Abstract
Liver injury is a characteristic feature of human immunodeficiency virus (HIV) infection, which is the second most common cause of mortality in HIV-infected patients. Now it is recognized that liver plays a key role in HIV infection pathogenesis. Antiretroviral therapy (ART), which suppresses HIV infection in permissive immune cells, is less effective in hepatocytes, thereby making these cells a silent reservoir of HIV infection. In addition to direct hepatotoxic effects of HIV, certain ART treatment modalities provide hepatotoxic effects. The exact mechanisms of HIV-triggered chronic hepatitis progression are not elucidated, but the liver is adversely affected by HIV-infection and liver cells are prominently involved in HIV-elicited injury. These effects are potentiated by second hits like alcohol. Here, we will focus on the incidence of HIV, clinical evidence of HIV-related liver damage, interactions between HIV and liver cells and the role of alcohol and co-infection with hepatotropic viruses in liver inflammation and fibrosis progression.
Keywords: Liver cells, Antiretroviral therapy, Apoptosis, Inflammation, Fibrosis, Immunodeficiency virus, Alcohol
Core tip: Here, we summarized the literature and our recent findings on human immunodeficiency virus (HIV)-related liver damage. Liver injury is the second and most frequent cause of HIV patients’ death after acquired immune deficiency syndrome. The results of clinical studies support close association between HIV severity and liver disease progression. It is clear now that both liver parenchymal and non-parenchymal cells play a significant role in HIV-triggered liver inflammation and fibrosis pathogenesis and might serve as reservoirs of HIV-infection. Hepatotoxicity comes from the direct interactions between HIV and liver cells as well as from harmful effects of anti-retroviral therapy potentiated by second insults, like alcohol.
INTRODUCTION
Incidence of human immunodeficiency virus-infection
The United Nations reported that at the end of 2017, worldwide about 36.9 million people are living with human immunodeficiency virus (HIV) infections, which include 1.8 million children[1]. Annual number of HIV infections in the United States peaked in the mid-1980s, then decreased through the early 1990s, and remained relatively stable through 2010[2,3]. The Centers for Disease Control and Prevention estimated that at the end of 2015, 1.1 million persons aged 13 and older were living with HIV infection in the United States, including 162500 (15%) persons whose infection had never been diagnosed. From 1987 to 2015, about 507351 people died from HIV disease[4]. The highest incidence of HIV in United States is in southern states, which are home to around 45% of all people living with HIV. This accounts for about 50% of the new diagnoses every year in the United States[5]. Around 70% of annual new HIV infections occur among gay and other men who have sex with men, among whom African American men are most affected, followed by Latino/Hispanic men. Heterosexual African American women and transgender women of all ethnicities are also disproportionately afflicted[4].
Clinical evidence of HIV-related liver damage
Liver disease is now recognized as the most common non-acquired immune deficiency syndrome (AIDS) related cause of death among HIV-infected patients and accounts for 14%-18% of all deaths in this group[6-8]. Approximately 50% of deaths among hospitalized HIV-infected patients in the antiretroviral therapy (ART)-era have been attributed to liver problems[9,10]. While HIV mono-infection elevates liver transaminase levels, the exacerbation of liver injury is even more prominent when HIV patients are co-infected with hepatotropic viruses [hepatitis C virus (HCV) and hepatitis B virus (HBV)] or are exposed to liver-damaging substances, such as alcohol[11]. Thus, managing the liver complications is very important during the care of HIV-infected individuals[12].
After 30 years of HIV pandemic, with the wide use of effective ART that increased HIV-infected patient survival, liver injury became recognized as an important clinical manifestation of HIV-infection affecting its outcomes[13]. It has been shown that the liver plays a key role in the clearance of circulating viral particles in the blood as supported by data obtained in simian immunodeficiency virus (SIV)-infected monkeys[14-17]. Liver transaminases are frequently elevated in the sera of HIV-infected patients even in the absence of accompanying viral hepatitis[18]. In addition, there is an association between HIV RNA content and liver fibrosis[19].
Liver manifestations accompanying HIV-infection may exist with no relationship to ART treatment outcomes and thus, whether HIV infection itself, immunodeficiency, and long-term ART are associated with the development of chronic liver injury is still a matter of debate. Previously, HIV-related liver injury was interpreted as a consequence of opportunistic infections or AIDS-induced malignancies, but recent data indicate that HIV itself may affect the liver. In HIV-monoinfected patients, high HIV-1 load was shown to be an independent risk factor for the development of chronic elevated alanine aminotransferase (ALT)[20,21] and steatosis[22]. Furthermore, a detectable HIV load combined with an aspartate aminotransferase (AST)-to-platelet ratio index (APRI) greater than 1.5 is the risk factor of liver disease development and is surrogative for significant fibrosis[23]. The impact of HIV viremia on liver fibrosis has been also described in HIV/HCV-coinfected patients[24]. Nevertheless, many clinical and epidemiological studies reported that HIV directly induces liver fibrinogenesis[25,26] in patients without viral hepatitis co-infection. A large North American clinical investigation demonstrated that HIV RNA plasma levels were associated with increased Fibrosis-4 (FIB-4) score in the absence of HBV, HCV, ART or alcohol use[27]. Studies from three groups using transient liver elastography detected liver injury in HIV-monoinfected patients[28-32], which correlates with high plasma HIV RNA levels[20]. Immunodeficiency-associated liver injury was also reported in a retrospective analysis of HIV-monoinfected patients with increased ALT values and a CD4+ cell count less than 200 cells/mL[21]. In a cross-sectional study, CD4+ cells level less than 200 /mL was considered as a predictor for abnormal liver stiffness[30].
