Abstract
Objective
To evaluate the effect of dual use of VA/Medicare Part D drug benefits on antihypertensive medication supply in older Veterans with dementia.
Data Sources/Study Setting
National, linked 2007–2010 Veterans Affairs (VA) and Medicare utilization and prescription records for 50,763 dementia patients with hypertension.
Study Design
We used inverse probability of treatment (IPT)‐weighted multinomial logistic regression to examine the association of dual prescription use with undersupply and oversupply of antihypertensives.
Data Collection/Extraction Methods
Veterans Affairs and Part D prescription records were used to classify patients as VA‐only, Part D‐only, or dual VA/Part D users of antihypertensives and summarize their antihypertensive medication supply in 2010: (1) appropriate supply of all prescribed antihypertensive classes, (2) undersupply of ≥1 class with no oversupply of another class, (3) oversupply of ≥1 class with no undersupply, or (4) both undersupply and oversupply.
Principal Findings
Dual prescription users were more likely than VA‐only users to have undersupply only (aOR = 1.28; 95 percent CI = 1.18–1.39), oversupply only (aOR = 2.38; 95 percent CI = 2.15–2.64), and concurrent under‐ and oversupply (aOR = 2.89; 95 percent CI = 2.53–3.29), versus appropriate supply of all classes.
Conclusions
Obtaining antihypertensives through both VA and Part D was associated with increased antihypertensive under‐ and oversupply. Efforts to understand how best to coordinate dual‐system prescription use are critically needed.
Keywords: Dementia, hypertension, medication use, Veterans, Medicare Part D
Millions of Veterans who obtain health care from the U.S. Department of Veterans Affairs (VA) also seek care from non‐VA sources (U.S. Department of Veterans Affairs 2016). Veterans aged 65 and older have enhanced access to non‐VA care through almost‐universal eligibility for Medicare. Use of non‐VA care among Medicare‐eligible VA patients is common (Petersen et al. 2010), amounting annually to an average of 10 visits to non‐VA providers on top of six outpatient VA visits (Liu et al. 2011). Moreover, 21 percent of Medicare‐eligible VA patients report having Part D prescription drug coverage, the majority of whom use this coverage to obtain medications from non‐VA sources (Stroupe et al. 2013).
The availability of Medicare Part D coverage may allow Veterans to more easily access medications. Although many Medicare‐eligible Veterans do not enroll in Part D primarily because they believe they have enough prescription coverage through VA or private insurance, Veterans who enroll in Part D do so to achieve prescription cost savings, to obtain medications not available through VA, as a “backup” in case VA benefits change, or due to VA distance barriers or appointment wait times (Stroupe et al. 2013). However, this dual use of prescription drug coverage may also constitute a type of care fragmentation, in that non‐VA prescribers may not easily share information with VA prescribers, which may compromise prescribing quality and safety. Research on the effect of dual use of VA and Medicare Part D drug benefits on prescribing is limited, but has shown increases in overall medication utilization (Stroupe et al. 2017), use of high‐risk medications in the elderly (Thorpe et al. 2017), and potentially unsafe opioid use (Gellad et al. 2017b, 2018) associated with dual use.
Dual‐system prescription use may also impede patients’ ability to effectively manage and adhere to prescribed regimens. Patients obtaining prescriptions from multiple, disconnected providers may receive conflicting treatment plans and instructions or experience worse communication with providers, which may hinder their ability to consistently take and/or refill medications for chronic conditions (Chen, Tseng, and Cheng 2013; Hong and Kang 2014; Hansen et al. 2015). Dual VA and non‐VA prescription use may also lead patients to receive more medication than is required (i.e., medication oversupply), if prescribers who are unaware of one another issue duplicate prescriptions (Farley et al. 2011). Receiving oversupplies of medications could result in adverse events if excess medications are actually taken, and wasted health care resources and increased patient and health system costs even if not taken (Stroupe et al. 2004, 2006; Krigsman et al. 2007).
Therefore, obtaining medications from both VA and non‐VA systems may represent a unique type of care fragmentation for Veterans that undermines extensive efforts VA has taken to enhance care coordination within the VA through their implementation of Patient‐Aligned Care Teams (PACT), VA's version of the patient‐centered medical home (Nelson et al. 2014; Schectman and Stark 2014; Yano et al. 2014). The risk of undersupply and/or oversupply due to dual use of VA and Part D drug benefits may be especially high among complex patients with multiple conditions, who have increased needs for medications and specialty care, but reduced functional and cognitive capacity to navigate multiple health care systems.
Our objective was to evaluate the effect of obtaining chronic disease medications through VA and Part D, versus VA‐only, on medication undersupply and oversupply in older Veterans with dementia. We focused on Veterans with dementia because of their increased risk for medication underuse (Smith et al. 2017; El‐Saifi et al. 2018) and higher potential vulnerability to care fragmentation. We focused on antihypertensive agents because hypertension is the most common chronic condition affecting patients with dementia (Maslow 2004; Schubert et al. 2006). A secondary objective was to compare antihypertensive medication supplies for Veterans obtaining all antihypertensive agents through Part D, relative to VA‐only users.
Methods
This study was a secondary analysis of 2007–2010 health care utilization and prescription drug–dispensing data from the VA and Centers for Medicare & Medicaid Services (CMS), for a national cohort of Veterans with dementia dually enrolled in VA and Medicare fee‐for‐service (FFS) benefits. The Institutional Review Boards at the VA Pittsburgh Healthcare System and the Durham VA Medical Center approved this study.
Data Sources
The VA Medical SAS files (VIReC 2015a,b) provided data on outpatient, inpatient, and nursing home encounters in VA facilities, including dates of service, diagnoses, and patient demographics. VA Pharmacy Benefits Management (PBM) records (U.S. Department of Veterans Affairs 2008) summarized outpatient medications dispensed from VA, providing drug name, dispensing date, quantity, and days’ supply dispensed. The VA Information Research Center (VIReC) provided linked Medicare and VA enrollee files for CY2007–2010 (Hynes et al. 2007; U.S. Department of Veterans Affairs). CMS medical claims (U.S. Department of Veterans Affairs) included the Medicare Provider Analysis and Review (MedPAR) file, providing data (dates of service, diagnoses) on hospital and skilled nursing facility stays; outpatient facility claims submitted by institutional providers, and carrier claims submitted from physicians and other noninstitutional providers. The Medicare Part D prescription drug event “slim” file provided drug name, National Drug Code (NDC), dispensing date, quantity, and days’ supply for medications dispensed through Part D. The Medicare Master Beneficiary Summary File (MBSF) provided patient demographics, chronic condition diagnoses, and periods of enrollment in Medicare and Medicaid benefits. VA and CMS data files were linked to one another via the patient's scrambled social security number (SSN). We used the Area Health Resources File (AHRF) (U.S. Department of Health and Human Services, Health Resources & Services Administration, Bureau of Health Professions 2012–2013) to link county‐level access to care covariates not available in VA data to Veteran's county of residence. The VA National Drug File (NDF), maintained by VA Pharmacy Benefits Management Services, was used to group drugs in PBM and Part D files into a common set of therapeutic classes (Pharmacy Benefits Management Services 2016) (class list available at http://www.pbm.va.gov/nationalformulary.gov).
Sample
Figure 1 shows the process of sample selection. VA and CMS data files were obtained for all Veterans aged ≥68 years or older as of January 1, 2010, who had at least one dementia ICD‐9 diagnosis code in Medical SAS files or Medicare claims in any of the prior three years (2007–2009). We used the CMS algorithm for Alzheimer's disease or a related disorder to identify diagnosis codes for dementia (Buccaneer Computer Systems & Service, Inc. 2013), in order to have good sensitivity and specificity compared to a gold‐standard clinical dementia assessment (Taylor et al. 2009) (Table S1). To ensure complete data availability for covariate development, we excluded Veterans not continuously enrolled in both VA and Medicare Parts A and B over 2007–2010 (or if they died in 2010, up until their death date). Patients with other drug coverage through Medicare Advantage or an employer‐sponsored drug plan in 2010 were excluded, as were patients with no prescriptions in either VA PBM outpatient records or Medicare Part D in 2010, and a very small number of patients residing outside of the United States. We further limited the sample to patients with a hypertension diagnosis in either VA records or Medicare claims (Buccaneer Computer Systems & Service, Inc. 2013). To ensure the ability to calculate 2010 adherence values for all patients, we required them to have at least one antihypertensive medication dispensed through VA or Part D in the last 120 days of 2009 and at least once in 2010. We excluded patients with fewer than 90 total days in 2010 when they were alive and not in an inpatient setting. The final sample consisted of 50,763 Veterans with dementia actively treated for hypertension in VA and/or Medicare Part D.
