Abstract
Key points
Presynaptic CaV2 voltage‐gated calcium channels link action potentials arriving at the presynaptic terminal to neurotransmitter release. Hence, their regulation is essential to fine tune brain circuitry.
CaV2 channels are highly sensitive to G protein‐coupled receptor (GPCR) modulation. Our previous data indicated that growth hormone secretagogue receptor (GHSR) constitutive activity impairs CaV2 channels by decreasing their surface density.
We present compelling support for the impact of CaV2.2 channel inhibition by agonist‐independent GHSR activity exclusively on GABA release in hippocampal cultures. We found that this selectivity arises from a high reliance of GABA release on CaV2.2 rather than on CaV2.1 channels.
Our data provide new information on the effects of the ghrelin–GHSR system on synaptic transmission, suggesting a putative physiological role of the constitutive signalling of a GPCR that is expressed at high levels in brain areas with restricted access to its natural agonist.
Abstract
Growth hormone secretagogue receptor (GHSR) displays high constitutive activity, independent of its endogenous ligand, ghrelin. Unlike ghrelin‐induced GHSR activity, the physiological role of GHSR constitutive activity and the mechanisms that underlie GHSR neuronal modulation remain elusive. We previously demonstrated that GHSR constitutive activity modulates presynaptic CaV2 voltage‐gated calcium channels. Here we postulate that GHSR constitutive activity‐mediated modulation of CaV2 channels could be relevant in the hippocampus since this brain area has high GHSR expression but restricted access to ghrelin. We performed whole‐cell patch‐clamp in hippocampal primary cultures from E16‐ to E18‐day‐old C57BL6 wild‐type and GHSR‐deficient mice after manipulating GHSR expression with lentiviral transduction. We found that GHSR constitutive activity impairs CaV2.1 and CaV2.2 native calcium currents and that CaV2.2 basal impairment leads to a decrease in GABA but not glutamate release. We postulated that this selective effect is related to a higher CaV2.2 over CaV2.1 contribution to GABA release (∼40% for CaV2.2 in wild‐type vs. ∼20% in wild‐type GHSR‐overexpressing cultures). This effect of GHSR constitutive activity is conserved in hippocampal brain slices, where GHSR constitutive activity reduces local GABAergic transmission of the granule cell layer (intra‐granule cell inhibitory postsynaptic current (IPSC) size ∼−67 pA in wild‐type vs. ∼−100 pA in GHSR‐deficient mice), whereas the glutamatergic output from the dentate gyrus to CA3 remains unchanged. In summary, we found that GHSR constitutive activity impairs IPSCs both in hippocampal primary cultures and in brain slices through a CaV2‐dependent mechanism without affecting glutamatergic transmission.
Keywords: Synapse, GPCR, ghrelin, primary cultures, inhibitory postsynaptic current, GABA, electrophysiology, brain slices
Key points
Presynaptic CaV2 voltage‐gated calcium channels link action potentials arriving at the presynaptic terminal to neurotransmitter release. Hence, their regulation is essential to fine tune brain circuitry.
CaV2 channels are highly sensitive to G protein‐coupled receptor (GPCR) modulation. Our previous data indicated that growth hormone secretagogue receptor (GHSR) constitutive activity impairs CaV2 channels by decreasing their surface density.
We present compelling support for the impact of CaV2.2 channel inhibition by agonist‐independent GHSR activity exclusively on GABA release in hippocampal cultures. We found that this selectivity arises from a high reliance of GABA release on CaV2.2 rather than on CaV2.1 channels.
Our data provide new information on the effects of the ghrelin–GHSR system on synaptic transmission, suggesting a putative physiological role of the constitutive signalling of a GPCR that is expressed at high levels in brain areas with restricted access to its natural agonist.