In addition to HIV-induced hepatotoxicity in ART non-exposed individuals, long-term treatment of HIV-patients with ART might be hepatotoxic, and especially this is a case for the old drugs, like azidothymidine, nevirapine and nucleoside analogs[33-35]. Previous observational data investigating risks for death suggested possible chronic ART-related hepatotoxicity[36,37]. Importantly, recent Spanish cross-sectional study found that prolonged ART exposure caused advanced liver fibrosis in HIV-monoinfected patients, but conversely, was protective for advanced fibrosis in HCV-coinfected patients. Didanosine and stavudine were identified as being of particular risk for fibrosis in both HIV-monoinfected and HCV-coinfected persons[38]. This observation was confirmed by several cross-sectional studies[20,30,39,40]. With further development of anti-HIV drugs, modern ART became less hepatotoxic. However, there are still reports on ART-associated hepatic syndromes, such as nonalcoholic fatty liver disease and non-cirrhotic portal hypertension in HIV-positive individuals[41]. Hepatotoxic effects of ART are developed via multiple mechanisms, including mitochondrial damage, generation of liver-toxic products by drug metabolism, drug-induced hypersensitivity reactions and immunosuppression[42,43].
Viral load is essential to trigger liver damage. In fact, close association between AST levels and HIV viral load has been shown in HIV-infected patients[18]. In addition, mathematical modeling of liver enzyme elevation in HIV-mono-infection also revealed that significant elevation of alanine aminotransferase coincides with increased HIV-load[44]. These data were confirmed by experimental studies on HIV-infected humanized mice demonstrating that the decrease in human albumin levels correlated with a decline in CD4+ cells in the liver and with an increase of HIV-1 viral load[45].
HIV AND LIVER CELLS
The liver contains parenchymal cells (hepatocytes) and various non-parenchymal cells. Hepatocytes can produce a characteristic protein response to noxious stimuli, termed the acute-phase response, which in turn, regulates immune cell responses. Non-parenchymal cells, such as Kupffer cells, sinusoidal endothelial cells, hepatic stellate cells (HSC), dendritic cells, and liver-associated lymphocytes play a role in immunologic surveillance within the hepatic sinusoids. Kupffer cells are located mainly in the periportal area, which allows them to phagocytose and eliminate pathogens entering the liver via the portal blood flow[46].
There is growing evidence to suggest that HIV may interact with several hepatic cell types. However, detailed evaluation of HIV replication in liver tissue has not been addressed to date. To this end, several possibilities can exist. First, viral antigens per se may engage liver cell populations without the need for viral infections. Liver cells may respond to viral antigens, which are the part of infectious virions (full viral particle) or defective virions that are unable to productively infect any cell type. These viral proteins may also represent antigens that have been shed from virions and are circulating freely[47]. In the case of HIV, these soluble antigens consist largely of the envelope glycoprotein 120 (gp120) and the trans-activator protein Tat. Other HIV proteins from the lysis of HIV-infected cells may be at a low concentration since they are diluted in the systemic circulation and therefore, unlikely demonstrate any appreciable effect in vivo. In addition to interactions between viral antigens and liver cells, there is evidence to suggest that several distinct liver cell populations, including hepatocytes, hepatic stellate cells, and Kupffer cells also support productive HIV infection[47].
HIV RNA has been detected in primary human hepatocytes both ex vivo[48-50] and in vitro[51,52]. We and others have shown that many hepatocyte cell lines are permissive to low level of HIV infection in vitro[53-55]. However, the nature of receptors for viral entry on hepatocytes are still under debate. As an option, it might be CXCR4 and CCR5 in Huh7.5 cells, which serve for HIV attachment/entering[56]. In fact, several studies confirmed that HIV-1 productive infection in primary hepatocytes and hepatoma cell lines is CD4-independent[55,57-59]. Another optional entry for HIV in hepatocytes is through plasma membrane glycosphingolipids, such as Glycolipid galactosyl ceramide[60]. Usually, the level of HIV-infection in hepatocytes is low, but can be significantly potentiated by second hits like co-infection[53] or alcohol. Our laboratory recently found increased HIV RNA levels in primary human hepatocytes and Huh7.5 cells that express CYP2E1, which becomes very visible upon exposure of infected cells to ethanol. In these cells, ethanol metabolism and specifically, ethanol metabolite acetaldehyde increased HIV RNA levels[53].
Resident liver macrophages, Kupffer cells, have higher infection levels than hepatocytes. They were shown to be infected by HIV in vivo[48-50]. In vitro studies suggested that this infection is productive[51,52]. Thus, Jiang et al[61] detected intracellular expression of p24 antigen in Kupffer cells, endothelial cells and hepatocytes. Lang et al[62], also demonstrated HIV infection in Kupffer cells and intrahepatic lymphocytes by immunostaining for HIV proteins.
Infection of Kupffer cells and hepatocytes with HIV was confirmed by several approaches. First, in vivo studies support the presence of HIV pro-viral DNA in liver tissue, particularly, in Kupffer cells and isolated hepatocytes[63]. Second, the presence of HIV proteins has been detected in parenchymal and non-parenchymal liver cells by immunohistochemistry[26]. The direct interactions occur between HIV and various liver cells, including hepatocytes, Kupffer cells, inflammatory mononuclear cells, and sinusoidal cells[48,64].
In addition to hepatocytes and Kupffer cells, HIV replicates in HSC, which plays a significant role in HIV-infection pathogenesis[26,65]. Further, it was a direct correlation between the expression of HIV in liver cells and severity of liver damage in HIV-infected patients.
