Abstract
Key points
A new caged nicotinic acetylcholine receptor (nAChR) agonist was developed, ABT594, which is photolysed by one‐ and two‐photon excitation.
The caged compound is photolysed with a quantum yield of 0.20.
One‐photon uncaging of ABT594 elicited large currents and Ca2+ transients at the soma and dendrites of medial habenula (MHb) neurons of mouse brain slices.
Unexpectedly, uncaging of ABT594 also revealed highly Ca2+‐permeable nAChRs on axons of MHb neurons.
Abstract
Photochemical release of neurotransmitters has been instrumental in the study of their underlying receptors, with acetylcholine being the exception due to its inaccessibility to photochemical protection. We caged a nicotinic acetylcholine receptor (nAChR) agonist, ABT594, via its secondary amine functionality. Effective photolysis could be carried out using either one‐ or two‐photon excitation. Brief flashes (0.5–3.0 ms) of 410 nm light evoked large currents and Ca2+ transients on cell bodies and dendrites of medial habenula (MHb) neurons. Unexpectedly, photorelease of ABT594 also revealed nAChR‐mediated Ca2+ signals along the axons of MHb neurons.
Keywords: medial habenula, uncaging, acetylcholine
Key points
A new caged nicotinic acetylcholine receptor (nAChR) agonist was developed, ABT594, which is photolysed by one‐ and two‐photon excitation.
The caged compound is photolysed with a quantum yield of 0.20.
One‐photon uncaging of ABT594 elicited large currents and Ca2+ transients at the soma and dendrites of medial habenula (MHb) neurons of mouse brain slices.
Unexpectedly, uncaging of ABT594 also revealed highly Ca2+‐permeable nAChRs on axons of MHb neurons.
Introduction
Caged neurotransmitters have been widely used as optical probes for understanding the chemical biology of transmitter receptors on neurons and glia (Eder et al. 2004; Ellis‐Davies, 2007, 2013). Biologically inert before irradiation, a brief flash of light releases a natural ligand that activates native signalling cascades in target cells. Almost all of the most important neurotransmitters (glutamate, GABA, glycine, dopamine, serotonin, etc.) have been studied using this method (Ellis‐Davies, 2013), with the exception being acetylcholine. The reasons for this exception lie in the chemical structure of acetylcholine itself: all heteroatom valences are fully saturated. Therefore, chemical biologists have turned to caged drug analogues as surrogates to enable photostimulation of the important receptor classes that respond to acetylcholine (Brieke et al. 2012; Hansen et al. 2015). In fact ortho‐nitrobenzyl (NB)‐carbamoylcholine was the first ‘caged neurotransmitter’ to be reported, as early as 1986 (Walker et al. 1986a). This optical probe proved useful for the study of the neurophysiology of acetylcholine (Khiroug et al. 2003; Yakel, 2013). However, carbamoylcholine, like acetylcholine, activates both ionotropic and G‐protein‐coupled receptors, but with lower affinity than the native ligand, thus requiring the use of NB‐carbamoylcholine at high probe concentration. Given that NB‐carbamoylcholine is antagonistic towards nicotinic acetylcholine receptors (nAChRs) (Walker et al. 1986a) and requires photolysis with ultraviolet light (Walker et al. 1986a), we chose to develop a new caged drug (ABT594) for the selective photostimulation of nAChRs with visible light. ABT594 has a high affinity for α3/4‐containing nAChRs while being much less potent at α7 homomeric receptors (Donnelly‐Roberts et al. 1998).
In the CNS, neurons of the medial habenula (MHb) exhibit very high expression levels of nAChRs, especially of the subunits α3/4 and β2/4 (Shih et al. 2014). The MHb is a small brain region in the epithalamus sending long‐range axonal projections via the fasciculus retroflexus to the interpeduncular nucleus (IPN). Considering its high expression of nAChRs, it is not surprising that this pathway has been implicated among others in nicotine addiction (De Biasi & Dani, 2011). However, due to lack of appropriate tools, the subcellular distribution of functional nAChRs in MHb neurons has not been investigated so far. Therefore, we developed a nitrobenzyl‐caged ABT594 and validated its effectiveness on MHb neurons by local uncaging. Uncaging combined with two‐photon Ca2+ imaging revealed the presence of highly Ca2+‐permeable nAChRs on the soma and dendrites of MHb neuron in living mouse brain slices. Unexpectedly, use of our new optical probe revealed nAChR‐mediated Ca2+ transients in axons of these neurons. These data show that caged ABT594 is well suited to photostimulation of nAChRs and can enable discovery of potentially new forms of Ca2+ signalling.
Methods
Ethical approval
All animal experiments were conducted according to Mount Sinai IACUC (protocol number LA10‐00201). Mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and bred in‐house. Animals were housed on a 12 h light–dark cycle and allowed free access to food and water. Mice were deeply anaesthetized with isoflurane and then euthanized by decapitation. Experiments were carried out on 3‐ to 7‐week‐old C57BL/6J mice of both sexes.
Synthesis of caged compounds
All chemicals were purchased from commercial sources unless otherwise noted. Reactions were monitored by thin‐layer chromatography on Merck KGaA glass silica gel plates (60 F254, New York, NY, USA) and were visualized with UV light or potassium permanganate staining followed by heating. Flash chromatography was performed using Agela Technologies industrial grade silica (200‐300 mesh, 40–60 μm, Willmington, DE, USA). HPLC analyses were performed on a Waters Acquity Arc system with a 2998 PDA Detector (Milford, MA, USA). Proton nuclear magnetic resonance (1H‐NMR) spectra were recorded on an Varian 300 MHz NMR spectrometer (Varian, Palo Alto, CA, USA) and the chemical shifts are reported in ppm using the solvent peak as the internal standard (CDCl3 at 7.26 ppm). Peaks are reported as: s, singlet; d, doublet; t, triplet; q, quartet; dd, doublet of doublets; m, multiplet. Proton‐decoupled carbon nuclear magnetic resonance (13C‐NMR) spectra were recorded on an Oxford 300 MHz NMR spectrometer and the chemical shifts are reported in ppm using the solvent peak as the internal standard (CDCl3 at 77.0 ppm). High resolution mass spectral data were obtained using a USAG1969A time of flight liquid chromatograph–mass spectrometer (Agilent Technologies, Santa Clara, CA, USA).