Introduction
Growth hormone secretagogue receptor (GHSR; or growth hormone secretagogue type 1a receptor) is a G protein‐coupled receptor (GPCR) with two different activation modes: one constitutive and the other agonist‐induced (Holst et al. 2003). The relevance of its ghrelin‐induced active mode in energy balance and in hedonic and addictive aspects of eating has been well studied (Perello et al. 2010; Abizaid, 2011; Henderson et al. 2013; Hsu, 2015; Perello & Dickson, 2015). On the other hand, the function of GHSR's constitutively active mode is less understood due to its chronic nature and the scarcity of specific inverse agonists (Holst et al. 2007). Few studies have unmasked a clear physiological role of GHSR constitutive activity. In this regard, the influence of this chronic active receptor on overeating behaviour after fasting in mice has recently been shown (Fernandez et al. 2018). At the cellular level we have demonstrated that GHSR constitutive activity severely reduces presynaptic voltage‐gated calcium (CaV) channel trafficking to the plasma membrane (Lopez Soto, 2015; Mustafá et al. 2017) by promoting the retention of the CaV complex in the endoplasmic reticulum. CaV channels generate the calcium influx that is triggered by depolarization in excitable cells. In particular CaV2.1 and CaV2.2 channels are responsible for calcium‐induced neurotransmitter release at presynaptic terminals (Catterall & Few, 2008). Because of their critical role in neurotransmission, there are many physiological mechanisms aimed at controlling CaV2 activity, GPCR activation being one of the most effective. GPCR‐mediated modulation of CaV channels can lead to dramatic changes in channel kinetics and in membrane channel density (Zhang et al. 2008; Zamponi & Currie, 2013). Thus, we reason that inhibition of CaV channels by GHSR constitutive activity could be relevant in brain areas with high GHSR expression level but restricted access to ghrelin (Cabral et al. 2014, 2015).
The hippocampus is a highly plastic glutamatergic neuronal circuit influenced by many diverse GABAergic inhibitory interneurons (Maccaferri & Lacaille, 2003; Houser, 2007; Mann & Paulsen, 2007). This area is far from the sites that ghrelin utilizes to access the brain, such as the median eminence and the circumventricular organs (Cabral et al. 2015, 2017; Abizaid & Edwards, 2017). Despite this, GHSR is widely expressed at relatively high levels in the hippocampus, particularly in the dentate gyrus (Zigman et al. 2006; Cabral et al. 2013; Mani et al. 2014, 2017), where exogenous ghrelin application modulates behavioural responses such as the reward value of food, response to stress, memory retention, spatial memory and learning behaviour (Diano et al. 2006; Atcha et al. 2009; Carlini et al. 2010; Chen et al. 2011; Davis et al. 2011; Wang, 2013; Cahill et al. 2014; Zhao et al. 2014; Kanoski & Grill, 2015; Kent et al. 2015; Hsu et al. 2016). The molecular mechanisms implicated in these effects include dendritic spine formation and long‐term potentiation development (Diano et al. 2006), increased AMPA receptor trafficking (Ribeiro et al. 2014) and enhanced activity of NMDA receptors (Cuellar & Isokawa, 2011; Ghersi et al. 2015).
Despite that ghrelin‐mediated effects at the hippocampus mainly target glutamatergic neurons, GHSR is expressed in glutamatergic but also in GABAergic neurons in several brain regions. The areas where GABA neurons are influenced by ghrelin action include the hypothalamus (Wang, 2013; Lopez Soto, 2015), the area postrema (Cabral et al. 2017) and the central nucleus of the amygdala (Cruz et al. 2012).
In contrast, the effect of GHSR constitutive activity on the brain remains unclear. Here we aimed to study the effect of GHSR constitutive activity on presynaptic CaV currents and synaptic transmission in neurons from the hippocampus, a brain area with restricted ghrelin access.
Methods
Ethical approval
All experimentation in this study received approval from the ethical committee of IMBICE in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Research Council, USA and all efforts were made to minimize suffering. (Reference number from Ethical Committee of IMBICE: 03‐09‐16 – ‘Caracterización de la modulación de los canales de calcio por receptores acoplados a proteína G’.) We understand the ethical principles under which The Journal of Physiology operates and ensured that our work complied with the policies and regulations regarding animal ethics.
Origin and source of the animals
This study was performed using wild‐type (WT) mice on a pure C57BL/6 background and GHSR‐deficient mice, which fail to express GHSR (Zigman et al. 2005) and were derived from crosses between heterozygous animals back‐crossed >10 generations onto a C57BL/6 genetic background. Mice of both sexes were bred at the animal facility of the IMBICE. Mice were housed in a 12 h light/dark cycle in a climate‐controlled room (22°C) with ad libitum access to water and food.