Role of HIV-induced hepatocyte apoptosis in inflammation/fibrosis development
HIV induces hepatocyte cell death. It is not clear yet whether pro-apoptotic effects and release of pro-inflammatory cytokines by these cells come from intracellular HIV replication or simply from hepatocyte interactions with HIV antigens[26,66]. However, parenchymal cell apoptosis serves as a basis for inflammation/fibrosis promotion. As known, HIV glycoproteins stimulate hepatocyte expression of the tumor necrosis factor (TNF)-related apoptosis inducing ligand, which mediates apoptosis[67]. Gp120 can also activate the hepatic expression of interleukin (IL)-8, an important mediator of hepatic inflammation[68]. In vivo evidence of HIV-specific hepatocyte apoptosis come from the studies on humanized mice models. Thus, study from our group[45] explored the immunopathogenesis of HIV-1-induced depletion of human hepatocytes in HIV-1-infected humanized mice dually reconstituted with human hepatocytes and human immune system. This advanced mouse model recapitulates multiple components of liver damage by HIV-1- infection in humans, including: (1) HIV-1-induced depletion in liver CD4+ cells; (2) decreased albumin levels; (3) liver immune activation; and (4) human hepatocytes death. In a different study, chronically HIV-1-infected humanized mice also showed reduction of albumin levels and restoration of liver synthetic function by ART[69], indicating the significance of replicating virus. Collectively, these findings suggest that HIV is expressed in human hepatocytes. However, the level of HIV-infection in parenchymal cells is low when compared to HIV permissive immune cells. This indicates that hepatocytes may serve as a silent reservoir of HIV-infection. Furthermore, Fromentin et al[70], reported that hepatocytes can bind and internalize HIV-1 particles as well as transmit cell surface-associated virus to CD4+ T cells in the liver through intercellular cell adhesion molecule-1/lymphocyte function-associated antigen-1 interactions. In addition, we observed that apoptotic bodies generated from hepatocytes with removed surface structures, can still infect HIV-permissive cells (unpublished data), indicating that HIV penetrates inside of hepatocytes and is not always only attached to cell surface. This cell-to-cell exchange of infectious cargo, in turn, might facilitate viral dissemination in the liver and throughout the whole organism. Moreover, HIV-infected hepatocytes can activate natural killer T cells as this cell population is enriched in the liver and is susceptible to productive HIV-1 infection[71].
Kupffer cells are known to play a key role in induction of hepatocyte apoptosis and to be involved in promotion of steatosis[72]. Moreover, recent studies showed that SIV and HIV-infected macrophage/monocytic cells secrete elevated levels of transforming growth factor beta, which, in turn, activate HSCs to induce fibrosis[17,73]. Engulfment of apoptotic hepatocytes may serve as the activators for Kupffer cells[74], thereby contributing to liver inflammation development.
Not only macrophages, but HIV-specific T cells may also promote liver fibrosis, but the evaluation of intrahepatic virus-specific T cell responses is difficult due to the low frequency of these cells in the liver and limited availability of liver biopsy material from patients[75]. A study performed in HIV/HCV co-infected patients demonstrated the presence of both HCV-specific and HIV-specific T cells in the liver[76]. As also demonstrated in this study, HIV-specific T cells were more functional than the HCV-specific T cells, and therefore, better equipped to promote HSCs activation. Once HSCs are activated, they exhibit properties of professional antigen-presenting cells and stimulate T lymphocyte proliferation by endocytosing the peripheral particles[77,78]. Studies from two different laboratories reported that like dendritic cells, HIV-infected HSCs can transfer the virus to infect the susceptible CD4+ lymphocytes[79,80]. The association of HSCs with lymphocytes during liver inflammation, their localization below the fenestrated sinusoidal endothelium and the direct in vivo interaction with lymphocytes has been confirmed by confocal microscopy[81,82]. Additionally, when compared to cell free virus, cell-associated HIV-1 enter CD4+ T cells, thereby causing effective infection. Finally, HIV- infected HSCs were shown to activate the neighboring cells, such as hepatocytes by providing an important intrahepatic source of HIV proteins[56,83].
Hepatocyte apoptosis and inflammation may also contribute to HSC activation, thereby causing liver fibrosis progression. Hepatocyte apoptosis induces pro-fibrotic activity of hepatic stellate cells in both HIV/HBV and HIV/HCV co-infections[65]. We have recently shown that apoptotic bodies from HIV/HCV pathogen-expressing hepatocytes trigger pro-fibrotic and to a lesser extent, pro-inflammatory changes in HSC, thereby promoting fibrosis development[53]. Furthermore, in HCV-HIV coinfected hepatocytes, the level of HCV RNA and HIV RNA is higher than in monoinfected hepatocytes, which becomes evident only when HCV-HIV-induced cell apoptosis is blocked by pan-caspase inhibitor. These in vitro pro-apoptotic effects of co-infection are more prominent when hepatocytes are plated on gels that mimic increased liver stiffness[53]. In this study, we have also demonstrated that increased liver stiffness potentiates apoptotic cell death in co-infected hepatocytes and thus, the progression of liver disease may be faster if HCV co-infection occurs in HIV+ patients with already fibrotic liver.
HIV-induced liver injury and specifically, hepatocyte apoptosis is significantly potentiated by alcohol exposure. Alcohol was shown to cause liver damage via oxidative stress accompanied by the formation/release of free reactive oxygen species that activate Kupffer cells. In addition, oxidative stress induces macromolecular damage to hepatocytes finally leading to apoptosis. In HIV-infected patients, alcohol causes increased activation of macrophages and hepatocyte apoptosis, which promote HSCs activation via nuclear factor kappa-beta (NF-κB) and activator protein 1, thereby increasing production of proinflammatory and profibrotic cytokines[53,84]. Figure 1, depicts the possible mechanisms of interactions between the HIV- infected liver cells.
Figure 1.