Compound 6
A mixture of 4 (Wehlauch et al. 2012; 220 mg, 1.16 mmol), 5 (alkyl bromide; Karimi et al. 2014; 757 mg, 3.35 mmol), potassium iodide (326 mg, 1.96 mmol) and potassium carbonate (421 mg, 2.73 mmol) in dry N,N‐dimethylformamide (DMF; 5 mL) was heated at 80°C for 2 h. The reaction mixture was cooled to ambient temperature and extracted with CH2Cl2 (30 mL × 3). The combined organic extract was dried over Na2SO4, filtered and concentrated in vacuo. The residue was further purified by flash column chromatography (gradient, 40% ethyl acetate/hexane to 100% ethyl acetate) to yield 6 as a yellow liquid (500 mg, 88%). 1H‐NMR (300 MHz, CDCl3): δ 7.58 (s, 1H), 6.76 (s, 1H), 4.22–4.14 (m, 4H), 3.87–3.75 (m, 4H), 3.68–3.61 (m, 4H), 3.60–3.52 (m, 8H), 3.48–3.41 (m, 4H), 3.28 (s, 3H), 3.26 (s, 3H), 2.39 (s, 3H). 13C‐NMR (75 MHz, CDCl3): δ 199.74, 153.73, 149.19, 138.38, 132.67, 110.55, 109.20, 71.81, 70.87, 70.57, 70.47, 70.45, 69.37, 69.34, 69.27, 58.91, 30.23. Electrospray ionization mass spectrometry (ESI‐MS) (m/z) for C22H36NO11: calculated, 490.2288; found, 490.2278 [M+H]+.
Compound 7
A solution of 6 (400 mg, 0.82 mmol) in methanol (3 mL) was cooled to 0°C and NaBH4 (31 mg, 0.82 mmol) was added. The reaction mixture was further stirred at room temperature for 5 h and was then quenched with saturated aqueous NH4Cl (50 mL) and extracted with CH2Cl2 (50 mL × 3). The combined organic extract was dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was further purified by flash column chromatography (1% methanol/ethyl acetate) to give 7 as a yellow oil (360 mg, 89%). 1H‐NMR (300 MHz, CDCl3): δ 7.52 (s, 1H), 7.34 (s, 1H), 5.43 (q, J = 6.1 Hz, 1H), 4.29–4.19 (m, 2H), 4.18–4.10 (m, 2H), 3.85–3.78 (m, 4H), 3.72–3.63 (m, 4H), 3.63–3.52 (m, 8H), 3.51–3.41 (m, 4H), 3.29 (s, 3H), 3.27 (s, 3H), 3.12 (bs, 1H), 1.42 (d, J = 6.2 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 153.64, 146.99, 139.37, 137.81, 110.78, 110.20, 71.84, 71.80, 70.84, 70.73, 70.60, 70.47, 70.32, 69.65, 69.47, 69.14, 68.77, 65.39, 58.93, 24.42. ESI‐MS (m/z) for C22H39NO12: calculated, 509.2472; found, 509.2719 [M+H2O]+.
Compound 8
A solution of 7 (350 mg, 0.71 mmol) in CH2Cl2 (4 mL) was cooled to 0°C and PBr3 (27 μL, 0.28 mmol) was added dropwise over 5 min. The reaction was then stirred at ambient temperature for 16 h. The reaction mixture was quenched with saturated aqueous NaHCO3 and extracted with CH2Cl2 (50 mL × 3). The combined organic extract was further washed with brine, dried over Na2SO4, filtered and concentrated to obtain 8 as a yellow oil (325 mg, 82%). 1H‐NMR (300 MHz, CDCl3): δ 7.50 (s, 1H), 7.30 (s, 1H), 5.97 (q, J = 6.8 Hz, 1H), 4.230–4.26 (m, 2H), 4.22–4.17 (m, 2H), 3.92–3.83 (m, 4H), 3.76–3.68 (m, 4H), 3.68–3.59 (m, 8H), 3.54–3.48 (m, 4H), 3.34 (s, 6H), 2.01 (d, J = 6.8 Hz, 3H). 13C‐NMR (75 MHz, CDCl3): δ 153.12, 148.08, 139.67, 132.61, 132.58, 113.39, 109.99, 109.84, 77.49, 77.06, 76.64, 71.88, 70.92, 70.65, 70.55, 70.53, 69.44, 69.42, 69.21, 69.09, 59.01, 43.11, 27.47. ESI‐MS (m/z) for C22H38NO11: calculated, 571.1628; found, 571.1856 [M+H2O]+.
di‐polyethyleneglycol‐nitrobenzyl (DPNB)‐ABT594 (compound 1)
To a solution of 8 (70 mg, 0.13 mmol) in dry DMF (3 mL) under N2 atmosphere was added K2CO3 (72 mg, 0.52 mmol) and ABT594 (Holladay et al. 1998; 2; 39.6 mg, 0.20 mmol) and this mixture was heated at 50°C for 6 h. After cooling to ambient temperature, the reaction mixture was diluted and extracted with CH2Cl2 (30 mL × 2). The combined organic extract was dried over Na2SO4, filtered and concentrated. The residue was further purified by silica gel column chromatography (gradient, 80% ethyl acetate/hexane to 3% methanol/ethyl acetate) to yield DPNB‐ABT (1) as a yellow liquid (18 mg, 21%). 1H‐NMR (300 MHz, CDCl3): δ 7.77 (d, J = 3.0 Hz, 1H), 7.39 (s, 1H), 7.29 (s, 1H), 7.14 (d, J = 8.7 Hz, 1H), 6.85 (dd, J = 9, 3 Hz, 1H), 4.14–4.08 (m, 3H), 4.07–4.01 (m, 1H), 3.92–3.87 (m, 1H), 3.86–3.82 (m, 2H), 3.74 = 3.69 (m, 4H), 3.69–3.61 (m, 10H), 3.56–3.51 (m, 1H), 3.51–3.45 (m, 2H), 3.36 (s, 3H), 3.35 (3H, s), 3.03 (q, J, 8 Hz, 1H), 2.18–1.92 (m, 3H), 1.22 (d, J = 6 Hz, 3H). 13C‐NMR (75 MHz, CDCl3): δ 153.89, 152.48, 147.08, 142.34, 141.74, 136.37, 134.71, 124.48, 124.29, 112.48, 109.51, 71.89, 70.88, 70.82, 70.76, 70.65, 70.60, 70.54, 70.50, 69.48, 69.12, 69.06, 68.66, 64.95, 61.31, 59.01, 49.93, 20.65, 19.43. ESI‐MS (m/z) for C31H47ClN3O11: calculated, 672.2899; found, 672.2850 [M+H]+.
