patient and total 15 years within institution, respectively)[8,9]. These findings are aligned with the results of previous studies of all perianal fistulas in CD. In a US-population based study, the frequency of perianal fistulas in CD was reported as 12% at 1 year, 15% at 5 years, 21% at 10 years, and 26% at 20 years[2]. In New Zealand, the cumulative incidence at 20 years of any perianal fistula in CD was reported as 28.3%[55]. No published studies on the prevalence of CPF in CD were identified in this review.
This review identified only one study evaluating the costs of CPF in patients with CD in Europe, highlighting a significant gap in the literature. A 2010 retrospective study conducted in Spain estimated the direct costs of CPF to be €8289 per patient per year, with biologic treatment costs a main cost driver, representing 61.4% of direct costs[56]; however, it is difficult to separate the costs attributable directly to CPF vs costs related to the underlying CD. A 2008 United States study found that patients with fistulising CD had higher mean direct costs than patients without fistula ($35373 vs $15564 per patient per year; P < 0.0001)[57]. As both studies considered only direct health care costs and are outdated, the reported costs are likely an underestimation of current total costs of CPF in patients with CD. Moreover, because the resolution of CPF in CD may take years, long-term total costs are likely to exceed published estimates of annual estimated costs. Further research is needed to characterise the economic burden of CPF in patients with CD.
Several European clinical guidelines related to treatment of CPF in patients with CD are currently available. Although recommendations vary slightly between guidelines, a combination of surgical and medical treatment is generally recommended. Recently, a new treatment algorithm for perianal fistulising disease, including CPF, in CD has been proposed that recommends a top-down approach for patients with CPF and/or proctitis[58]. The authors suggest local injection of mesenchymal stem cells can be considered in patients without proctitis who do not respond to conventional therapies. In general, surgery may still be required in a high proportion of patients and should not be delayed when criteria of drug failure are met. Timely assessment of response to medical therapy is essential to initiate alternative therapies or surgery[58].
This review also highlights low response rates with most treatments, and the considerable unmet needs that remain for patients with CD and CPF. A total of 45 studies reporting treatment outcomes for this population was identified, most of which were small observational studies. Differences in study methodologies, populations, definition of endpoints, and duration of follow-up in each study make comparison across studies difficult and emphasise the need for studies in larger CPF populations with long-term follow-up. Definitions for outcomes assessment differed considerably between studies, and few studies included assessments of response by MRI (a recently published review details the issues in assessment of fistula disease activity[58]). Due to large differences in the follow-up time and lack of specificity in published results, it is not possible to differentiate between primary failure and loss of response with the treatments evaluated in this review. Moreover, the effect of placebo response must be considered when interpreting response rates[55] Bearing in mind these challenges in interpretation, response rates ranged from 20% to 100% across all studies; for most studies, they were between 50% and 85% (Supplementary Table 4). Treatment failure rates varied greatly, ranging from 0% to 80% across studies; rates of relapse also varied, ranging from 0% to 66% (Figure 4). These findings suggest that many patients experience inadequate response to treatment, fail treatment altogether, or go on to experience relapse, highlighting the need for more effective treatments.
Expanded ASCs may be a viable treatment option for patients with CD and treatment-refractory CPF - a group of patients for whom treatment options are currently limited. In a randomised controlled trial, the eASC treatment darvadstrocel has shown significant improvement in combined remission rates relative to control treatment at 24 wks in patients who were treatment refractory[3]. Importantly, the response was durable; a study of the long-term efficacy of eASCs (published after this review was conducted) found that the proportion of patients in combined remission 52 wk after the single injection remained stable (eASCs 56%, placebo 39%, P = 0.010)[59].
In addition, there is evidence from a recent study that higher infliximab trough levels are associated with perianal fistula healing in patients with CD[60]. In this cross-sectional study enrolling 117 patients with CD who had (any) perianal fistula and were treated with infliximab for at least 24 wk, patients who achieved fistula healing had significantly higher median serum infliximab levels (≥ 10.1 μg/mL) compared with patients with active fistulas. Moreover, there was an incremental gain in fistula healing with higher infliximab levels[60]. Further research is needed to estimate clinical utility of infliximab trough levels and costs associated with dose escalation in the treatment of CPF in patients with CD.