Figure 1.
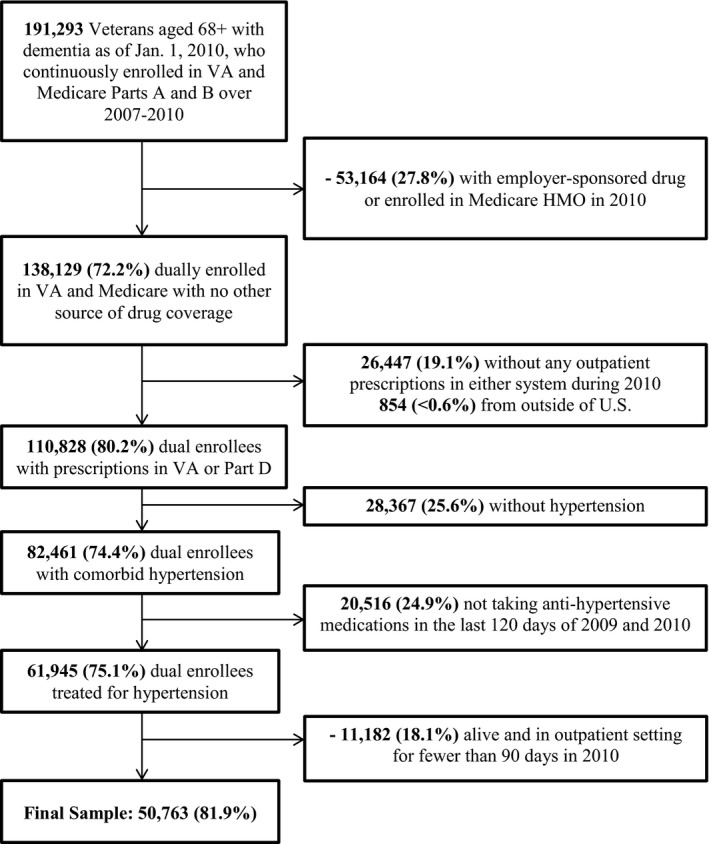
Construction of the study sample: 50,673 VA‐Medicare fee‐for‐service (FFS) dual enrollees with dementia, who were actively treated for hypertension in 2009 and 2010
Measures
Dependent Variable
The primary dependent variable was patients’ 2010 antihypertensive medication supply, considering both VA and Part D prescriptions. Because providers may choose from several antihypertensive classes and often prescribe more than one, we sought to create one patient‐level variable that summarized their annual supply of all antihypertensive classes prescribed at the start of 2010. To create this summary variable, we first identified antihypertensive classes for which patients had at least one fill in either VA or Part D during the last 120 days of 2009 and calculated class‐specific 2010 medication supply values for these classes. A 120‐day window was used because even patients receiving 90‐day prescriptions with some intermittent nonadherence would be expected to have refills during this time frame. Antihypertensive classes and specific agents included were defined using the VA NDF (Pharmacy Benefits Management Services 2016); see Table S2. We searched for all VA and Part D prescription records for fills associated with generic drug names and/or National Drug Codes (NDCs) corresponding to these classes. Fills for combination products were included in calculations for each class in the combination product.
For each class patients obtained in the last 120 days of 2009, we used dispensing date and days’ supplied for the 2009 index fill and all subsequent 2010 refills to calculate the proportion of days in 2010 for which the patient had medication in their possession, divided by 365 days, per previously published methods (Bryson et al. 2007; Thorpe et al. 2009, 2015). For patients who entered an inpatient setting or died in 2010, the denominator of the class‐specific supply values was decreased to include only days the patient was alive and not in an inpatient setting. Extra days’ supply from refills near the end of the year in which days’ supply dispensed exceeded number of days left in the year were not included. From these class‐specific values, we categorized patients using the following thresholds based on prior research (Stroupe et al. 2000, 2006; Morris et al. 2006; Krigsman et al. 2007; Yang, Barner, and Worchel 2007; Thorpe et al. 2009, 2015; Hedna et al. 2013; Chen, Blank, and Cheng 2014): appropriate supply (≥80 percent to <120 percent of days covered) of all classes, undersupply (<80 percent) of at least one antihypertensive class with no oversupply of another class, oversupply (>120 percent) of at least one class with no undersupply of another class, or both undersupply and oversupply of at least one class.
Primary Independent Variable
The primary independent variable was the patient's antihypertensive drug benefit user group, based on where patients filled antihypertensive prescriptions in 2010: 100 percent from VA (“VA‐only”); 100 percent from Part D (“Part D‐only); or at least one antihypertensive from both VA and Part D (“Dual Use”).
Covariates
Past research on dual health care system use (Petersen et al. 2010; Liu et al. 2011) and the Andersen Behavioral Model of Health Service Use (Andersen 1995) guided selection of covariates to consider as predictors of antihypertensive user group and potential confounders of the relationship between user group and antihypertensive medication supply. The Andersen model was chosen based on its past use in studies of predictors of medication utilization (Blalock 2011) and supply (Thorpe et al. 2015). VA encounter records provided data on predisposing factors, including age (68–74, 75–79, 80–84, or ≥85), sex, and race/ethnicity (white, non‐Hispanic; black, non‐Hispanic; Hispanic; other). When race/ethnicity was missing in VA data, the Medicare RTI race variable was used (Eicheldinger and Bonito 2008). The RTI race variable uses the beneficiary's race code in Social Security Administration records together with an algorithm using beneficiary first and last name to identify patients’ race and has established validity (Eicheldinger and Bonito 2008). VA enrollment priority status, an enabling factor, was categorized into four groups associated with generosity of VA health benefits: high disability, low/moderate disability, low income, and no service‐connected disability/low income. We used the Medicare MBSF to determine whether patients were enrolled in Medicaid, the Part D low‐income subsidy (LIS) but not Medicaid, or neither, since both Medicaid and the LIS reduce patient cost‐sharing. County‐level enabling variables which may impact the availability of both VA and non‐VA health care services were defined using the AHRF and included census region (United States Census Bureau 2017) and rurality (U.S. Department of Agriculture Economic Research Service 2013). Distance from the centroid of the patient's ZIP code to the nearest VA facility was obtained from the VA Planning Systems Support Group database (Pizer and Gardner 2011) and classified into quartiles, as an additional geographic indicator of Veterans’ access to VA services. Medical need factors included the Elixhauser Comorbidity Index (Elixhauser et al. 1998), based on diagnosis codes in 2008–2009 inpatient and outpatient VA and Medicare records. We excluded indicators for hypertension and neurological conditions because of redundancy with conditions required for sample inclusion, and peptic ulcer disease, drug abuse, and AIDS because of low prevalence (<1 percent). We also included the total count of Elixhauser conditions (0–2, 3–4, or ≥5). Use of the antidementia drug memantine in 2009 was used as a proxy for dementia stage, as this drug is indicated for moderate to later stage dementia (Food and Drug Administration 2010; VA Pharmacy Benefits Management Services 2014). Other medical need factors included number of inpatient stays and emergency department visits in 2009 (Research Data Assistance Center 2008; Hastings et al. 2011; Wagner, Chow, and Barnett 2011), use of VA home‐based primary care in 2009, and number of days the patient was alive in 2010. We also counted the total number of unique generic medications (0–5, 6–10, 11–15, ≥15) patients used in 2010. Finally, we included 21 indicators representing the Veteran Integrated Service Network (VISN) of the Veteran's preferred VA facility, as VISN is the main administrative unit in the VHA; each VISN is responsible for service planning and allocation of resources at facilities in their region.
Analytic Approach
Analyses were conducted using Stata v14.2 (StataCorp, College Station, TX, USA). To address missing data for <0.1 percent of patients on three variables (driving distance to VA, census region, and rurality), we used hotdeck imputation, in which missing values are replaced with values from randomly selected sample members with no missing data (Schonlau 2017). We generated descriptive statistics for all covariates and used chi‐square and t‐tests to test for differences across drug benefit user groups.
