Abstract
Many chronic diseases, including those classified as cardiovascular, neurodegenerative, or autoimmune, are characterized by persistent inflammation. The origin of this inflammation is mostly unclear, but it is typically mediated by inflammatory biomarkers, such as cytokines, and affected by both environmental and genetic factors. Recently circulating bacterial inflammagens such as lipopolysaccharide (LPS) have been implicated. We used a highly selective mouse monoclonal antibody to detect bacterial LPS in whole blood and/or platelet poor plasma of individuals with Parkinson’s Disease, Alzheimer’s type dementia, or Type 2 Diabetes Mellitus. Our results showed that staining is significantly enhanced (P < 0.0001) compared to healthy controls. Aberrant blood clots in these patient groups are characterized by amyloid formation as shown by the amyloid-selective stains thioflavin T and Amytracker™ 480 or 680. Correlative Light-Electron Microscopy (CLEM) illustrated that the LPS antibody staining is located in the same places as where amyloid fibrils may be observed. These data are consistent with the Iron Dysregulation and Dormant Microbes (IDDM) hypothesis in which bacterial inflammagens such as LPS are responsible for anomalous blood clotting as part of the aetiology of these chronic inflammatory diseases.
Introduction
Many chronic diseases, including those classified as autoimmune, cardiovascular, or neurodegenerative, are associated with persistent inflammation. Although typically mediated by ‘inflammatory’ cytokines and affected by both environmental and genetic factors, the origin of this inflammation is mostly unclear. Since the recognition that most peptic ulcers, other than those caused by non-steroidal anti-inflammatory drugs (NSAIDs), have a microbial basis1,2, there is a significant body of literature that suggests many other supposedly non-communicable diseases might actually have a bacterial and/or viral origin. For instance, Herpes simplex virus type 1 (HSV1), Chlamydia pneumoniae, and several types of spirochaetes are specific microbes that have been implicated in the aetiology of Alzheimer’s Disease (AD)3–14. A bacterial link has also been suggested for Parkinson’s Disease (PD)3,15–21, and microbes have been associated with ageing in general22. The presence of an aberrant blood microbiome, as assessed by sequencing, has also been implicated in Type 2 Diabetes (T2D) and cardiovascular events23–25.
These microbes are not detected by standard microbiological tests involving replicative culture because they are dormant (e.g.26–30). The exit from dormancy has been linked to dysregulation of iron metabolism, and/or stress hormones31–33, in part because free iron is required for the reactivation and/or growth of the microbes in question. This reactivation of low levels of bacteria can then release highly potent inflammagens such as lipopolysaccharide (LPS) from Gram-negative organisms and lipoteichoic acids (LTA) from Gram-positive organisms. Therefore, in a series of papers, as summarised in a review30, we refer to these dormancy/iron/inflammation events as the Iron Dysregulation and Dormant Microbes hypothesis (IDDM). In this hypothesis we argue that microorganisms and their circulating products represent an important external stimulus in inflammatory conditions such as PD, AD, T2D as well as in other diseases such as pre-eclampsia34,35, overlaid on any genetic disease predisposition and exposure to environmental stressors.
LPS and LTA have recently been shown to be capable of triggering hypercoagulation or aberrant blood clotting into an amyloid form36–39. Hypercoagulation is a well-known hallmark of inflammation40 and is caused by pathological levels of circulating inflammatory molecules, including pro-inflammatory cytokines36. Importantly, during inflammation, erythrocytes (RBCs) and platelets are also involved in the pathological clotting process, where circulating mediators of inflammation cause the membranes of these cells to become altered into procoagulant surfaces41–46. Also, it was recently reported that von Willebrand factor plays a role in erythrocyte endothelial adhesion, where eryptotic erythrocytes may interact with von Willebrand factor fibres47. In inflammatory conditions, including PD, AD and T2D, a changed erythrocyte and platelet structure, together with close interactions with pathological fibrin (atypical fibrin fibre formation), were previously reported48–51. Such a hypercoagulable state and anomalous blood clotting go hand in hand37,52,53, resulting in pathological clotting, and is one of the main causes contributing to myocardial infarction and thrombo-embolic strokes54,55. Therefore these bacterial inflammagens (LPS and LTA) may have a much larger role in inflammatory conditions, such as PD, AD, T2D and others, than is presently conceived.
Specifically, we have shown that the addition of minute concentrations (highly substoichiometric amounts (10−8 molar ratio)) of LPS and LTA to healthy blood plasma, together with thrombin to create a clot, can result in fibrin(ogen) plasma proteins adopting an amyloid form37–39. We also described the reversal of this aberrant clotting by the addition of LPS-binding protein to healthy blood. Similarly, we have shown blood clots with an amyloid form in PD, AD and T2D37,39,49,56–59. We could also reverse this amyloid state, by adding LPS-binding protein to plasma of these patients37,39,49,56–59. This is consistent with the view that LPS and/or LTA have a causal role in these diseases.
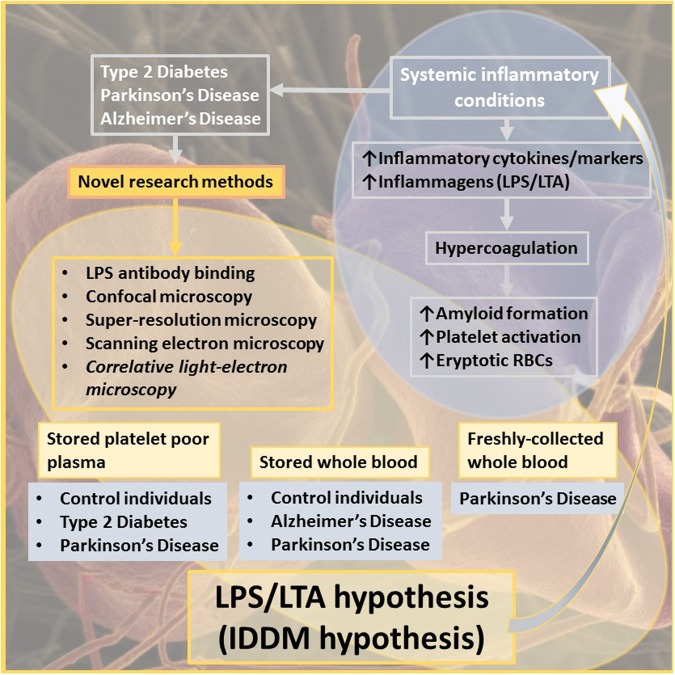
The current ELISA methods to determine LPS concentration, based on a Limulus amoebocyte lysate or its recombinant factor C60, have limitations, as the results can be variable, not least since LPS in plasma is bound to proteins such as apoE61. We therefore investigated a novel fluorescence antibody-based technique to detect and measure the levels or amount of LPS in blood. The current paper focuses on developing this method, by first adding various concentrations of LPS to healthy platelet poor plasma (PPP), enriched in fibrinogen, followed by fluorescence antibody detection. After we optimized this method on healthy PPP with added LPS, we used our technique to detect LPS in PPP and/or whole blood (WB) samples of PD, AD and T2D individuals. We used confocal microscopy and super-resolution structured illumination microscopy (SR-SIM) to visualize antibody binding. Furthermore, we used a novel technique, referred to as correlative light-electron microscopy (CLEM), where samples are imaged separately, first using the fluorescence microscopy modality (confocal or super-resolution), and then using a Shuttle and Find functionality, imaging exactly the same area using a high-resolution scanning electron microscope (SEM)62–64. We could thereby detect an increased presence of LPS in the blood of individuals with PD, AD and T2D, compared to that of healthy individuals. Furthermore, in this paper, Amytracker™ and thioflavin T (ThT) were used to confirm amyloid formation in PD. Previously, we showed amyloid formation with these fluorescent stains in T2D (refer to the link included in the paper to access raw data: https://1drv.ms/f/s!AgoCOmY3bkKHvEigbzhPJ-gPv1Vr)58. We conclude here that this amyloid formation in fibrin(ogen) is, at least in part, a major consequence of the presence of circulating LPS. The corollary of this is that if one could remove or decrease the levels of the circulating LPS, the attendant coagulopathies and hence severity of these diseases might be ameliorated. Figure 1 gives an overview of this paper.