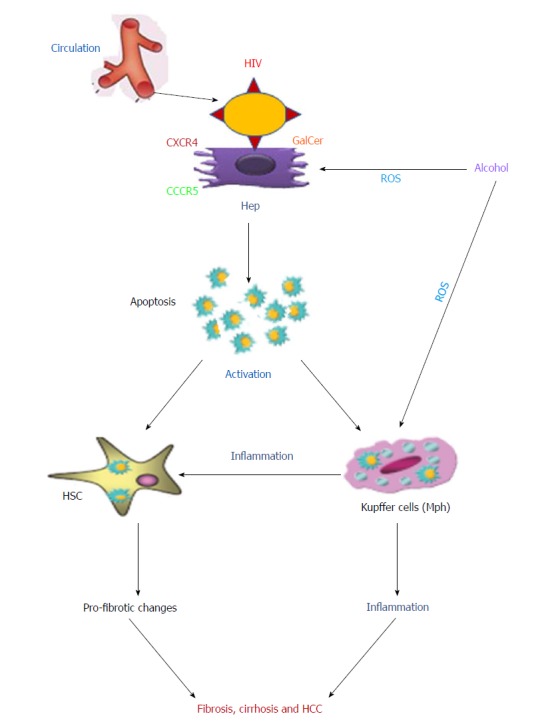
Possible mechanisms of interaction between human immunodeficiency virus-infected liver parenchymal and non-parenchymal cells. Human immunodeficiency virus (HIV) infected immune cells are trapped by the liver. HIV envelope proteins interact with hepatocytes using the co-receptors CXCR4/CCR5 or GalCer to induce apoptosis. Apoptotic bodies from infected hepatocytes are captured by both hepatic stellate cells (HSC) and Kupffer cells. This process activates both cell types, which induce the profibrotic changes and inflammation, respectively. In addition, activated Kupffer cells, in turn, regulate HSCs activation. The second hit, alcohol, potentiates inflammation and fibrosis development by oxidative stress-induced hepatocyte apoptosis enhanced by HIV-infected Kupffer cells. All these combined events may lead to fibrosis, cirrhosis and hepatocellular carcinoma development. All HIV related events can be suppressed by antiretroviral therapy. HIV: Human immunodeficiency virus; ART: Antiretroviral therapy; GalCer: Galactosyl ceramide; Hep: Hepatocytes; ROS: Reactive oxygen species; HCC: Hepatocellular carcinoma.
ROLE OF MICROBIAL TRANSLOCATION AND MICROBIOTA IN HIV INDUCED LIVER DAMAGE
It became clear now that microbial translocation is an important determinant of clinical manifestations and HIV disease progression[76]. Damage to the intestinal mucosa in patients with HIV leads to disruption of the gut epithelial barrier, which facilitates leakage of luminal microbial products to translocate into the portal and systemic circulation[85]. Gut microbial translocation leads to hepatic injury primarily via increased hepatic levels of bacterial lipopolysaccharides (LPS), causing hepatic inflammation by one of three mechanisms: (1) Recruitment and activation of inflammatory cells; (2) Indirectly induced systemic immune responses and promotion of hepatocyte cell death[86,87]; and (3) Production of cytokines and acute phase cytokines, such as transforming growth factor beta 1, IL-6 and IL-10[88,89]. As our understanding of gut-liver axis has improved, this pathological translocation of bacterial degradation products is recognized as a significant mechanism for triggering progressive liver damage in many disease states, including alcoholic and non-alcoholic liver diseases.
Further, HIV-infection directly targets gut lymphocyte tissue and preferentially depletes CD22+, CD4+ cells and TH17 cells[90-93]. In addition, HIV viral proteins increase the production of inflammatory cytokines by gut epithelium, leading to enhanced apoptosis of epithelial cells and breakdown of tight junctions[94-96]. Notably, this gut barrier dysfunction appears to persist even after successful treatment with ART, since soluble CD14 (a surrogate marker for the presence of bacterial LPS) remains elevated even after systemic markers of infection (such as viral load and IL-6) have normalized[97,98]. In both cases, this leads to increased intestinal permeability and hepatic exposure to LPS. LPS exerts its effect in the liver primarily by activating the Kupffer cells and HSCs via the Toll-Like-receptor 4 (TLR4)-mediated signaling pathway[46,99,100], which further activates three major transcriptional complexes: NF-κB, activator protein 1 and interferon regulatory factors. The specific actions of these transcriptional complexes are poorly understood, but the net effect of their activation is an upregulation of the inflammatory/fibrotic HSC phenotype and potentiation/increase in longevity of HSC cell lines[98]. In the case of Kupffer cell activation via TLR signaling, the mediators upregulated by the TLR4 pathway include TNF-α, IL-1, and IL-6, which promote liver fibrosis by directly activating HSCs or by priming and recruiting other inflammatory leukocyte populations[99,100].
ALCOHOL-INDUCED LIVER INJURY IN HIV-INFECTED PATIENTS
In United States, about 50% of deaths from HIV-induced liver disease are due to alcohol-induced liver damage but the mechanisms, by which alcohol potentiates HIV-infection are not clear[12]. Chronic alcohol consumption is observed in 30% of HIV-infected patients[101,102] and may result in alcoholic hepatitis, which leads to liver deterioration[103,104]. Alcohol, via its metabolic effects, depletes liver-protecting factors (like major anti-oxidant glutathione), thereby enhancing the drug-related toxicity[105-108]. The immunosuppressive properties of chronic alcohol consumption enhance viral replication, and the combination of alcohol and chronic infection with hepatotropic viruses has synergistic and deleterious effects[109,110]. Both alcohol misuse and addiction significantly enhance the risk of advanced fibrosis and cirrhosis in HIV mono-infection as well as in HIV/HCV co-infection[111]. These observations were confirmed by Chaudhry et al[112]. Alcohol use was categorized according to National Institute on Alcohol Abuse and Alcoholism instructions, and APRI was used to classify the liver function: APRI greater than 1.5 is considered as a significant liver disease[112,113]. It has been clearly demonstrated that alcohol drinking is an important, independent and variable risk factor for liver fibrosis in HIV-infected patients. In this regard, ART/alcohol associations are significant factors influencing liver function. For instance, in alcohol misusing patients with compromised liver function, proper dosing of ART becomes challenging since the liver also metabolizes these drugs. Thus, the doses should be adjusted properly, otherwise liver dysfunction could lead to harmful accumulation of some anti-HIV drugs. Even though there are only a small number of drug interaction studies assessing the outcome of alcohol misuse on ART, a few of them have indeed documented an increased risk of ART- induced hepatotoxicity in alcohol abusing patients, particularly co-infected with HCV ones[101,114]. So far, little attention has been paid to the mechanisms underlying ART hepatotoxicity in combination with alcohol or other cofactors. Some recent studies have demonstrated that liver injury induced by alcohol and ART shares numerous potential mechanisms. It is strongly believed that both alcohol and ART can adversely affect the same cellular targets (mitochondria, cytokines, and proteasomes)[111]. Hence, there is a critical need for studies that describe mechanisms of ART-induced liver toxicity, particularly, in combination with alcohol. These studies will be helpful in evaluating potential therapeutic interventions. However, ART-induced drug toxicity is not the only mechanism, by which alcohol exacerbates liver injury in HIV- infection. Alcohol also potentiates HIV-induced apoptosis of hepatocytes[8], thereby causing intensive activation of Kupffer cells and HSC and promotion of inflammation and apoptosis in HIV-infected alcohol-abusing patients[54].