To test our hypothesis that dual use of VA and Part D prescription benefits to obtain antihypertensives would increase likelihood of medication undersupply and oversupply, we used multinomial logistic regression. We used inverse probability of treatment (IPT) weights to balance the three drug benefit user groups on all covariates. Specifically, we estimated the predicted probability of each “treatment” (VA‐only antihypertensive use, Part D‐only use, dual use) for each patient, via multinomial logistic regression including all covariates and converted these probabilities into stabilized, inverse probability of treatment weights (Thoemmes and Ong 2015). These weights were then applied in a weighted multinomial logistic regression model examining the relationship between drug benefit user group and patient‐level medication supply categories. We used the “margins” command to calculate the adjusted, average predicted probability of being in each medication supply category for each drug benefit user group.
Sensitivity Analyses
We examined robustness of our analyses to two alternative specifications of the medication supply variable: (1) categorizing patient‐level summary medication supply using a 110 percent cutoff for defining oversupply (Stroupe et al. 2004; Farley et al. 2011; Chen, Blank, and Cheng 2014); and (2) categorizing patient‐level summary medication supply using the mean of all class‐specific medication supply values for each patient. Also, because descriptive analyses revealed enrollment in Medicaid and/or the Part D low‐income subsidy to be a very strong predictor of dual‐system antihypertensive use, we re‐estimated unadjusted and IPT‐weighted models after stratifying by Medicaid/LIS enrollment. Finally, to examine whether associations between drug benefit user group and medication supply may generalize to other conditions, we conducted a parallel set of analyses on the subsample of Veterans with dementia who had diabetes and were taking oral hypoglycemic agents (OHAs) and otherwise met inclusion/exclusion criteria shown in Figure 1 (except they were required to have diabetes instead of hypertension; n = 13,857).
Results
Sample Characteristics
Table 1 shows descriptive characteristics of the sample, overall and by antihypertensive drug benefit user group. The majority was male, White, and aged 80 or older. Only 38 percent had no service‐connected disability or low‐income status, and a minority was simultaneously enrolled in Medicaid (12 percent) or the LIS (2 percent). A majority of patients used ≥10 unique generic medications in 2010 and most used multiple antihypertensive classes.
Table 1.
Characteristics of 50,763 Veterans With Dementia and Hypertension, Overall and by Antihypertensive Drug Benefit User Group
| Full Sample, N = 50,763 | Dual Prescription User, n = 5,194 (10.2%) | VA‐Only, n = 35,647 (70.2%) | Part D‐Only, n = 9,922 (19.6%) | p‐Value | |
|---|---|---|---|---|---|
| Age in years | |||||
| 68–74 | 6,075 (12%) | 632 (12%) | 4,585 (13%) | 858 (9%) | <.0001 |
| 75–79 | 10,975 (22%) | 1,178 (23%) | 7,928 (22%) | 1,869 (19%) | |
| 80–84 | 15,057 (30%) | 1,650 (32%) | 10,498 (30%) | 2,909 (29%) | |
| 85+ | 18,656 (37%) | 1,734 (33%) | 12,636 (36) | 4,286 (43%) | |
| Male | 49,473 (98%) | 5,085 (98%) | 34,768 (98%) | 9,620 (97%) | .001 |
| Race/ethnicity | |||||
| White | 44,981 (89%) | 4,682 (90%) | 31,309 (88%) | 8,990 (91%) | <.0001 |
| Black | 3,875 (8%) | 317 (6%) | 3,004 (8%) | 554 (6%) | |
| Hispanic | 1,180 (2%) | 130 (3%) | 840 (2%) | 210 (2%) | |
| Other | 727 (1%) | 65 (1%) | 494 (1%) | 168 (2%) | |
| VA priority statusa | |||||
| High disability | 14,474 (29%) | 1,216 (23%) | 11,746 (33%) | 1,512 (15%) | <.0001 |
| Low/mod disability | 7,774 (15%) | 775 (15%) | 5,134 (14%) | 1,865 (19%) | |
| Low income | 9,045 (18%) | 1,151 (.2%) | 6,677 (19%) | 1,217 (12%) | |
| No service‐connected disability or low income | 19,470 (38%) | 2,052 (40%) | 12,090 (34%) | 5,328 (54%) | |
| Medicaid/low‐income subsidy enrollment | |||||
| No Medicaid or LIS | 43,479 (86%) | 3,476 (67%) | 32,918 (92%) | 7,085 (71%) | <.0001 |
| LIS only | 990 (2%) | 226 (4%) | 475 (1%) | 289 (3%) | |
| Medicaid | 6,294 (12%) | 1,492 (29%) | 2,254 (6%) | 2,548 (26%) | |
| Driving distance to closest VA facilitya | |||||
| Quartile 1 | 12,884 (25%) | 1,268 (24%) | 9,117 (26%) | 2,499 (25%) | .461 |
| Quartile 2 | 12,682 (25%) | 1,342 (26%) | 8,826 (25%) | 2,514 (25%) | |
| Quartile 3 | 12,770 (25%) | 1,303 (25%) | 8,964 (25%) | 2,503 (25%) | |
| Quartile 4 | 12,427 (25%) | 1,281 (25%) | 8,740 (25%) | 2,406 (24%) | |
| Census region of countrya | |||||
| Northeast | 9,218 (18%) | 1,060 (20%) | 5,807 (16%) | 2,351 (24%) | <.0001 |
| Midwest | 12,593 (25%) | 1,185 (23%) | 9,158 (26%) | 2,250 (23%) | |
| South | 22,698 (45%) | 2,361 (45%) | 16,397 (46%) | 3,940 (40%) | |
| West | 6,254 (12%) | 588 (11%) | 4,285 (12%) | 1,381 (14%) | |
| Ruralitya | |||||
| Large metropolitan | 20,351 (40%) | 2,060 (40%) | 13,784 (39%) | 4,507 (45%) | <.0001 |
| Small metro metropolitan | 17.718 (35%) | 1,825 (35%) | 12,772 (36%) | 3,121 (32%) | |
| Micropolitan | 6,780 (13%) | 706 (14%) | 4,863 (14%) | 1,211 (12%) | |
| Noncore rural | 5,914 (12%) | 603 (12%) | 4,228 (12%) | 1,083 (11%) | |
| Number of Elixhauser comorbidities | |||||
| 0–2 | 7,371 (15%) | 489 (9%) | 5,613 (16%) | 1,269 (13%) | <.0001 |
| 3–4 | 15,087 (30%) | 1,241 (24%) | 11,201 (31%) | 2,645 (27%) | |
| 5+ | 28,305 (56%) | 3,464 (67%) | 18,833 (53%) | 6,008 (61%) | |
| Elixhauser comorbidities | |||||
| CHF | 14,599 (29%) | 1,963 (38%) | 9,242 (26%) | 3,394 (34%) | <.0001 |
| Valve disorder | 9,773 (19%) | 1,273 (25%) | 6,278 (18%) | 2,222 (22%) | <.0001 |
| Pulmonary‐circulation disease | 2,386 (5%) | 332 (6%) | 1,556 (4%) | 498 (5%) | <.0001 |
| Peripheral vascular disease | 15,725 (31%) | 1,980 (38%) | 10,107 (28%) | 3.638 (37%) | <.0001 |
| Paralysis | 2,997 (6%) | 352 (7%) | 1,976 (6%) | 669 (7%) | <.0001 |
| Chronic pulmonary disease | 15,889 (31%) | 1,985 (38%) | 10,728 (30%) | 3,176 (32%) | <.0001 |
| Diabetes without complications | 15,918 (31%) | 1,805 (35%) | 11,165 (31%) | 2,948 (30%) | <.0001 |
| Diabetes with complications | 9,945 (20%) | 1,345 (26%) | 6,815 (19%) | 1,785 (18%) | <.0001 |
| Hypothyroidism | 9,353 (18%) | 1,081 (21%) | 6,173 (17%) | 2,099 (21%) | <.0001 |
| Renal failure | 12,437 (25%) | 1,530 (30%) | 8,338 (23%) | 2,569 (26%) | <.0001 |
| Liver disease | 624 (1%) | 84 (2%) | 406 (1%) | 134 (1%) | .007 |
| Lymphoma | 605 (1%) | 65 (1%) | 415 (1%) | 125 (1%) | .678 |
| Metastases | 657 (1%) | 74 (1%) | 416 (1%) | 167 (2%) | <.0001 |
| Solid tumor | 10,539 (21%) | 1,152 (22%) | 7,385 (21%) | 2,002 (20%) | .015 |
| Rheumatoid arthritis | 1,934 (4%) | 245 (5%) | 1,269 (4%) | 420 (4%) | <.0001 |
| Coagulopathy | 3,859 (8%) | 506 (10%) | 2,483 (7%) | 870 (9%) | <.0001 |
| Obesity | 4,030 (8%) | 511 (10%) | 3,048 (9%) | 471 (5%) | <.0001 |
| Weight loss | 4,064 (8%) | 439 (9%) | 2,629 (7%) | 996 (10%) | <.0001 |
| Electrolyte disorder | 13,414 (26%) | 1,647 (32%) | 8,557 (24%) | 3,210 (32%) | <.0001 |
| Chronic blood loss anemia | 1,211 (2%) | 162 (3%) | 718 (2%) | 331 (3%) | <.0001 |