Figure 1.
Overview of this paper, focusing on systemic inflammation in various inflammatory conditions, the presence of inflammagens such as LPS, and its contribution to hypercoagulation and amyloid formation, along with a list of novel research methods employed.
Materials and Methods
Ethical statement
Ethical clearance for the collection of blood from all individuals was obtained from the Health Sciences Ethical Committee of the University of Pretoria, as well as from the Health Research Ethics Committee (HREC) of Stellenbosch University (ethical numbers: 80/2013 and reapproved 2015; 81/2013 and reapproved 2015; 298/2016; 1952 and 6592). Written informed consent was obtained from all individuals (available on request). The methods were carried out in accordance with the approved guidelines. Blood was collected and methods were carried out in accordance with the relevant guidelines of the ethics committee. We adhered strictly to the Declaration of Helsinki.
Sample population
Healthy individuals without known inflammatory conditions and individuals diagnosed with Parkinson’s Disease (PD), Alzheimer’s Disease (AD) and Type 2 Diabetes (T2D) were included in this study. The exclusion criteria for the healthy population included inflammatory conditions such as asthma, smoking, and (if female) being on contraceptive or hormone replacement treatment. These individuals did not use chronic medication nor take any anti-inflammatory medication. The PD individuals were diagnosed by a neurologist with the use of the Unified Parkinson’s Disease Rating Scale (UPDRS)65,66. The AD patients were also diagnosed by a neurologist and individuals with vascular dementia were excluded. Exclusion criteria for the individuals with the various inflammatory conditions were smoking and the use of contraceptives or hormone replacement treatment (if female). Conditions including asthma, human immunodeficiency virus (HIV) and tuberculosis also formed part of the exclusion criteria.
Sample preparation
Whole blood (WB) from ten healthy individuals, eleven individuals diagnosed with PD and ten individuals diagnosed with AD were included in this study. WB was either frozen at −80 °C or used at room temperature, directly after collection. In addition, platelet poor plasma (PPP) was prepared from eleven healthy individuals, eleven individuals diagnosed with PD and ten individuals diagnosed with T2D. WB of all the participants was collected in citrate tubes. PPP was prepared by centrifuging the citrated blood samples at 3000 g for 15 minutes at room temperature (±21 °C), followed by storage at −80 °C.
Confocal microscopy of clots prepared from stored healthy and Parkinson’s Disease (PD) platelet poor plasma (PPP)
Previously, we showed amyloid formation in T2D using the fluorescent stains, thioflavin T (ThT) and Amytracker™ 480 and 680. We also showed amyloid formation in PD, but we only used ThT to show this58,59. In this paper, to confirm the presence of amyloid formation in PD PPP, we used ThT as well as Amytracker™ 480 and 680. On the day of analysis, the −80 °C-stored PPPs were brought to room temperature. ThT (final concentration of 5 µM) and Amytracker™ 480 and 680 (final exposure concentration: 0.1 μL stock solution into 100 μL PPP) were added to the healthy and PD PPP samples, followed by incubation for 30 minutes (protected from light) at room temperature. A small volume (10 µl) of the stained PPP sample was transferred to a microscope slide, after which thrombin (provided by the South African National Blood Service) was added in the ratio 1:2 (5 µL thrombin: 10 µL PPP) and slightly mixed to form a clot and to create extensive fibrin networks. After 30 seconds a coverslip was placed over the prepared clot, and samples were viewed immediately using a Zeiss LSM 780 with ELYRA PS1 confocal microscope with a Plan-Apochromat 63×/1.4 Oil DIC objective. The following settings were used:
For ThT: 488 nm excitation laser, and emission measured at 508–570 nm.
For Amytracker™ 480: 405 nm excitation laser, and emission measured at 478–539 nm.
For Amytracker™ 680: 561 nm excitation laser, with emission measured at 597–695 nm.
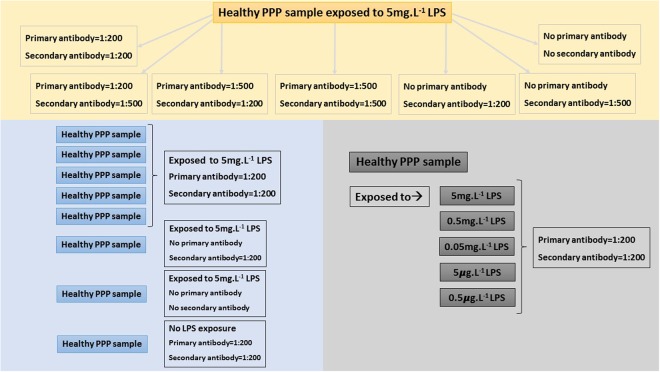
Optimisation of LPS antibody binding by using platelet poor plasma (PPP) from healthy individuals with added LPS
Optimal concentrations of the primary antibody, Anti-E.coli LPS antibody [2D7/1] (mouse monoclonal IgG, ab35654, Abcam), and secondary antibody, Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) (ab150113, Abcam), were determined, using PPP smears from a healthy individual exposed to 5 mg.L−1 LPS (from E. coli O111:B4 (Sigma, L2630)). A primary antibody concentration of 1:200 and secondary antibody concentration of 1:200 gave the optimal fluorescence signal. Secondary antibody and non-stained controls were also included.