CONCLUSION
HIV induces liver injury by direct interactions with parenchymal and non-parenchymal liver cells. These HIV-infected liver cells, in turn cross-talk with uninfected cells, thereby causing the spread of liver injury. Multiple mechanisms can be attributed to the role of HIV in promoting liver inflammation and fibrosis. The most important are pro-apoptotic effects of HIV on hepatocytes and HIV-associated microbial translocation and microbiota changes. In addition to cytotoxic effects of HIV on hepatocytes and activation of non-parenchymal liver cells, ART also promotes liver injury due to drug toxicity and/or drug metabolism, mitochondrial damage, immunosuppression and drug hypersensitivity reactions. The effects of HIV-infection on liver cells are tremendously potentiated by second hits, and alcohol is most important of them. Proper management of HIV-infected patients requires recognition of liver injury conditions for effective targeted diagnosis and treatment.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: August 28, 2018
First decision: October 9, 2018
Article in press: October 21, 2018
P- Reviewer: Gassler N, Li Y S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Murali Ganesan, Research Service, Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE 68105, United States; Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE 68105, United States.
Larisa Y Poluektova, Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE 68198, United States.
Kusum K Kharbanda, Research Service, Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE 68105, United States; Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE 68105, United States.
Natalia A Osna, Research Service, Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE 68105, United States; Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE 68105, United States. nosna@unmc.edu.
References
- 1.UNAIDS. 2018. Global HIV AIDS statistics - 2018 fact sheet. [Google Scholar]
- 2.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, Karon J, Brookmeyer R, Kaplan EH, McKenna MT, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2012. Estimated HIV incidence in the United States, 2007-2010. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2018. Estimated HIV incidence and prevalence in the United States, 2010-2015. [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2017. HIV Surveillance Report. [Google Scholar]
- 6.Palella FJ Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD; HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 7.Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, Monforte Ad, Kirk O, Friis-Moller N, Phillips A, Reiss P, El Sadr W, Pradier C, Worm SW. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–1548. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 8.Han H, He Y, Hu J, Lau R, Lee H, Ji C. Disrupted ER-to-Golgi Trafficking Underlies Anti-HIV Drugs and Alcohol-Induced Cellular Stress and Hepatic Injury. Hepatol Commun. 2017;1:122–139. doi: 10.1002/hep4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 10.Martín-Carbonero L, Soriano V, Valencia E, García-Samaniego J, López M, González-Lahoz J. Increasing impact of chronic viral hepatitis on hospital admissions and mortality among HIV-infected patients. AIDS Res Hum Retroviruses. 2001;17:1467–1471. doi: 10.1089/08892220152644160. [DOI] [PubMed] [Google Scholar]
- 11.Pandrea I, Happel KI, Amedee AM, Bagby GJ, Nelson S. Alcohol’s role in HIV transmission and disease progression. Alcohol Res Health. 2010;33:203–218. [PMC free article] [PubMed] [Google Scholar]
- 12.Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penton PK, Blackard JT. Analysis of HIV quasispecies suggests compartmentalization in the liver. AIDS Res Hum Retroviruses. 2014;30:394–402. doi: 10.1089/aid.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Dailey PJ, Gettie A, Blanchard J, Ho DD. The liver is a major organ for clearing simian immunodeficiency virus in rhesus monkeys. J Virol. 2002;76:5271–5273. doi: 10.1128/JVI.76.10.5271-5273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mates JM, Yao Z, Cheplowitz AM, Suer O, Phillips GS, Kwiek JJ, Rajaram MV, Kim J, Robinson JM, Ganesan LP, et al. Mouse Liver Sinusoidal Endothelium Eliminates HIV-Like Particles from Blood at a Rate of 100 Million per Minute by a Second-Order Kinetic Process. Front Immunol. 2017;8:35. doi: 10.3389/fimmu.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesan LP, Mohanty S, Kim J, Clark KR, Robinson JM, Anderson CL. Rapid and efficient clearance of blood-borne virus by liver sinusoidal endothelium. PLoS Pathog. 2011;7:e1002281. doi: 10.1371/journal.ppat.1002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher BS, Green RR, Brown RR, Wood MP, Hensley-McBain T, Fisher C, Chang J, Miller AD, Bosche WJ, Lifson JD, et al. Liver macrophage-associated inflammation correlates with SIV burden and is substantially reduced following cART. PLoS Pathog. 2018;14:e1006871. doi: 10.1371/journal.ppat.1006871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata-Marín JA, Gaytán-Martínez J, Grados-Chavarría BH, Fuentes-Allen JL, Arroyo-Anduiza CI, Alfaro-Mejía A. Correlation between HIV viral load and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross-sectional study. Virol J. 2009;6:181. doi: 10.1186/1743-422X-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L, Cardona Maya W, Moreno-Fernandez ME, Ma G, Shata MT, Sherman KE, Chougnet C, Blackard JT. Low-level HIV infection of hepatocytes. Virol J. 2012;9:157. doi: 10.1186/1743-422X-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovari H, Ledergerber B, Battegay M, Rauch A, Hirschel B, Foguena AK, Vernazza P, Bernasconi E, Mueller NJ, Weber R. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin Infect Dis. 2010;50:502–511. doi: 10.1086/649922. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53:1375–1382. