Abstract
The rewarding properties of social interactions are essential for the expression of social behavior and the development of adaptive social relationships. Here, we review sex differences in social reward, and more specifically, how oxytocin (OT) acts in the mesolimbic dopamine system (MDS) to mediate the rewarding properties of social interactions in a sex-dependent manner. Evidence from rodents and humans suggests that same-sex social interactions may be more rewarding in females than in males. We propose that there is an inverted U relationship between OT dose, social reward, and neural activity within structures of the MDS in both males and females, and that this dose–response relationship is initiated at lower doses in females than males. As a result, depending on the dose of OT administered, OT could reduce social reward in females, while enhancing it in males. Sex differences in the neural mechanisms regulating social reward may contribute to sex differences in the incidence of a large number of psychiatric and neurodevelopmental disorders. This review addresses the potential significance of a sex-dependent inverted U dose–response function for OT’s effects on social reward and in the development of gender-specific therapies for these disorders.
Subject terms: Empathy, Reward, Motivation, Motivation, Autism spectrum disorders
Introduction
Many social interactions are rewarding, as defined by their ability to elicit approach responses [1], and the neural mechanisms mediating social reward play an essential role in social motivation [2, 3]. Social reward is critical for the formation and maintenance of adaptive social relationships, such as pair bonding and dominate/subordinate relationships [4–7]. Indeed, the powerful rewarding properties of social interactions are evident even in species that are not overly gregarious [8]. Most of what we know about social reward has come from studies of males. Yet, social interactions are rewarding in both males and females [9], and some basic questions about the rewarding nature of social interactions in males and females have not been addressed. For example, although there are data indicating sex differences in the rewarding properties of other stimuli (e.g., food and drugs), little is known about whether there are sex differences in the rewarding properties of social interaction. As discussed below, there is now evidence to suggest that women find positive social interactions with same-sex partners to be more rewarding than men do [10]. Similarly, recent animal studies have described sex differences in the rewarding properties of social interactions in rodents, e.g., female Syrian hamsters display higher levels of social reward following same-sex social interactions than do males. While much more needs to be learned, these data indicate that more comprehensive studies of sex differences in social reward may be essential for defining the basic mechanisms underlying many different types of social behavior. In addition, this knowledge has significant translational importance, as several lines of evidence point to deficits in social reward as one of the central symptoms and causes of psychiatric disorders, such as autism spectrum disorder, antisocial personality disorder, and attention-deficit disorder. The prominent sex differences in the incidence of these and other psychiatric disorders [11–14] may be based in part on sex differences in social reward. Therefore, sex differences in social reward and its underlying neural mechanisms are critical areas for future research.
Sex differences in the brain
The existence of sex differences in the brain was recognized less than 60 years ago [15], and much remains to be learned about their physiological mechanisms and functional significance [16, 17]. Sex differences in the brain not only underlie sex differences in reproductive behavior, but also play an important role in the expression of social behaviors, such as aggression [18, 19], social play [20, 21], and social communication [22, 23]. A major focus of the study of sex differences in the brain has been neurochemical signaling, revealing sex differences in the distributions of signaling molecules and their receptors [24, 25]. The first, and perhaps the most pervasive, sex difference identified in the mammalian brain to date is the distribution of arginine-vasopressin (AVP), a peptide of the arginine-vasopressin/oxytocin family of nonapeptides (i.e., peptides composed of nine amino acids). For example, AVP immunoreactivity is greater in cell bodies in the bed nucleus of the stria terminalis and medial amygdala in male rats than in females [26]. Although the functional significance of this sex difference in AVP immunoreactivity is not fully understood, sex differences in the AVP system do play an important role in regulating sex differences in at least some social behaviors [27, 28]. For example, AVP regulates aggression by acting within the hypothalamus in opposite ways in males and females; AVP stimulates aggression in males and inhibits aggression in females [18, 29–31]. Another member of this family of peptides, oxytocin (OT), also plays a major role in the regulation of social behavior, especially those behaviors related to social bonding in particular [32, 33] and social reward in general, e.g., parental behavior, pair bonding, and trust [34, 35]. Given the likely importance of OT for regulating social reward in males and females, this review explores the literature on sex differences in the neurobehavioral mechanisms mediating social reward, with a focus on the role of OT. We also put forth a new hypothesis that an inverted U shape describes the relationship between social stimulation and social reward, perhaps mediated by OT.
OT and AVP signaling and social behavior
The AVP/OT family of peptides is evolutionarily ancient, and their structures are remarkably similar [36]. In mammals, the structure of the two most important members of this peptide family, OT and AVP differ in only two amino acids. The canonical receptors for these peptides include OTR for OT, and V1aR, V1bR, and V2R for AVP, with V1aR serving as the dominant AVP receptor in the brain. As in the similarity of the structure of these peptides, these receptors share at least 25% of their primary structure [37]. As a result, it is not surprising that substantial cross-talk has been identified, through which OT activates AVP receptors and vice versa [38–40]. Therefore, the functions of the OT system cannot be entirely divorced from the functions of the AVP system. While exogenously administered OT activates AVP receptors, and exogenously administered AVP activates OTRs in many cases, the extent to which endogenously released OT or AVP activate each other’s canonical receptors is not known [41]. These complexities in cross-talk must be considered and explored in the context of sex differences as well. Although the focus of this review will be on OTRs, a full understanding of the effects of OT on social behavior and reward in males and females will also require studies of potential sex differences in the activation of AVP receptors by OT.
OTRs are members of an evolutionarily ancient superfamily of G protein-coupled receptors composed of seven putative transmembrane domains [37, 42]. OTRs are coupled to Gqand Gi protein complexes, and their activation can result in a range of complex cellular effects that remain to be fully delineated [43, 44]. Interestingly, however, the coupling of OTRs to different G proteins can result in diverse, even opposing, effects within the cell [45]. Moreover, the concentration of OT appears to determine the coupling of OTRs to different subtypes of G proteins [46]. Therefore, the concentration-dependent behavioral effects of OT might be the result of concentration-dependent effects on the coupling of OTRs to different G protein subtypes (see below). It will be important to consider that diverse types of OTR coupling may occur in neurons of differing phenotypes, and that these differences are likely to have functional consequences in the control of social behavior. Indeed, there is evidence of sex differences in the strength of coupling of neuropeptide receptors with their G protein transduction mechanisms [47].
Central distribution of OT and AVP
Because of the potential for AVP, as well as OT, to activate OTRs, it is important to consider sex differences in the levels of AVP and OT expressed within specific brain regions that may contribute to the regulation of social behavior. The distribution of OT- and AVP-containing neurons have been reviewed recently [48, 49], so they are summarized only briefly here. High levels of OT and AVP are expressed in magnocellular neurons of the hypothalamus (e.g., paraventricular nucleus (PVN), supraoptic nucleus (SON), and nucleus circularis). Both OT and AVP are also produced by smaller populations of parvocellular neurons in several brain regions, e.g., amygdala. Sex differences in the levels of OT are described for just a few brain regions to date, although the findings are not consistent across species. For example, higher levels of OT immunoreactivity have been reported in neuronal cell bodies in the PVN and SON of females compared to males in several species such as mice [50, 51], but no sex differences in OT immunoreactivity in neurons of these regions has been reported in several other species, including humans [52–56]. As noted above, major sex differences in the number of AVP-containing cell bodies have been reported for the amygdala and bed nucleus of the stria terminalis and in their projections in rats [26, 57]. Subsequent studies detailed similar sex differences in AVP levels in the extended amygdala, PVN, and SON of several other species as well [50, 53, 56, 58, 59]. Although these sex differences are not uniformly present in all species [55, 60, 61], it is noteworthy that when sex differences were observed in the regions producing the highest levels of OT and AVP (i.e., the PVN and SON) higher levels were seen in females.
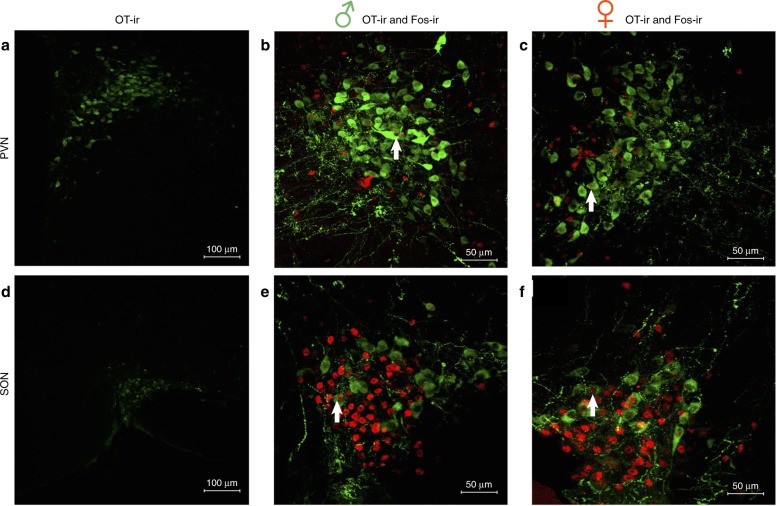
The factors regulating the release of OT and AVP are not fully understood, but likely involve both synaptic and non-synaptic mechanisms [28, 62–64]. OT and AVP have been identified in dense core vesicles in synaptic regions of neurons in a variety of brain sites, and these peptides can be released in a calcium-dependent manner [65–67]. Thus, local activation of AVP/OT receptors could be produced by the synaptic release of these peptides, or possibly by their release from axons in passage. In contrast, when AVP/OT are released from non-synaptic regions of neurons (e.g., dendrites), the peptides are thought to spread far more widely than following synaptic release although the distance of spread is not clear [64, 68, 69]. Non-synaptic release of AVP/OT has been most extensively studied in magnocellular neurons, but non-synaptic release can also occur in parvocellular neurons [70]. Importantly, social interactions result in significant levels of activity in OT-containing magnocellular neurons of the PVN and SON in males and females (Fig. 1). Because these magnocellular neurons produce some of the largest amounts of OT in the brain, their activation by social interactions likely results in substantial elevations of OT throughout the brain.