Evidence on the efficacy of thiopurines for the treatment of CPF in CD is limited. Most studies evaluating thiopurines in this review evaluated them in combination with an anti-TNF-α agent. Earlier studies of thiopurines conducted before anti-TNF-α agents were marketed are inconclusive regarding the efficacy of thiopurines for fistula healing in general and provide no outcomes data for CPF patients or use as surgical pre-treatment[61]. Moreover, a Cochrane systematic literature review and meta-analysis of randomised controlled trials for azathioprine or 6-mercaptopurine induction therapy, focusing on subgroups of CD patients with perianal fistulas, indicated a lack of efficacy in terms of fistula improvement or healing[61] (fistula healing was not assessed in a parallel Cochrane review of use of thiopurine in maintenance[62]).
No identified studies presented specific data for dose escalations with infliximab, and there is a lack of evidence on how biologics impact the remission of CPF in real-world settings. Recent published evidence suggests that a treat-to-target approach using dose escalation may improve outcomes in patients with CD[60]. Moreover, there is a need for randomised controlled trials assessing outcomes of medical, surgical, and combination treatment of CPF in patients with CD. Few studies assessed factors predicting response to therapy (including activity of luminal CD), and those that did found few or no correlations[58]. No identified studies assessed the influence of active proctitis on treatment decisions and outcomes in patients with CD and CPF, although the association of proctitis with negative outcomes is well-documented for patients with CD and any perianal fistula[17,58]. Additionally, few studies reported the proportion of patients with CD with CPF who developed incontinence. It has previously been reported that HRQOL is significantly affected by faecal incontinence related to inflammatory bowel disease[63]. In another study, patients were highly motivated to relieve anal incontinence, with 85% inclined to accept a stoma for relief of the symptom[64]. Finally, time between diagnosis of CD and development of CPF was not frequently reported. In a Dutch retrospective single-centre study, 66 of 232 (28.4%) patients with CD and perianal fistulas (78% had CPF) were diagnosed with perianal fistulas within 6 mo after diagnosis of CD[13]. Among patients who were diagnosed after 6 mo, median time to diagnosis of perianal CD was 7.0 (range, 0.7-38.0) years (although it must be noted that this study excluded patients who were diagnosed with perianal disease before diagnosis of CD, which was approximately 10% of the 436 patients initially assessed for eligibility)[13]. In a retrospective Israeli study, diagnosis of perianal disease preceded diagnosis of CD in 5 of 52 (9.6%) patients, was evident at presentation (it is assumed this refers to diagnosis of CD) in 27 of 52 (52%) patients, and was confirmed during disease course in 20 of 52 (38.4%) patients[14].
The study results should be interpreted in view of several strengths and limitations. The study followed the robust methodology of a systematic literature review, and the scope was broad and included all treatment types and clinical studies across regions. Nevertheless, a particular limitation of this review is that CD patients with CPF constitute a rare, small, geographically disparate, and clinically heterogenous patient population, and it is challenging to evaluate outcomes in a large number of patients and control for their heterogeneity. Accordingly, many identified studies on treatment outcomes were small, retrospective, and/or non-comparative. As study methods, populations, definition of endpoints, and duration of follow-up varied widely, meaningful comparison among these studies is difficult. Definitions of CPF were particularly variable across studies (see Supplementary Table 4 for details); for instance, some studies included rectovaginal fistulas as part of their definition of CPF, whereas others did not (outcomes of rectovaginal fistulas, if not included as part of the overall definition of CPF, were not a specific focus of this review). Consensus on valid definitions for CPF and CPF outcomes would be valuable for future studies. Articles were categorised as reporting outcomes for medical therapy only, surgical therapy only (with major and minor surgeries classified accordingly for the purposes of this review), or combination therapy (and in refractory patients or not) as reported by the author; however, across all studies, most patients received multiple prior/current treatments.