| Deficiency anemia | 19,374 (38%) | 2,326 (45%) | 12,631 (35) | 4,417 (45%) | <.0001 |
| Alcohol abuse | 1,669 (3%) | 195 (4%) | 1,165 (3%) | 309 (3%) | <.0001 |
| Psychoses | 8,385 (17%) | 969 (19%) | 5,746 (16%) | 1,670 (17%) | <.0001 |
| Depression | 12,964 (26%) | 1,463 (28%) | 9,163 (26%) | 2,338 (24%) | <.0001 |
| Epilepsy | 1,824 (4%) | 216 (4%) | 1,220 (3%) | 388 (4%) | .005 |
| Syncope | 5,053 (10%) | 568 (11%) | 3,264 (9%) | 1,221 (12%) | <.0001 |
| Memantine use in 2009 | 6,856 (14%) | 883 (17%) | 4,025 (11%) | 1,948 (20%) | <.0001 |
| Home‐based primary care in 2009 | 3,152 (6%) | 251 (5%) | 2,831 (8%) | 70 (1%) | <.0001 |
| Number of inpatient stays in 2009 | |||||
| 0 | 29,623 (58%) | 2,649 (51%) | 21,722 (60.9%) | 5,252 (53%) | <.0001 |
| 1 | 11,249 (22%) | 1,216 (23%) | 7.716 (21.7%) | 2,317 (23%) | |
| 2+ | 9,891 (20%) | 1,329 (26%) | 6,209 (17.4%) | 2,353 (24%) | |
| Number of emergency department visits in 2009 | |||||
| 0 | 28,887 (57%) | 2,748 (53%) | 20,365 (57%) | 5,774 (58%) | <.0001 |
| 1 | 11,960 (24%) | 1,271 (25%) | 8,301 (23%) | 2,388 (24%) | |
| 2+ | 9,916 (20%) | 1,175 (23%) | 6,981 (20%) | 1,760 (18%) | |
| Days alive in 2010, M(SD) | 358.7 (29) | 358.8 (27) | 360.6 (23) | 351.8 (45) | <.0001 |
| Number of unique generic drugs in 2010 | |||||
| 0–5 | 4,544 (9%) | 55 (1%) | 3,828 (11%) | 661 (7%) | <.0001 |
| 6–10 | 15,533 (31%) | 652 (13%) | 11,861 (33%) | 3,020 (30%) | |
| 11–15 | 11,957 (24%) | 1,126 (22%) | 8,188 (23%) | 2,643 (27%) | |
| ≥15 | 18,729 (37%) | 3,361 (65%) | 11,770 (33%) | 3,598 (36%) | |
| Total number of antihypertensive agents taken in last 120 days of 2009 | |||||
| 1 | 18,401 (36%) | 1,403 (27%) | 13,322 (37%) | 3,676 (37%) | <.0001 |
| 2 | 17,749 (35%) | 1,826 (35%) | 12,455 (35%) | 3,468 (35%) | |
| 3 | 10,528 (21%) | 1,312 (25%) | 7,191 (20%) | 2,025 (20%) | |
| 4 or more | 4,085 (8%) | 653 (13%) | 2,679 (8%) | 753 (8%) | |
| Use of specific antihypertensive classes | |||||
| ACE/ARBs/renin inhibitors | 30,101 (59%) | 3,213 (62%) | 21,373 (60%) | 5,515 (56%) | <.0001 |
| Beta blockers | 26,405 (52%) | 3,027 (58%) | 17,971 (50%) | 5,407 (55%) | <.0001 |
| Calcium channel blockers | 18,084 (36%) | 2,049 (40%) | 12,921 (36%) | 3,114 (31%) | <.0001 |
| Loop diuretics | 12,689 (25%) | 1,729 (33%) | 7,830 (22%) | 3,130 (32%) | <.0001 |
| Thiazide diuretics | 10,134 (20%) | 1,073 (21%) | 7,339 (21%) | 1.722 (17%) | <.0001 |
| Potassium‐sparing diuretics | 2,663 (5%) | 307 (6%) | 1,921 (5%) | 435 (4%) | <.0001 |
| Vasodilators/Centrally acting agents | 2.431 (5%) | 343 (7%) | 1,569 (4%) | 519 (5%) | <.0001 |
Variables with <1% missing data. Missing values were imputed using hotdeck imputation prior to all analyses.
LIS, low‐income subsidy.
Ten percent of patients were dual VA/Part D users of antihypertensives, 70 percent obtained all antihypertensives from VA, and 20 percent obtained all antihypertensives from Part D. Patients differed significantly across drug benefit user groups on all covariates, except for driving distance to nearest VA and presence of lymphoma. The largest magnitudes of differences across user groups were seen for VA priority status, Medicaid/LIS enrollment, health status variables, and medication utilization variables. Dual prescription users (40 percent) and Part D‐only users (54 percent) were more likely than VA‐only users (34 percent) to not have service‐connected disability or low‐income status, and were more likely to be enrolled in Medicaid (29 percent and 26 percent vs. 6 percent, respectively). Dual prescription users and Part D‐only users had more comorbidities compared to VA‐only users, and dual prescription users were more likely than VA‐only and Part D‐only users to use ≥15 total medications (65 percent vs. 33 percent and 36 percent).
Forty‐five percent of Veterans had an appropriate supply of all prescribed antihypertensives; 41 percent had undersupply for ≥1 class (with no oversupply of another class); 10 percent had oversupply for ≥1 class (with no undersupply of another class); and 4 percent simultaneously had oversupply of at least one class and undersupply of another class (Table 2). Medication supply differed according to drug benefit user group status. Just 32 percent of dual prescription users had an appropriate supply of all prescribed classes, compared to 47 percent of VA‐only users and 45 percent of Part D‐only users. Dual prescription users were less likely than VA‐only users to have undersupply with no oversupply (38 percent vs. 40 percent), but were more likely to have oversupply with no undersupply (19 percent vs. 9 percent), as well as simultaneous oversupply and undersupply (10 percent vs. 4 percent). Part D‐only users were more likely to have undersupply with no oversupply (47 percent) but less likely to have oversupply with (3 percent) or without undersupply (5 percent). This pattern remained consistent when using the alternative specifications for the summary medication supply variable.
Table 2.
Antihypertensive Medication Supply by VA Patients With Dementia, Overall and by Hypertension Medication User Group Status, 2010
| Overall Hypertension Sample, N = 50,763 | Dual Prescription User, n = 5,194 | VA‐Only, n = 35,647 | Part D‐Only, n = 9,922 | p‐Value | |
|---|---|---|---|---|---|
| Primary outcome: medication supply categories, 120% oversupply cutoff | |||||
| Appropriate supply of all classes | 22,938 (45%) | 1,672 (32%) | 16,788 (47%) | 4,478 (45%) | <.0001 |
| Undersupply for at least one class, but no oversupply | 20,906 (41%) | 1,996 (38%) | 14,277 (40%) | 4,633 (47%) | |
| Oversupply for at least one class, but no undersupply | 4,802 (10%) | 997 (19%) | 3,284 (9%) | 521 (5%) | |
| Oversupply and undersupply for at least one class, respectively | 2,117 (4%) | 529 (10%) | 1,298 (4%) | 290 (3%) | |
| Sensitivity analysis: medication supply categories, 110% oversupply cutoff | |||||
| Appropriate supply of all classes | 19,951 (39%) | 1,252 (24%) | 14,658 (41%) | 4,040 (41%) | <.0001 |
| Undersupply for at least one class, but no oversupply | 19,599 (39%) | 1,759 (34%) | 13,406 (38%) | 4,434 (45%) | |
| Oversupply for at least one class, but no undersupply | 7.78 (15%) | 1,417 (27%) | 5,413 (15%) | 959 (10%) | |
| Oversupply and undersupply for at least one class, respectively | 3,424 (7%) | 766 (15%) | 2,169 (6%) | 489 (5%) | |
| Sensitivity analysis: medication supply categories, based on average of all classes used by a patient | |||||
| Appropriate supply | 29,834 (59%) | 2,775 (53%) | 21,562 (61%) | 5,497 (55%) | <.0001 |
| Undersupply | 17,449 (34%) | 1,675 (32%) | 11,729 (33%) | 4,045 (41%) | |
| Oversupply | 3,480 (7%) | 744 (14%) | 2,356 (7%) | 380 (4%) | |
IPT‐Weighted Regression Models
The application of IPT weights dramatically improved covariate balance across drug benefit user groups (Table S3). The only covariates showing statistically significant differences after IPT‐weighting included hypothyroidism, psychoses, and total number of unique drugs; however, differences in these characteristics across groups after weighting were very small in magnitude (e.g., after weighting, 36 percent of dual prescription users used ≥15 drugs, compared to 37 percent of VA‐only users and 32 percent of Part D‐only users).