We also prepared PPP smears of five more healthy individuals, all exposed to 5 mg.L−1 LPS. Secondary antibody and non-stained controls were also included, as well as a healthy PPP smear with no LPS exposure (negative control). Ultimately, healthy PPP was exposed to decreasing concentrations of LPS, to the point of extinction of any signal. PPP smears from the same healthy individual were exposed to the following LPS concentrations: 5 mg.L−1, 0.5 mg.L−1, 0.05 mg.L−1, 5 μg.L−1 and 0.5 μg.L−1 respectively. See Fig. 2 for an overview of the optimisation of LPS antibody binding.
Figure 2.
Overview of optimization of LPS antibody binding, using healthy platelet poor plasma (PPP) samples exposed to LPS.
Fluorescent detection of LPS in platelet poor plasma (PPP) and whole blood (WB) (freshly collected and/or stored) from healthy individuals and individuals diagnosed with Parkinson’s Disease (PD), Alzheimer’s Disease (AD) and Type 2 Diabetes (T2D)
A smear of either PPP or WB was prepared on a microscope slide, using 5 μL of PPP or WB. The slide was then air dried for 45 minutes. The sample was fixed with 10% neutral buffered formalin (NBF) for 3–5 minutes, followed by three GibcoTM phosphate-buffered saline (PBS) (pH = 7.4) washes. Next, the sample was blocked with 5% goat serum (prepared in PBS), after which it was stained with primary antibody (1:200 prepared in blocking buffer), Anti-E.coli LPS antibody [2D7/1] (mouse monoclonal IgG, ab35654, Abcam), for one hour at room temperature. After the sample was again washed three times with PBS, the sample was stained with secondary antibody (1:200 prepared in PBS), Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) (ab150113, Abcam), for one hour at room temperature in the dark. Ultimately, following the last wash step, a coverslip was mounted with a drop of Dako fluorescence mounting medium.
The prepared smears were stored at −20 °C (protected from light), and viewed using a Zeiss LSM 780 with ELYRA PS1 confocal microscope with a Plan-Apochromat 63x/1.4 Oil DIC objective. The 488 nm Argon excitation laser was used, with emission measured with a GaAsP detector at 493–630 nm.
Correlative light-electron microscopy (CLEM)
CLEM is a novel, albeit expensive and tedious procedure, where a confocal micrograph is correlated on a scanning electron microscope (SEM) micrograph, via a shuttle-and-find system62–64. Therefore, we selected stored healthy WB, stored PPP from T2D and PD, as well as freshly-collected PD WB. For CLEM preparation, the same procedure was followed as described above, but a smear of 3 μL PPP or WB was made on a cover slip instead of a microscope slide. The prepared smear was stored at 4 °C overnight, immersed in double distilled H20.
The coverslip was mounted in the Shuttle and Find Coverslip holder (Zeiss, Germany), which is equipped with a marked coordinate system. Prior to imaging the sample, the microscope is calibrated according to these coordinates, using the Shuttle and Find modality of the ZEN 2012 software (Zeiss, Germany).
For confocal imaging, the microscope setup which was used for quantification remained the same for CLEM. However, for improved resolution and subsequent correlation, the PD WB (fresh) sample was imaged with the super-resolution structured illumination microscopy (SR-SIM) platform. A 488 nm 100 mW laser was for excitation and emission detected with a BP 495–550 filter and captured with an Andor EM-CCD camera iXon DU 885 for SIM. Z-stack micrographs were processed with the ZEN 2012 software, applying an optimised noise filtering algorithm.
Following capturing of the fluorescence micrographs, SEM sample preparation was immediately performed with the cover slip still mounted in the Shuttle and Find Coverslip Holder. Firstly, the sample was fixed with 4% paraformaldehyde in PBS for 30 minutes. The sample was then washed three times (three minutes each) with PBS and incubated with 1% osmium tetroxide in double distilled H20 for 15 minutes. Following fixation, the sample was again washed three times with PBS. The sample was dehydrated using a standard series of ethanol dilutions: 30%, 50%, 70%, 90% and 100% (3x) for three minutes each. The sample was then covered with 99.9% hexamethyldisilazane (HMDS) for 30 minutes to complete sample dehydration. Ultimately, one final drop of HMDS was directly placed onto the sample, after which the sample was left to air dry in a fume hood overnight (±16 hours).
The sample, still mounted in the sample holder, was coated with a thin (~5 nm thick) layer of carbon prior to analysis, using a Quorum Q150T coater by performing carbon rod evaporation. SEM ultrastructural analysis of the selected samples was performed on the Zeiss MERLINTM field emission scanning electron microscope located in the Central Analytical Facility (CAF) Electron Microbeam Unit, Stellenbosch University. The microscope was first calibrated according to the coordinates of the sample holder after which the areas of interest were located using the Shuttle and Find modality of the ZEN 2012 software. Micrographs were captured with the SmartSEM software (Zeiss, Germany), using high resolution InLens capabilities at 1 kV accelerating voltage of the beam, a working distance of 4.2 mm and a beam current of 86 pA.
After the SEM micrographs were captured, they were overlaid with the fluorescence images with the Shuttle and Find functionality, by identification of three identical points in the SEM and fluorescence micrographs respectively.
Statistical analysis
Data were analysed and tested for normality using the Shapiro-Wilk normality test. To quantify LPS antibody binding in healthy, PD and T2D PPP, the mean fluorescence intensity of all the images was determined in ImageJ (FIJI)67. Analyses were performed using either the Welch’s t test or Mann-Whitney test (depending on the normality of the data), in GraphPad/Prism v7. Statistical significance was accepted at P < 0.05.
Ethics approval and consent to participate
Ethical clearance was obtained from the Health Sciences Ethical Committee of the University of Pretoria (Ethics References: 80/2013 and reapproved 2015; 81/2013 and reapproved 2015; 298/2016), as well as from the Health Research Ethics Committee (HREC) of Stellenbosch University (Ethics References: 1952 and 6592). A written form of informed consent was obtained from all donors. Blood was collected and methods were carried out in accordance with the relevant guidelines of the ethics committees. We adhered strictly to the Declaration of Helsinki.
Results
Healthy individuals and individuals diagnosed with PD, AD and T2D were age-correlated and participants included both genders (Table 1). The Hoehn and Yahr scale (used to identify or rate the severity of PD), as well as the HbA1c levels for the individuals diagnosed with T2D are indicated in Table 1.