The identification of a lack of quality evidence and high variation in response rates is consistent with previous reviews of publications in fistulising CD[7,65]. An American Gastroenterological Association review of 29 studies involving patients with high or complex fistulas reported largely heterogeneous results, with postoperative healing rates ranging from 0% to 100%, recurrence rates from 0% to 75%, and proctectomy rates from 0% to 60%[65-74].
In conclusion, there is a paucity of data on the epidemiology and burden of CPF in patients with CD. CPF is associated with reduced HRQOL in patients with CD. Current treatment options include a combination of medical and surgical interventions. Based on results from various small observational studies reviewed, rates of failure and relapse are generally high. Consequently, there is a need for effective treatment options for CPF in patients with CD, especially those refractory to anti-TNF-α agents.
ARTICLE HIGHLIGHTS
Research background
Perianal fistulas occur in an estimated 26% of patients with Crohn’s disease (CD) in the two decades following diagnosis, and 70% to 80% of these fistulas are complex perianal fistulas (CPF). CPF involve the upper part of the sphincter complex, have multiple external openings (tracts), are associated with pain or fluctuation suggesting a perianal abscess, and/or are associated with a rectovaginal fistula or anorectal stricture. The available evidence, while limited, suggests that CPF can result in significantly diminished health-related quality of life (HRQOL) and considerable disease burden. Understanding disease burden and unmet needs for patients with CPF may inform the development of more effective treatment strategies in this population.
Research motivation
To date, no systematic literature review evaluating the epidemiology, burden, and management of CPF in patients with CD has been conducted.
Research objectives
The objective of this study was to systematically review the literature on epidemiology, disease burden, and treatment outcomes for CD patients with CPF, thus improving understanding of the burden of CPF.
Research methods
A systematic search of electronic medical literature databases and relevant conferences was conducted in November 2016. Combinations of free text and Medical Subject Headings terms were used for the searches. The population of interest was adult (≥ 18 years) patients with CD and/or CPF undergoing medical or surgical therapy. CPF were generally defined as fistulas with intersphincteric, transsphincteric, suprasphincteric, extrasphincteric, or horseshoe tracts, although definitions of CPF varied between studies. Relevant studies reported on the epidemiology, burden, costs, treatment patterns, and/or treatment outcomes. Articles were selected by two independent researchers. Titles and abstracts of identified articles were first reviewed for relevance according to the inclusion and exclusion criteria. Full-text articles selected were then reviewed for relevance using the same criteria. Data were extracted from the included studies.
Research results
A total of 62 relevant studies were identified. Most included studies were small observational studies. The cumulative incidence of CPF in CD from two referral-centre studies was 12%-14%. CPF result in significant impairments in HRQOL. Up to 59% of patients are at risk of faecal incontinence, an outcome with significant effects on HRQOL in inflammatory bowel disease. Treatments for CPF include combinations of medical and surgical interventions and expanded allogeneic adipose-derived stem cells. High proportions of patients experience lack of or inadequate response to treatments commonly reported in the literature. Failure and relapse rates, respectively, for medical therapies were 12%-73% and 0%-41%; for surgical therapies were 0%-100% and 11%-20%; for combined medical and surgical therapies were 0%-80% and 0%-50%; and for allogenic adipose-derived stem cell therapies were 29%-47% and not reported). Few studies (1 of infliximab; 3 of surgical interventions) have been conducted in treatment-refractory patients, a population with considerable unmet needs.
Research conclusions
CPF in patients with CD are associated with considerable clinical and humanistic burden. Effective treatments are needed, especially for patients refractory to anti- tumour necrosis factor alpha agents, as evidenced by high failure and relapse rates with therapies evaluated in the literature.
Research perspectives
Most studies reviewed were small, observational studies. Based on the available evidence, patients with CPF face significant burden; however future research should more fully characterise the epidemiology and burden of CPF in patients with CD.
