Serotonin (5-hydroxytryptamine; 5-HT) was identified in the mammalian brain in the 1950s. We now recognize brain 5-HT to actualize flexible and complex actions directed by binding to at least 14 5-HT receptors as well as the serotonin reuptake transporter. The 5-HT2C receptor (5-HT2CR) is a G protein-coupled receptor (GPCR) that has been implicated in psychiatric and neurological disorders, including anxiety, depression, schizophrenia, and substance use disorders as well as obesity. The orthosteric 5-HT2CR site, at which the endogenous agonist binds, has been a primary target for ligand discovery for obesity based upon the role of 5-HT2CR in the control of feeding, energy and glucose homeostasis, yielding the first-in-class 5-HT2CR agonist lorcaserin (Belviq®) approved by the FDA for weight loss. While 5-HT2CR antagonism potentially mediates the side effect of some antipsychotics (e.g., clozapine) to increase weight gain, the development of 5-HT2CR antagonists has been pursued for the treatment of anxiety disorders and depression. Importantly, selective agonists and antagonists have advanced knowledge of 5-HT2CR neuropsychopharmacology.
Binding of 5-HT to the 5-HT2CR results in a conformational change that catalyzes the diffusion of multiple second messenger effectors and G protein-dependent signaling. An allosteric modulator is a ligand that binds to a spatially distinct allosteric site(s) and alters the receptor conformation to modulate its interaction with other ligands and/or signal transduction molecules [1]. Higher sequence divergence for allosteric sites is expected across receptor subtypes relative to the highly conserved orthosteric domain. Such allosteric modulation is saturable (comes to a finite magnitude when the allosteric site is fully occupied) and probe-dependent (varies dependent upon the orthosteric ligand) with the prospects for separate control of affinity and efficacy. A positive- (PAM) or negative-allosteric modulator (NAM) can enhance or inhibit the functional response to an orthosteric agonist, while silent allosteric ligands are suggested to compete with PAMs or NAMs at the allosteric binding site. Additionally, allosteric ligands can be antagonists or agonists with, or without, PAM or NAM activity (e.g., ago-PAMs, ligands that potentiate agonists and display intrinsic efficacy).
Small molecule 5-HT2CR PAMs would not act as an orthosteric agonists (as does lorcaserin), but instead act at distinct allosteric site(s) on the 5-HT2CR and potentiate signaling of an orthosteric ligand (Fig. 1). The first identified 5-HT2CR PAM was the fatty acid oleamide [2], which lacked selectivity against other GPCRs. In 2003, chemical library screening resulted in the discovery of an analogue of the antibiotic lincomycin, PNU-69176E, as a selective 5-HT2CR PAM [3]. In 2012, Zhou and colleagues optimized the synthetic route to generate PNU-69176E and its diastereomer [4], and we have recently designed, synthesized and characterized a series of new chemical entities as selective 5-HT2CR PAMs [5]. Several molecules potentiated 5-HT2CR-evoked signaling in vitro without displaying 5-HT2CR efficacy or altering 5-HT2AR signaling. CYD-1-79 exhibited a favorable overall pharmacokinetic profile, potentiated 5-HT2CR-mediated behaviors, and attenuated impulsive action and sensitivity to cocaine-associated cues in a preclinical self-administration model. This series of 5-HT2CR PAMs is complemented by the discovery of a 5-HT2CR PAM, which suppressed food intake in rodents [6]. Thus, the chemical space for discovery of novel 5-HT2CR neuroprobes and therapeutics is now expanding to include allosteric 5-HT2CR modulators, providing the opportunity to explore cellular mechanisms of action, functional selectivity, neurochemical mechanisms, and neural sites of action for 5-HT2CR to control behavior.
Fig. 1.
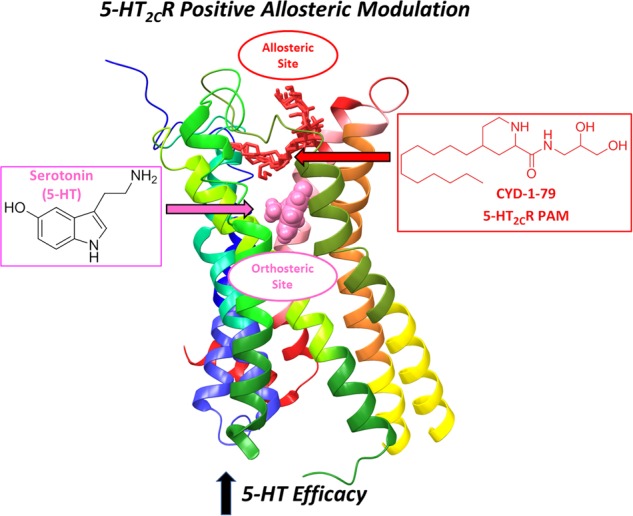
The orthosteric and allosteric sites on the 5-HT2C receptor are distinct. Serotonin binds to the orthosteric site to generate downstream signaling. The 5-HT2CR PAM CYD-1-79 (cis-4-alkylpiperidine-2-carboxamide series) increases 5-HT efficacy through actions at the allosteric site [5]
Acknowledgments
Thanks to our outstanding team of faculty, mentees and staff who contributed to this work. The research described is supported by R01 DA038446.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia Zhou, Email: jizhou@utmb.edu.
Kathryn A. Cunningham, Email: kcunning@utmb.edu
References
- 1.Christopoulos A, Changeux JP, Catterall WA, Fabbro D, Burris TP, Cidlowski JA, et al. International Union of Basic and Clinical Pharmacology. XC. Multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev. 2014;66:918–47. doi: 10.1124/pr.114.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huidobro-Toro JP, Valenzuela CF, Harris RA. Modulation of GABAA receptor function by G protein-coupled 5- HT2C receptors. Neuropharmacology. 1996;35:1355–63. doi: 10.1016/S0028-3908(96)00084-6. [DOI] [PubMed] [Google Scholar]
- 3.Im WB, Chio CL, Alberts GL, Dinh DM. Positive-allosteric modulator of the human 5-HT2C receptor. Mol Pharmacol. 2003;64:78–84. doi: 10.1124/mol.64.1.78. [DOI] [PubMed] [Google Scholar]
- 4.Ding C, Bremer NM, Smith TD, Seitz PK, Anastasio NC, Cunningham KA, et al. Exploration of synthetic approaches and pharmacological evaluation of PNU-69176E and its stereoisomer as 5-HT2C receptor allosteric modulators. ACS Chem Neurosci. 2012;3:538–45. doi: 10.1021/cn300020x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild CT, Miszkiel JM, Wold EA, Soto CA, Ding C, Hartley RM, et al. Design, synthesis, and characterization of 4-undecylpiperidine-2-carboxamides as positive-allosteric modulators of the serotonin (5-HT) 5-HT2C receptor. J Med Chem. 2018 10.1021/acs.jmedchem.8b00401 [DOI] [PMC free article] [PubMed]
- 6.Garcia-Carceles J, Decara JM, Vazquez-Villa H, Rodriguez R, Codesido E, Cruces J, et al. A positive-allosteric modulator of the serotonin 5-HT2C receptor for obesity. J Med Chem. 2017;60:9575–84. doi: 10.1021/acs.jmedchem.7b00994. [DOI] [PubMed] [Google Scholar]