Mouse neuronal primary culture
Hippocampal neuronal cultures were obtained from WT and GHSR‐deficient mice at embryonic days 16–18. A total of 17 pregnant females were subjected to cervical dislocation between days 16 and 18 of pregnancy in order to quickly remove embryos. Embryos were placed immediately in cold Hanks’ solution. Embryos’ brains were exposed and the hippocampi removed, placed in sterile Hanks’ solution and rinsed twice. Then, cells were dissociated at 37°C for 20 min with 0.25 mg ml−1 trypsin (Microvet, Buenos Aires, Argentina). Next, 300 μl of fetal bovine serum (FBS, Internegocios, Mercedes, Buenos Aires, Argentina) to stop the enzyme digestion and 0.28 mg ml−1 deoxyribonuclease I from bovine pancreas (Sigma‐Aldrich, Buenos Aires, Argentina) were added. Cells were mechanically dissociated using several glass pipettes with consecutive smaller‐tip diameters. About 60,000 cells were plated on 12 mm‐diameter glass coverslips treated previously with poly‐l‐lysine (Sigma‐Aldrich) and laid over 24‐well plates. Cells were incubated at 37°C in a 95% air −5% CO2 atmosphere with Dulbecco's modified Eagle's medium/F12 (DMEM/F12) (Microvet) 1:1 medium supplemented with B27 supplement (1:50, Gibco, Thermo Fisher Scientific, Buenos Aires, Argentina), 10% FBS, 0.25% glucose, 2 mm glutamine (Gibco), 3.3 μg ml−1 insulin (Novo Nordisk Pharmaceutical Industries, Inc., Buenos Aires, Argentina), 40 μg ml−1 gentamicin sulfate salt (Richet, Buenos Aires, Argentina), and 1% vitamin solution (Microvet). On the fourth day in culture, half of the incubating medium was replaced with fresh medium containing cytosine β‐d‐arabinofuranoside (Sigma‐Aldrich) to reach a final concentration of 5 μm.
Lentiviral trasduction
tsA201 cells were grown in DMEM (Gibco) with 10% FBS and subcultured when 80% confluent. tsA201 cells were plated in a 100 mm‐diameter dish; 24 h later they were co‐transfected with plasmids coding for a third generation lentiviral system using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Buenos Aires, Argentina) reagent. The culture medium was changed 1 h prior to transfection. A total of 20 mg of plasmids DNA was used for the transfection of one dish: 3.5 mg of the envelope plasmid pCMV‐VSV‐G, 6.5 mg of packaging plasmids pMDLg/pRRE and pRSV‐Rev, and 10 mg of transfer vector plasmid containing GHSR or a natural mutant lacking constitutive activity, GHSRA204E. Conditioned medium was collected after 24 h, cleared by low‐speed centrifugation, filtered through 0.22 μm pore size filters and used to replace growth medium. After transfection, cells were kept in culture to allow lentiviral production for 48 h. Then, the medium was collected and centrifuged at room temperature at 1000 rpm. The supernatant was filtered through 0.45 μm pore size filters and 500 μl was used to replace the growth medium of each well containing the primary neuron culture for 4 h.
Hippocampal slice preparation
Adult WT and GHSR‐deficient mice were anaesthetized with isofluorane (2%) and immediately decapitated. Brains were quickly removed and hippocampal horizontal brain slices (300 μm) containing the hippocampus (Bischofberger et al. 2006; Xiong et al. 2017) were prepared in bubble ice‐cold 95% O2–5% CO2‐equilibrated solution containing (in mm): 110 choline chloride, 25 glucose, 25 NaHCO3, 7 MgCl2, 11.6 ascorbic acid, 3.1 sodium pyruvate, 2.5 KCl, 1.25 NaH2PO4 and 0.5 CaCl2. Slices were then stored at room temperature in 95% O2–5% CO2‐equilibrated artificial cerebrospinal fluid (aCSF) containing (in mm): 124 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2 and 1 NaH2PO4.
Drugs
In some patch‐clamp recordings on mouse neuronal primary culture the CaV2.1 blocker ω‐agatoxin‐IVA (0.2 μm, Peptides International, Louisville, KY, USA) and the CaV2.2 blocker ω‐conotoxin‐GVIA (1 μm, Alomone Labs, Jerusalem, Israel) were used.
Electrophysiology
Ion channel currents were recorded with an Axopatch 200 (Molecular Devices, San Jose, CA, USA) or an EPC7 (HEKA Electonik, Lambrecht/Pfalz, Germany) amplifier. Data were sampled at 20 kHz and filtered at 10 kHz (−3 dB) using pCLAMP8.2 (Molecular Devices) or PatchMaster (HEKA) software. Recording electrodes with resistances between 2 and 5 MΩ were used and filled with internal solution. Series resistances of less than 6 MΩ were admitted and compensated 80% with a 10 μs lag time. Current leak was subtracted on‐line using a P/−4 protocol. All recordings were obtained at room temperature (∼24°C).