Table 1.
Demographics of the individuals included in this study. Data expressed as mean ± SEM.
| Sample type | Stored platelet poor plasma | ||
|---|---|---|---|
| Healthy individuals (n = 11) | Type 2 Diabetes individuals (n = 10) | Parkinson’s Disease individuals (n = 11) | |
| Gender | Male (n = 5); Female (n = 6) | Male (n = 5); Female (n = 5) | Male (n = 8); Female (n = 3) |
| Age (years) | 48 ± 3.240 (n = 11) | 57.5 [51–67.5]* (n = 10) | 71 ± 1.404 (n = 11) |
| HbA1c (%) | 7.4 ± 0.456 (n = 10) | ||
| Hoehn and Yahr scale | 2.5 [2.5–3]* (n = 11) | ||
| Sample type | Stored whole blood | ||
| Healthy individuals (n = 10) | Alzheimer’s Disease individuals (n = 10) | Parkinson’s Disease individuals (n = 10) | |
| Gender | Male (n = 2); Female (n = 8) | Male (n = 7); Female (n = 3) | Male (n = 7); Female (n = 3) |
| Age (years) | 68 ± 5.696 (n = 10) | 66 ± 4.740 (n = 10) | 71 ± 1.133 (n = 10) |
| Hoehn and Yahr scale | 1.5 (n = 10) | ||
| Sample type | Freshly-collected whole blood | ||
| Parkinson’s Disease individual (n = 1) | |||
| Gender | Female | ||
| Age (years) | 69 | ||
| Hoehn and Yahr scale | 2 | ||
*Data expressed as median and [interquartile ranges].
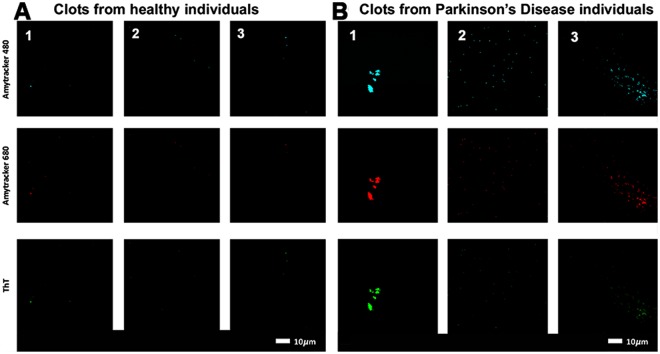
The pathologic assembly of amyloidogenic fibrin(ogen) is a direct cause of abnormal clotting or hypercoagulation. We therefore investigated amyloid formation in PPP clots, which reflects pathological clotting. Representative confocal micrographs of (A) clots prepared from PPP of healthy individuals versus (B) clots prepared from PPP of individuals diagnosed with PD are shown in Fig. 3. PPP was incubated with three specific amyloidogenic fluorescent markers, thioflavin T (binding to open hydrophobic areas on fibrin) and Amytracker™ 480 and 680 (staining classical amyloid structures)68.
Figure 3.
Typical range of confocal micrographs of platelet poor plasma (PPP) with added thrombin, showing the fluorescence amyloid signal of (A) healthy individuals and (B) Parkinson’s Disease (PD) individuals. Platelet poor plasma (PPP) from each individual was incubated with three specific amyloidogenic fluorescent markers, thioflavin T and Amytracker™ 480 and 680.
The fluorescence amyloid signal in samples taken from PD individuals, in comparison with those from healthy individuals, was greatly enhanced. This suggests that there is more amyloid when clots are formed with fibrin(ogen) of PD individuals than with fibrin(ogen) of healthy individuals. This was also previously found in fibrin(ogen) of individuals diagnosed with T2D and AD57–59,69.
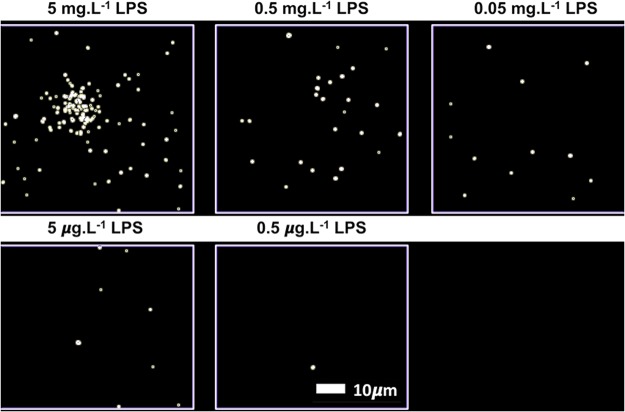
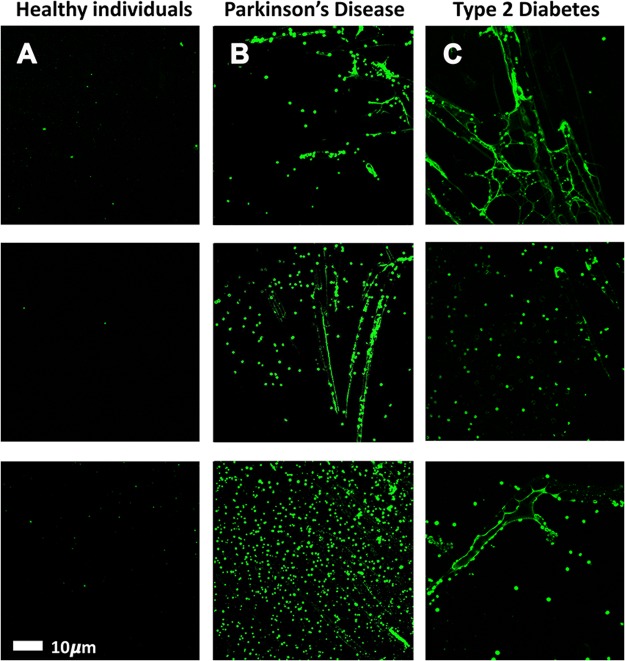
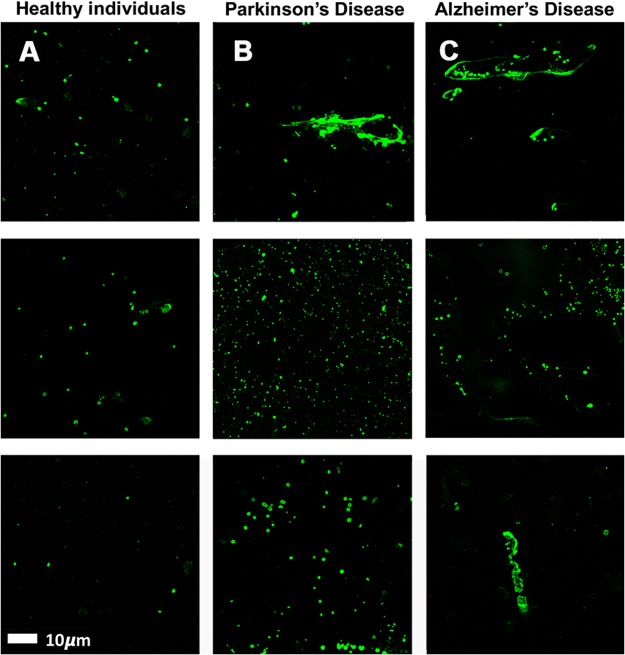
PPP smears from the same healthy individual were exposed to decreasing LPS concentrations. This formed part of optimizing the method for determining the presence of LPS in PPP, via anti-E.coli LPS antibody and a secondary antibody (see Fig. 4 for examples). So far as we are aware, this has not previously been done in PPP. We studied antibody binding in PPP from healthy individuals, PD individuals and T2D individuals (see Fig. 5 for representative micrographs). Figure 6 illustrates the distribution of the (normalised) mean fluorescence intensity of the confocal micrographs of the healthy, PD and T2D PPP. There is a significant increase in the fluorescence LPS signal in PPP from PD individuals, in comparison with healthy individuals (P value < 0.0001). There is also a significant increase in antibody binding in PPP taken from T2D individuals, in comparison with those of healthy individuals (P value < 0.0001). See Fig. 7 for representative confocal micrographs of antibody binding in WB from healthy individuals, PD individuals and AD individuals. This is a confirmation that LPS can be detected with antibody staining in both PPP and WB samples.