4,5‐Dimethoxy‐2‐nitrobenzyl‐ABT594 (compound 3)
ABT594 (Holladay et al. 1998; 14.5 mg, 0.073 mmol) was dissolved in anhydrous dichloromethane (1 mL). To this solution was added 4,5‐dimethoxy‐2‐nitrobenzaldehyde (15.4 mg, 0.073 mmol) and titanium (IV) isopropoxide (21.6 μL, 0.073 mmol) and the resulting mixture was stirred at 25 °C. After 3 h, sodium cyanoborohydride (5.0 mg, 0.080 mmol) was added and the reaction mixture was stirred at ambient temperature for 24 h, after which time the reaction was quenched with saturated aqueous NaHCO3 (20 mL). The organics were extracted with CH2Cl2 (3 × 20 mL), washed with brine (30 mL), dried over Na2SO4, filtered and concentrated. The product was purified by silica gel chromatography (gradient, 30:68:2 to 75:23:2 ethyl acetate/hexanes/triethylamine), resulting in the isolation of 10.2 mg of 4,5‐dimethoxy‐2‐nitrobenzyl (DMNB)‐ABT594 (3; 36% yield). 1H‐NMR (300 MHz, CDCl3): δ 8.00 (d, J = 3.1 Hz, 1H), 7.58 (s, 1H), 7.24–7.16 (m, 2H), 7.11 (dd, J = 8.7, 3.0 Hz, 1H), 4.24 (d, J = 16.3 Hz, 1H), 4.06–3.98 (m, 3H), 3.91 (s, 6H), 3.79–3.66 (m, 1H), 3.51–3.40 (m, 1H), 3.00–2.84 (m, 1H), 2.15 (td, J = 8.0, 3.4 Hz, 2H). ESI‐MS (m/z) for C18H20ClN3O5: calculated, 394.1165; found, 394.1164 [M+H]+.
Quantum yield of photolysis of DPNB‐ABT594
A solution of DPNB‐ABT594 and DM‐nitrophen in equal concentration (0.1 mM each, total absorption at 365 nm 0.28 in a 3 mL quartz cuvette) in HEPES buffer with saturating Ca2+ was photolysed with a 365 nm LED (Thorlabs, Newton, NJ, USA). Since the absorption spectra of the caged compounds are identical in this region, the relative rate of photolysis allows the quantum yield of uncaging of DPNB‐ABT594 to be calculated. The time course of photolysis was followed by HPLC monitored at 250 and 365 nm with inosine included as a photochemically inert standard. Using a linear gradient from a Waters Acuity of 10–100% acetonitrile with water with 0.1% trifluoroacetic acid, DM‐nitrophen eluted at 2.09 min and DPNB‐ABT594 at 3.19 min on a Waters Cortecs C‐18 column (4.6 × 50 mm, 2.7 μm).
Brain slice preparation
Mice were anaesthetized with isoflurane and the brain quickly removed. Coronal brain slices (250 μm) were cut in ice‐cold cutting solution containing (in mM): 60 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 26 NaHCO3, 10 glucose, 100 sucrose, 3 sodium pyruvate, 1.3 sodium ascorbate equilibrated with 95% O2–5% CO2 (pH 7.3‐7.4). The brain slices were then incubated for 15–20 min at 33°C in artificial cerebrospinal fluid (ACSF, mM: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, 10 glucose, 3 sodium pyruvate, 1.3 sodium ascorbate; 95% O2–5% CO2, pH 7.3–7.4) before being stored at room temperature.
Electrophysiological recordings
Hemisected brain slices were transferred to the recording chamber of a BX‐61 microscope (Olympus, Penn Valley, PA, USA) and superfused with ACSF at room temperature. Neurons of the lateral portion of the ventral MHb were visualized with a ×60 objective (Olympus) and infrared differential interference contrast optics. Whole‐cell recordings were made with an EPC‐9 amplifier (HEKA Instruments, Bellmore, NY, USA) in voltage‐clamp mode (V hold = −60 mV). Patch pipettes were filled with an internal solution containing (in mM): 135 potassium gluconate, 4 MgCl2, 10 HEPES, 4 Na2‐ATP, 0.4 Na2‐GTP, 10 Na2‐phosphocreatine, pH 7.35. The red fluorescent dye Alexa 594 (0.05 mM, Thermo Fisher Scientific, Waltham, MA, USA) was added to visualize the morphology of neurons, and the fluorescent Ca2+ indicator fluo‐4FF (0.5 mM) was added to measure Ca2+ transients. Currents were recorded at 20 kHz and filtered at 3 kHz with Pulse (HEKA). All experiments were conducted in the presence of the voltage‐gated Na+ channel blocker TTX (1 μM). The nAChR blocker dihydro‐β‐erythroidine (3 μM) and mecamylamine (3 μM) were added as indicated.
Two‐photon imaging and one‐/two‐photon uncaging
Dye‐filled patch‐clamped neurons were imaged with a Prairie Technologies (Middleton, WI, USA) Ultima dual galvanometer scan head equipped with a Chameleon Ultra II and a Verdi‐Mira laser (Coherent Inc., Palo Alto, CA, USA). Alexa 594 and fluo‐4FF were excited with the imaging laser tuned to 820 nm and red/green emission simultaneously monitored by two photomultiplier tubes. Laser intensities were modulated by Pockels cells (Conoptics, Danby, CT, USA). Frame‐scan Ca2+ imaging (∼7.5 or 9.1 Hz) was performed at 128 × 128 pixels resolution with a pixel dwell time of 4 μs. Two‐photon uncaging was carried out at 720 nm, and one‐photon uncaging with a 410 nm laser (CUBE, Coherent). Light power was measured with a photometer (S120VC, Thorlabs) at the exit of the objective prior to the experiment. All time‐correlated imaging, uncaging and electrophysiology experiments were controlled and triggered via PrairieView 5.3 (Prairie Technologies). For one‐photon uncaging, DPNB‐ABT594 (20 μM) was applied via the recirculating perfusion system (7 ml); for two‐photon uncaging the compound was applied at higher concentration (400 μM) by local pressure application from a patch pipette (Picospritzer III, Parker Instrumentation, Fairfield, NJ, USA). The concentration of caged compound was determined with a UV spectrophotometer (Cary 50 Bio, Varian, Palo Alto, CA, USA) based on the extinction coefficient (ε350 = 5000).
Data analysis and statistics
Image analysis was performed with ImageJ. Regions of interest (ROIs) were manually selected and drawn based on morphological reconstruction of the red fluorescence (Alexa 594). Data are given as ΔF/F 0, based on the equation ΔF/F 0 = (Ft – F 0)/(F 0 – F dark), where F t is the fluo‐4FF fluorescence intensity at time t, F 0 is the average fluorescence intensity of the baseline recorded for 10 s prior to uncaging and F dark is the average systems background dark fluorescence intensity with the imaging laser shutter closed. Displayed current and Ca2+ traces were low‐pass 1 kHz filtered for clarity. Analysis of current and Ca2+ transients was carried out using FitMaster (HEKA), Microsoft Excel and IGOR Pro (WaveMetrics, Lake Oswego, OR, USA). Rise times (20–80%) and decay times (37%) were measured with NeuroMatic for IGOR Pro (WaveMetrics). Data are presented as mean ± SEM.