Fig. 1.
Activation of oxytocin (OT) immunoreactive (ir) neurons (green) (as indicated by colocalization with cfos-ir (red)) in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus 1 h after 10 min same-sex social interactions in Syrian male and female hamsters. a Representative image of OT-ir neurons in the PVN, b double labeling of OT- and fos-ir in the PVN of a male hamster, c double labeling of OT- and fos-ir in the PVN of a female hamster, d representative image of OT-ir neurons in the SON, e double labeling of OT- and fos-ir in the SON of a male hamster, and f double labeling of OT- and fos-ir in the SON of a female hamster. Neuronal activity in OT-ir neurons is increased in the PVN and SON of both male and female hamsters following the 10 min social interaction, although no sex differences were observed in the number of OT-ir-activated neurons (Borland and Albers, unpublished). White arrows illustrate examples of the colocalization of OT- and fos-ir
Central distribution of OTRs
Sex differences in the distribution and number of receptors for OT have been observed within specific brain regions, although these sex differences, like those for the expression levels of OT and AVP, are not entirely consistent across species [48, 49]. The distribution of OTR binding tends to be greater in males compared to females in forebrain regions, such as the nucleus accumbens (NAc), where sex differences have been reported in a number of rodent species including rats, mice, and prairie vole [48, 49, 71–73]. However, these male/female differences are not always consistent. For example, OTR binding in the CA1 region of the hippocampus is higher in males than females in some species (i.e., rats) [72] but lower in males than females in others (i.e., mice) [72, 74]. Gonadal hormones also influence OTR binding in specific brain regions in a species-specific manner [75–78]. For example, estrogen selectively influences OTR binding in the anterior olfactory nucleus in prairie voles and in the ventromedial hypothalamus in rats [79, 80]. Of significant note, OTRs are distributed in key structures of what has been called the social decision-making network that includes structures important for social behavior and reward [81, 82].
OT and social reward—animal studies
OT in the mesolimbic dopamine system
There is strong support for the role of OT in regulating social reward by its actions in the mesolimbic dopamine system. This system is critical for the rewarding properties of many stimuli, including food [83], water [84], drugs of abuse, and various social interactions [85–87]. The primary pathway of the mesolimbic circuitry is dopamine (DA)-containing neurons in the ventral tegmental area (VTA) that project to the NAc [88–90]. Social interactions can increase neuronal activity in both the VTA and NAc in male hamsters, rats, and mice [8, 85, 91], and selective activation of this DA projection increases social motivation in mice [87]. Indeed, the activation of OTRs in the mesolimbic circuit appears to be necessary for social interactions to be rewarding in male rodents. For example, activation of OTRs in the NAc appears necessary for social reward in male mice [92], and activation of OTRs in the VTA also appears necessary for social reward in male hamsters and mice [93, 94]. OT-containing projections innervate structures in the mesolimbic DA system and play an important role in mediating the rewarding properties of a range of stimuli. OT is synthesized in several hypothalamic nuclei including the PVN and SON, and OT-containing fibers project from these sites to structures such as the NAc and VTA [95–97] (Fig. 2a). In the VTA, OTRs are found on DA-containing neurons that project to the NAc, as well as to other structures [98]. These fibers appear to stimulate DA activity, as exogenous OT injected into the caudal VTA leads to DA efflux in the NAc [95] (Fig. 2b). Finally, OT-containing neurons in the PVN projecting to the VTA are necessary for social reward in male mice [94].
Fig. 2.
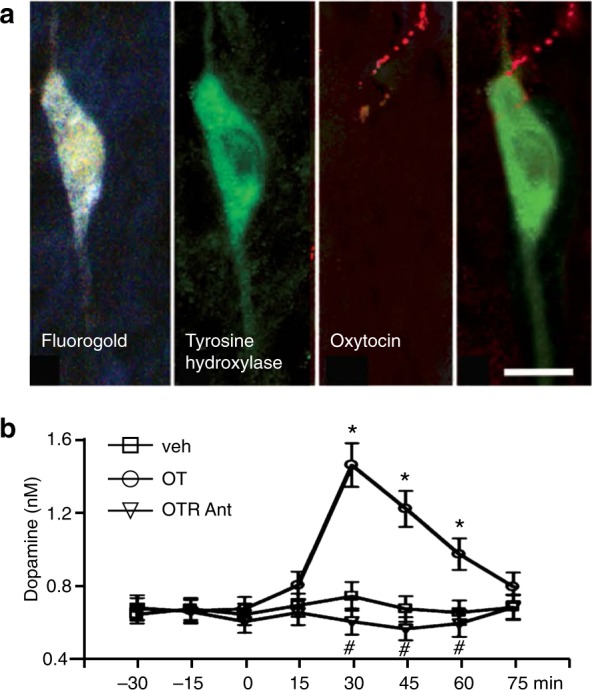
a Injections of Fluorogold in the nucleus accumbens resulted in retrograde labeling of neurons in the ventral tegmental area in male rats. These neurons were colocalized with tyrosine hydroxylase (TH)-immunoreactive neurons. Of these colocalized Fluorogold and TH-labeled neurons, one-third had oxytocin (OT) immunoreactive axons impinging or in close apposition. b Injections of oxy into the caudal VTA resulted in an increase in dopamine efflux in the nucleus accumbens in male rats, and this effect was attenuated when oxytocin was co-injected with an oxytocin receptor antagonist (OT Ant.) Scale bar is 20 μm. Reproduced from ref. [95]
Sex differences in mesolimbic dopamine signaling
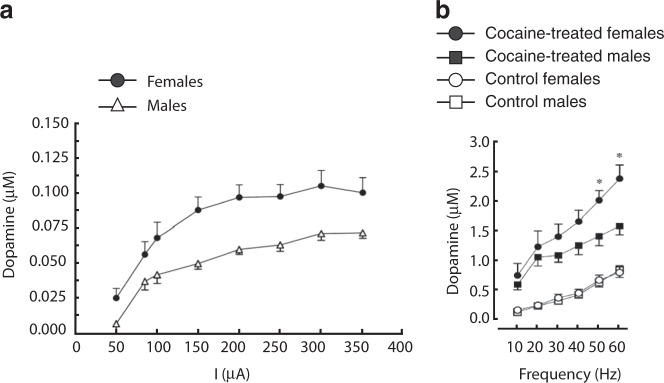
The structure and function of the mesolimbic system are sexually differentiated, with many reports suggesting higher basal and stimulated activity in the system among females compared with males (for a review see ref. [99]). For example, morphological sex differences include more cell bodies containing DA and greater volume in the VTA of female rats compared to males [100], as well as sex differences in the projections of DA-containing VTA neurons [101]. On a functional level, the same degree of electrical stimulation of medial forebrain bundle fibers leads to greater DA efflux in the NAc of female rats compared to males [102]. Moreover, basal extracellular levels of DA in the NAc are higher in female rats compared to males [103], and females display a faster rate of DA uptake and release [104]. When stimulated by drugs blocking or reversing the DA transporter, higher levels of extracellular DA are seen in females than males [104] (Fig. 3), although an interesting dichotomy was reported in one study, such that amphetamine increased extracellular DA in male rats while decreasing it in females [103]. This increased sensitivity among females to psychostimulant drug effects is also associated with greater immediate early gene expression across the middle and caudal striatum [105]. Postsynaptically in the NAc, the balance between the D1 and D2 families of receptors may vary across the sexes and across the estrous cycle in females [106]. Similar sex differences extend to humans [107]; e.g., women have a higher synaptic concentration of DA in the striatum than men [108], as well as a stronger ventral striatum response to prosocial decisions [109]. At least some of these sex differences are mediated by estrogen, as variations across estrous and menstrual cycles suggest increased activation of DA pathways when estrogen levels are high or rising, while ovariectomy attenuates sex differences [110, 111]. On the other hand, gonadectomy has little effect in males [112–114].
Fig. 3.
a Over a range of current intensities, electrical stimulation of the medial forebrain bundle resulted in greater extracellular dopamine in the caudate nucleus of female rats compared to males [102]. b Administration of 40 mg/kg cocaine i.p. resulted in a greater increase in evoked extracellular dopamine in the striatum in female rats compared to males. Reproduced from ref. [104]
Sex differences in social reward
As mentioned above, the rewarding nature of social interactions has recently been compared explicitly between males and females, using Syrian hamsters as an animal model. While not a great deal is known about the social behavior of hamsters in the field, laboratory studies show social interactions to be highly rewarding in this species [8, 115, 116]. Hamsters have robust skills in social recognition of, and communication with, conspecifics [117, 118]. Unlike other laboratory rodents, female as well as male hamsters rapidly form and maintain hierarchical dominance relationships and display many of the fundamental features of social organizations often seen in primates [119–121]. As such, hamsters are an excellent preclinical model with which to explore potential sex differences in reward, using social interaction as the rewarding stimulus.
Using both classical and operant conditioning methods, we demonstrate that same-sex social interactions are more rewarding in female hamsters than in males even though the social behaviors observed during these same-sex social interactions are quite similar in both sexes. In a conditioned place preference (CPP) test, females spent about twice as much time in the chamber associated with a same-sex stimulus hamster, as males spent in the chamber with a same-sex stimulus hamster (Fig. 4a). Similarly, in a novel operant social preference test, the rewarding
Fig. 4.