Figure 4.
Representative confocal micrographs of healthy platelet poor plasma (PPP) with added LPS to show optimization of detection of LPS, by using anti-E.coli LPS antibody and a secondary antibody (1:200 dilution for primary and secondary antibodies). We used five different LPS concentrations, and estimated that 0.5 μgL−1 LPS is the lowest detectable concentration. For clarity, we inverted the micrographs, followed by applying the “find edges” function in ImageJ (FIJI), to show the decreasing fluorescence LPS signal with decreasing concentrations added.
Figure 5.
Representative platelet poor plasma (PPP) smears with added anti-E.coli LPS antibody and secondary antibody, from (A) healthy individuals, (B) Parkinson’s Disease (PD) individuals and (C) Type 2 Diabetes (T2D) individuals (1:200 dilution for primary and secondary antibodies).
Figure 6.
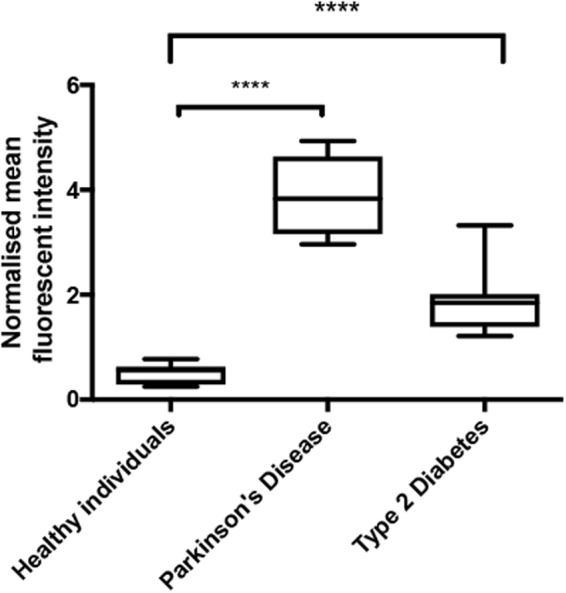
Boxplot of the distribution of the mean fluorescence intensity, normalised by dividing the mean fluorescence intensity values of the healthy individuals, Parkinson’s Disease (PD) individuals and Type 2 Diabetes (T2D) individuals, by the mean fluorescence intensity values of the corresponding secondary antibody control. In this way, we accounted for non-specific secondary antibody binding; ****P < 0.0001.
Figure 7.
Representative whole blood (WB) smears with added anti-E.coli LPS antibody and secondary antibody, from (A) healthy individuals, (B) Parkinson’s Disease (PD) individuals and (C) Alzheimer’s Disease (AD) individuals (1:200 dilution for primary and secondary antibodies).
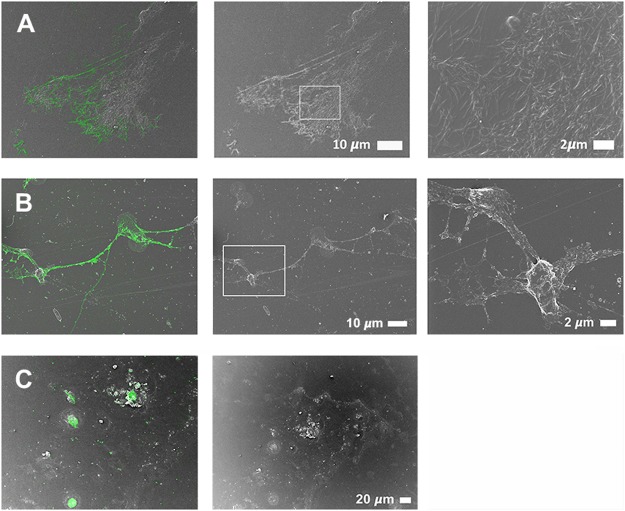
CLEM, which allows the overlay of a confocal or super-resolution (fluorescence) micrograph onto a scanning electron microscope micrograph, by using a shuttle-and-find technique, was employed in order to indicate the location of the fluorescence LPS signal on the actual ultrastructure of the samples analysed. The fluorescence signal shows the presence of LPS in the PPP or WB, by direct antibody binding, while SEM shows the ultrastructure of the fibrin(ogen) or cellular structure. CLEM analysis therefore pinpoints the antibody-bound fluorescence signal to a specific area on the ultrastructure. Importantly, SR-SIM allows a higher resolution micrograph than confocal microscopy. In the CLEM analysis, we found that the fluorescence LPS signal is merged and fused into the dense matted fibrin(ogen) deposits, and we suggest that this incorporation of LPS into the fibrin(ogen) strands is an additional spatial and visual confirmation of the extent to which LPS is the causative agent of the presence of amyloid in inflammatory conditions such as PD, AD and T2D. Figure 8A shows a correlative micrograph of confocal and SEM micrographs overlaid of PPP from a T2D patient, while a correlative micrograph of SR-SIM and SEM micrographs overlaid of freshly-collected WB from a PD patient are shown in Fig. 8B. Due to the limit of resolution with confocal microscopy, the localisation of LPS seemed to be extended beyond that of the fibres (see Fig. 8A). With the improved resolution of SR-SIM, we showed that the LPS signal clearly correlates with the fibre structures, showing that LPS is closely associated with the actual fibres (see also Fig. 9). Citrated WB from a PD patient was also analysed (Fig. 8C). The storage method of the sample caused cell lysis and only cellular remnants are thus visible. Correlation of confocal and SEM micrographs illustrates that LPS antibody staining localises with the cellular content. Furthermore, SR-SIM, SEM and correlated micrographs of freshly-collected WB from a PD patient show that the fibrin(ogen) fibres are forming a network between and on top of the red blood cells (Fig. 9). The nature of the fibrin(ogen) fibres demonstrates hypercoagulability, with LPS antibody staining located in the same places as where amyloid fibrils may be observed. The binding of the antibody to fibre-like structures suggests that LPS plays a role in anomalous blood clotting.