Table 3 shows the results of multinomial regression models for the relationship between antihypertensive drug benefit user group and medication supply. In the IPT‐weighted model, dual prescription users (relative to VA‐only users) had higher odds of undersupply with no oversupply (OR = 1.28, 95 percent CI = 1.13, 1.44; adjusted predicted probability [APP] of 40.5 percent vs. 40.1 percent), oversupply with no undersupply (OR = 2.00, 95 percent CI = 1.75, 2.29; APP of 15.7 percent vs. 9.9 percent), and simultaneous oversupply and undersupply (OR = 2.40, 95 percent CI = 2.00, 2.88; APP 7.5 percent vs. 4.0 percent). Relative to VA‐only users, Part D‐only users also exhibited higher odds of undersupply with no oversupply (OR = 1.13, 95 percent CI = 1.03, 1.25; APP of 46.8 percent vs. 40.1 percent), but lower odds of oversupply with no undersupply (OR = 0.39, 95 percent CI = 0.32, 0.47; APP of 4.0 vs. 9.9 percent) and simultaneous undersupply and oversupply (OR = 0.48, 95 percent CI = 0.40, 0.57; APP of 1.9 percent vs. 4.0 percent).
Table 3.
Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Medication Supply Categories, for Dual Prescription Users (n = 5,194) and Part D‐only Users (n = 9,922), Relative to VA‐only Users (n = 35,647)
| Unadjusted odds ratios and 95% confidence intervals | Adjusted odds ratios and 95% confidence intervals (IPT‐weighted Model)a | Adjusted predicted probabilities and 95% confidence intervals | |||||
|---|---|---|---|---|---|---|---|
| Dual prescription users | Part D‐only users | Dual prescription users | Part D‐only users | VA‐only users | Dual prescription users | Part D‐only users | |
| Appropriate supply of all classes | ref | ref | ref | ref | 46.0% (45.3–46.6) | 36.3% (34.0–38.7) | 47.3% (45.1–49.5) |
| Undersupply for at least one class, but no oversupply | 1.40 (1.31, 1.50) | 1.22 (1.16, 1.27) | 1.28 (1.13, 1.44) | 1.13 (1.03, 1.25) | 40.1% (39.5–40.8) | 40.5% (38.1–42.9) | 46.8% (44.6–48.9) |
| Oversupply for at least one class, but no undersupply | 3.05 (2.79, 3.32) | 0.59 (0.53, 0.66) | 2.00 (1.75, 2.29) | 0.39 (0.32, 0.47) | 9.9% (9.5–10.3) | 15.7% (14.2–17.1) | 4.0% (3.3–4.6) |
| Oversupply and undersupply for at least one class | 4.09 (3.66, 4.58) | 0.84 (0.73, 0.96) | 2.40 (2.00, 2.88) | 0.48 (0.40, 0.57) | 4.0% (3.7–4.3) | 7.5% (6.5–8.6) | 1.9% (1.6–2.2) |
The following variables were included in the IPT weights: age, sex, race/ethnicity, VA priority group, Medicaid/LIS enrollment, distance from VA, region, rural/urban, number and specific type of Elixhauser comorbidities, memantine use as proxy for dementia severity, use of home‐based primary care, baseline inpatient visits, baseline emergency room visits, days alive in 2010, total number of unique medications, and VISN indicators.
Sensitivity Analyses
The two alternative IPT‐weighted multinomial regression models using a less conservative threshold for defining oversupply and medication supply categories based on mean medication supply values yielded substantively similar results (Tables S4 and S5). The pattern of results was also substantively similar in stratified analyses within the Medicaid/LIS‐enrollees and nonenrollees (Table S6). In analyses for Veterans with dementia and comorbid diabetes (n = 13,857), both unadjusted and IPT‐weighted regression models for the relationship between OHA user group status and OHA medication supply categories (Tables S7 and S8) yielded similar results compared to hypertension analyses. The only divergent finding was that there was no difference in odds of having simultaneous undersupply and oversupply of OHAs in Part D‐only users compared to VA‐only users.
Discussion
In a national sample of Veterans with dementia dually enrolled in VA and fee‐for‐service Medicare, we found that Veterans obtaining antihypertensive prescriptions through both VA and Medicare Part D rather than VA alone were less likely to obtain appropriate supplies of antihypertensive medications. Only 32 percent of dual prescription users of antihypertensive agents had an appropriate supply of all prescribed classes in 2010, compared to 47 percent of VA‐only users and 45 percent of Part D‐only users. In adjusted analyses, dual prescription users were more likely than VA‐only users to exhibit undersupply alone, oversupply alone, and simultaneous undersupply and oversupply. Part D‐only users were more likely than VA‐only users to have undersupply, but less likely to have oversupply. These results were robust to sensitivity analyses using alternative methods to calculate medication supply, stratification by Medicaid/LIS enrollment, and an alternative dependent variable of OHA medication supply instead of antihypertensive supply.
We believe this study is the first to examine the effect of dual use of VA and Medicare Part D prescription drug benefits—a potential source of care fragmentation—on patients’ under‐ and overutilization of chronic disease medications. Although only 10 percent of older, Medicare‐enrolled Veterans with dementia obtained antihypertensive medications through both sources, our findings suggest that previously documented risks associated with dual use of VA and Part D drug benefits may extend beyond reducing prescribing safety (Thorpe et al. 2017; Gellad et al. 2018) to interfere with patients’ ability to maintain appropriate supplies of chronic medications. Our findings are consistent with past studies showing that more outpatient providers (Chen, Tseng, and Cheng 2013; Hong and Kang 2014) and prescribers (Farley et al. 2011; Hansen et al. 2015) are associated with medication undersupply and oversupply. The effect of dual prescription use was present for both undersupply and oversupply, suggesting that dual prescription use may negatively impact patients’ appropriate acquisition of medication through multiple mechanisms (e.g., duplicate prescribing, confusing/conflicting instructions, and worse patient–provider communication). The large magnitude of effect of dual versus VA‐only prescription use on oversupply suggests that duplicate prescribing within classes may be a particular concern. Future research with family caregivers and health care providers of Veterans with dementia who obtain prescriptions from both VA and Part D, regarding care coordination problems introduced by dual prescription use, may provide insight into which of these potential mechanisms are at play.
It is notable that 20 percent of dually enrolled Veterans with dementia and hypertension obtained all antihypertensives from non‐VA sources through Part D. Among Part D‐only users, undersupply was slightly more common than in VA‐only users; however, oversupply was less common. These results are consistent with prior studies, in which prevalence of oversupply has been reported to be common among Veterans using the VA (Thorpe et al. 2009) but relatively uncommon among non‐Veteran Medicare beneficiaries (Thorpe et al. 2015). The higher rate of undersupply in Part D‐only versus VA‐only users may again be explained by greater potential for care fragmentation due to receiving antihypertensive prescriptions from multiple, unconnected non‐VA prescribers and/or pharmacies (Marcum et al. 2014) through Part D benefits. On the other hand, duplicate prescriptions from multiple, unconnected non‐VA prescribers would likely be identified before they were dispensed when the pharmacy attempts to bill the Part D plan, as most payers employ computerized utilization management systems designed to prevent dispensing of oversupplies (Centers for Medicare & Medicaid Services 2016).