Results
Synthesis of caged ABT594
A range of agonists specific for nAChRs were developed by Abbott (Holladay et al. 1998) in the 1990s, and one of the most potent, ABT594, activated nAChRs containing the α4β2 subunits with good selectivity versus the α7 subunit. This drug has a secondary amine functionality ripe for caging with a nitrobenzyl chromophore, as the N‐methyl derivative is 100‐fold less potent than ABT594 (Holladay et al. 1998; Fig. 1 A). No longer commercially available, ABT594 required synthesis itself, and for this we followed the published protocol (Holladay et al. 1998). Initially, we caged ABT594 (2, Fig. 1 A) by reductive amination with the known 4,5‐dimethoxy‐2‐nitrobenzyl (DMNB) aldehyde to give DMNB‐ABT594 (3, Fig. 1 A). We selected neurons in the MHb for probe evaluation, as these are known to express nAChRs (Fowler et al. 2011). While our initial biological evaluation of 3 was encouraging, photoevoked responses were highly variable (data not shown). We surmised this could be caused by the hydrophobic nature of 3, so we synthesized a more water‐soluble di‐polyethylene glycol (DP) version of 3 called DPNB‐ABT594 (1, Fig. 1 A). The synthesis started with the known nitrocachetol 4, which was alkylated with a polyethylene glycol moiety 5 to give ketone 6. This was then transformed into bromide 8 via reduction to alcohol 7 in an overall yield of 64% for three steps. ABT594 was then alkylated with bromide 8 to give the desired caged compound 1. Note, the DPNB photochemical protecting group is a novel, water‐soluble version of the DMNB chromophore. We could make solutions of 1 in HEPES buffer of at least 1 mM. We compared the rate of photolysis of DPNB‐ABT594 with DM‐nitrophen, which we had previously shown by photon counting methods to have a quantum yield of 0.18 (Kaplan & Ellis‐Davies, 1988). Since DM‐nitrophen and DPNB‐ABT594 have similar chromophores, we compared the relative rates of photolysis of these compounds when they were irradiated simultaneously with near‐UV light. We determined that our new caged compound was photolysed with a quantum yield of 0.20 (Fig. 1 B).
Figure 1. Synthesis and photolysis of caged ABT594.
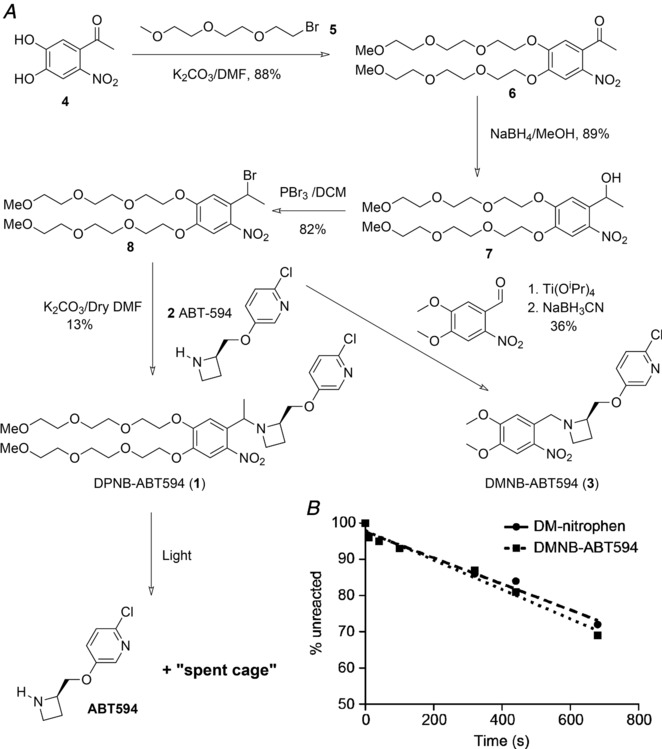
(A) Synthesis of DMNB‐ and DPNB‐ABT594. (B) A solution in HEPES buffer of DPNB‐ABT594 and DM‐nitrophen was photolysed with a 365 nm LED. The time course of photolysis was followed by HPLC monitored at 250 and 365 nm. DPNB‐ABT594 photolysed at a slightly faster rate than DM‐nitrophen (quantum yield 0.18; Kaplan & Ellis‐Davies, 1988), corresponding to a quantum yield of 0.20.
One‐photon uncaging of DPNB‐ABT594 on MHb neurons
To evaluate the photopharmacology of DPNB‐ABT594, we selected neurons in the lateral portion of the ventral MHb as these are known to express very high levels of nAChRs, including α3β4 and α4β2 subtypes (McLaughlin et al. 2017). Since ABT594 is a high affinity agonist for nAChRs, we were able to apply DPNB‐ABT594 at a low concentration (20 μM) to brain slices acutely isolated from mice. Neurons were patch‐clamped with a solution containing the morphological marker Alexa 594 and the Ca2+ indicator fluo‐4FF (Fig. 2 A). These cells exhibited large inward currents and Ca2+ transients in response to laser uncaging at 410 nm at the soma (Fig. 2 B), which increased linearly with energy dosage as expected for one‐photon excitation (1 mW, 0.5–3 ms, n = 6 cells; Fig. 2 C). These responses were blocked by the nAChR blocker DHβE (3 μM) and mecamylamine (3 μM) indicating activation of a pure nAChR‐mediated conductance (Fig. 2 D).
Figure 2. One‐photon uncaging of DPNB‐ABT594 on medial habenula neurons with violet light.
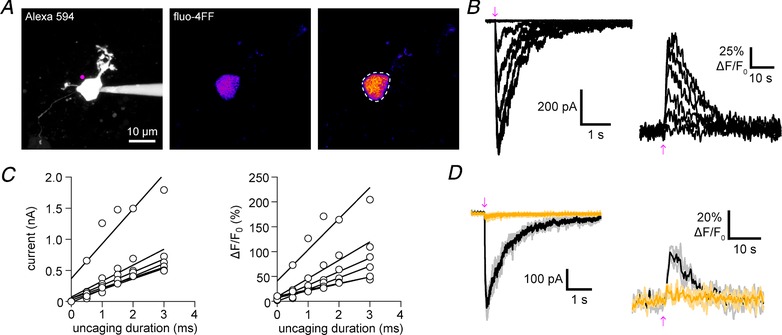
A, two‐photon fluorescence maximum projection image of a patch‐clamped medial habenula (MHb) neuron. Left: morphology of a MHb neuron filled with Alexa 594 with the position of uncaging labelled (violet). Middle and right: pseudo‐colour images showing the increase in fluorescence of fluo‐4FF (right compared to baseline, middle) caused by DPNB‐ABT594 (20 μM, bath‐applied) one‐photon uncaging. Dashes indicate region of interest used for analysis. B, amplitudes of inward current (left) and fluo‐4FF fluorescence (right) in response to increased duration of irradiation (410 nm, 1 mW, 0.5–3.0 ms). C, uncaging‐evoked currents and Ca2+ signals increase linearly with uncaging duration. Inward current amplitudes (left) and fluo‐4FF fluorescence amplitudes (right) plotted against duration of irradiation for each cell (n = 5 cells; 410 nm, 1 mW). Linear regression lines were fitted. D, currents and fluo‐4FF fluorescent signals were blocked (yellow traces) by the nAChR antagonists DHβE (3 μM) and mecamylamine (3 μM). Black/dark yellow traces are averages of three consecutive trials (grey/light yellow). [Color figure can be viewed at http://wileyonlinelibrary.com]
Subcellular distribution of nAChRs on MHb neurons
Next, we asked whether functional Ca2+‐permeable nAChRs were also present on dendrites of MHb neurons. Uncaging DPNB‐ABT594 elicited currents and large local Ca2+ transients in dendrites of all cells tested (n = 12; Fig. 3 A and B). Compared to principal neurons from other brain regions, MHb neurons display a morphology comprising a round cell soma with several short, mostly aspiny dendrites of varying diameter that often display a striking tortuousity (Kim & Chang, 2005a ; Fig. 2, 3, 4, 5 A). This overlap could cause currents recorded from dendrites using one‐photon uncaging to be a sum of several axial locations. However, two‐photon imaging has much better axial resolution and allows local Ca2+ transients to be specified with excellent spatial precision. This approach allowed us to define the lateral resolution of one‐photon uncaging (Fig. 3). Uncaging at two positions separated by 5 μm along a dendrite caused concomitant changes in Ca2+ signals at position 1–3 (Fig. 3 A and B). However, moving 5 μm away from position 2 reduced the Ca2+ signal at position 2 considerably (Fig. 3 C). Signals at further distances (position 1 and 3) were not detectable, suggesting one‐photon photolysis has a lateral resolution of 3–5 μm.