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan P, Blanco F, García-Gascó P, García-Merchán J, Vispo E, Barreiro P, Labarga P, González-Lahoz J, Soriano V. Predictors of severe hepatic steatosis using abdominal ultrasound in HIV-infected patients. HIV Med. 2009;10:53–59. doi: 10.1111/j.1468-1293.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 23.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V 3rd. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bräu N, Salvatore M, Ríos-Bedoya CF, Fernández-Carbia A, Paronetto F, Rodríguez-Orengo JF, Rodríguez-Torres M. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Blackard JT, Hiasa Y, Smeaton L, Jamieson DJ, Rodriguez I, Mayer KH, Chung RT. Compartmentalization of hepatitis C virus (HCV) during HCV/HIV coinfection. J Infect Dis. 2007;195:1765–1773. doi: 10.1086/518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–330. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackard JT, Welge JA, Taylor LE, Mayer KH, Klein RS, Celentano DD, Jamieson DJ, Gardner L, Sherman KE. HIV mono-infection is associated with FIB-4 - A noninvasive index of liver fibrosis - in women. Clin Infect Dis. 2011;52:674–680. doi: 10.1093/cid/ciq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han SH, Kim SU, Kim CO, Jeong SJ, Park JY, Choi JY, Kim DY, Ahn SH, Song YG, Han KH, et al. Abnormal liver stiffness assessed using transient elastography (Fibroscan®) in HIV-infected patients without HBV/HCV coinfection receiving combined antiretroviral treatment. PLoS One. 2013;8:e52720. doi: 10.1371/journal.pone.0052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasson H, Merli M, Galli L, Gallotta G, Carbone A, Messina E, Bagaglio S, Morsica G, Salpietro S, Castagna A, et al. Non-invasive fibrosis biomarkers - APRI and Forns - are associated with liver stiffness in HIV-monoinfected patients receiving antiretroviral drugs. Liver Int. 2013;33:1113–1120. doi: 10.1111/liv.12159. [DOI] [PubMed] [Google Scholar]
- 30.Merchante N, Pérez-Camacho I, Mira JA, Rivero A, Macías J, Camacho A, Gómez-Mateos J, García-Lázaro M, Torre-Cisneros J, Pineda JA; Grupo Andaluz para el Estudio de las Hepatitis Víricas de la Sociedad Andaluza de Enfermedades Infecciosas. Prevalence and risk factors for abnormal liver stiffness in HIV-infected patients without viral hepatitis coinfection: role of didanosine. Antivir Ther. 2010;15:753–763. doi: 10.3851/IMP1612. [DOI] [PubMed] [Google Scholar]
- 31.Morse CG, McLaughlin M, Proschan M, Koh C, Kleiner DE, Heller T, Kovacs JA. Transient elastography for the detection of hepatic fibrosis in HIV-monoinfected adults with elevated aminotransferases on antiretroviral therapy. AIDS. 2015;29:2297–2302. doi: 10.1097/QAD.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benmassaoud A, Ghali P, Cox J, Wong P, Szabo J, Deschenes M, Osikowicz M, Lebouche B, Klein MB, Sebastiani G. Screening for nonalcoholic steatohepatitis by using cytokeratin 18 and transient elastography in HIV mono-infection. PLoS One. 2018;13:e0191985. doi: 10.1371/journal.pone.0191985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tikoo K, Tamta A, Ali IY, Gupta J, Gaikwad AB. Tannic acid prevents azidothymidine (AZT) induced hepatotoxicity and genotoxicity along with change in expression of PARG and histone H3 acetylation. Toxicol Lett. 2008;177:90–96. doi: 10.1016/j.toxlet.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Claudio GA, Martin AF, de Dios Perrino S, Velasco AA. DRESS syndrome associated with nevirapine therapy. Arch Intern Med. 2001;161:2501–2502. doi: 10.1001/archinte.161.20.2501. [DOI] [PubMed] [Google Scholar]
- 35.Moyle GJ, Datta D, Mandalia S, Morlese J, Asboe D, Gazzard BG. Hyperlactataemia and lactic acidosis during antiretroviral therapy: relevance, reproducibility and possible risk factors. AIDS. 2002;16:1341–1349. doi: 10.1097/00002030-200207050-00005. [DOI] [PubMed] [Google Scholar]
- 36.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 37.Mocroft A, Soriano V, Rockstroh J, Reiss P, Kirk O, de Wit S, Gatell J, Clotet B, Phillips AN, Lundgren JD; EuroSIDA Study Group. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117–2125. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
- 38.Blanco F, Barreiro P, Ryan P, Vispo E, Martín-Carbonero L, Tuma P, Labarga P, Medrano J, González-Lahoz J, Soriano V. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2011;18:11–16. doi: 10.1111/j.1365-2893.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 39.Sulkowski MS, Mehta SH, Torbenson M, Afdhal NH, Mirel L, Moore RD, Thomas DL. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–592. doi: 10.1097/01.aids.0000163935.99401.25. [DOI] [PubMed] [Google Scholar]
- 40.McGovern BH, Ditelberg JS, Taylor LE, Gandhi RT, Christopoulos KA, Chapman S, Schwartzapfel B, Rindler E, Fiorino AM, Zaman MT, et al. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006;43:365–372. doi: 10.1086/505495. [DOI] [PubMed] [Google Scholar]
- 41.Kovari H, Weber R. Influence of antiretroviral therapy on liver disease. Curr Opin HIV AIDS. 2011;6:272–277. doi: 10.1097/COH.0b013e3283473405. [DOI] [PubMed] [Google Scholar]
- 42.Puoti M, Nasta P, Gatti F, Matti A, Prestini K, Biasi L, Carosi G. HIV-related liver disease: ARV drugs, coinfection, and other risk factors. J Int Assoc Physicians AIDS Care (Chic) 2009;8:30–42. doi: 10.1177/1545109708330906. [DOI] [PubMed] [Google Scholar]
- 43.Soriano V, Puoti M, Garcia-Gascó P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 44.Nampala H, Luboobi LS, Mugisha JY, Obua C. Mathematical modeling of liver enzyme elevation in HIV mono-infection. Math Biosci. 2013;242:77–85. doi: 10.1016/j.mbs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Dagur RS, Wang W, Cheng Y, Makarov E, Ganesan M, Suemizu H, Gebhart CL, Gorantla S, Osna N, Poluektova LY. Human hepatocyte depletion in the presence of HIV-1 infection in dual reconstituted humanized mice. Biol Open. 2018:7. doi: 10.1242/bio.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care. 2001;7:99–104. doi: 10.1097/00075198-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Bansal MB, Blackard JT. Sherman KE, editor HIV and liver disease. New York: Springer; 2012. Effects of HIV on Liver Cell Populations; pp. 81–90. [Google Scholar]