ACKNOWLEDGMENTS
Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Takeda.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: This study was conducted under a research contract between RTI Health Solutions and Takeda and was funded by Takeda. The preparation of this paper was funded by Takeda. Shahnaz Khan and Joan Forns are salaried employees of RTI Health Solutions. Ewa Rupniewska was a salaried employee of RTI Health Solutions when this research was conducted. Haridarshan Patel, Javaria Mona Khalid, Daniela Bojic are salaried employees of Takeda. Julian Panes and Walter Reinisch are consultants for Takeda.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: July 26, 2018
First decision: August 27, 2018
Article in press: October 21, 2018
P- Reviewer: Lara FJ, Taxonera C S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Julian Panes, Department of Gastroenterology, Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona 08036, Spain.
Walter Reinisch, Medical University of Vienna, Vienna 1090, Austria.
Ewa Rupniewska, RTI Health Solutions, Manchester M20 2LS, United Kingdom.
Shahnaz Khan, RTI Health Solutions, Research Triangle Park, NC 27709, United States. skhan@rti.org.
Joan Forns, RTI Health Solutions, Barcelona 08028, Spain.
Javaria Mona Khalid, Takeda, Deerfield, IL 60015, United States.
Daniela Bojic, Takeda, Deerfield, IL 60015, United States.
Haridarshan Patel, Takeda, Deerfield, IL 60015, United States.
References
- 1.Taxonera C, Schwartz DA, García-Olmo D. Emerging treatments for complex perianal fistula in Crohn’s disease. World J Gastroenterol. 2009;15:4263–4272. doi: 10.3748/wjg.15.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 3.Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 4.American Gastroenterological Association Clinical Practice Committee. American Gastroenterological Association medical position statement: perianal Crohn’s disease. Gastroenterology. 2003;125:1503–1507. doi: 10.1016/j.gastro.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A. Management of complex perianal Crohn’s disease. Ann Gastroenterol. 2017;30:33–44. doi: 10.20524/aog.2016.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzo M, Felice C, Pugliese D, Andrisani G, Mocci G, Armuzzi A, Guidi L. Management of perianal fistulas in Crohn’s disease: an up-to-date review. World J Gastroenterol. 2015;21:1394–1403. doi: 10.3748/wjg.v21.i5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasscock ME 3rd. Pediatric otology. J Tenn Med Assoc. 1971;64:19–28. [PubMed] [Google Scholar]
- 8.Chaparro M, Burgueno P, Vera I, Bermejo F, Marin-Jimenez I, Yela C, Lopez P, Martin M, Taxonera C, Botella B, et al. Epidemiological study of perianal fistulas in patients with Crohn’s disease. Gastroenterology 201; 140 Suppl: S736 [Google Scholar]
- 9.Morete M, Puig P, Fuster L, Vila G, Ollero V, Echarri A. Inflammatory bowel disease and complicated perianal fistulas: a 15 year follow-up. J Crohns Colitis. 2013;7 Suppl:S299. [Google Scholar]
- 10.Haennig A, Staumont G, Lepage B, Faure P, Alric L, Buscail L, Bournet B, Moreau J. The results of seton drainage combined with anti-TNFα therapy for anal fistula in Crohn’s disease. Colorectal Dis. 2015;17:311–319. doi: 10.1111/codi.12851. [DOI] [PubMed] [Google Scholar]
- 11.Alessandroni L, Papi C, Papparella L, Addarii M, Kohn A. Clinical course of perianal fistulas in Crohn’s disease: a retrospective study. J Crohns Colitis. 2013;7 Suppl:S125–S126. [Google Scholar]
- 12.Riss S, Schwameis K, Mittlböck M, Pones M, Vogelsang H, Reinisch W, Riedl M, Stift A. Sexual function and quality of life after surgical treatment for anal fistulas in Crohn’s disease. Tech Coloproctol. 2013;17:89–94. doi: 10.1007/s10151-012-0890-x. [DOI] [PubMed] [Google Scholar]