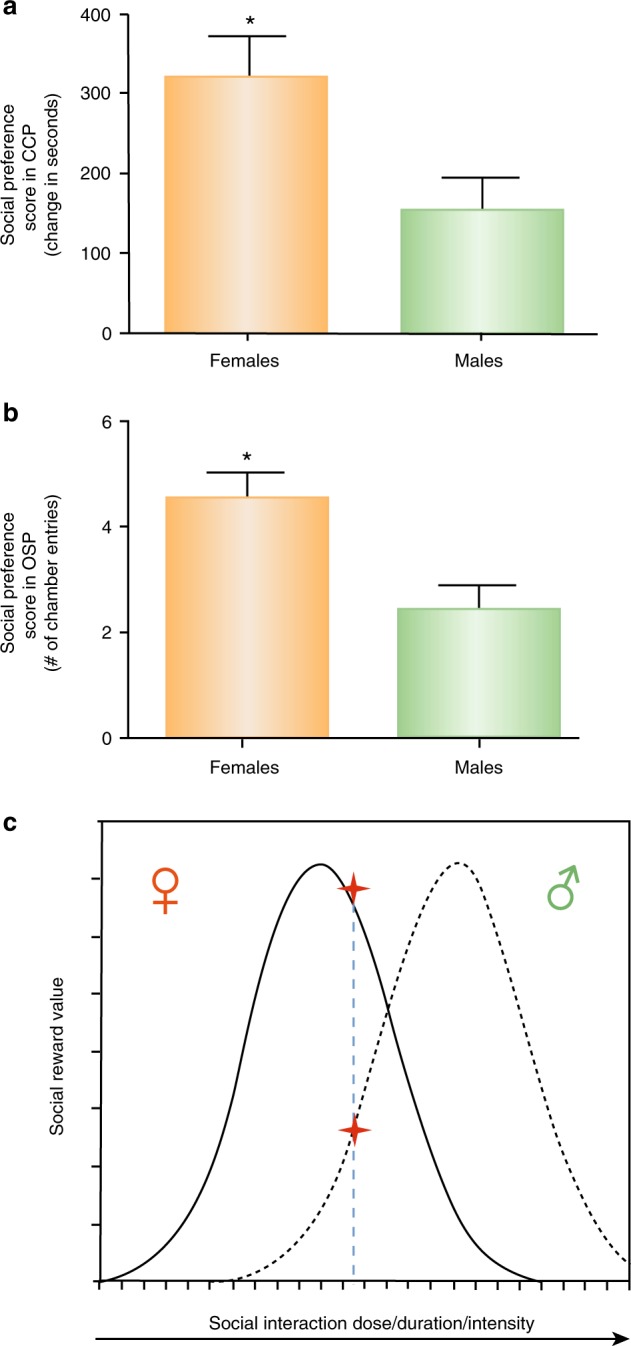
Female Syrian hamsters find same-sex social interactions more rewarding than males. a Female hamsters displayed a greater social preference score (time spent in the social interaction associated chambers minus the time spent in the no social interaction chambers) following social conditioning sessions in a conditioned place preference test compared to males (* indicates significant differences p < 0.05). b Female hamsters also displayed a greater social preference score (the number of entries into chambers containing same-sex stimulus hamsters minus the number of entries into the empty chambers) in the operant social preference task compared to males. c Hypothesis of the inverted U dose–response function of the relationship between social reward value and the dose (e.g., duration) of same-sex social interaction in males and females. Females are predicted to be more sensitive to the rewarding and aversive properties of social interactions than males (Borland and Albers, unpublished)
properties of social interactions were significantly greater in females compared to males. Specifically, hamsters were placed in a three-chambered apparatus and allowed access to either a chamber containing an unrestrained same-sex stimulus hamster or an empty chamber, accessed through one-way entry, vertical-swing doors [122]. Females made about twice as many entries into the chamber containing the stimulus hamster as did males. In other models, the opportunity for social interactions appears to compete effectively with other rewarding stimuli to determine behavioral outcomes, perhaps to a greater extent in females than males. For example, social stimuli might trigger greater DA release in females than males, as shown among control groups in a study of rats exposed to same-sex stimulus animals after a period of social isolation [86]. Furthermore, in female rats, DOPAC (DA metabolite) levels in the striatum were elevated during same-sex social interactions, while in males no difference in DOPAC levels were observed [123]. In addition, social housing can reduce drug intake in operant conditioning models, compared to social isolation housing [124–127], an effect that is more consistent in females than in males and may be mediated by OT in mesolimbic regions [128, 129]. OT itself also decreases drug-related reward and reinforcement [130], with greater effects in females than males [131, 132]. The mechanisms underlying the sex differences in social reward are not known, although sex differences in gonadal hormones are likely to play a role, as they do in mediating sex differences in drug reward [106, 133, 134]. In translation to the human condition, it appears clear that social support reduces drug use, ameliorates stress, and predicts better outcomes in the treatment of various disorders [135, 136], although sex differences in this arena are not well documented.
Parameters regulating social reward in males and females
To evaluate the parameters regulating social reward in animal models, we recently explored the relationship between the reward value of social interactions (e.g., duration of interaction) and the frequency of choosing social interaction and found that it mimics the relationship between the reward value of drugs (e.g., dose of drug) and the frequency of drug intake [122]. In both cases, as the reward value increases, the number of rewards obtained in a test session decreases [122, 137, 138]. Another well-known relationship drawn from the literature on rewarding properties of drugs is the inverted U-shaped dose–response curve between drug dose and reward value [139]. Initially, as dose increases, reward value also rises, but only to a point. Once this peak is reached, increasing drug dose further begins to drive down the reward value. We raise the possibility here that a similar relationship exists between the “dose” of social interaction and the value of the social reward. In this case, the “dose” of social interaction might be defined by duration (e.g., time spent in an environment where social interaction is possible) or intensity (e.g., number of conspecifics available for interactions, or time since last social interaction). The concept of an inverted U relationship between the duration or intensity of social interaction and its rewarding properties may provide a framework for describing how social stimuli can transition from positive to negative valence (Fig. 4c), as ocurrs in a variety of psychiatric disorders, such as social anxiety, agoraphobia, enochlophobia, and autism spectrum disorder. If so, as duration and/or intensity of social interactions increase, the rewarding properties of those interactions would be initially increased, then ultimately reduced. Consistent with this possibility are the findings in rats that brief social interactions are more rewarding than longer interactions [140, 141], and that rodents housed in social isolation find brief social interactions more rewarding than do rodents housed in groups, where social interactions occur continuously [9, 142]. Furthermore, using the operant social preference test, we have found that increasing the duration of social interaction decreases the rate of seeking social interaction, i.e., entering chambers with stimulus conspecifics. Additionally, increasing the duration of social interaction increases the latency to re-enter chambers containing conspecifics in males [122]. Because females appear to be more sensitive to the rewarding properties of social interaction than males, the inverted U function would be displaced such that less social interaction would be required in females to produce the same levels of social reward, compared to males (Fig. 4c). The differences in the inverted U functions in males and females may be useful in predicting sex differences in the responses to social interactions, and as a guide to understanding how the neural mechanisms that mediate social reward differ in males and females (see below). Indeed, one of the major challenges in the development of new treatments for psychiatric disorders is understanding the transition from assigning positive value to social stimuli to assigning negative value to social stimuli, or at least omitting positive value, as in psychiatric disorders that include social impairments. Treatments intended to improve or restore positive social attributions may need to account for these “dose-effect” relationships, and potential sex differences therein.
An inverted U hypothesis to interpret sex differences in OT effects
A sex-dependent inverted U function has been hypothesized for the relationship between brain OT levels and neural activity in human studies [10] (see below). Here, we extend the inverted U hypothesis to explain various effects of OT on social behaviors in male and female subjects. OT administered peripherally or centrally appears to have some rewarding properties of its own in both male and female rodents. Rodents spend more time in the chambers associated with OT treatment than control chambers, and will self-administer OT intravenously [143, 144]. OT may mediate social reward by its actions in the mesolimbic DA system, especially in the VTA where activation of OTRs is essential for the rewarding properties of social interactions in male hamsters and mice [93, 94, 122]. As noted above, the ability of OT to reduce responses to drug reward may be more robust in female rats than males [132], and we have shown recently that OTRs specifically in the VTA may play a critical role in the sex differences in social reward in hamsters (Fig. 5a). Injection of a selective OTR antagonist significantly reduced the rewarding properties of same-sex social interactions by more than 50% in both males and females, thus supporting the hypothesis that OTRs mediate social reward through their actions in the VTA in both sexes. Surprisingly, however, injection of OT itself or a highly selective OTR agonist into the VTA has opposite effects on social reward in males and females (Fig. 5b); whereas the OTR agonist in the VTA significantly increases social reward in males, it significantly reduces social reward in females. One interpretation of these results is that females are closer to the peak of an inverted U dose-effect relationship between social interaction and reward value at baseline, and that further elevation of reward value by activation of OTRs with an exogenous agonist pushes reward value past peak levels, thereby causing a decline in reward value (Fig. 5c). In contrast, because males experience lower levels of social reward at baseline, a further elevation of reward value by activation of OTRs in the VTA simply increases the rewarding properties of the interaction. If this interpretation is correct, OT would be predicted to enhance the rewarding properties of social interactions in females in situations where the social reward of those interactions had not peaked (i.e., a low “dose” of social interaction). To test this hypothesis, the “dose” of social reward was lowered in females by reducing the number of social interaction trials during CPP from the three used in the prior experiments to a single trial. When social reward was reduced in females using this approach, OT injected into the VTA significantly increased the rewarding properties of that single social interaction trial (Borland et al., unpublished data).