- 48.Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Housset C, Boucher O, Girard PM, Leibowitch J, Saimot AG, Bréchot C, Marche C. Immunohistochemical evidence for human immunodeficiency virus-1 infection of liver Kupffer cells. Hum Pathol. 1990;21:404–408. doi: 10.1016/0046-8177(90)90202-g. [DOI] [PubMed] [Google Scholar]
- 50.Hufert FT, Schmitz J, Schreiber M, Schmitz H, Rácz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J Acquir Immune Defic Syndr. 1993;6:772–777. [PubMed] [Google Scholar]
- 51.Gendrault JL, Steffan AM, Schmitt MP, Jaeck D, Aubertin AM, Kirn A. Interaction of cultured human Kupffer cells with HIV-infected CEM cells: an electron microscopic study. Pathobiology. 1991;59:223–226. doi: 10.1159/000163650. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt MP, Gendrault JL, Schweitzer C, Steffan AM, Beyer C, Royer C, Jaeck D, Pasquali JL, Kirn A, Aubertin AM. Permissivity of primary cultures of human Kupffer cells for HIV-1. AIDS Res Hum Retroviruses. 1990;6:987–991. doi: 10.1089/aid.1990.6.987. [DOI] [PubMed] [Google Scholar]
- 53.Ganesan M, Dagur RS, Makarov E, Poluektova LI, Kidambi S, Osna NA. Matrix stiffness regulate apoptotic cell death in HIV-HCV co-infected hepatocytes: Importance for liver fibrosis progression. Biochem Biophys Res Commun. 2018;500:717–722. doi: 10.1016/j.bbrc.2018.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganesan M, Dagur RS, Poluektova LY, Osna NA. Alcohol Exacerbates HIV Infection In Liver Cells: Possible Mechanisms. Hepatology. 2017;66:414. [Google Scholar]
- 55.Iser DM, Warner N, Revill PA, Solomon A, Wightman F, Saleh S, Crane M, Cameron PU, Bowden S, Nguyen T, et al. Coinfection of hepatic cell lines with human immunodeficiency virus and hepatitis B virus leads to an increase in intracellular hepatitis B surface antigen. J Virol. 2010;84:5860–5867. doi: 10.1128/JVI.02594-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, Kim SS, Borges CB, Shao RX, Chung RT. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee R, Sperber K, Pizzella T, Mayer L. Inhibition of HIV-1 productive infection in hepatoblastoma HepG2 cells by recombinant tumor necrosis factor-alpha. AIDS. 1992;6:1127–1131. doi: 10.1097/00002030-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Cao YZ, Friedman-Kien AE, Huang YX, Li XL, Mirabile M, Moudgil T, Zucker-Franklin D, Ho DD. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990;64:2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao P, Usami O, Suzuki Y, Ling H, Shimizu N, Hoshino H, Zhuang M, Ashino Y, Gu H, Hattori T. Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. AIDS. 2008;22:1749–1757. doi: 10.1097/QAD.0b013e328308937c. [DOI] [PubMed] [Google Scholar]
- 60.Fromentin R, Tardif MR, Tremblay MJ. Inefficient fusion due to a lack of attachment receptor/co-receptor restricts productive human immunodeficiency virus type 1 infection in human hepatoma Huh7.5 cells. J Gen Virol. 2011;92:587–597. doi: 10.1099/vir.0.028746-0. [DOI] [PubMed] [Google Scholar]
- 61.Jiang TJ, Zhao M, Zhao JM, Zhou GD, Pan D, Wang J, Zhang YH, Zhou ZP. Immunohistochemical evidence for HIV-1 infection in the liver of HIV-infected patients. Zhonghua Shiyan He Linchuangbing Duxue Zazhi. 2005;19:152–154. [PubMed] [Google Scholar]
- 62.Lang ZW, Dao WB, Zhang FJ, Shi XH, Ma ZC, Ma PQ, Shen B, Lü HB. A pathological study on liver tissues of patients with HIV infection. Zhonghua Ganzangbing Zazhi. 2005;13:930–932. [PubMed] [Google Scholar]
- 63.Hosseini SY, Tayeri K, Teimoori A, Baesi K. HIV and Hepatitis C virus co-infection: A closer view of their interactions and clinical consequences. In: SeyedAlinagh SA, editor Frontiers in HIV Research: Current Studies in HIV Research: Bentahm Science Publishers; 2016. pp. 117–142. [Google Scholar]
- 64.Housset C, Lamas E, Bréchot C. Detection of HIV1 RNA and p24 antigen in HIV1-infected human liver. Res Virol. 1990;141:153–159. doi: 10.1016/0923-2516(90)90017-d. [DOI] [PubMed] [Google Scholar]
- 65.Crane M, Iser D, Lewin SR. Human immunodeficiency virus infection and the liver. World J Hepatol. 2012;4:91–98. doi: 10.4254/wjh.v4.i3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455–1460. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 67.Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV induces TRAIL sensitivity in hepatocytes. PLoS One. 2009;4:e4623. doi: 10.1371/journal.pone.0004623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 69.Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, Gelbard HA, McMillan J, Gorantla S, Poluektova LY. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 2012;26:2135–2144. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fromentin R, Tardif MR, Tremblay MJ. Human hepatoma cells transmit surface bound HIV-1 to CD4+ T cells through an ICAM-1/LFA-1-dependent mechanism. Virology. 2010;398:168–175. doi: 10.1016/j.virol.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195:869–879. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel P, Khan N, Rani M, Gupta D, Jameel S. The expression of HIV-1 Vpu in monocytes causes increased secretion of TGF-β that activates profibrogenic genes in hepatic stellate cells. PLoS One. 2014;9:e88934. doi: 10.1371/journal.pone.0088934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganesan M, Natarajan SK, Zhang J, Mott JL, Poluektova LI, McVicker BL, Kharbanda KK, Tuma DJ, Osna NA. Role of apoptotic hepatocytes in HCV dissemination: regulation by acetaldehyde. Am J Physiol Gastrointest Liver Physiol. 2016;310:G930–G940. doi: 10.1152/ajpgi.00021.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrett L, Trehanpati N, Poonia S, Daigh L, Sarin SK, Masur H, Kottilil S. Hepatic compartmentalization of exhausted and regulatory cells in HIV/HCV-coinfected patients. J Viral Hepat. 2015;22:281–288. doi: 10.1111/jvh.12291. [DOI] [PubMed] [Google Scholar]