- 13.Molendijk I, Nuij VJ, van der Meulen-de Jong AE, van der Woude CJ. Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm Bowel Dis. 2014;20:2022–2028. doi: 10.1097/MIB.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 14.Lahat A, Assulin Y, Beer-Gabel M, Chowers Y. Endoscopic ultrasound for perianal Crohn’s disease: disease and fistula characteristics, and impact on therapy. J Crohns Colitis. 2012;6:311–316. doi: 10.1016/j.crohns.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bell SJ, Williams AB, Wiesel P, Wilkinson K, Cohen RC, Kamm MA. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1145–1151. doi: 10.1046/j.1365-2036.2003.01561.x. [DOI] [PubMed] [Google Scholar]
- 16.Mueller MH, Geis M, Glatzle J, Kasparek M, Meile T, Jehle EC, Kreis ME, Zittel TT. Risk of fecal diversion in complicated perianal Crohn’s disease. J Gastrointest Surg. 2007;11:529–537. doi: 10.1007/s11605-006-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. doi: 10.1136/gutjnl-2013-306709. [DOI] [PubMed] [Google Scholar]
- 18.Arberas-Diez B, Chaparro M, Burgueno P, Vera I, Bermejo F, Marin-Jimenez I, Yela C, Lopez P, Martin M, Taxonera C, et al. P536 Effectiveness of the available therapeutic options in the treatment of perianal fistulas in patients with Crohn’s disease (CD) J Crohns Colitis. 2013;7 Suppl:S225. [Google Scholar]
- 19.Gionchetti P, Dignass A, Danese S, Dias FJM, Rogler G, Lakatos PL, Adamina M, Ardizzone S, Buskens CJ, Sebastian S. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: Surgical management and special situations. J Crohns Colitis. 2016 doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 20.Orlando A, Armuzzi A, Papi C, Annese V, Ardizzone S, Biancone L, Bortoli A, Castiglione F, D’Incà R, Gionchetti P, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: The use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1–20. doi: 10.1016/j.dld.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Preiß JC, Bokemeyer B, Buhr HJ, Dignaß A, Häuser W, Hartmann F, Herrlinger KR, Kaltz B, Kienle P, Kruis W, et al. [Updated German clinical practice guideline on “Diagnosis and treatment of Crohn’s disease” 2014] Z Gastroenterol. 2014;52:1431–1484. doi: 10.1055/s-0034-1385199. [DOI] [PubMed] [Google Scholar]
- 22.Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 23.EMA. Ustekinumab summary of product characteristics. Available from: http: //www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000958/WC500058513.pdf. December 11, 2017.
- 24.Erzin Y, Hatemi I, Ercaliskan K, Eyice D, Baca B, Celik A. P304 Anti-TNF based treatment regimens (triple: anti- TNF, azathioprine, antibiotic vs double: anti-TNF, azathioprine or anti-TNF, antibiotic) on complex perianal fistula healing in Crohn’s disease patients: a retrospective clinical data assessment. J Crohns Colitis. 2013;7 Suppl:S130–S131. [Google Scholar]
- 25.Erzin Y, Ercaliskan A, Hatemi I, Eyice D, Baca B, Demir N, Celik A. P295 What is our success on complex perianal fistula healing in the clinic? From antibiotic to combined anti-TNF based treatment, ending with or without ileostomy. J Crohns Colitis. 2014;8 Suppl:S186–S187. [Google Scholar]
- 26.Erzin Y, Ercaliskan K, Hatemi I, Atay K, Bozcan S, Demir N, Celik A. P334 Evolution of a long-term follow-up cohort of Crohn’s disease with complex perianal fistula: from antibiotic to combined AZA and anti-TNF based treatment ending up clinical and radiological healing with or without stoma. J Crohns Colitis. 2016;10 Suppl:S262. [Google Scholar]
- 27.Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595–600. doi: 10.1007/s00384-011-1350-1. [DOI] [PubMed] [Google Scholar]