Barium currents of primary neuronal cultures
Mouse‐cultured neurons of 5–21 days in vitro (DIV) were patched‐clamped in voltage‐clamp whole‐cell mode at a holding potential of −80 mV applying squared test pulses to 0 mV for 20 ms every 10 s. (Raingo et al. 2007). Internal pipette solution contained (in mm): 134 CsCl, 10 EGTA, 1 EDTA, 10 Hepes and 4 MgATP, pH 7.2 with CsOH. Neurons were bathed with high sodium external solution containing (in mm): 135 NaCl, 4.7 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 Hepes and 10 glucose, pH 7.4 with NaOH. After attaining the whole‐cell configuration, CaV currents were recorded replacing the external solution by a high barium solution containing (in mm): 10 BaCl2, 110 choline chloride, 20 tetraethylammonium chloride, 1 MgCl2, 10 Hepes, 10 glucose and 0.001 tetrodotoxin (TTX; Sigma‐Aldrich), pH 7.4 with CsOH.
Postsynaptic currents of primary neuronal cultures
Mouse neurons of 12–21 DIV were patch‐clamped in voltage‐clamp whole‐cell mode at a holding potential of −80 mV. Internal pipette solution contained (in mm): 115 caesium methanesulfonate, 10 CsCl, 5 NaCl, 10 Hepes, 20 tetraethylammonium chloride, 4 Mg‐ATP, 0.3 NaGTP, 0.6 EGTA and 10 lidocaine N‐ethyl bromide (pH 7.2 with CsOH). The external solution used was the high sodium solution described above, containing 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX, 10 μm Alomone Labs) and (2R)‐amino‐5‐phosphonopentanoate (APV, 100 μm Alomone Labs) or picrotoxin (50 μm, Sigma‐Aldrich) in order to isolate inhibitory or excitatory postsynaptic currents, respectively (IPSCs and EPSCs). Electrical stimulation through parallel platinum electrodes (duration, 1 ms; amplitude, 20 mA) was delivered while neurons were held at −80 mV to elicit evoked responses. TTX (1 μm) was added in order to record miniature IPSCs and EPSCs (mIPSCs and mEPSCs). For Fig. 3, paired‐pulse responses were recorded delivering paired electrical stimulation and varying the inter‐stimulus space between 20 and 200 ms. For Fig. 4, the amount of CaCl2 in the external solution was modified from 0.5 to 10 mm.
Figure 3. Paired pulse ratio (PPR) is modified by GHSR expression levels only for GABAergic transmission.
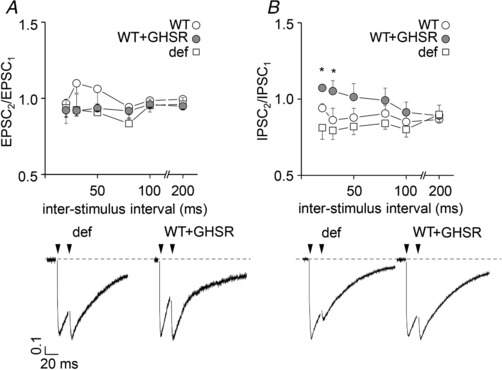
Average EPSC (A) and IPSC (B) PPR at different inter‐stimulus intervals (20–200 ms) from >14 DIV GHSR‐deficient (def) and wild‐type (WT) hippocampal neurons transduced with lentiviral plasmids encoding GHSR (WT+GHSR) or not (WT) and examples of paired pulses for WT overexpressing GHSR (WT+GHSR) and GHSR deficient (def) conditions evoked by 1 ms depolarizing stimuli at a 20 ms inter‐stimulus interval. Electrical stimuli are indicated by arrowheads. Statistical significance evaluated by Kruskal–Wallis ANOVA and Dunn's post hoc test (n = 283, 4–11 per mean data point).
Figure 4. Increased IPSC size due to the absence of GHSR is conserved at different Ca2+ concentrations.