- 76.Vali B, Yue FY, Jones RB, Sheth PM, Kaul R, Betts MR, Wong D, Kovacs C, Loutfy M, Common A, et al. HIV-specific T-cells accumulate in the liver in HCV/HIV co-infection. PLoS One. 2008;3:e3454. doi: 10.1371/journal.pone.0003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 78.Viñas O, Bataller R, Sancho-Bru P, Ginès P, Berenguer C, Enrich C, Nicolás JM, Ercilla G, Gallart T, Vives J, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 79.Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, Chen P, Chen BK, Klotman ME, Bansal MB. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–622. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705–714. doi: 10.1053/j.gastro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Muhanna N, Horani A, Doron S, Safadi R. Lymphocyte-hepatic stellate cell proximity suggests a direct interaction. Clin Exp Immunol. 2007;148:338–347. doi: 10.1111/j.1365-2249.2007.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 84.Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. 2017;4:e000166. doi: 10.1136/bmjgast-2017-000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 86.Sacchi P, Cima S, Corbella M, Comolli G, Chiesa A, Baldanti F, Klersy C, Novati S, Mulatto P, Mariconti M, et al. Liver fibrosis, microbial translocation and immune activation markers in HIV and HCV infections and in HIV/HCV co-infection. Dig Liver Dis. 2015;47:218–225. doi: 10.1016/j.dld.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS. 2011;6:472–477. doi: 10.1097/COH.0b013e32834bbc71. [DOI] [PubMed] [Google Scholar]
- 88.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 90.Dandekar S, George MD, Bäumler AJ. Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS. 2010;5:173–178. doi: 10.1097/COH.0b013e328335eda3. [DOI] [PubMed] [Google Scholar]
- 91.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, Shahabi K, Benko E, Loutfy M, Ostrowski M, et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol. 2013;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 93.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 94.Canani RB, Cirillo P, Mallardo G, Buccigrossi V, Secondo A, Annunziato L, Bruzzese E, Albano F, Selvaggi F, Guarino A. Effects of HIV-1 Tat protein on ion secretion and on cell proliferation in human intestinal epithelial cells. Gastroenterology. 2003;124:368–376. doi: 10.1053/gast.2003.50056. [DOI] [PubMed] [Google Scholar]
- 95.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hattab S, Guihot A, Guiguet M, Fourati S, Carcelain G, Caby F, Marcelin AG, Autran B, Costagliola D, Katlama C. Comparative impact of antiretroviral drugs on markers of inflammation and immune activation during the first two years of effective therapy for HIV-1 infection: an observational study. BMC Infect Dis. 2014;14:122. doi: 10.1186/1471-2334-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martínez-Maza O, Bream JH. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010;3:21. doi: 10.1186/1755-1536-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corbitt N, Kimura S, Isse K, Specht S, Chedwick L, Rosborough BR, Lunz JG, Murase N, Yokota S, Demetris AJ. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. Am J Pathol. 2013;182:180–191. doi: 10.1016/j.ajpath.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pol S, Lamorthe B, Thi NT, Thiers V, Carnot F, Zylberberg H, Berthelot P, Bréchot C, Nalpas B. Retrospective analysis of the impact of HIV infection and alcohol use on chronic hepatitis C in a large cohort of drug users. J Hepatol. 1998;28:945–950. doi: 10.1016/s0168-8278(98)80341-3. [DOI] [PubMed] [Google Scholar]
- 102.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 103.Lapoile E, Vona G, Canioni D, Chaix ML, Nalpas B, Fontaine C. Factors participating in severe HCV-related liver disease in HIV/HCV coinfection. J Hepatol. 2002;36 Suppl 1:172. [Google Scholar]
- 104.Lipsky JJ. Antiretroviral drugs for AIDS. Lancet. 1996;348:800–803. doi: 10.1016/S0140-6736(95)12333-4. [DOI] [PubMed] [Google Scholar]
- 105.Prescott LF. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 2000;49:291–301. doi: 10.1046/j.1365-2125.2000.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–773. [PubMed] [Google Scholar]
- 107.Bilal U, Lau B, Lazo M, McCaul ME, Hutton HE, Sulkowski MS, Moore RD, Chander G. Interaction Between Alcohol Consumption Patterns, Antiretroviral Therapy Type, and Liver Fibrosis in Persons Living with HIV. AIDS Patient Care STDS. 2016;30:200–207. doi: 10.1089/apc.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kahler CW, Liu T, Cioe PA, Bryant V, Pinkston MM, Kojic EM, Onen N, Baker JV, Hammer J, Brooks JT, et al. Direct and Indirect Effects of Heavy Alcohol Use on Clinical Outcomes in a Longitudinal Study of HIV Patients on ART. AIDS Behav. 2017;21:1825–1835. doi: 10.1007/s10461-016-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Degott C, Valla D, Erlinger S, Rueff B. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 110.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- 111.Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, Joshi-Barve S, McClain CJ. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33:229–236. [PMC free article] [PubMed] [Google Scholar]
- 112.Chaudhry AA, Sulkowski MS, Chander G, Moore RD. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10:133–142. doi: 10.1111/j.1468-1293.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Price JC, Seaberg EC, Stosor V, Witt MD, Lellock CD, Thio CL. AST-to-platelet ratio index increases significantly 3 years prior to liver-related death in HIV-hepatitis-coinfected men. AIDS. 2018 doi: 10.1097/QAD.0000000000001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23–31. doi: 10.1086/341084. [DOI] [PubMed] [Google Scholar]