- 28.Wiese DM, Beaulieu D, Slaughter JC, Horst S, Wagnon J, Duley C, Annis K, Nohl A, Herline A, Muldoon R, et al. Use of Endoscopic Ultrasound to Guide Adalimumab Treatment in Perianal Crohn’s Disease Results in Faster Fistula Healing. Inflamm Bowel Dis. 2015;21:1594–1599. doi: 10.1097/MIB.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz DA, White CM, Wise PE, Herline AJ. Use of endoscopic ultrasound to guide combination medical and surgical therapy for patients with Crohn’s perianal fistulas. Inflamm Bowel Dis. 2005;11:727–732. doi: 10.1097/01.mib.0000172811.57242.18. [DOI] [PubMed] [Google Scholar]
- 30.Duff S, Sagar PM, Rao M, Dolling S, Sprakes M, Hamlin PJ. Infliximab and surgical treatment of complex anal Crohn’s disease. Colorectal Dis. 2012;14:972–976. doi: 10.1111/j.1463-1318.2011.02811.x. [DOI] [PubMed] [Google Scholar]
- 31.Ardizzone S, Maconi G, Colombo E, Manzionna G, Bollani S, Bianchi Porro G. Perianal fistulae following infliximab treatment: clinical and endosonographic outcome. Inflamm Bowel Dis. 2004;10:91–96. doi: 10.1097/00054725-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Alessandroni L, Kohn A, Cosintino R, Marrollo M, Papi C, Monterubbianesi R, Tersigni R. Local injection of infliximab in severe fistulating perianal Crohn’s disease: an open uncontrolled study. Tech Coloproctol. 2011;15:407–412. doi: 10.1007/s10151-011-0759-4. [DOI] [PubMed] [Google Scholar]
- 33.Castaño-Milla C, Chaparro M, Saro C, Barreiro-de Acosta M, García-Albert AM, Bujanda L, Martín-Arranz MD, Carpio D, Muñoz F, Manceñido N, et al. Effectiveness of adalimumab in perianal fistulas in crohn’s disease patients naive to anti-TNF therapy. J Clin Gastroenterol. 2015;49:34–40. doi: 10.1097/MCG.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 34.Chung W, Ko D, Sun C, Raval MJ, Brown CJ, Phang PT. Outcomes of anal fistula surgery in patients with inflammatory bowel disease. Am J Surg. 2010;199:609–613. doi: 10.1016/j.amjsurg.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Schaden D, Schauer G, Haas F, Berger A. Myocutaneous flaps and proctocolectomy in severe perianal Crohn’s disease--a single stage procedure. Int J Colorectal Dis. 2007;22:1453–1457. doi: 10.1007/s00384-007-0337-4. [DOI] [PubMed] [Google Scholar]
- 36.Baldin A. Fistula tract transposition for extrasphincteric perianal fistulae in Crohn’s Disease. Gastroenterology. 2016;150 Suppl:S1263. [Google Scholar]
- 37.Roumeguère P, Bouchard D, Pigot F, Castinel A, Juguet F, Gaye D, Capdepont M, Zerbib F, Laharie D. Combined approach with infliximab, surgery, and methotrexate in severe fistulizing anoperineal Crohn’s disease: results from a prospective study. Inflamm Bowel Dis. 2011;17:69–76. doi: 10.1002/ibd.21405. [DOI] [PubMed] [Google Scholar]
- 38.Sciaudone G, Di Stazio C, Limongelli P, Guadagni I, Pellino G, Riegler G, Coscione P, Selvaggi F. Treatment of complex perianal fistulas in Crohn disease: infliximab, surgery or combined approach. Can J Surg. 2010;53:299–304. [PMC free article] [PubMed] [Google Scholar]
- 39.Antakia R, Shorthouse AJ, Robinson K, Lobo AJ. Combined modality treatment for complex fistulating perianal Crohn’s disease. Colorectal Dis. 2013;15:210–216. doi: 10.1111/j.1463-1318.2012.03124.x. [DOI] [PubMed] [Google Scholar]