Fig. 5.
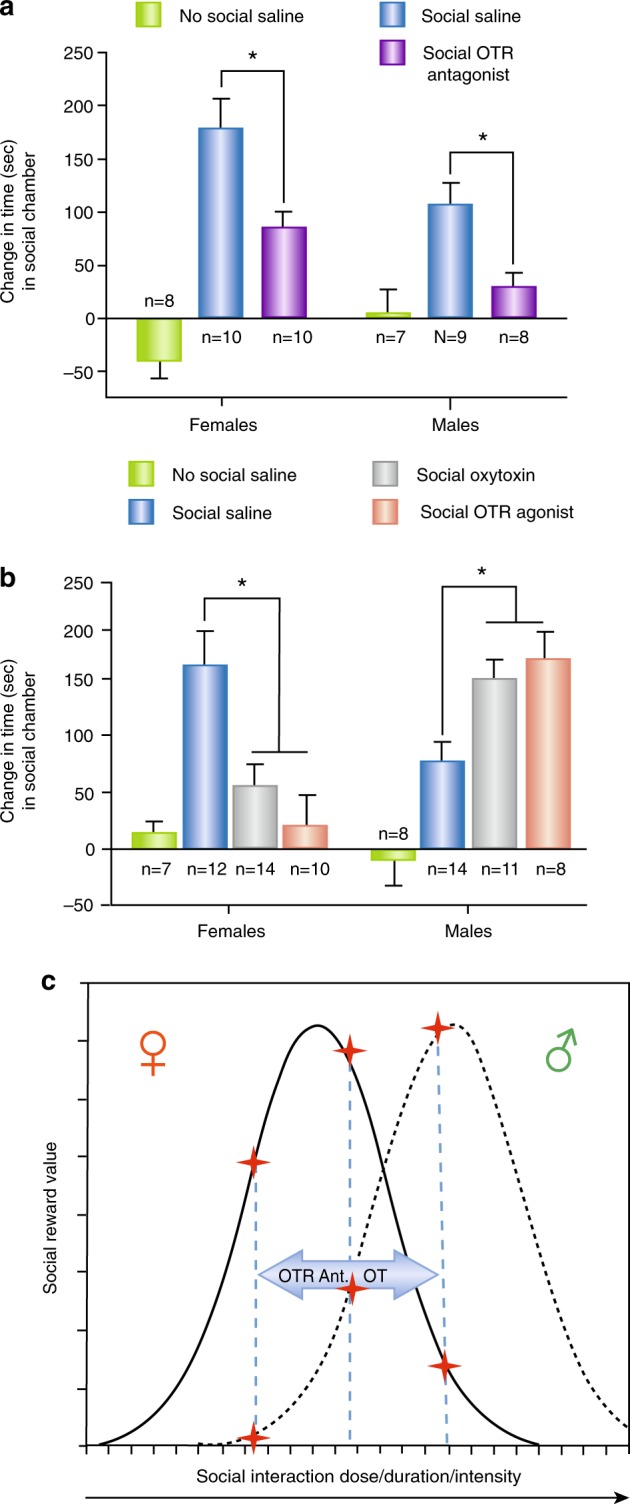
Effects of oxytocin (OT), a selective OT receptor (OTR) agonist and a selective OTR antagonist, on the rewarding properties of same-sex social interactions in Syrian hamsters. a Injection of a selective OTR antagonist into the caudal VTA before each of three social conditioning sessions decreased social reward (the time spent in the chambers associated with social interaction during the social preference posttest) in both males and females. b Injection of OT and a selective OTR agonist into the caudal VTA before social conditioning sessions decreased the rewarding properties of social interactions in females but increased social reward in males. c Predicted effects of OT and OTR antagonist on social reward value in males and females based on the inverted U hypothesis of the relationship between social reward value and the dose (e.g., duration) of same-sex social interaction in males and females. Females are predicted to be more sensitive to the rewarding and aversive properties of social interactions than males. OT and OTR antagonists (OT Ant.) can increase or decrease social reward depending on the sex-dependent inverted U function of the relationship between social reward value and the dose (e.g., duration) of social interaction (* indicated significant difference p > 0.05) (Borland and Albers, unpublished)
Data sets from other rodent studies are also consistent with this inverted U relationship. In female mice, intracerebroventricular injections of OT induce a conditioned social preference for female stimulus mice at lower concentrations, but this preference is lost at higher concentrations [145]. Using intranasal administration of OT, sex differences in the rewarding properties of social interactions have also been studied [146]. In female mice, pairing 12 μg intranasal OT with the presence of a same-sex stimulus mouse induced a preference over another same-sex stimulus mouse not paired with OT administration. Interestingly, when the concentration of OT was increased to 36 μg and the trials extended, the initial preference for the stimulus mouse was eliminated, and the stimulus mouse appeared to become aversive. In contrast, in males, administration of 12 μg OT had no effect on the preference for same-sex stimulus mice. These data support the hypothesis that males are less sensitive to the reward-enhancing effects of OT, and that increasing concentrations of OT initially increase reward, but can subsequently lead to an aversive response at higher concentrations.
Taken together, the data summarized above suggest that OT in the VTA is a primary neural signal through which social stimuli trigger the mesolimbic DA pathway to assign salience to social interactions, thereby making them rewarding. The data further support the contention that females exhibit higher sensitivity than males to this process, perhaps through one of three mechanisms: higher levels of OT release triggered by the same social stimuli, greater response among VTA neurons to the same level of OT release, and/or a heightened postsynaptic impact of DA projections to forebrain nuclei such as the NAc.
OT and social reward—human studies
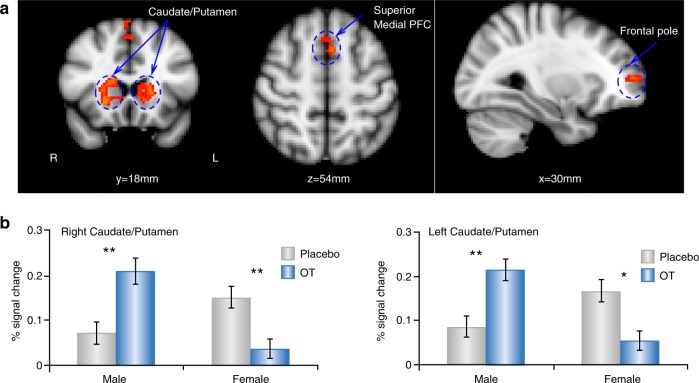
In humans, OT effects on social reward processing have been investigated by examining the effects of intranasal OT administration on behavior and brain function, as measured by functional magnetic resonance imaging (fMRI). While multiple studies have examined the effects of intranasal OT administration on social reward processing in either men or women [35, 147–152], few have compared men and women in the same study. In one double-blind, placebo-controlled, pharmaco-fMRI study, men and women were randomized to treatment with either 24IU intranasal OT administration or placebo ~40 min before they received an fMRI scan, while playing a dyadic social interaction task involving positive (reciprocated cooperation) and negative (unreciprocated cooperation) social interactions with same-sex partners. At baseline, women but not men showed bilateral activation in NAc in response to positive social interactions. Although caution must be exercised in inferring psychological processes from neural activations (i.e., reverse inference) [153] this result suggests that women may find positive social interactions with same-sex partners to be more rewarding or more salient than men do at baseline. However, pre-treatment with 24IU intranasal OT administration significantly increased the NAc response to positive social interactions in men, along with the caudate and putamen. On the other hand, pre-treatment with 24IU intranasal OT administration significantly decreased activation across many regions in women, including the putamen. A direct statistical comparison between men and women showed that intranasal OT administration increased the caudate and putamen response in men to a greater extent than in women. More specifically, the right caudate/putamen response to reciprocated cooperation was larger in women than in men at baseline (Fig. 6), but intranasal OT administration treatment increased the response in men to the level of women in the placebo group. On the other hand, intranasal OT treatment decreased the response of women to the level of men in the placebo group [10, 154]. These results, coupled with evidence that women have higher baseline CSF OT levels [155], support the hypothesis of an inverted U-shaped dose–response relationship between brain OT levels and neural response, whereby raising brain OT levels in men would augment the caudate/putamen response, moving them closer to the maximum. On the other hand, raising OT levels in women might displace them to the right of the response maximum, decreasing the brain response. This hypothesis is supported by studies demonstrating non-linear dose–response properties of intranasal OT administration in human males. 24IU intranasal OT administration, as compared with placebo reduced men’s cortisol response to physical stress, enhanced autobiographical memory retrieval, and promoted the retrieval of social affiliation memories associated with more positive feelings [156, 157]. On the other hand, the 48IU dose did not differ from the placebo and was also significantly lower than the 24IU dose for memory retrieval.
Fig. 6.