Figure 3. DPNB‐ABT594 uncaging evoked Ca2+ signals in dendrites of medial habenula neurons with good lateral resolution.
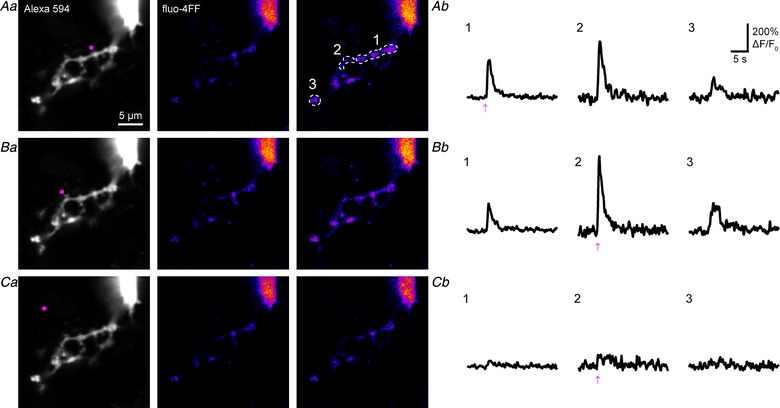
Aa–Ca, high resolution images of local uncaging on dendrites (A and B) and ∼5 μm away from dendrite (C). Left: morphology of the targeted subregion with the position of uncaging labelled (violet). Middle and right: pseudo‐colour images showing the increase in fluorescence of fluo‐4FF (right compared to baseline, middle) caused by DPNB‐ABT594 (20 μM, bath‐applied) one‐photon uncaging (410 nm, 2 mW, 1 ms). Dashes indicate region of interest used for analysis (Ab–Cb) Ca2+ transients evoked by local uncaging at the regions indicated in Aa–Ca. Scales and regions of interest in A also apply to B and C. Note, moving the uncaging point ∼5 μm away from the dendrite evoked almost no response (C). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4. Uncaging evoked currents and Ca2+ signals in dendrites and axons of medial habenula neurons.
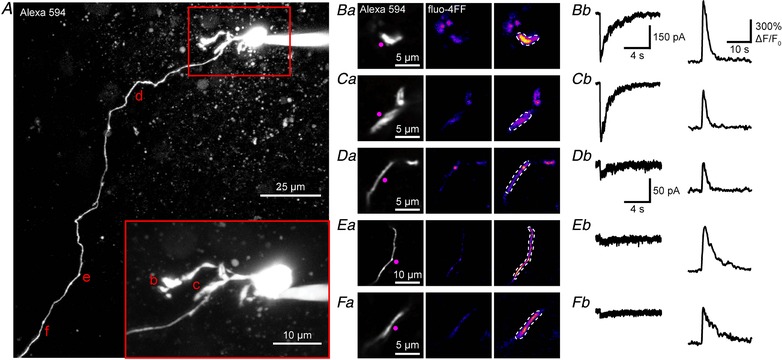
A, two‐photon fluorescence maximum projection image of a patch‐clamped medial habenula (MHb) neuron filled with Alexa 594 and fluo‐4FF. Soma and dendrites shown at higher magnification (inset). Regions selected for uncaging and imaging are marked by the letters b–f and are shown in Ba–Fa, respectively. Ba–Fa, high resolution images of local uncaging on dendrites (B and C) and the axon (D–F) at locations indicated by b–f, respectively, in A. Left: morphology of the targeted subregion with the position of uncaging labelled (violet). Middle and right: pseudo‐colour images showing the increase in fluorescence of fluo‐4FF (right compared to baseline, middle) caused by DPNB‐ABT594 (20 μM, bath‐applied) one‐photon uncaging (410 nm, 2 mW, 1 ms). Dashes indicate region of interest used for analysis. (Bb–Fb, current responses (left) and Ca2+ transients (right) evoked by local uncaging at the regions indicated in Ba–Fa. Current scale in B also applies to C, the scale in D to E and F. Fluorescence scale in B also applies to C–F. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5. Two‐photon uncaging of DPNB‐ABT594 on medial habenula neurons.