Our results add to growing evidence regarding the importance of improving timely exchange of linked data about VA and non‐VA prescriptions across systems of care for both research and clinical care, given nontrivial numbers of Veterans obtaining medications from non‐VA sources (Nguyen et al. 2017; Thorpe et al. 2017; Gellad et al. 2018) and the consistent association of dual prescription use and worse prescribing outcomes (Thorpe et al. 2017; Gellad et al. 2018). VA's partnership with CMS to provide researchers with access to Part D records for Veterans is essential for accurately evaluating prescribing quality in Veterans and supporting research to improve their pharmacotherapy. However, conducting timely research to improve care for Medicare‐enrolled Veterans is currently hindered by a two‐year lag in availability of Veterans’ Part D data for researchers. From a clinical perspective, VA clinicians currently rely almost entirely on patients or their informal caregivers to communicate information about prescriptions from non‐VA sources. Early efforts to facilitate health information exchange between VA and non‐VA electronic health records (EHRs) for use by clinicians, through the VA Virtual Lifetime Electronic Record (VLER), have shown promise (Byrne et al. 2014) and may be a key strategy in reducing care fragmentation and associated prescribing problems. However, further efforts are needed to expand VLER implementation across VA and evaluate its impact on prescribing quality and safety. In addition, pharmacist medication therapy management interventions and nurse case management have both shown promise for improving medication adherence and appropriateness in complex patients (Viswanathan et al. 2012, 2015) and should be considered as a strategy to help reduce medication undersupply and oversupply associated with dual prescription use in Veterans with dementia.
Our findings have implications for current efforts to expand Veterans’ access to non‐VA care through the Veterans Choice Program (VCP) and highlight the need to ensure that VCP use does not undermine VA's efforts to deliver coordinated care. As the VCP allows Veterans to see non‐VA providers when they experience VA appointment delays or geographic barriers (Mattocks and Yehia 2017), it may also lead to Veterans being prescribed medications by non‐VA providers to be filled at VA pharmacies. Like non‐VA providers seen via Medicare benefits, VCP providers do not have direct, point‐of‐care access to Veterans’ VA EHR to obtain complete information on current prescriptions. As such, they may unknowingly issue duplicate prescriptions or conflicting instructions. Because VCP prescriptions are filled at VA pharmacies and recorded in the EHR, there is an opportunity for VA clinicians to identify and correct prescribing duplications or inefficiencies that does not exist for non‐VA prescriptions filled at outside the VA through Part D. However, suboptimal patient–provider communication may also contribute to nonadherence, if prescriptions are issued by new non‐VA providers with whom the Veteran does not have an established relationship. Future research should examine the impact of VCP use on medication undersupply and oversupply, as well as pharmacist workload, given preliminary evidence of increased workload for VA pharmacists to resolve prescribing errors and inefficiencies associated with non‐VA VCP prescriptions (Gellad et al. 2017a).
This research has several limitations. First, it is unknown how results generalize to Veterans with other sources of non‐VA drug coverage or dual VA‐Medicare enrollees without dementia. It is likely that dual prescription use would have a similarly negative impact on medication supply in other older adults with complex medication needs; for example, the vast majority of older adults with multiple chronic conditions. However, these effects may not generalize to younger Veterans with less complex care needs and/or greater functional/cognitive capacity. Research examining the effect of dual VA‐Part D prescription use in other Veteran populations is needed, as are studies using linked data for Veterans with other sources of drug coverage.
Second, our measurement of medication supply relies on the assumption that prescriptions filled in the last 120 days of 2009 reflect specific classes of medications patients were instructed to take while outpatients throughout 2010 and does not account for switching from one antihypertensive class to another. When switching between classes occurs, medication supply may be underestimated. However, potential cases of cross‐class switching were rare in this sample, occurring in <2 percent of patient‐class records. We also have no way of knowing whether oversupplies were actually ingested. Third, although IPT‐weighting achieved good balance on covariates, we cannot rule out the possibility that unmeasured confounding factors may bias results. Fourth, due to the lack of prescriber information in the Part D “slim” file, we were unable to examine the role of number of prescribers within a system in addition to dual health system use. And finally, we did not assess for adverse events associated with oversupply or undersupply.
Despite these limitations, this study suggests that utilization of Medicare Part D drug benefits to obtain chronic disease medications may contribute to medication undersupply and oversupply when used simultaneously with VA drug benefits. In addition, patients who solely rely on non‐VA prescriptions through Part D rather than VA may also be at greater risk for undersupply. These findings highlight the importance of considering both VA and non‐VA prescription drug data when examining the quality of care for Veterans, and suggest that increased efforts by VA and CMS to coordinate prescriptions across systems may be needed to reduce inefficient prescribing and nonadherence associated with dual use of VA‐Part D drug benefits.
Supporting information
Appendix SA1: Author Matrix.
Table S1. ICD‐9‐CM Diagnosis Codes Used to Identify Alzheimer's Disease or Related Disorder (ADRD).
Table S2. Antihypertensive Classes and Agents Included in Refill Adherence Calculations.
Table S3. Covariate Balance Achieved After Applying Stabilized Inverse Probability of Treatment Weights.
Table S4. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Mutually Exclusive MPR Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users, Using 110% Cutoff for Oversupply (N = 50,763).
Table S5. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in MPR Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users, Using Average Refill Adherence Values Across Classes (N = 50,763).
Table S6. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Antihypertensive Medication Supply Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users, in Medicaid/LIS Enrollees and Non‐Enrollees.
Table S7. Oral Hypoglycemic Agent (OHA) Use by VA Patients with Dementia and Diabetes, Overall and by OHA Medication User Group Status, 2010.
Table S8. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Diabetes Medication Supply Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users (N = 13,857).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was funded by a grant from the U.S. Department of Veterans Affairs (IIR 12‐379, PI: Joshua M. Thorpe). Dr. Niznik was funded by a T32 award from the National Institute on Aging (T32AG021885) and Dr. Carico was funded by a postdoctoral fellowship through the Veterans Administration Office of Academic Affairs. The authors have no potential financial conflict of interests to report. The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs.
Disclosures: None.
Disclaimer: None.