Sex differences in the effects of oxytocin (OT) on brain activations in response to reciprocated cooperation from human partners. a Activations revealed by whole brain analysis, voxel-wise threshold of p < 0.001 in conjunction with cluster-wise threshold of p < 0.05 FWE-corrected; b Region of interest plot from a illustrating the caudate/putamen response as a function of sex and drug treatment. OT-augmented neural response to reciprocated cooperation outcomes from human partners among males, whereas the opposite was found for females. Error bars represent one standard error. *p < 0.05, **p < 0.005 (Bonferroni corrected). Reproduced from ref. [10]
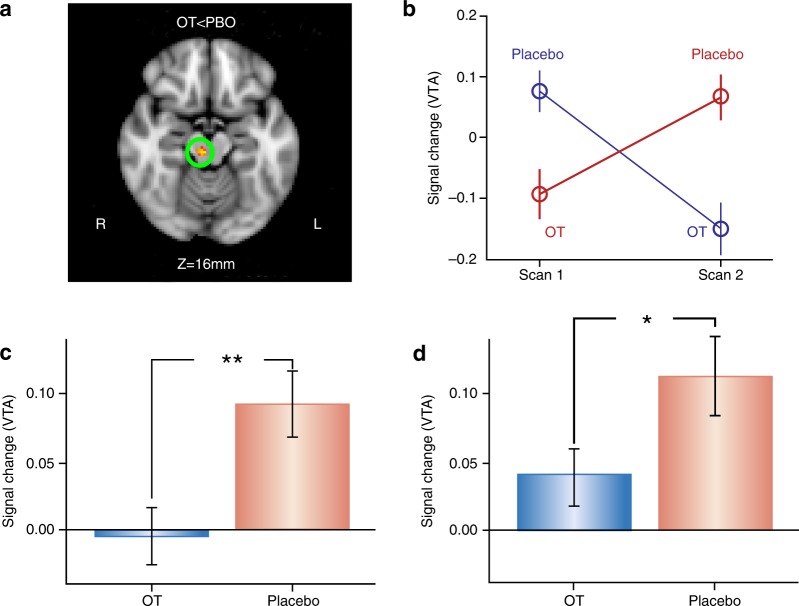
Collectively, these results show that 24IU intranasal OT administration modulates the neural response to reciprocated cooperation very differently in men and women. In men, OT increases the response to social reward associated with reciprocated cooperation within areas involved in reward and salience, such as the caudate/putamen. Importantly, these modulatory effects of OT were specific to interactions with human partners—similar effects were not found with computer partners. In women, intranasal OT administration actually decreased the neural response to positive social interactions (reciprocated cooperation) across widespread brain regions [10, 154]. Further analysis indicated that effects of intranasal OT administration on the caudate nucleus response to reciprocated cooperation were driven by individuals with the GG genotype at OXTR SNP rs53576 [154]. A subset of the participants in this study returned for a second session as part of a within-subject design to evaluate OT effects. While OT did not increase the striatal response to positive social interactions in men, it robustly decreased the VTA response to positive social interactions in women (Fig. 7) [158]. This result parallels the effects of injections of OT into the VTA on social reward in female hamsters described above.
Fig. 7.
Effects of oxytocin (OT) on the ventral tegmental area (VTA) response to reciprocated cooperation among women in a within-subject design. a OT attenuated the BOLD response to reciprocated cooperation in the VTA of female participants (FWE-corrected cluster p < .05 with voxel p < .001). b Average BOLD signal change in VTA (mean ± s.e.m.) as a function of scan number for each drug administration order. c This OT effect also replicates in cross-sectional data using the same task in a much larger cohort. d The OT group still has significantly less VTA activation relative to the placebo (PBO) after excluding subjects from the cross-sectional sample who were also in the within-subject sample.**p < 0.005 (1-tail); *p < 0.05 (1-tail). Reproduced from ref. [158]
In addition to examining how intranasal OT administration modulated activation within individual brain areas, data from this same study were analyzed to determine whether intranasal OT administration modulated functional connectivity across a neural network that animal studies implicate in social behavior. Intranasal OT administration induced widespread increases in functional connectivity in response to positive social interactions among men and widespread decreases in functional connectivity in response to negative social interactions among women. Regions known to receive mesolimbic DA projections such as the NAc and lateral septum were hubs for intranasal OT administration effects on functional connectivity, again consistent with the notion that 24IU intranasal OT administration enhances social reward processing in men but not women [159]. It should be noted however that fMRI does not measure DA signaling directly, nor can it reveal if activations within areas like the NAc necessarily involve the mesolimbic DA system.
As mentioned above, same-sex social interactions are more rewarding for female than male hamsters, and OT can significantly increase social reward in males while decreasing social reward in females. The human results described above closely parallel these findings in hamsters. Nevertheless, other evidence suggests that human sex differences in intranasal OT administration effects on social reward processing may be context or relationship-specific. For example, one study showed that 24IU intranasal OT administration enhanced the pleasantness of a romantic partners’ touch and also increased neural responses to the partners’ touch in the NAc and anterior cingulate cortex [160]. In contrast to the above study, there was no evidence for sex differences in OT effects on the perceived pleasantness of partner touch, and OT effects on the NAc response were actually stronger among women than men. These effects of intranasal OT administration were specific to touch from an assumed romantic partner and did not generalize to touch from an unfamiliar person. In fact, intranasal OT administration actually decreased the NAc response to touch by a stranger. The same research team has shown that intranasal OT administration increases the perceived attractiveness of, and NAc response to, male romantic partners among women [148]. However, this effect was not observed when women viewed pictures of familiar men who were not their partner. Two other studies in women have shown intranasal OT administration to increase VTA responses to pictures of infant and sexual images [151], and to cues that predict presentation of friendly faces [35]. Thus, the sex differences in the effects of intranasal OT administration on social reward processing mentioned above, including decreased activation in social reward processing regions in women, may be specific to positive social interactions with same-sex and/or unfamiliar individuals. Alternatively, OT attenuation of social reward in women may only apply for social stimuli that are sufficiently rewarding at baseline (i.e., near the peak of the dose–response function). For less rewarding social stimuli on the ascending portion of the inverted U function, OT would instead increase social reward. Thus, for example, if a woman has habituated to her partner’s touch, it may not be highly rewarding at baseline, but OT treatment may render it more so.
Synthesis
As is the case in most areas of social neuroscience, the mechanisms underlying social reward have been investigated more extensively in males than in females. The existing data in rodents and humans, however, suggest that females find same-sex interactions to be more rewarding than males do. Given that activation of OTRs in the VTA and other areas of the mesolimbic circuitry is necessary for social reward processing in both males and females, the sex differences in social reward could result from sex differences in the action of OT in these or related brain circuits. As outlined above, sex differences could be related to endogenous levels of OT, neuronal responses to OT, and/or downstream signaling in reward circuitry. Because OT/AVP can be released locally from presynaptic neuronal terminals, as well as more globally from non-synaptic regions of neurons, it is difficult to know if there are sex differences in the amount of AVP/OT reaching OTRs in the mesolimbic system. It is noteworthy, however, that women have higher baseline CSF OT levels [155]. Although OTRs have been identified throughout the neural circuitry controlling social behavior, there is no compelling evidence for the presence of higher concentrations of OTRs in females than in males. In fact, the opposite may be true in some regions, as a greater number of OTRs has been reported in subregions of the striatum for males than females in rodents. Nevertheless, possible sex differences in the signaling triggered by OTR binding could exist, such as in sex differences in the effectiveness of coupling to G proteins or the specific array of G proteins available for coupling. Another possibility is that OT activation within the mesolimbic system is not sexually differentiated, but the higher basal and stimulated DA activity in females compared with males is the explanatory mechanism.
Based on data from rodents and humans, we propose that there is an inverted U relationship between the duration and/or intensity of social stimuli and their associated social reward value, perhaps mediated or at least modulated by OT in the VTA or other regions of the mesolimbic DA system. Further, we propose although OTR activation in the mesolimbic DA system is necessary for the rewarding properties of social interactions in both males and females, the inverted U relationship between OT dose, social reward, and neural activity is initiated at lower doses in females than males (Fig. 4c). As a result, depending on the dose of OT administered, OT could reduce social reward in females, while enhancing it in males (Fig. 5c). Although the hypothesis of an inverted U dose–response relationship for the effects of mesolimbic OT is speculative, precedence exists for an inverted U dose–response function for the effects of OT. Previously, an inverted U dose–response for OT was identified following systemic administration or injection of OT directly into the VTA [95, 161, 162]. For example, injections of OT into the caudal VTA of male rats induce penile erections when given at intermediate concentrations (i.e., 40 or 80 ng) but not lower (i.e., 20 ng) or higher concentrations (i.e., 100 ng). The potential cellular and molecular mechanisms underlying an inverted U dose–response are not known. One possibility is that as OT concentrations increase they activate AVP receptors in addition to OTRs and the activation of AVP receptors reduces the effects of OTR activation. This possibility may be unlikely, however, because administration of concentrations of OT that activate V1a AVP receptors (90 μM OT) do not reduce responses mediated by OT activation of OTRs [39, 40, 122]. Another interesting possibility, however, relates to the finding that the coupling of OTRs to different G proteins can result in different, or even opposing, effects within the cell [45]. Because the concentration of OT determines the coupling of OTRs to different G protein subtypes [46], perhaps the inverted U-shaped dose–response might be the result of concentration-dependent effects on the coupling of OTRs to different G protein subtypes.