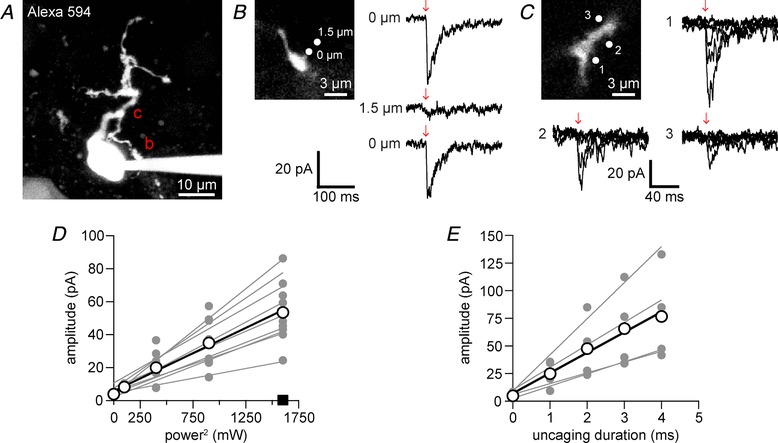
A, two‐photon fluorescence image of a patch‐clamped medial habenula (MHb) neuron filled with Alexa 594 (maximum projection of 25 μm). Regions selected for two‐photon uncaging at 720 nm of DPNB‐ABT594 (400 μM, locally applied) are marked by the letters b and c and are shown in B and C at higher resolution. B, current response (right, top) caused two‐photon uncaging (2 ms, 30 mW) on the dendrite (top left, zoomed image of dendrite b in A). Current response (right, middle) from uncaging at 1.5 μm away from the dendrite. Redirecting the laser to the dendrite restored the current response (right, bottom). Current traces are averages of 3–4 trials. C, current recordings in response to increasing power (0–40 mW) with constant duration (2 ms) at locations 1–3 (top left, zoomed image of dendrite c in A). D, two‐photon uncaging‐evoked currents increased quadratically with power (n = 9 cells with 3 power curves each; 2 ms, 0–40 mW). Uncaging on cells without DPNB‐ABT594 (×10, 40 mW, 2 ms, at 5 Hz) evoked no current (square, n = 4). E, two‐photon uncaging‐evoked currents showed a linear dependence on photolysis duration (0–4 ms) at constant power (20 mW, n = 4 cells with 3 power curves each). Linear regression lines in D and E were fitted for each cell (grey points and lines) and the average across cells (white points and black lines). [Color figure can be viewed at http://wileyonlinelibrary.com]
In several cases, we were also able to fill the small diameter axon and track it over considerable distances. The axon either emerged directly from the soma or from a dendrite (Kim & Chang, 2005b ), as seen in Fig. 4 A. The subcellular distribution of nAChRs is much more complex than that of ionotropic glutamate receptors. Most crucial in skeletal muscle postsynaptic physiology (Katz, 1979; Colquhoun & Sakmann, 1998), they are also known to play an important role at presynaptic terminals in the CNS, where Ca2+ entry through nAChRs can enhance neurotransmitter release (Nashmi & Lester, 2006). Such terminals normally appear as bulbous varicosities arising from thin, non‐myelinated axons (De Paola et al. 2006). Unexpectedly, uncaging ABT594 at long bouton‐free axonal compartments of patch‐clamped MHb neurons elicited large local Ca2+ transients (n = 5 cells; Fig. 4 A and D–F). We could also detect currents measured at the soma from these photostimulations; however, these were much smaller than dendritic currents in the same cell (Fig. 4 B and C), likely due to ‘axonal filtering’ of the evoked currents.
Two‐photon uncaging of DPNB‐ABT594 on MHb neurons
Next, we examined the two‐photon uncaging of DPNB‐ABT594 using a mode‐locked Ti:sapphire laser tuned to 720 nm. Due to the comparatively low two‐photon absorption of the nitrobenzyl chromophore, we locally applied DPNB‐ABT594 at much higher concentrations (∼400 μM). Short pulses of two‐photon light (2 ms, 30 mW) directed to at a single location at a dendrite of a dye‐filled MHb neuron (Fig. 5 A and B) induced fast nAChR‐mediated currents (Fig. 5 B, right top). Moving the uncaging point 1.5 μm away from the dendrite eliminated the current (Fig. 5 B, right middle) while moving it back to the original location restored the current response (Fig. 5 B, right bottom) demonstrating the expected high resolution of two‐photon excitation.
When the laser was directed to the soma or dendrite of single MHb neurons (Fig. 5 A and C), we could evoke currents that increased either with energy for a fixed shutter duration or with shutter duration with a fixed energy of photolysis. In the former case we used 2 ms shutter exposures and increased the energy from 0–40 mW. The evoked currents increased with power according the expected (Denk et al. 1990) quadratic relationship for two‐photon excitation (Fig. 5 C and D). We tested if 200 pulses at 720 nm for 2 ms with 40 mW of power caused any phototoxic side effects on MHb in brain slices (n = 3). We could detect no change in baseline fluorescence with fluo‐4FF, nor any changes in resting membrane potential or access resistance. These data are consistent with our previous study which used similar energies, but longer duration for intracellular uncaging of IP3 in vivo, during which we detected no phototoxicity (Crowe et al. 2010). Finally, when we kept the power fixed (20 mW) and simply increased the shutter time (0–5 ms), we observed a linear increase in evoked current (Fig. 5 E).
Discussion
Even though acetylcholine was the first neurotransmitter to be discovered, considerable technical challenges remain for the study of its synaptic physiology. The challenge for the neurotransmitter itself is that all valances, including the nitrogen lone pair, are fully satisfied. In contrast, carbamoylcholine has N–H bonds free for derivatization with photochemical protecting groups such as the NB or carboxy‐ (C)NB chromophores (Walker et al. 1986b; Milburn et al. 1989; Khiroug et al. 2003; Yakel, 2013). Further, in 1994 Denk was able to use CNB‐carbamoylcholine for two‐photon uncaging experiments on living cells (Denk, 1994). However, since the simple NB chromophore has a very low two‐photon cross section (0.001 units of Goeppert‐Mayer (GM); Brown et al. 1999), a short wavelength (640 nm) was needed for successful uncaging, one that was subsequently reported to be challenging to cells (Kiskin et al. 2002). This study suggested that more electron‐rich chromophores could be used for successful two‐photon uncaging at wavelengths >700 nm, for example (Ellis‐Davies, 1999; Matsuzaki et al. 2000). These probes, along with other electron‐rich caged compounds, all have required quadratic relationship for two‐photon uncaging (DelPrincipe et al. 1999; Matsuzaki et al. 2001, 2010; Momotake et al. 2006; Ellis‐Davies et al. 2007; Olson et al. 2013; Amatrudo et al. 2014; Agarwal et al. 2016, 2017; Passlick & Ellis‐Davies, 2017; Richers et al. 2017).
Consistent with these studies, DPNB‐ABT594 also shows power‐squared relationship of evoked current and incident power (Fig. 5). In contrast, a recent report using a 7‐aminocoumarin‐caged nicotine showed a linear relationship with power (Banala et al. 2018). This new caged compound used the DCAC chromophore, which we had previously shown to be truly two‐photon effective in 2010 (Kantevari et al. 2010).
Molecules with a relatively low two‐photon cross section, such as the DMNB chromophore, are probably much better suited to linear excitation. In this context, a key property of caged compounds that makes them practical for the study of living cells is the efficiency of use of the absorbed light, in other words, the quantum yield of photolysis should be high (Kaplan et al. 1978; Kaplan & Ellis‐Davies, 1988; Walker et al. 1988; Ellis‐Davies & Kaplan, 1994; Fedoryak et al. 2005; Ellis‐Davies et al. 2007; Matsuzaki et al. 2010). DPNB‐ABT594 has a quantum yield of photolysis of 0.20, making it extremely effective in its use of incident light compared to a recently published caged nicotine, which has a quantum yield of 0.009 (Banala et al. 2018). Our high quantum yield allowed us to use brief durations and low power of uncaging, with as little as 0.5 ms and 1 mW of 410 nm light evoking rapid responses from neurons (Figs 2 and 4). In contrast, inefficient uncaging requires longer pulse durations (Banala et al. 2018) potentially harmful for living cells.