References
- Andersen, R. M. 1995. “Revisiting the Behavioral Model and Access to Medical Care: Does It Matter?” Journal of Health and Social Behavior 36 (1): 1‐10. [PubMed] [Google Scholar]
- Blalock, S. J. 2011. “The Theoretical Basis for Practice‐Relevant Medication use Research: Patient‐Centered/Behavioral Theories.” Research in Social Administrative Pharmacy 7 (4): 317‐29. [DOI] [PubMed] [Google Scholar]
- Bryson, C. L. , Au D. H., Young B., McDonell M. B., and Fihn S. D.. 2007. “A Refill Adherence Algorithm for Multiple Short Intervals to Estimate Refill Compliance (ReComp).” Medical Care 45 (6): 497‐504. [DOI] [PubMed] [Google Scholar]
- Buccaneer Computer Systems & Service, Inc . 2013. Chronic Condition Data Warehouse Medicare Administrative Data User Guide.Warrenton, VA: Buccaneer Computer Systems & Services, Inc. [Google Scholar]
- Byrne, C. M. , Mercincavage L. M., Bouhaddou O., Bennett J. R., Pan E. C., Botts N. E., Olinger L. M., Hunolt E., Banty K. H., and Cromwell T.. 2014. “The Department of Veterans Affairs’ (VA) Implementation of the Virtual Lifetime Electronic Record (VLER): Findings and Lessons Learned From Health Information Exchange at 12 Sites.” International Journal of Medical Informatics 83 (8): 537‐47. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services . 2016. Medicare Prescription Drug Benefit Manual: Chapter 6 – Part D Drugs and Formulary Requirements. Washington, DC: D. o. H. a. H. Services. [Google Scholar]
- Chen, C. C. , Blank R. H., and Cheng S. H.. 2014. “Medication Supply, Healthcare Outcomes and Healthcare Expenses: Longitudinal Analyses of Patients with Type 2 Diabetes and Hypertension.” Health Policy 117 (3): 374‐81. [DOI] [PubMed] [Google Scholar]
- Chen, C. C. , Tseng C. H., and Cheng S. H.. 2013. “Continuity of Care, Medication Adherence, and Health Care Outcomes among Patients with Newly Diagnosed Type 2 Diabetes: A Longitudinal Analysis.” Medical Care 51 (3): 231‐7. [DOI] [PubMed] [Google Scholar]
- Eicheldinger, C. , and Bonito A.. 2008. “More Accurate Racial and Ethnic Codes for Medicare Administrative Data.” Health Care Financing Review 29 (3): 27‐42. [PMC free article] [PubMed] [Google Scholar]
- Elixhauser, A. , Steiner C., Harris D. R., and Coffey R. N.. 1998. “Comorbidity Measures for use with Administrative Data.” Medical Care 36 (1): 8‐27. [DOI] [PubMed] [Google Scholar]
- El‐Saifi, N. , Moyle W., Jones C., and Tuffaha H.. 2018. “Medication Adherence in Older Patients with Dementia: A Systematic Literature Review.” Journal of Pharmacy Practice 31: 322‐34. [DOI] [PubMed] [Google Scholar]
- Farley, J. F. , Wang C. C., Hansen R. A., Voils C. I., and Maciejewski M. L.. 2011. “Continuity of Antipsychotic Medication Management for Medicaid Patients with Schizophrenia.” Psychiatric Services 62 (7): 747‐52. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . 2010. Drug Approval Package: Namenda XR (Memantine Hydrochloride) 7 mg, 14 mg, 21 mg, & 28 mg Extended Release Capsules. Washington, DC: Food and Drug Administration. [Google Scholar]
- Gellad, W. F. , Cunningham F. E., Good C. B., Thorpe J. M., Thorpe C. T., Bair B., Roman K., and Zickmund S. L.. 2017a. “Pharmacy Use in the First Year of the Veterans Choice Program: A Mixed‐Methods Evaluation.” Medical Care 55: S26‐S32. [DOI] [PubMed] [Google Scholar]
- Gellad, W. F. , Zhao X., Thorpe C. T., Thorpe J. M., Sileanu F. E., Cashy J. P., Mor M., Hale J. A., Radomski T., Hausmann L. R., Fine M. J., and Good C. B.. 2017b. “Overlapping Buprenorphine, Opioid, and Benzodiazepine Prescriptions among Veterans Dually Enrolled in Department of Veterans Affairs and Medicare Part D.” Substance Abuse 38 (1): 22‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellad, W. F. , Thorpe J. M., Zhao X., Thorpe C. T., Sileanu F. E., Cashy J. P., Hale J. A., Mor M. K., Radomski T. R., Hausmann L. R. M., Donohue J. M., Gordon A. J., Suda K. J., Stroupe K. T., Hanlon J. T., Cunningham F. E., Good C. B., and Fine M. J.. 2018. “Impact of Dual use of VA and Medicare Part D Drug Benefits on Potentially Unsafe Opioid use.” American Journal of Public Health 108 (2): 248‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, R. A. , Voils C. I., Farley J. F., Powers B. J., Sanders L. L., Sleath B., and Maciejewski M. L.. 2015. “Prescriber Continuity and Medication Adherence for Complex Patients.” Annals of Pharmacotherapy 49 (3): 293‐302. [DOI] [PubMed] [Google Scholar]
- Hastings, S. N. , Smith V. A., Weinberger M., Schmader K. E., Olsen M. K., and Oddone E. Z.. 2011. “Emergency Department Visits in Veterans Affairs Medical Facilities.” American Journal of Managed Care 17(6 Spec No.): e215‐23. [PMC free article] [PubMed] [Google Scholar]
- Hedna, K. , Hagg S., Andersson Sundell K., Petzold M., and Hakkarainen K. M.. 2013. “Refill Adherence and Self‐Reported Adverse Drug Reactions and sub‐Therapeutic Effects: A Population‐Based Study.” Pharmacoepidemiology and Drug Safety 22 (12): 1317‐25. [DOI] [PubMed] [Google Scholar]
- Hong, J. S. , and Kang H. C.. 2014. “Relationship between Continuity of Ambulatory Care and Medication Adherence in Adult Patients with Type 2 Diabetes in Korea: A Longitudinal Analysis.” Medical Care 52 (5): 446‐53. [DOI] [PubMed] [Google Scholar]
- Hynes, D. M. , Koelling K., Stroupe K., Arnold N., Mallin K., Sohn M. W., Weaver F. M., Manheim L., and Kok L.. 2007. “Veterans’ Access to and use of Medicare and Veterans Affairs Health Care.” Medical Care 45 (3): 214‐23. [DOI] [PubMed] [Google Scholar]
- Krigsman, K. , Melander A., Carlsten A., Ekedahl A., and Nilsson J. L.. 2007. “Refill non‐Adherence to Repeat Prescriptions Leads to Treatment Gaps or to High Extra Costs.” Pharmacy World and Science 29 (1): 19‐24. [DOI] [PubMed] [Google Scholar]
- Liu, C. F. , Manning W. G., Burgess J. F. Jr, Hebert P. L., Bryson C. L., Fortney J., Perkins M., Sharp N. D., and Maciejewski M. L.. 2011. “Reliance on Veterans Affairs Outpatient Care by Medicare‐Eligible Veterans.” Medical Care 49 (10): 911‐7. [DOI] [PubMed] [Google Scholar]
- Marcum, Z. A. , Driessen J., Thorpe C. T., Gellad W. F., and Donohue J. M.. 2014. “Effect of Multiple Pharmacy use on Medication Adherence and Drug‐Drug Interactions in Older Adults with Medicare Part D.” Journal of the American Geriatrics Society 62 (2): 244‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow, K. 2004. “Dementia and Serious Coexisting Medical Conditions: A Double Whammy.” Nursing Clinics of North America 39 (3): 561‐79. [DOI] [PubMed] [Google Scholar]
- Mattocks, K. M. , and Yehia B.. 2017. “Evaluating the Veterans Choice Program: Lessons for Developing a High‐Performing Integrated Network.” Medical Care 55 Suppl 7 Suppl 1: 1‐3. [DOI] [PubMed] [Google Scholar]
- Morris, A. B. , Li J., Kroenke K., Bruner‐England T. E., Young J. M., and Murray M. D.. 2006. “Factors Associated with Drug Adherence and Blood Pressure Control in Patients with Hypertension.” Pharmacotherapy 26 (4): 483‐92. [DOI] [PubMed] [Google Scholar]
- Nelson, K. , Sun H., Dolan E., Maynard C., Beste L., Bryson C., Schectman G., and Fihn S. D.. 2014. “Elements of the Patient‐Centered Medical Home Associated with Health Outcomes among Veterans: The Role of Primary Care Continuity, Expanded Access, and Care Coordination.” Journal of Ambulatory Care Management 37 (4): 331‐8. [DOI] [PubMed] [Google Scholar]
- Nguyen, K. A. , Haggstrom D. A., Ofner S., Perkins S. M., French D. D., Myers L. J., Rosenman M., Weiner M., Dixon B. E., and Zillich A. J.. 2017. “Medication Use among Veterans across Health Care Systems.” Applied Clinical Informatics 8 (1): 235‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, L. A. , Byrne M. M., Daw C. N., Hasche J., Reis B., and Pietz K.. 2010. “Relationship between Clinical Conditions and use of Veterans Affairs Health Care among Medicare‐Enrolled Veterans.” Health Services Research 45 (3): 762‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmacy Benefits Management Services . 2016. “VA National Drug File.” Department of Veterans Affairs – Veterans Health Administration.