Although the rewarding properties of many types of social interaction are self-evident, the importance of social reward in the expression of adaptive and maladaptive behavior, and in the establishment and maintenance of social relationships, remains to be fully appreciated. There is increasing evidence that dysfunctions in the mechanisms mediating reward can play a substantial role in the expression of various psychiatric disorders, including substance abuse, affective disorders, and obsessive–compulsive disorders, as well as in a range of neurodevelopmental disorders including autism spectrum disorder, schizophrenia, and attention-deficit/hyperactivity disorders. Indeed, it has been proposed that dysfunctions in the circuits mediating reward (e.g., mesolimbic DA system) may be present in many different psychiatric and neurodevelopmental disorders and represent a common target for their treatment [163]. Recently, the National Institute of Mental Health has developed the Research Domain Criteria (RDoC) as a “research framework for new approaches to investigating mental disorders” and has defined two of these domains as “Negative valence systems” that are primarily responsible for responses to aversive situations and “Positive valence systems” that are primarily responsible for responses to positive motivational situations. Not infrequently, however, a characteristic of mental disorders is that social stimuli that normally have a positive valence can become less rewarding or even aversive. It is interesting to consider whether an inverted U function of the rewarding properties of the social duration and/or intensity of social interactions could contribute to changing the valence of social stimuli from positive to negative, and thereby contribute to the symptomology of mental disorders. One possibility is that the perceived value of social interactions could be abnormally increased or decreased in specific disorders. If so, the direction and magnitude of the change in perceived value of social interaction would determine the sex-dependent change in the valence of social stimuli.
While there are substantial sex differences in the incidence of many psychiatric disorders, relatively little is known about the underlying causes of these differences [164, 165]; it seems likely that sex differences in the rewarding properties of social interactions are likely contributors. For example, the possibility that social interactions are less rewarding in men than women at baseline could pre-dispose men to be more susceptible to disorders such as autism, which is characterized by diminished social motivation and reward, and which does indeed occur four times more frequently in men than women [166]. It also seems likely that as more research is conducted in females, more sex differences will be found in the factors that contribute mental disorders. For example, social stress is a significant factor in many types of mental disorders, and it is now clear that females are more susceptible to social stress than males [167]. Taken together, these data suggest that the causes of mental disorders such as stress and a diminished capacity for social reward may interact in complex ways that differ in men and women and indicate the importance of the development of gender-based treatments.
OT has been proposed to be a promising treatment for several psychiatric disorders including substance abuse, autism spectrum disorders, anxiety, stress-related disorders, and schizophrenia [168–172]. If the inverted U hypothesis of a sex-dependent relationship between OT dose and its effects on social reward (and perhaps other factors important in the etiology of mental disorders) is correct, then consideration of gender differences in OT administration will be particularly important. Indeed, giving the same dose of OT in men and women could have the opposite effect producing a positive clinical outcome in one sex while producing a negative clinical outcome in the other. While considerable effort is underway to develop drugs that can act selectively on OTRs in the brain, at present the most common route of administration of OT in humans is intranasal. Intranasal OT administration is thought to produce supraphysiological levels [173], making it particularly important to examine the dose-dependent effects of OT in both men and women.
Future areas of research
Because social reward plays such a key role in the expression of social behavior, more comprehensive studies of the rewarding nature of social interactions and their neural mechanisms in males and females are needed. In particular, it will be important to define what specific characteristics of social interactions alter the rewarding properties of those interactions. For example, while the duration of social interactions have been shown to alter reward value, the conditions under which the intensity of social interactions might alter their reward value are less clear. It will also be important to determine if different types of social interactions are more rewarding in one sex than the other across species. For example, aggression may be more rewarding in males than in females. In addition, some types of social interactions may elicit different states of arousal in males and females, resulting in social experiences that are qualitatively different. Particularly in humans, same-sex interactions may be more complex and represent more than just sex differences in the rewarding properties of those interactions. For example, same-sex social interactions could also be influenced by motivation to avoid agonistic interactions, which may have more severe consequences for one sex than the other (e.g., the more aggressive sex). If so, OT might also influence social behavior by modulating the impact of negative social interactions. For example, OT has been shown to attenuate the amygdala response to negative social interactions in men, perhaps signifying decreased stress or anxiety in this context [174, 158].
Investigation of the role of the AVP/OT family of peptides in social neuroscience will likely continue at a rapid pace because of its importance for understanding the basic neural mechanisms of social behavior and their translational significance. Understanding the action of these peptides will require studies of the functional significance of the cross-talk between OT and AVP and their receptors in both males and females. It will also be important to define the critical sex differences in AVP/OT signaling. What are the roles of sex differences in the amount and distribution of peptide release (e.g., synaptic and non-synaptic release), the number and distribution of their receptors and/or in the cellular, and/or network events precipitated following receptor activation? It will also be important to determine the role of gonadal hormones in mediating sex differences in social reward, particularly because they play an important role in sex differences in drug reward.
Another key area of future research will be to define the dynamic interactions between OT and the many other neurochemical signals found within the mesolimbic DA system that contribute to reward, and how these interactions differ in males and females. Of course, interactions with DA will be of central importance but in addition it will be critical to determine the roles of the many other neurochemical signals, that have not been discussed in this review, but likely play an important role such as serotonin, GABA, and glutamate. It will be interesting to fully test the inverted U hypothesis of the rewarding properties of the duration/intensity of social interactions and determine the extent to which they can account for changing the valence of social stimuli from positive to negative (or vice versa) thereby contributing to the symptomology (and potentially treatments) of mental disorders in men and women. If the inverted U hypothesis of a sex-dependent relationship between OT dose and its effects on social reward and perhaps other factors important in the etiology of mental disorders is correct then consideration of gender differences in OT administration will be particularly significant. It will be particularly important to examine the effects of OT dose on social reward as well as its utility in developing gender-specific OT treatments for a range of psychiatric and neurodevelopmental disorders.
Acknowledgements
We would like to thank Dr. Maurice Manning for his generous gift of the OT receptor agonist and antagonist.
Funding
This work was supported by an NIH predoctoral fellowship F31MH113367 to JMB, NIH grants MH109302 and MH110212 to HEA, NIH grant MH084068 to JKR, and funds from the Brains and Behavior Program at Georgia State University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.White NM. Reward or reinforcement: what’s the difference? Neurosci Biobehav Rev. 1989;13:181–6. doi: 10.1016/S0149-7634(89)80028-4. [DOI] [PubMed] [Google Scholar]
- 2.Panksepp J, Yovell Y. Preclinical modeling of primal emotional affects (Seeking, Panic and Play): gateways to the development of new treatments for depression. Psychopathology. 2014;47:383–93. doi: 10.1159/000366208. [DOI] [PubMed] [Google Scholar]
- 3.Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011;1:444–58. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 5.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114:173–83. doi: 10.1037/0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 6.Gray CL, Norvelle A, Larkin T, Huhman KL. Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus) Behav Brain Res. 2015;286:22–8. doi: 10.1016/j.bbr.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC. Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur J Neurosci. 2015;42:3081–94. doi: 10.1111/ejn.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil M, Nguyen NT, McDonald M, Albers HE. Social reward: interactions with social status, social communication, aggression, and associated neural activation in the ventral tegmental area. Eur J Neurosci. 2013;38:2308–18. doi: 10.1111/ejn.12216. [DOI] [PubMed] [Google Scholar]
- 9.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 10.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, et al. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2015;9:754–64. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- 11.Novacek DM, Gooding DC, Pflum MJ. Hedonic capacity in the broader autism phenotype: should social anhedonia be considered a characteristic feature? Front Psychol. 2016;7:666. doi: 10.3389/fpsyg.2016.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49:217–28 e211-213. [PMC free article] [PubMed] [Google Scholar]
- 13.Stavropoulos KK, Carver LJ. Research review: social motivation and oxytocin in autism–implications for joint attention development and intervention. J Child Psychol Psychiatry. 2013;54:603–18. doi: 10.1111/jcpp.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7:160–72. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. Surprising origins of sex differences in the brain. Horm Behav. 2015;76:3–10. doi: 10.1016/j.yhbeh.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–8. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 18.Terranova JI, Song Z, Larkin TE, 2nd, Hardcastle N, Norvelle A, Riaz A, et al. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci USA. 2016;113:13233–8. doi: 10.1073/pnas.1610446113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–84. doi: 10.1016/S0018-506X(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 20.Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38:2554–61. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telgkamp P, Combs N, Smith GT. Serotonin in a diencephalic nucleus controlling communication in an electric fish: sexual dimorphism and relationship to indicators of dominance. Dev Neurobiol. 2007;67:339–54. doi: 10.1002/dneu.20356. [DOI] [PubMed] [Google Scholar]