- 40.Talbot C, Sagar PM, Johnston MJ, Finan PJ, Burke D. Infliximab in the surgical management of complex fistulating anal Crohn’s disease. Colorectal Dis. 2005;7:164–168. doi: 10.1111/j.1463-1318.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 41.Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn’s disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis. 2003;9:98–103. doi: 10.1097/00054725-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Löffler T, Welsch T, Mühl S, Hinz U, Schmidt J, Kienle P. Long-term success rate after surgical treatment of anorectal and rectovaginal fistulas in Crohn’s disease. Int J Colorectal Dis. 2009;24:521–526. doi: 10.1007/s00384-009-0638-x. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Kang S, Park K, Jung H, Song I. Are combined medical and surgical treatments optimal therapy for complex perianal fistula in Crohn’s disease? 6th Congress of the European Crohn’s and Colitis Organisation. Dublin, Ireland; 2011. [Google Scholar]
- 44.EMA. Alofisel (darvadstrocel), 2018 [Google Scholar]
- 45.de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 47.Lozynskyy YS. Treatment algorithms in the case of perianal complications of Crohn’s disease. Dig Dis. 2009;27:565–570. doi: 10.1159/000233299. [DOI] [PubMed] [Google Scholar]
- 48.Maeda Y, Heyckendorff-Diebold T, Tei TM, Lundby L, Buntzen S. Gracilis muscle transposition for complex fistula and persistent nonhealing sinus in perianal Crohn’s disease. Inflamm Bowel Dis. 2011;17:583–589. doi: 10.1002/ibd.21311. [DOI] [PubMed] [Google Scholar]
- 49.Mennigen R, Laukötter M, Senninger N, Rijcken E. The OTSC(®) proctology clip system for the closure of refractory anal fistulas. Tech Coloproctol. 2015;19:241–246. doi: 10.1007/s10151-015-1284-7. [DOI] [PubMed] [Google Scholar]
- 50.Gingold DS, Murrell ZA, Fleshner PR. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn’s disease. Ann Surg. 2014;260:1057–1061. doi: 10.1097/SLA.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 51.van der Hagen SJ, Baeten CG, Soeters PB, Russel MG, Beets-Tan RG, van Gemert WG. Anti-TNF-alpha (infliximab) used as induction treatment in case of active proctitis in a multistep strategy followed by definitive surgery of complex anal fistulas in Crohn’s disease: a preliminary report. Dis Colon Rectum. 2005;48:758–767. doi: 10.1007/s10350-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 52.Galis-Rozen E, Tulchinsky H, Rosen A, Eldar S, Rabau M, Stepanski A, Klausner JM, Ziv Y. Long-term outcome of loose seton for complex anal fistula: a two-centre study of patients with and without Crohn’s disease. Colorectal Dis. 2010;12:358–362. doi: 10.1111/j.1463-1318.2009.01796.x. [DOI] [PubMed] [Google Scholar]
- 53.Göttgens KW, Smeets RR, Stassen LP, Beets GL, Pierik M, Breukink SO. Treatment of Crohn’s disease-related high perianal fistulas combining the mucosa advancement flap with platelet-rich plasma: a pilot study. Tech Coloproctol. 2015;19:455–459. doi: 10.1007/s10151-015-1311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jarrar A, Church J. Advancement flap repair: a good option for complex anorectal fistulas. Dis Colon Rectum. 2011;54:1537–1541. doi: 10.1097/DCR.0b013e31822d7ddd. [DOI] [PubMed] [Google Scholar]
- 55.Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum. 2012;55:773–777. doi: 10.1097/DCR.0b013e31825228b0. [DOI] [PubMed] [Google Scholar]
- 56.Chaparro M, Zanotti C, Burgueño P, Vera I, Bermejo F, Marín-Jiménez I, Yela C, López P, Martín MD, Taxonera C, et al. Health care costs of complex perianal fistula in Crohn’s disease. Dig Dis Sci. 2013;58:3400–3406. doi: 10.1007/s10620-013-2830-7. [DOI] [PubMed] [Google Scholar]
- 57.Cohen RD, Waters HC, Tang B, Rahman MI. Effects of fistula on healthcare costs and utilization for patients with Crohn’s disease treated in a managed care environment. Inflamm Bowel Dis. 2008;14:1707–1714. doi: 10.1002/ibd.20530. [DOI] [PubMed] [Google Scholar]
- 58.Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:652–664. doi: 10.1038/nrgastro.2017.104. [DOI] [PubMed] [Google Scholar]