- Pizer, S. D. , and Gardner J. A.. 2011. “Is Fragmented Financing bad for Your Health?” Inquiry 48 (2): 109‐22. [DOI] [PubMed] [Google Scholar]
- Research Data Assistance Center . 2008. How to Identify Emergency Room Services in the Medicare Claims Data. Minneapolis, MN: University of Minnesota. [Google Scholar]
- Schectman, G. , and Stark R.. 2014. “Orchestrating Large Organizational Change in Primary Care: The Veterans’ Health Administration Experience Implementing a Patient‐Centered Medical Home.” Journal of General Internal Medicine 29 (Suppl 2): S550‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonlau, M . 2017. Creating imputed variables through single hotdeck imputation. Stata Version 14 College Station, TX: StataCorp. [Google Scholar]
- Schubert, C. C. , Boustani M., Callahan C. M., Perkins A. J., Carney C. P., Fox C., Unverzagt F., Hui S., and Hendrie H. C.. 2006. “Comorbidity Profile of Dementia Patients in Primary Care: Are They Sicker?” Journal of the American Geriatrics Society 54 (1): 104‐9. [DOI] [PubMed] [Google Scholar]
- Smith, D. , Lovell J., Weller C., Kennedy B., Winbolt M., Young C., and Ibrahim J.. 2017. “A Systematic Review of Medication non‐Adherence in Persons with Dementia or Cognitive Impairment.” PLoS ONE 12 (2): e0170651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe, K. T. , Murray M. D., Stump T. E., and Callahan C. M.. 2000. “Association between Medication Supplies and Healthcare Costs in Older Adults From an Urban Healthcare System.” Journal of the American Geriatrics Society 48 (7): 760‐8. [DOI] [PubMed] [Google Scholar]
- Stroupe, K. T. , Teal E. Y., Weiner M., Gradus‐Pizlo I., Brater D. C., and Murray M. D.. 2004. “Health Care and Medication Costs and use among Older Adults with Heart Failure.” American Journal of Medicine 116 (7): 443‐50. [DOI] [PubMed] [Google Scholar]
- Stroupe, K. T. , Teal E. Y., Tu W., Weiner M., and Murray M. D.. 2006. “Association of Refill Adherence and Health Care use among Adults with Hypertension in an Urban Health Care System.” Pharmacotherapy 26 (6): 779‐89. [DOI] [PubMed] [Google Scholar]
- Stroupe, K. T. , Smith B. M., Hogan T. P., St Andre J. R., Gellad W. F., Weiner S., Lee T. A., Burk M., Cunningham F., Piette J. D., Rogers T. J., Huo Z., and Weaver F. M.. 2013. “Medication Acquisition across Systems of Care and Patient‐Provider Communication among Older Veterans.” American Journal of Health‐System Pharmacy 70 (9): 804‐13. [DOI] [PubMed] [Google Scholar]
- Stroupe, K. T. , Smith B. M., Bailey L., Adas J., Gellad W. F., Suda K., Huo Z., Tully S., Burk M., and Cunningham F.. 2017. “Medication Acquisition by Veterans Dually Eligible for Veterans Affairs and Medicare Part D Pharmacy Benefits.” American Journal of Health‐System Pharmacy 74 (3): 140‐50. [DOI] [PubMed] [Google Scholar]
- Taylor Jr, D. H. , Ostbye T., Langa K. M., Weir D., and Plassman B. L.. 2009. “The Accuracy of Medicare Claims as an Epidemiological Tool: The Case of Dementia Revisited.” Journal of Alzheimer's Disease 17 (4): 807‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoemmes, F. , and Ong A. D.. 2015. “A Primer on Inverse Probability of Treatment Weighting and Marginal Structural Models.” Emerging Adulthood 4 (1): 40‐59. [Google Scholar]
- Thorpe, C. T. , Bryson C. L., Maciejewski M. L., and Bosworth H. B.. 2009. “Medication Acquisition and Self‐Reported Adherence in Veterans with Hypertension.” Medical Care 47 (4): 474‐81. [DOI] [PubMed] [Google Scholar]
- Thorpe, C. T. , Johnson H., Dopp A. L., Thorpe J. M., Ronk K., Everett C. M., Palta M., Mott D. A., Chewning B., Schleiden L., and Smith M. A.. 2015. “Medication Oversupply in Patients with Diabetes.” Research in Social & Administrative Pharmacy 11(3): 382‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe, J. M. , Thorpe C. T., Gellad W. F., Good C. B., Hanlon J. T., Mor M. K., Pleis J. R., Schleiden L. J., and Van Houtven C. H.. 2017. “Dual Health Care System Use and High‐Risk Prescribing in Patients with Dementia: A National Cohort Study.” Annals of Internal Medicine 166 (3): 157‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau . 2017. “Census Bureau Regions and Divisions with State FIPS Codes” [accessed on July 21, 2017]. Available at https://www2.census.gov/geo/docs/maps-data/maps/reg_div.txt
- U.S. Department of Agriculture Economic Research Service . 2013. Urban Influence Codes. Washington, DC: U.S. Department of Agriculture Economic Research Service. [Google Scholar]
- U.S. Department of Health and Human Services, Health Resources & Services Administration, Bureau of Health Professions . 2012. –2013. Area Health Resource File (AHRF). Rockville, MD: U.S. Department of Health and Human Services. [Google Scholar]
- U.S. Department of Veterans Affairs . “VA/CMS Data. VHA Directive 1153: Access to Centers for Medicare and Medicaid Services (CMS) and the United States Renal Data System (USRD) Data for Veterans Health Administration (VHA) Users within the Department of Veterans Affairs (VA) Information Technology (IT) Systems, April 15, 2016.”
- U.S. Department of Veterans Affairs, H. S. R. D . 2016. “Research Into Veterans’ Dual use of Healthcare Coverage” [accessed on November 7, 2017]. Available at https://www.hsrd.research.va.gov/news/feature/dual_use.cfm.
- U.S. Department of Veterans Affairs, V. I. R. C. V . 2008. VIReC Research User Guide: VHA Pharmacy Prescription Data, 2nd edn. Hines, IL: U.S. Department of Veterans Affairs, V. I. R. C. V. [Google Scholar]
- VA Pharmacy Benefits Management Services, M. A. P. a. V. P. E . 2014. Memantine to Treat Dementia: Updated 2014 Criteria for Use. Hines, IL: VA Pharmacy Benefits Management Services, M. A. P. a. V. P. E. [Google Scholar]
- VIReC . 2015a. VIReC Research User Guide: Fiscal Year 2014 Medical SAS Outpatient Datasets and Inpatient Encounters Dataset. Hines, IL: H. S. R. D. S. US Department of Veterans Affairs, VA Information Research Center. [Google Scholar]
- VIReC . 2015b. VIReC Research User Guide: Fiscal Year 2014 VHA Medical SAS Inpatient Datasets, 2nd edn. Hines, IL: H. S. R. D. S. US Department of Veterans Affairs, VA Information Research Center. [Google Scholar]
- Viswanathan, M. , Golin C. E., Jones C. D., Ashok M., Blalock S. J., Wines R. C., Coker‐Schwimmer E. J., Rosen D. L., Sista P., and Lohr K. N.. 2012. “Interventions to Improve Adherence to Self‐Administered Medications for Chronic Diseases in the United States: A Systematic Review.” Annals of Internal Medicine 157 (11): 785‐95. [DOI] [PubMed] [Google Scholar]
- Viswanathan, M. , Kahwati L. C., Golin C. E., Blalock S. J., Coker‐Schwimmer E., Posey R., and Lohr K. N.. 2015. “Medication Therapy Management Interventions in Outpatient Settings: A Systematic Review and Meta‐Analysis.” Journal of the American Medical Association Internal Medicine 175 (1): 76‐87. [DOI] [PubMed] [Google Scholar]
- Wagner, T. H. , Chow A., and Barnett P. G.. 2011. "HERC's Average Cost Datasets for VA Inpatient Care FY1998‐FY2010. Guidebook." Menlo Park, CA: VA Palo Alto, Health Economics Resource Center. [Google Scholar]
- Yang, M. , Barner J. C., and Worchel J.. 2007. “Factors Related to Antipsychotic Oversupply among Central Texas Veterans.” Clinical Therapeutics 29 (6): 1214‐25. [DOI] [PubMed] [Google Scholar]
- Yano, E. M. , Bair M. J., Carrasquillo O., Krein S. L., and Rubenstein L. V.. 2014. “Patient Aligned Care Teams (PACT): VA's Journey to Implement Patient‐Centered Medical Homes.” Journal of General Internal Medicine 29 (Suppl 2): S547‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Table S1. ICD‐9‐CM Diagnosis Codes Used to Identify Alzheimer's Disease or Related Disorder (ADRD).
Table S2. Antihypertensive Classes and Agents Included in Refill Adherence Calculations.
Table S3. Covariate Balance Achieved After Applying Stabilized Inverse Probability of Treatment Weights.
Table S4. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Mutually Exclusive MPR Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users, Using 110% Cutoff for Oversupply (N = 50,763).
Table S5. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in MPR Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users, Using Average Refill Adherence Values Across Classes (N = 50,763).
Table S6. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Antihypertensive Medication Supply Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users, in Medicaid/LIS Enrollees and Non‐Enrollees.
Table S7. Oral Hypoglycemic Agent (OHA) Use by VA Patients with Dementia and Diabetes, Overall and by OHA Medication User Group Status, 2010.
Table S8. Results of Multinomial Logistic Regression Model Comparing Odds of Membership in Diabetes Medication Supply Categories, for Dual Prescription Users and Part D‐only Users Relative to VA‐only Users (N = 13,857).