- 23.Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–92. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Panzica G, Melcangi RC. Structural and molecular brain sexual differences: a tool to understand sex differences in health and disease. Neurosci Biobehav Rev. 2016;67:2–8. doi: 10.1016/j.neubiorev.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain–presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 27.Terranova JI, Ferris CF, Albers HE. Sex differences in the regulation of offensive aggression and dominance by arginine-vasopressin. Front Endocrinol. 2017;8:308. doi: 10.3389/fendo.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004;46:444–9. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Ferris CF, Melloni RH, Jr., Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) Eur J Neurosci. 2010;31:1655–63. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell Heather K. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. The Neuroscientist. 2017;23(5):517–528. doi: 10.1177/1073858417708284. [DOI] [PubMed] [Google Scholar]
- 33.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–6. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 34.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 35.Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Grunder G, et al. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172–9. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Acher R, Chauvet J. The neurohypophysial endocrine regulatory cascade: precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995;16:237–89. doi: 10.1006/frne.1995.1009. [DOI] [PubMed] [Google Scholar]
- 37.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 38.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–84. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Z, Larkin TE, Malley MO, Albers HE. Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus) Horm Behav. 2016;81:20–7. doi: 10.1016/j.yhbeh.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50C:14–9. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Zhimin, Albers H. Elliott. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Frontiers in Neuroendocrinology. 2018;51:14–24. doi: 10.1016/j.yfrne.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazell GG, Hindmarch CC, Pope GR, Roper JA, Lightman SL, Murphy D, et al. G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei–serpentine gateways to neuroendocrine homeostasis. Front Neuroendocrinol. 2012;33:45–66. doi: 10.1016/j.yfrne.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Burg EH, Neumann ID. Bridging the gap between GPCR activation and behaviour: oxytocin and prolactin signalling in the hypothalamus. J Mol Neurosci. 2011;43:200–8. doi: 10.1007/s12031-010-9452-8. [DOI] [PubMed] [Google Scholar]
- 44.Busnelli Marta, Chini Bice. Behavioral Pharmacology of Neuropeptides: Oxytocin. Cham: Springer International Publishing; 2017. Molecular Basis of Oxytocin Receptor Signalling in the Brain: What We Know and What We Need to Know; pp. 3–29. [DOI] [PubMed] [Google Scholar]
- 45.Gravati M, Busnelli M, Bulgheroni E, Reversi A, Spaiardi P, Parenti M, et al. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J Neurochem. 2010;114:1424–35. doi: 10.1111/j.1471-4159.2010.06861.x. [DOI] [PubMed] [Google Scholar]
- 46.Busnelli M, Sauliere A, Manning M, Bouvier M, Gales C, Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem. 2012;287:3617–29. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldwell HK. Oxytocin and sex differences in behavior. Curr Opin Behav Sci. 2018;23:13–28. doi: 10.1016/j.cobeha.2018.02.002. [DOI] [Google Scholar]
- 49.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiao X, Yan Y, Wu R, Tai F, Hao P, Cao Y, et al. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014;200:149–59. doi: 10.1007/s00359-013-0870-2. [DOI] [PubMed] [Google Scholar]
- 51.Haussler HU, Jirikowski GF, Caldwell JD. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J Chem Neuroanat. 1990;3:271–6. [PubMed] [Google Scholar]
- 52.Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–17. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–37. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 55.Caffe AR, Van Ryen PC, Van der Woude TP, van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–25. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- 56.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab. 1999;84:4637–44. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- 57.van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–4. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109:305–11. doi: 10.1037/0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- 59.Delville Y, Koh ET, Ferris CF. Sexual differences in the magnocellular vasopressinergic system in golden hamsters. Brain Res Bull. 1994;33:535–40. doi: 10.1016/0361-9230(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 60.Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, et al. Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–34. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albers HE, Rowland CM, Ferris CF. Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus) Brain Res. 1991;539:137–42. doi: 10.1016/0006-8993(91)90696-S. [DOI] [PubMed] [Google Scholar]
- 62.Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chini B, Verhage M, Grinevich V. The action radius of oxytocin release in the mammalian CNS: from single vesicles to behavior. Trends Pharmacol Sci. 2017 doi: 10.1016/j.tips.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Buijs RM. Vasopressin and oxytocin - their role in neurotransmission. Pharmacol Ther. 1983;22:127–41. doi: 10.1016/0163-7258(83)90056-6. [DOI] [PubMed] [Google Scholar]
- 66.Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res. 1979;204:355–65. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- 67.Buijs RM, Van Heerikhuize JJ. Vasopressin and oxytocin release in the brain–a synaptic event. Brain Res. 1982;252:71–6. doi: 10.1016/0006-8993(82)90979-9. [DOI] [PubMed] [Google Scholar]
- 68.Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–32. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol. 2000;85:125S–30S. doi: 10.1111/j.1469-445X.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 70.Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. Neuroreport. 1996;7:543–7. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- 71.Donovan M, Liu Y, Wang Z. Anxiety-like behavior and neuropeptide receptor expression in male and female prairie voles: the effects of stress and social buffering. Behav Brain Res. 2018;342:70–8. doi: 10.1016/j.bbr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Guoynes CD, Simmons TC, Downing GM, Jacob S, Solomon M, Bales KL. Chronic intranasal oxytocin has dose-dependent effects on central oxytocin and vasopressin systems in prairie voles (Microtus ochrogaster) Neuroscience. 2018;369:292–302. doi: 10.1016/j.neuroscience.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–30. doi: 10.1016/0306-4522(91)90321-E. [DOI] [PubMed] [Google Scholar]
- 75.Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 1990;511:129–40. doi: 10.1016/0006-8993(90)90232-Z. [DOI] [PubMed] [Google Scholar]
- 76.De Kloet ER, Voorhuis TAM, Elands J. Estradiol induces oxytocin binding sites in rat hypothalamic ventromedial nucleus. Eur J Pharmacol. 1986;118:185–6. doi: 10.1016/0014-2999(85)90679-X. [DOI] [PubMed] [Google Scholar]
- 77.Bale TL, Dorsa DM, Johnston CA. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J Neurosci. 1995;15:5058–64. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson AE, Coirini H, Ball GF, McEwen BS. Anatomical localization of the effects of 17ß-estradiol on oxytocin receptor binding in the ventromedial hypothalamic nucleus. Endocrinology. 1989;124:207–11. doi: 10.1210/endo-124-1-207. [DOI] [PubMed] [Google Scholar]
- 79.Witt DM, Carter CS, Lnsel TR. Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. J Neuroendocrinol. 1991;3:155–61. doi: 10.1111/j.1365-2826.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 80.Bale TL, Dorsa DM. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology. 1995;136:27–32. doi: 10.1210/endo.136.1.7828541. [DOI] [PubMed] [Google Scholar]
- 81.O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–39. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 82.Caldwell HK, Albers HE. Oxytocin, vasopressin, and the motivational forces that drive social behaviors. Curr Top Behav Neurosci. 2016;27:51–103. doi: 10.1007/7854_2015_390. [DOI] [PubMed] [Google Scholar]
- 83.Wei D, Lee D, Li D, Daglian J, Jung KM, Piomelli D. A role for the endocannabinoid 2-arachidonoyl-sn-glycerol for social and high-fat food reward in male mice. Psychopharmacology. 2016;233:1911–9. doi: 10.1007/s00213-016-4222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mikhailova MA, Bass CE, Grinevich VP, Chappell AM, Deal AL, Bonin KD, et al. Optogenetically-induced tonic dopamine release from VTA-nucleus accumbens projections inhibits reward consummatory behaviors. Neuroscience. 2016;333:54–64. doi: 10.1016/j.neuroscience.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kummer KK, El Rawas R, Kress M, Saria A, Zernig G. Social interaction and cocaine conditioning in mice increase spontaneous spike frequency in the nucleus accumbens or septal nuclei as revealed by multielectrode array recordings. Pharmacology. 2015;95:42–9. doi: 10.1159/000370314. [DOI] [PubMed] [Google Scholar]
- 86.Grotewold SK, Wall VL, Goodell DJ, Hayter C, Bland ST. Effects of cocaine combined with a social cue on conditioned place preference and nucleus accumbens monoamines after isolation rearing in rats. Psychopharmacology. 2014;231:3041–53. doi: 10.1007/s00213-014-3470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–34. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El Rawas R, Klement S, Kummer KK, Fritz M, Dechant G, Saria A, et al. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front Behav Neurosci. 2012;6:63. doi: 10.3389/fnbeh.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–84. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–72. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–11. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–35. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- 96.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 98.Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol. 2017;525:1094–108. doi: 10.1002/cne.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gillies GE, Virdee K, McArthur S, Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience. 2014;282:69–85. doi: 10.1016/j.neuroscience.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology. 2007;32:1462–76. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- 101.Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–35. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–70. doi: 10.1016/S0306-4522(99)00500-X. [DOI] [PubMed] [Google Scholar]
- 103.Virdee K, McArthur S, Brischoux F, Caprioli D, Ungless MA, Robbins TW, et al. Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology. 2014;39:339–50. doi: 10.1038/npp.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- 105.Castner SA, Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1996;712:245–57. doi: 10.1016/0006-8993(95)01429-2. [DOI] [PubMed] [Google Scholar]
- 106.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–9. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 108.Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52:759–63. doi: 10.1016/S0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- 109.Soutschek A, Beharelle AR, Burke CJ, Schreiber R, Weber SC, Karipidis II, ten Velden J, Weber B, Haker H, Kalenscher T, Tobler PN. The dopaminergic reward system underpins gender differences in social preferences. Nat Human Behav. 2017;1:819–27. doi: 10.1038/s41562-017-0226-y. [DOI] [PubMed] [Google Scholar]