Figure 8.
Representative CLEM and SEM micrographs of (A) stored platelet poor plasma (PPP) from a Type 2 Diabetes (T2D) individual, (B) freshly-collected whole blood (WB) from a Parkinson’s Disease (PD) individual and (C) stored whole blood (WB) from a Parkinson’s Disease (PD) individual (1:200 dilution for primary and secondary antibodies). The fluorescence microscopy modalities used were super-resolution (SR-SIM) for (B) and confocal for (A) and (C).
Figure 9.
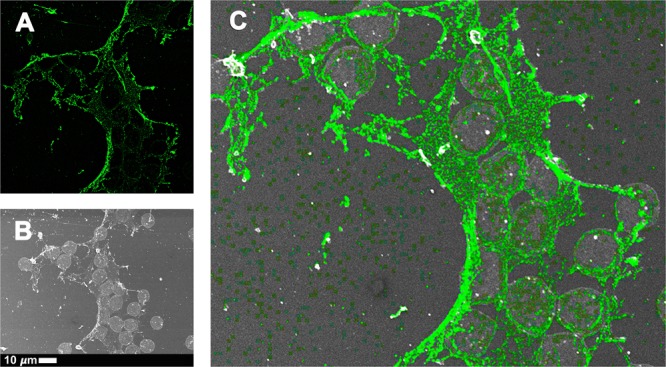
Representative (A) super-resolution (SR-SIM), (B) SEM and (C) CLEM micrographs of freshly-collected whole blood (WB) from a Parkinson’s Disease (PD) individual (1:200 dilution for primary and secondary antibodies). LPS antibody staining is closely associated with the fibre-like structures. Micrograph C colour was enhanced for publication clarity, by adjusting the vibrancy, brightness and contrast in Adobe Photoshop CS6.
Discussion
Previously, we showed that the addition of highly substoichiometric levels of LPS caused fibrin(ogen) proteins in whole blood and platelet poor plasma from healthy individuals to polymerize into an amyloid form, with a greatly increased amount of ß-sheets. Healthy fibrin(ogen) protein structure shows more α-coils and fewer ß-sheets, but the addition of LPS changes the protein conformation37–39. This structural change both causes and reflects pathological clotting. We have also shown that in conditions like PD, AD and T2D, where there is an intrinsic, increased presence of circulating inflammagens, and an upregulation of inflammatory biomarkers (including various pro-inflammatory cytokines), pathological clotting is present30,36,38,49,50,56,58,70. We could show that such aberrant clotting is accompanied by amyloid formation in the fibrin(ogen) of these patients, and that this aberrant clotting can be reversed by adding LPS-binding protein57–59,69. In this paper, we confirmed the presence of increased amyloid in PD plasma (Fig. 3).
Extremely low levels of LPS can thus cause a cascade of events that lead to pathological clotting, and in this paper we sought to use a direct fluorescence LPS antibody-based technique to detect the presence of LPS in PD, AD and T2D. We found increased binding of antibody compared to controls. The binding of the antibody to fibre-like structures in the clots was further investigated using CLEM technology. With CLEM, it was confirmed that LPS strongly associated with all fibres in the field of view (Figs 8 and 9). This is the first use of such correlative microscopy to investigate the presence and location of LPS in WB and PPP. Super-resolution microscopy (Fig. 9), with its improved resolution, showed that the LPS signal clearly correlates with the fibre structures.
The specificity of antibodies is always a cause for concern71,72. However, cross-reactivity of the anti-E.coli LPS antibody (ab35654) was absent or minimal, as evident from the healthy controls in our experiments, as little LPS presence was detected. Because anti-E. coli LPS (ab35654) is a mouse monoclonal antibody, it will detect a specific epitope. Results obtained by Zhan and co-workers in 2016 also suggest little cross-reactivity with human proteins73. They found that LPS colocalized with Aβ1–40/42 in amyloid plaques and with Aβ1-40/42 around vessels in AD brains. DNA sequencing confirmed E. coli DNA in human control and AD brains73. Furthermore, based on the staining pattern found in rat intestine, this antibody does not seem to cross-react with other mammalian membrane proteins (see website link: http://www.abcam.com/E-coli-LPS-antibody-2D71-ab35654/reviews/24711). Grover and co-workers in 2012 also used ab35654 on yeast lysates and found no cross-reactive band in these cells74. Considering this, we suggest that our LPS antibody binding results are not influenced by cross-reactivity issues.
In conclusion, we showed that LPS is present in PPP and WB of patients with PD, AD and T2D; most importantly CLEM confirmed that it is closely associated in the fibrin fibre strands. Therefore we suggest that LPS could (at least in part) be a primary causative agent of abnormal clotting in PD, AD and T2D. This is a further confirmation that bacterial inflammagens can fuel the inflammatory processes in both systemic and neuroinflammatory conditions. It also implies strongly that decreasing (or removing completely) the availability of ‘free’ LPS could provide significant clinical and therapeutic benefits.
Acknowledgements
Funders include the Biotechnology and Biological Sciences Research Council (grant BB/L025752/1) as well as the National Research Foundation (NRF) of South Africa (91548: Competitive Program) and the Medical Research Council of South Africa (MRC) (Self-Initiated Research Program) for supporting this collaboration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This is paper 17 in the series “a dormant blood microbiome in chronic, inflammatory diseases”.
Author Contributions
G.d.W.: Confocal analysis, SEM, statistical analysis, co-writing of paper; L.E.: technical assistance with confocal and CLEM, editing of paper; T.D.: developing of antibody protocol; W.d.V.: editing of paper; D.B.K.: editing of paper; E.P.: study leader and corresponding author, writing of paper.