The favourable properties of our new caged compound allowed us to examine the subcellular distribution of nAChRs in MHb neurons. We found that dendrites of MHb neurons express functional nAChRs (Figs 3 and 4) in line with recent results (Banala et al. 2018). More importantly, by combining one‐photon uncaging with two‐photon Ca2+ imaging, we demonstrated that activation of dendritic nAChRs gives rise to large local Ca2+ transients (Figs 3 and 4). These local Ca2+ signals may mediate diverse functions in these neurons ranging from regulation of cytosolic Ca2+ levels to changes in gene expression (Shen & Yakel, 2009).
Our data suggest that functional nAChRs are expressed on MHb axons projecting to the IPN where they might exert a modulatory function on the relay of information between the MHb and the IPN and thereby influence behaviours that are controlled by these brain structures, such as nicotine addiction (Fowler et al. 2011), encoding of fear memories (Zhang et al. 2016) and resolution of social conflict (Chou et al. 2016).
In conclusion, we developed a new caged drug which specifically activates nAChRs with high affinity using one‐ and two‐photon light. This compound enabled us to map the distribution of nAChRs on MHb neurons and uncover the unexpected presence of these receptors on MHb axons. Little is known about the Ca2+ signalling of MHb neurons. Our new compound will help gain a deeper understanding of the nAChR‐mediated Ca2+ signalling in the MHb and its implications for nicotine addiction and other complex behavioural processes.
Additional information
Competing interests
None.
Author contributions
E.R.T. made DPNB‐ABT594. M.T.R. made DMNB‐ABT594. S.P. performed and analysed all the physiological experiments. G.C.R.E.‐D. conceived of caged ABT594 and supervised all aspects of the project. G.C.R.E.‐D. and S.P. wrote the paper. All authors edited the MS. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by NIH grants to G.C.R.E.‐D. S.P. was supported by a DFG fellowship.
Biographies
Stefan Passlick received his PhD from the Institute of Cellular Neurosciences at the University of Bonn where he studied the physiology of NG2 glial cells. Currently, he is a postdoctoral fellow at the Mount Sinai School of Medicine in New York, where he is developing and implementing new optical tools to probe neuronal function. His work in New York has been supported, in part, by a Deutsche Forschungsgemeinschaft fellowship.

Ek Raj Thapaliya received his MSc in chemistry from Tribhuvan University (Nepal) in 2006 and a PhD in chemistry under the guidance of Franscisco Raymo from the University of Miami in 2017. His graduate research involved the synthesis and analysis of photoactivatable fluorophores and fluorescent amphiphilic polymers designed for bioimaging applications. He joined the Ellis‐Davies laboratory in May 2017 as a postdoctoral fellow.
S. Passlick and E. R. Thapaliya were equal contributors
Edited by: Ian Forsythe & William Taylor
References
- Agarwal HK, Janicek R, Chi SH, Perry JW, Niggli E & Ellis‐Davies GCR (2016). Calcium uncaging with visible light. J Am Chem Soc 138, 3687−3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal HK, Zhai S, Surmeier DJ & Ellis‐Davies GCR (2017). Intracellular uncaging of cGMP with blue light. ACS Chem Neurosci 8, 2139–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatrudo JM, Olson JP, Lur G, Chiu CQ, Higley MJ & Ellis‐Davies GCR (2014). Wavelength‐selective one‐ and two‐photon uncaging of GABA. ACS Chem Neurosci 5, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banala S, Arvin MC, Bannon NM, Jin XT, Macklin JJ, Wang Y, Peng C, Zhao G, Marshall JJ, Gee KR, Wokosin DL, Kim VJ, McIntosh JM, Contractor A, Lester HA, Kozorovitskiy Y, Drenan RM & Lavis LD (2018). Photoactivatable drugs for nicotinic optopharmacology. Nat Methods 15, 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieke C, Rohrbach F, Gottschalk A, Mayer G & Heckel A (2012). Light‐controlled tools. Angew Chem Int Ed 51, 8446–8476. [DOI] [PubMed] [Google Scholar]
- Brown EB, Shear JB, Adams SR, Tsien RY & Webb WW (1999). Photolysis of caged calcium in femtoliter volumes using two‐photon excitation. Biophys J 76, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Amo R, Kinoshita M, Cherng BW, Shimazaki H, Agetsuma M, Shiraki T, Aoki T, Takahoko M, Yamazaki M, Higashijima S & Okamoto H (2016). Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science 352, 87–90. [DOI] [PubMed] [Google Scholar]
- Colquhoun D & Sakmann B (1998). From muscle endplate to brain synapses: a short history of synapses and agonist‐activated ion channels. Neuron 20, 381–387. [DOI] [PubMed] [Google Scholar]
- Crowe SE, Kantevari S & Ellis‐Davies GCR (2010). Photochemically initiated intracellular astrocytic calcium waves in living mice using two‐photon uncaging of IP3 . ACS Chem Neurosci 1, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M & Dani JA (2011). Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelPrincipe F, Egger M, Ellis‐Davies GC & Niggli E (1999). Two‐photon and UV‐laser flash photolysis of the Ca cage, dimethoxynitrophenyl‐EGTA‐4. Cell Calcium 25, 85–91. [DOI] [PubMed] [Google Scholar]
- Denk W ( 1994). Two‐photon scanning photochemical microscopy: mapping ligand‐gated ion channel distributions. Proc Natl Acad Sci U S A 91, 6629–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH & Webb WW (1990). Two‐photon laser scanning fluorescence microscopy. Science 248, 73–76. [DOI] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P & Svoboda K (2006). Cell type‐specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875. [DOI] [PubMed] [Google Scholar]
- Donnelly‐Roberts DL, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, Piattoni‐Kaplan M, McKenna DG, Wasicak JT, Holladay MW, Williams M & Arneric SP (1998). ABT‐594 [(R)‐5‐ (2‐azetidinylmethoxy)‐2‐chloropyridine]: a novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: I. In vitro characterization. J Pharmacol Exp Ther 285, 777–786. [PubMed] [Google Scholar]
- Eder M, Zieglgansberger W & Dodt HU (2004). Shining light on neurons – lucidation of neuronal functions by photostimulation. Rev Neurosci 15, 167–183. [DOI] [PubMed] [Google Scholar]
- Ellis‐Davies GCR ( 1999). Localized photolysis of caged compounds. J Gen Physiol 114, 1a.10398688 [Google Scholar]