- 110.Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–25. doi: 10.1016/S0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 111.White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–41. doi: 10.1016/S0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- 112.Robinson TE, Camp DM, Becker JB. Gonadectomy attenuates turning behavior produced by electrical stimulation of the nigrostriatal dopamine system in female but not male rats. Neurosci Lett. 1981;23:203–8. doi: 10.1016/0304-3940(81)90041-0. [DOI] [PubMed] [Google Scholar]
- 113.Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 114.Forgie ML, Stewart J. Six differences in the locomotor-activating effects of amphetamine: role of circulating testosterone in adulthood. Physiol Behav. 1994;55:639–44. doi: 10.1016/0031-9384(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 115.Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–8. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 116.Borland JM, Frantz KJ, Aiani LM, Grantham KN, Song Z, Albers HE. A novel operant task to assess social reward and motivation in rodents. J Neurosci Methods. 2017;287:80–8. doi: 10.1016/j.jneumeth.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heth G, Todrank J, Johnston RE. Kin recognition in golden hamsters: evidence for phenotype matching. Anim Behav. 1998;56:409–17. doi: 10.1006/anbe.1998.0747. [DOI] [PubMed] [Google Scholar]
- 118.Johnston RE, Peng A. Memory for individuals: hamsters (Mesocricetus auratus) require contact to develop multicomponent representations (concepts) of others. J Comp Psychol. 2008;122:121–31. doi: 10.1037/0735-7036.122.2.121. [DOI] [PubMed] [Google Scholar]
- 119.Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36:259–69. doi: 10.1163/156853970X00402. [DOI] [PubMed] [Google Scholar]
- 120.Drickamer LC, Vandenbergh JG. Predictors of social dominance in the adult female golden hamster (Mesocricetus auratus) Anim Behav. 1973;21:564–70. doi: 10.1016/S0003-3472(73)80017-X. [DOI] [PubMed] [Google Scholar]
- 121.Drickamer LC, Vandenbergh JG, Colby DR. Predictors of dominance in the male golden hamster (Mesocricetus auratus) Anim Behav. 1973;21:557–63. doi: 10.1016/S0003-3472(73)80016-8. [DOI] [PubMed] [Google Scholar]
- 122.Borland JM, Grantham KN, Aiani LM, Frantz KJ, Albers HE. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology. 2018;95:128–37. doi: 10.1016/j.psyneuen.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weiss VG, Hofford RS, Yates JR, Jennings FC, Bardo MT. Sex differences in monoamines following amphetamine and social reward in adolescent rats. Exp Clin Psychopharmacol. 2015;23:197–205. doi: 10.1037/pha0000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raz S, Berger BD. Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol. 2010;21:39–46. doi: 10.1097/FBP.0b013e32833470bd. [DOI] [PubMed] [Google Scholar]
- 125.Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12:1151–6. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bregolin T, Pinheiro BS, El Rawas R, Zernig G. Preventive strength of dyadic social interaction against reacquisition/reexpression of cocaine conditioned place preference. Front Behav Neurosci. 2017;11:225. doi: 10.3389/fnbeh.2017.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–78. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westenbroek C, Perry AN, Jagannathan L, Becker JB. Effect of social housing and oxytocin on the motivation to self-administer methamphetamine in female rats. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:903–7. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- 130.Carson DS, Guastella AJ, Taylor ER, McGregor IS. A brief history of oxytocin and its role in modulating psychostimulant effects. J Psychopharmacol. 2013;27:231–47. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- 131.Leong KC, Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp Clin Psychopharmacol. 2016;24:55–64. doi: 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–53. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Becker JB. Sex differences in addiction. Dialog Clin Neurosci. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE. Estradiol promotes the rewarding effects of nicotine in female rats. Behav Brain Res. 2016;307:258–63. doi: 10.1016/j.bbr.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dobkin PL, De CM, Paraherakis A, Gill K. The role of functional social support in treatment retention and outcomes among outpatient adult substance abusers. Addiction. 2002;97:347–56. doi: 10.1046/j.1360-0443.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 136.Havassy BE, Wasserman DA, Hall SM. Social relationships and abstinence from cocaine in an American treatment sample. Addiction. 1995;90:699–710. doi: 10.1111/j.1360-0443.1995.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 137.Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF. D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav. 1993;45:239–42. doi: 10.1016/0091-3057(93)90112-7. [DOI] [PubMed] [Google Scholar]
- 138.Doherty JM, Cooke BM, Frantz KJ. A role for the prefrontal cortex in heroin-seeking after forced abstinence by adult male rats but not adolescents. Neuropsychopharmacology. 2013;38:446–54. doi: 10.1038/npp.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uhl GR, Drgonova J, Hall FS. Curious cases: altered dose-response relationships in addiction genetics. Pharmacol Ther. 2014;141:335–46. doi: 10.1016/j.pharmthera.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 140.Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zernig G, Pinheiro BS. Dyadic social interaction inhibits cocaine-conditioned place preference and the associated activation of the accumbens corridor. Behav Pharmacol. 2015;26:580–94. doi: 10.1097/FBP.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matthews TJ, Abdelbaky P, Pfaff DW. Social and sexual motivation in the mouse. Behav Neurosci. 2005;119:1628–39. doi: 10.1037/0735-7044.119.6.1628. [DOI] [PubMed] [Google Scholar]
- 143.Liberzon I, Trujillo KA, Akil H, Young EA. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 1997;17:353–9. doi: 10.1016/S0893-133X(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 144.Donhoffner ME, Goings SP, Atabaki K, Wood, RI. Intracerebroventricular oxytocin self-administration in female rats. J Neuroendocrinol. 2016;28. 10.1111/jne.12416. [DOI] [PMC free article] [PubMed]
- 145.Kent K., Arientyl V., Khachatryan M. M., Wood R. I. Oxytocin Induces a Conditioned Social Preference in Female Mice. Journal of Neuroendocrinology. 2013;25(9):803–810. doi: 10.1111/jne.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kosaki Yutaka, Watanabe Shigeru. Conditioned social preference, but not place preference, produced by intranasal oxytocin in female mice. Behavioral Neuroscience. 2016;130(2):182–195. doi: 10.1037/bne0000139. [DOI] [PubMed] [Google Scholar]
- 147.Li T, Chen X, Mascaro J, Haroon E, Rilling JK. Intranasal oxytocin, but not vasopressin, augments neural responses to toddlers in human fathers. Horm Behav. 2017;93:193–202. doi: 10.1016/j.yhbeh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scheele D, Plota J, Stoffel-Wagner B, Maier W, Hurlemann R. Hormonal contraceptives suppress oxytocin-induced brain reward responses to the partner’s face. Soc Cogn Affect Neurosci. 2016;11:767–74. doi: 10.1093/scan/nsv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA. 2013;110:20308–13. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weisman Omri, Zagoory-Sharon Orna, Feldman Ruth. Oxytocin Administration to Parent Enhances Infant Physiological and Behavioral Readiness for Social Engagement. Biological Psychiatry. 2012;72(12):982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 151.Gregory R, Cheng H, Rupp HA, Sengelaub DR, Heiman JR. Oxytocin increases VTA activation to infant and sexual stimuli in nulliparous and postpartum women. Horm Behav. 2015;69:82–8. doi: 10.1016/j.yhbeh.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hecht EE, Robins DL, Gautam P, King TZ. Intranasal oxytocin reduces social perception in women: neural activation and individual variation. Neuroimage. 2017;147:314–29. doi: 10.1016/j.neuroimage.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 153.Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–7. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feng C, Lori A, Waldman ID, Binder EB, Haroon E, Rilling JK. A common oxytocin receptor gene (OXTR) polymorphism modulates intranasal oxytocin effects on the neural response to social cooperation in humans. Genes Brain Behav. 2015;14:516–25. doi: 10.1111/gbb.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Altemus M, Jacobson KR, Debellis M, Kling M, Pigott T, Murphy DL, et al. Normal CSF oxytocin and NPY levels in OCD. Biol Psychiatry. 1999;45:931–3. doi: 10.1016/S0006-3223(98)00263-7. [DOI] [PubMed] [Google Scholar]
- 156.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology. 2013;38:399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 157.Cardoso C, Orlando MA, Brown CA, Ellenbogen MA. Oxytocin and enhancement of the positive valence of social affiliation memories: an autobiographical memory study. Soc Neurosci. 2014;9:186–95. doi: 10.1080/17470919.2013.873079. [DOI] [PubMed] [Google Scholar]
- 158.Chen X, Gautam P, Haroon E, Rilling JK. Within vs. between-subject effects of intranasal oxytocin on the neural response to cooperative and non-cooperative social interactions. Psychoneuroendocrinology. 2017;78:22–30. doi: 10.1016/j.psyneuen.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rilling JK, Chen X, Chen X, Haroon E. Intranasal oxytocin modulates neural functional connectivity during human social interaction. Am J Primatol. 2018. In press. [DOI] [PMC free article] [PubMed]
- 160.Kreuder AK, Scheele D, Wassermann L, Wollseifer M, Stoffel-Wagner B, Lee MR, et al. How the brain codes intimacy: the neurobiological substrates of romantic touch. Hum Brain Mapp. 2017;38:4525–34. doi: 10.1002/hbm.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology. 1992;106:71–4. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- 162.Boccia MM, Kopf SR, Baratti CM. Effects of a single administration of oxytocin or vasopressin and their interactions with two selective receptor antagonists on memory storage in mice. Neurobiol Learn Mem. 1998;69:136–46. doi: 10.1006/nlme.1997.3817. [DOI] [PubMed] [Google Scholar]
- 163.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cover KK, Maeng LY, Lebron-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry. 2014;4:e422. doi: 10.1038/tp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gobinath AR, Choleris E, Galea LA. Sex, hormones, and genotype interact to influence psychiatric disease, treatment, and behavioral research. J Neurosci Res. 2017;95:50–64. doi: 10.1002/jnr.23872. [DOI] [PubMed] [Google Scholar]
- 166.Ferri SL, Abel T, Brodkin ES. Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep. 2018;20:9. doi: 10.1007/s11920-018-0874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–9. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 169.Rich ME, Caldwell HK. A role for oxytocin in the etiology and treatment of schizophrenia. Front Endocrinol. 2015;6:90. doi: 10.3389/fendo.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sippel Lauren M., Allington Casey E., Pietrzak Robert H., Harpaz-Rotem Ilan, Mayes Linda C., Olff Miranda. Oxytocin and Stress-related Disorders: Neurobiological Mechanisms and Treatment Opportunities. Chronic Stress. 2017;1:247054701668799. doi: 10.1177/2470547016687996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Benner S, Yamasue H. Clinical potential of oxytocin in autism spectrum disorder: current issues and future perspectives. Behav Pharmacol. 2018;29:1–2. doi: 10.1097/FBP.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 172.Gottschalk MG, Domschke K. Oxytocin and anxiety disorders. Curr Top Behav Neurosci. 2017 doi: 10.1007/7854_2017_25. [DOI] [PubMed] [Google Scholar]
- 173.Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biol Psychiatry. 2016;79:243–50. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 174.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]