- 59.Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, et al. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2018;154:1334–1342.e4. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D, Patel A, Best K, Fox C, Idstein K, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45:933–940. doi: 10.1111/apt.13970. [DOI] [PubMed] [Google Scholar]
- 61.Chande N, Townsend CM, Parker CE, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;10:CD000545. doi: 10.1002/14651858.CD000545.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015:CD000067. doi: 10.1002/14651858.CD000067.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norton C, Dibley LB, Bassett P. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. J Crohns Colitis. 2013;7:e302–e311. doi: 10.1016/j.crohns.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Mahadev S, Young JM, Selby W, Solomon MJ. Quality of life in perianal Crohn’s disease: what do patients consider important? Dis Colon Rectum. 2011;54:579–585. doi: 10.1007/DCR.0b013e3182099d9e. [DOI] [PubMed] [Google Scholar]
- 65.Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–1530. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 66.Michelassi F, Melis M, Rubin M, Hurst RD. Surgical treatment of anorectal complications in Crohn’s disease. Surgery. 2000;128:597–603. doi: 10.1067/msy.2000.108779. [DOI] [PubMed] [Google Scholar]
- 67.Guidi L, Ratto C, Semeraro S, Roberto I, De Vitis I, Papa A, Marzo M, Parello A, Foglietto G, Doglietto GB, et al. Combined therapy with infliximab and seton drainage for perianal fistulizing Crohn’s disease with anal endosonographic monitoring: a single-centre experience. Tech Coloproctol. 2008;12:111–117. doi: 10.1007/s10151-008-0411-0. [DOI] [PubMed] [Google Scholar]
- 68.Solina G, Renna S, Orlando E, Affronti M, Cottone M, Orlando A. P461 Clinical benefit of adalimumab after surgery in the treatment of complex perianal Crohn’s disease: a tertiary referral centre experience. J Crohns Colitis. 2016;10 Suppl:S334. [Google Scholar]
- 69.Solina G, Orlando A, Renna S, Mocciaro F, Gioia F, Olivo M, Rizzuto G, Sinagra E, Cottone M. P186 Treatment of perianal disease in Crohn’s disease: efficacy and safety of a multidisciplinary approach in a tertiary referral centre. Dig Liver Dis. 2011;43 Suppl:S215. [Google Scholar]
- 70.Schwartz DA, Cross R, Regueiro M, Ghazi LJ, Swoger JM, Beaulieu DB, Horst SN, Flasar M, Patil S, Herline A, et al. Sa1263 A prospective multicenter trial evaluating the benefit of initial seton placement prior to starting anti-TNF therapy for the treatment of Crohn’s perianal fistulas. Gastroenterology. 2015;148(4):S274–S275. [Google Scholar]
- 71.Spradlin NM, Wise PE, Herline AJ, Muldoon RL, Rosen M, Schwartz DA. A randomized prospective trial of endoscopic ultrasound to guide combination medical and surgical treatment for Crohn’s perianal fistulas. Am J Gastroenterol. 2008;103:2527–2535. doi: 10.1111/j.1572-0241.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- 72.Laureti S, Coscia M, Gentilini L, Ugolini F, Vitali G, Vittori L, Rizzello F, Gionchetti P, Calabrese C, Calafiore A, et al. P393 Combination of surgical therapy and local injections of adalimumab in treatment of complex perianal Crohn’s disease. J Crohns Colitis. 2012;6 Suppl:S166. [Google Scholar]
- 73.Poggioli G, Laureti S, Pierangeli F, Rizzello F, Ugolini F, Gionchetti P, Campieri M. Local injection of Infliximab for the treatment of perianal Crohn’s disease. Dis Colon Rectum. 2005;48:768–774. doi: 10.1007/s10350-004-0832-4. [DOI] [PubMed] [Google Scholar]
- 74.Echarri A, Castro J, Barreiro M, Carpio D, Pereira S, Lorenzo A. Evaluation of adalimumab therapy in multidisciplinary strategy for perianal Crohn’s disease patients with infliximab failure. J Crohns Colitis. 2010;4:654–660. doi: 10.1016/j.crohns.2010.07.012. [DOI] [PubMed] [Google Scholar]