Data Availability
Raw data, including original micrographs can be accessed at: https://1drv.ms/f/s!AgoCOmY3bkKHiM5kihHc4y7AfUm4HQ and on https://www.researchgate.net/profile/Etheresia_Pretorius.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Marshall BJ. Helicobacter pylori. The American journal of gastroenterology. 1994;89:S116–128. [PubMed] [Google Scholar]
- 3.De Chiara G, et al. Infectious agents and neurodegeneration. Molecular neurobiology. 2012;46:614–638. doi: 10.1007/s12035-012-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itzhaki RF. Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Frontiers in aging neuroscience. 2014;6:202. doi: 10.3389/fnagi.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itzhaki RF, Wozniak MA. Alzheimer’s disease, the neuroimmune axis, and viral infection. Journal of neuroimmunology. 2004;156:1–2. doi: 10.1016/j.jneuroim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Itzhaki RF, Wozniak MA. Could antivirals be used to treat Alzheimer’s disease? Future microbiology. 2012;7:307–309. doi: 10.2217/fmb.12.10. [DOI] [PubMed] [Google Scholar]
- 7.Itzhaki RF, et al. Microbes and Alzheimer’s Disease. Journal of Alzheimer’s disease: JAD. 2016;51:979–984. doi: 10.3233/jad-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balin BJ, Appelt DM. Role of infection in Alzheimer’s disease. The Journal of the American Osteopathic Association. 2001;101:S1–6. [PubMed] [Google Scholar]
- 9.Balin BJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Medical microbiology and immunology. 1998;187:23–42. doi: 10.1007/s004300050071. [DOI] [PubMed] [Google Scholar]
- 10.Balin BJ, et al. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2008;13:371–380. doi: 10.3233/JAD-2008-13403. [DOI] [PubMed] [Google Scholar]
- 11.Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease–role of Spirochetes. Journal of Alzheimer’s disease: JAD. 2008;13:381–391. doi: 10.3233/JAD-2008-13404. [DOI] [PubMed] [Google Scholar]
- 12.Miklossy J. Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. Journal of neuroinflammation. 2011;8:90. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miklossy J. Emerging roles of pathogens in Alzheimer disease. Expert reviews in molecular medicine. 2011;13:e30. doi: 10.1017/s1462399411002006. [DOI] [PubMed] [Google Scholar]
- 14.Miklossy J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Frontiers in aging neuroscience. 2015;7:46. doi: 10.3389/fnagi.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheperjans Filip, Aho Velma, Pereira Pedro A. B., Koskinen Kaisa, Paulin Lars, Pekkonen Eero, Haapaniemi Elena, Kaakkola Seppo, Eerola-Rautio Johanna, Pohja Marjatta, Kinnunen Esko, Murros Kari, Auvinen Petri. Gut microbiota are related to Parkinson's disease and clinical phenotype. Movement Disorders. 2014;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 16.Fasano A, et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2013;28:1241–1249. doi: 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]
- 17.Liu M, Bing G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s disease. 2011;2011:327089. doi: 10.4061/2011/327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tufekci KU, Genc S, Genc K. The endotoxin-induced neuroinflammation model of Parkinson’s disease. Parkinson’s disease. 2011;2011:487450. doi: 10.4061/2011/487450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsyth CB, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabrielli M, et al. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2011;26:889–892. doi: 10.1002/mds.23566. [DOI] [PubMed] [Google Scholar]
- 21.Tan AH, et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism & related disorders. 2014;20:535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Grosicki GJ, Fielding RA, Lustgarten MS. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcified tissue international. 2018;102:433–442. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amar J, et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: the D.E.S.I.R. study. PLoS One. 2013;8:e54461. doi: 10.1371/journal.pone.0054461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amar J, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54:3055–3061. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- 25.Sato J, et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes care. 2014;37:2343–2350. doi: 10.2337/dc13-2817. [DOI] [PubMed] [Google Scholar]
- 26.Kaprelyants AS, Kell DB. Dormancy in Stationary-Phase Cultures of Micrococcus luteus: Flow Cytometric Analysis of Starvation and Resuscitation. Applied and environmental microbiology. 1993;59:3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaprelyants AS, Gottschal JC, Kell DB. Dormancy in non-sporulating bacteria. FEMS microbiology reviews. 1993;10:271–285. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- 28.Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS microbiology reviews. 2015;39:567–591. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kell DB, Potgieter M, Pretorius E. Individuality, phenotypic differentiation, dormancy and ‘persistence’ in culturable bacterial systems: commonalities in environmental, laboratory, and clinical microbiology. F1000Review. 2015;4:179. doi: 10.12688/f1000research.6709.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kell Douglas B., Pretorius Etheresia. No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biological Reviews. 2018;93(3):1518–1557. doi: 10.1111/brv.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC medical genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kell DB. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Archives of toxicology. 2010;84:825–889. doi: 10.1007/s00204-010-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freestone PP, et al. The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. Journal of bacteriology. 2000;182:6091–6098. doi: 10.1128/JB.182.21.6091-6098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kell DB, Kenny LC. A Dormant Microbial Component in the Development of Preeclampsia. Frontiers in medicine. 2016;3:60. doi: 10.3389/fmed.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenny LC, Kell DB. Immunological tolerance, pregnancy and pre-eclampsia: the roles of semen microbes and the father. Front Med Obs Gynecol. 2018;4:239. doi: 10.3389/fmed.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kell DB, Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen) Integrative Biology. 2015;7:24–52. doi: 10.1039/C4IB00173G. [DOI] [PubMed] [Google Scholar]
- 37.Kell DB, Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progress in biophysics and molecular biology. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Pretorius Etheresia, Mbotwe Sthembile, Bester Janette, Robinson Christopher J., Kell Douglas B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. Journal of The Royal Society Interface. 2016;13(122):20160539. doi: 10.1098/rsif.2016.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pretorius E, et al. Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. Royal Society Interface. 2018;15:20170941. doi: 10.1098/rsif.2017.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lester PA, et al. Inflammation and thrombosis: new insights. Frontiers in bioscience (Scholar edition) 2012;4:620–638. doi: 10.2741/s289. [DOI] [PubMed] [Google Scholar]