- Ellis‐Davies GCR ( 2007). Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods 4, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis‐Davies GCR ( 2013). A chemist and biologist talk to each other about caged neurotransmitters. Beilstein J Org Chem 9, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis‐Davies GCR & Kaplan JH (1994). Nitrophenyl‐EGTA, a photolabile chelator that selectively binds Ca with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci U S A 91, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis‐Davies GCR, Matsuzaki M, Paukert M, Kasai H & Bergles DE (2007). 4‐Carboxymethoxy‐5,7‐dinitroindolinyl‐Glu: an improved caged glutamate for expeditious ultraviolet and two‐photon photolysis in brain slices. J Neurosci 27, 6601–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoryak OD, Sul JY, Haydon PG & Ellis‐Davies GCR (2005). Synthesis of a caged glutamate for efficient one‐ and two‐photon photorelease on living cells. Chem Commun (29), 3664–3666. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ & Kenny PJ (2011). Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MJ, Velema WA, Lerch MM, Szymanski W & Feringa BL (2015). Wavelength‐selective cleavage of photoprotecting groups: strategies and applications in dynamic systems. Chem Soc Rev 44, 3358–3377. [DOI] [PubMed] [Google Scholar]
- Holladay MW, Bai H, Li Y, Lin NH, Daanen JF, Ryther KB, Wasicak JT, Kincaid JF, He Y, Hettinger AM, Huang P, Anderson DJ, Bannon AW, Buckley MJ, Campbell JE, Donnelly‐Roberts DL, Gunther KL, Kim DJ, Kuntzweiler TA, Sullivan JP, Decker MW & Arneric SP (1998). Structure‐activity studies related to ABT‐594, a potent nonopioid analgesic agent: effect of pyridine and azetidine ring substitutions on nicotinic acetylcholine receptor binding affinity and analgesic activity in mice. Bioorg Med Chem Lett 8, 2797–2802. [DOI] [PubMed] [Google Scholar]
- Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H & Ellis‐Davies GCR (2010). Two‐color, two‐photon uncaging of glutamate and GABA. Nat Methods 7, 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH & Ellis‐Davies GCR (1988). Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A 85, 6571–6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH, Forbush B & Hoffman JF (1978). Rapid photolytic release of adenosine 5′‐triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry 17, 1929–1935. [DOI] [PubMed] [Google Scholar]
- Karimi B, Mansouri F & Vali H (2014). A highly water‐dispersible/magnetically separable palladium catalyst based on a Fe3O4@SiO2 anchored TEG‐imidazolium ionic liquid for the Suzuki‐Miyaura coupling reaction in water. Green Chemistry 16, 2587–2596. [Google Scholar]
- Katz B ( 1979). Elementary components of synaptic transmission. Naturwissenschaften 66, 606–610. [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D & Yakel JL (2003). Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci 23, 9024–9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U & Chang SY (2005a). Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol 483, 236–250. [DOI] [PubMed] [Google Scholar]
- Kim U & Chang SY (2005b). Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol 483, 236–250. [DOI] [PubMed] [Google Scholar]
- Kiskin NI, Chillingworth R, McCray JA, Piston D & Ogden D (2002). The efficiency of two‐photon photolysis of a “caged” fluorophore, o‐1‐ (2‐nitrophenyl)ethylpyranine, in relation to photodamage of synaptic terminals. Eur Biophys J 30, 588–604. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis‐Davies GCR & Kasai H (2000). Two‐photon uncaging of glutamate reveals AMPA receptors density expression at single spine heads. Society for Neuroscience Annual Meeting Abstract 426.12. [Google Scholar]
- Matsuzaki M, Ellis‐Davies GCR, Nemoto T, Miyashita Y, Iino M & Kasai H (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4, 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Hayama T, Kasai H & Ellis‐Davies GCR (2010). Two‐photon uncaging of gamma‐aminobutyric acid in intact brain tissue. Nat Chem Biol 6, 255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA & De Biasi M (2017). The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J Neurochem 142 (Suppl 2), 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn T, Matsubara N, Billington AP, Udgaonkar JB, Walker JW, Carpenter BK, Webb WW, Marque J, Denk W, Mccray JA & Hess GP (1989). Synthesis, photochemistry, and biological‐activity of a caged photolabile acetylcholine‐receptor ligand. Biochemistry 28, 49–55. [DOI] [PubMed] [Google Scholar]
- Momotake A, Lindegger N, Niggli E, Barsotti RJ & Ellis‐Davies GCR (2006). The nitrodibenzofuran chromophore: a new caging group for ultra‐efficient photolysis in living cells. Nat Methods 3, 35–40. [DOI] [PubMed] [Google Scholar]
- Nashmi R & Lester HA (2006). CNS localization of neuronal nicotinic receptors. J Mol Neurosci 30, 181–184. [DOI] [PubMed] [Google Scholar]
- Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL & Ellis‐Davies GCR (2013). Optically selective two‐photon uncaging of glutamate at 900 nm. J Am Chem Soc 135, 5954–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passlick S & Ellis‐Davies GCR (2017). Comparative one‐ and two‐photon uncaging of MNI‐glutamate and MNI‐kainate on hippocampal CA1 neurons. J Neurosci Methods 293, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richers MT, Amatrudo JM, Olson JP & Ellis‐Davies GCR (2017). Cloaked caged compounds: chemical probes for two‐photon optoneurobiology. Angew Chem Int Ed 56, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JX & Yakel JL (2009). Nicotinic acetylcholine receptor‐mediated calcium signaling in the nervous system. Acta Pharmacol Sin 30, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA & Drenan RM (2014). Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci 34, 9789–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JW, McCray JA & Hess GP (1986a). Photolabile protecting groups for an acetylcholine receptor ligand. Synthesis and photochemistry of a new class of o‐nitrobenzyl derivatives and their effects on receptor function. Biochemistry 25, 1799–1805. [DOI] [PubMed] [Google Scholar]
- Walker JW, McCray JA & Hess GP (1986b). Photolabile protecting groups for an acetylcholine receptor ligand. Synthesis and photochemistry of a new class of o‐nitrobenzyl derivatives and their effects on receptor function. Biochemistry 25, 1799–1805. [DOI] [PubMed] [Google Scholar]
- Walker JW, Reid GP, McCray JA & Trentham DR (1988). Photolabile 1‐ (2‐nitrophenyl)ethyl phosphate esters of adenine nucleotide analogs – synthesis and mechanism of photolysis. J Am Chem Soc 110, 7170–7177. [Google Scholar]
- Wehlauch R, Hoecker J & Gademann K (2012). Nitrocatechols as tractable surface release systems. Chempluschem 77, 1071–1074. [Google Scholar]
- Yakel JL ( 2013). Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch 465, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan L, Ren Y, Liang J, Lin R, Feng Q, Zhou J, Hu F, Ren J, Wei C, Yu T, Zhuang Y, Bettler B, Wang F & Luo M (2016). Presynaptic excitation via GABAB receptors in habenula cholinergic neurons regulates fear memory expression. Cell 166, 716–728. [DOI] [PubMed] [Google Scholar]