- 41.Qadri SM, Donkor DA, Nazy I, Branch DR, Sheffield WP. Bacterial neuraminidase-mediated erythrocyte desialylation provokes cell surface aminophospholipid exposure. European journal of haematology. 2018;100:502–510. doi: 10.1111/ejh.13047. [DOI] [PubMed] [Google Scholar]
- 42.Qadri SM, et al. Phosphatidylserine externalization and procoagulant activation of erythrocytes induced by Pseudomonas aeruginosa virulence factor pyocyanin. Journal of cellular and molecular medicine. 2016;20:710–720. doi: 10.1111/jcmm.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qadri SM, Bissinger R, Solh Z, Oldenborg PA. Eryptosis in health and disease: A paradigm shift towards understanding the (patho)physiological implications of programmed cell death of erythrocytes. Blood reviews. 2017;31:349–361. doi: 10.1016/j.blre.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Pretorius E. Erythrocyte deformability and eryptosis during inflammation, and impaired blood rheology. Clinical hemorheology and microcirculation. 2018;69:545–550. doi: 10.3233/ch-189205. [DOI] [PubMed] [Google Scholar]
- 45.Kempe-Teufel DS, et al. Cellular markers of eryptosis are altered in type 2 diabetes. Clinical chemistry and laboratory medicine. 2018;56:e177–e180. doi: 10.1515/cclm-2017-1058. [DOI] [PubMed] [Google Scholar]
- 46.Bester J, Pretorius E. Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. doi: 10.1038/srep32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolay JP, et al. Cellular stress induces erythrocyte assembly on intravascular von Willebrand factor strings and promotes microangiopathy. Sci Rep. 2018;8:10945. doi: 10.1038/s41598-018-28961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pretorius E, et al. Eryptosis as a marker of Parkinson’s disease. Aging. 2014;6:788–819. doi: 10.18632/aging.100695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pretorius E, et al. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovascular Diabetology. 2015;14:30. doi: 10.1186/s12933-015-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bester J, Soma P, Kell DB, Pretorius E. Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia, and the possible role of bacterial lipopolysaccharides (LPS) Oncotarget. 2015;6:35284–35303. doi: 10.18632/oncotarget.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bester J, Buys AV, Lipinski B, Kell DB, Pretorius E. High ferritin levels have major effects on the morphology of erythrocytes in Alzheimer’s disease. Frontiers in aging neuroscience. 2013;5:88. doi: 10.3389/fnagi.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levi M, van der Poll T. Inflammation and coagulation. Critical care medicine. 2010;38:S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 53.van der Poll T, de Boer JD, Levi M. The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis. 2011;24:273–278. doi: 10.1097/QCO.0b013e328344c078. [DOI] [PubMed] [Google Scholar]
- 54.Maino A, Rosendaal FR, Algra A, Peyvandi F, Siegerink B. Hypercoagulability Is a Stronger Risk Factor for Ischaemic Stroke than for Myocardial Infarction: A Systematic Review. PLoS One. 2015;10:e0133523. doi: 10.1371/journal.pone.0133523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegerink B, Maino A, Algra A, Rosendaal FR. Hypercoagulability and the risk of myocardial infarction and ischemic stroke in young women. Journal of thrombosis and haemostasis: JTH. 2015;13:1568–1575. doi: 10.1111/jth.13045. [DOI] [PubMed] [Google Scholar]
- 56.Pretorius E, Bester J, Kell DB. A Bacterial Component to Alzheimer’s-Type Dementia Seen via a Systems Biology Approach that Links Iron Dysregulation and Inflammagen Shedding to Disease. Journal of Alzheimer’s disease: JAD. 2016;53:1237–1256. doi: 10.3233/jad-160318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pretorius E, Mbotwe S, Kell DB. Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular co-morbidities. Sci Rep. 2017;7:9680. doi: 10.1038/s41598-017-09860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pretorius E, Page MJ, Engelbrecht L, Ellis GC, Kell DB. Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovascular diabetology. 2017;16:141. doi: 10.1186/s12933-017-0624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pretorius E, Page MJ, Mbotwe S, Kell DB. Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease: implications. PlosOne. 2018;13:e0192121. doi: 10.1371/journal.pone.0192121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding JL, Ho B. Endotoxin detection–from limulus amebocyte lysate to recombinant factor C. Sub-cellular biochemistry. 2010;53:187–208. doi: 10.1007/978-90-481-9078-2_9. [DOI] [PubMed] [Google Scholar]
- 61.Kell DB, Pretorius E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr Biol. 2015;7:1339–1377. doi: 10.1039/C5IB00158G. [DOI] [PubMed] [Google Scholar]
- 62.Goetz JG, Monduc F, Schwab Y, Vermot J. Using correlative light and electron microscopy to study zebrafish vascular morphogenesis. Methods in molecular biology (Clifton, N.J.) 2015;1189:31–46. doi: 10.1007/978-1-4939-1164-6_3. [DOI] [PubMed] [Google Scholar]
- 63.López CS, et al. A fully integrated, three-dimensional fluorescence to electron microscopy correlative workflow. Methods in cell biology. 2017;140:149–164. doi: 10.1016/bs.mcb.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Onouchi T, Shiogama K, Mizutani Y, Takaki T, Tsutsumi Y. Visualization of Neutrophil Extracellular Traps and Fibrin Meshwork in Human Fibrinopurulent Inflammatory Lesions: III. Correlative Light and Electron Microscopic Study. Acta histochemica et cytochemica. 2016;49:141–147. doi: 10.1267/ahc.16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.UPDRS. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Movement disorders: official journal of the Movement Disorder Society18, 738–750, 10.1002/mds.10473 (2003). [DOI] [PubMed]
- 66.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders: official journal of the Movement Disorder Society. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 67.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pretorius, E. et al. Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: Assessment with novel AmytrackerTM stains. Vol. 15 (2018). [DOI] [PMC free article] [PubMed]
- 69.Pretorius, E., Page, M. J., Bester, J. & Kell, D. B. Reversal of amyloid formation in the plasma fibrin of individuals with Alzheimer-type dementia using LPS-binding protein. Frontiers Aging Neurosci. 2018 Aug 22;10:257. 10.3389/fnagi.2018.00257.eCollection (2018). [DOI] [PMC free article] [PubMed]
- 70.Pretorius E, et al. Eryptosis as a marker of Parkinson’s disease. Aging-US. 2014;6:788–818. doi: 10.18632/aging.100695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker M. Reproducibility crisis: Blame it on the antibodies. Nature. 2015;521:274–276. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- 72.Uhlen M, et al. A proposal for validation of antibodies. Nature methods. 2016;13:823–827. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhan X, et al. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. doi: 10.1212/wnl.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grover RK, et al. The costimulatory immunogen LPS induces the B-Cell clones that infiltrate transplanted human kidneys. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6036–6041. doi: 10.1073/pnas.1202214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data, including original micrographs can be accessed at: https://1drv.ms/f/s!AgoCOmY3bkKHiM5kihHc4y7AfUm4HQ and on https://www.researchgate.net/profile/Etheresia_Pretorius.