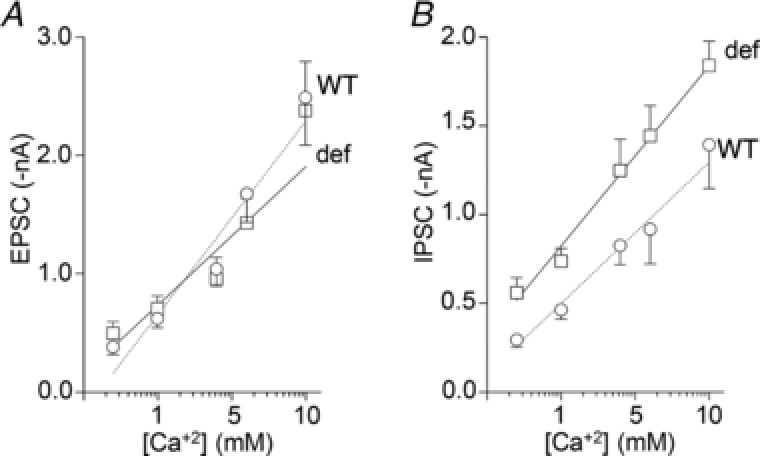
EPSC (A) and IPSC (B) average values at different Ca2+ concentrations (logarithmic scale) and linear regression plot from >14 DIV wild‐type (WT, open circles) and GHSR deficient (def, open squares) hippocampal neurons. Linear regression fitting parameters: EPSC slope test: F = 1.22978, degree of freedom for numerator (DFn) = 1, degree of freedom for denominator (DFd) = 75, P = 0.271, pooled slope = 0.576134 r 2 (WT) = 0.7167, r 2 (def) = 0.5260; intercepts test: F = 0.0203306, DFn = 1, DFd = 76, P = 0.887, pooled intercept = 2.77994. (n = 79, ranging between 3–12 per mean data point.) IPSC slope test: F = 0.699603, DFn = 1, DFd = 81, P = 0.4054, pooled slope = 0.501558, r 2 (WT) = 0.5170, r 2 (def) = 0.5888; intercepts test F = 29.3249, DFn = 1, DFd = 82, P < 0.0001, intercept (WT) = −0.50 ± 0.05 nA, intercept (def) = −0.82 ± 0.06 nA. (n = 85, ranging between 3–13 per mean data point.)
Hyperosmotic shock
Charge movement was recorded as a response to hyperosmotic shock, applied as a 0.5 m sucrose solution pulse while neurons were held at −80 mV. Average charge was measured as integrated area 3 s before and 3 s after peak response.
Postsynaptic currents from hippocampal slices
Recordings were made under a Zeiss Examiner.A1 microscope at 25°C in aCSF under a flow rate of 2.5 ml min−1. Access resistance and input resistance were monitored by a step of −10 mV. Experiments were discarded if the access resistance increased by >20%. The internal solution used to examine neuronal excitability contained (in mm): 140 potassium gluconate, 5 NaCl, 5 KCl, 4 MgCl2, 0.6 EGTA, 10 Hepes, 3 Na2ATP and 0.3 Na2GTP (pH 7.3 with KOH). Before recording postsynaptic currents, intrinsic properties of neurons were monitored by current‐clamp. Both IPSCs from granule cells and EPSCs from CA3 pyramidal cells were evoked by a concentric bipolar electrode placed in the granule cell layer of the hippocampus and recorded in whole‐cell voltage‐clamp configuration. Cells were maintained at −80 mV throughout the voltage clamp recording.
Statistics
Data were analysed using the OriginPro 8 (Origin‐Lab Corp., Northampton, MA, USA) and Prism 6 (GraphPad Software, Inc., San Diego, CA, USA) software. We used the Kolmogorov–Smirnov test to test for conformity to a normal distribution, and variance homogeneity was examined using Bartlett's (normally distributed data) and Brown–Forsythe's (non‐normally distributed data) test. P values were calculated from Student's t test or multiple comparison one‐way ANOVA with Tukey's post hoc test (normally distributed data), or from the Mann–Whitney test or the Kruskal–Wallis test with Dunn's post hoc test (non‐normally distributed data), after trying data transformation. The specific statistical test used and sample size are indicated for each data set in the figure legends. Data were expressed as mean ± SEM, and individual data points are represented as black dots.
Results
To assess whether GHSR constitutive activity could impact CaV currents in hippocampal neurons we performed voltage‐clamp whole‐cell recordings in primary cultured neurons from WT and GHSR‐deficient mice. We manipulated GHSR levels by infecting the cultures with lentiviruses expressing GHSR tagged with green fluorescent protein (GFP). We found that the total CaV current in hippocampal neurons significantly increased after 7 DIV (Fig. 1 A) and that GHSR overexpression occluded the CaV current increase in both genotypes (between 5 and 14 DIV). Notably, the increase in current amplitude was higher in neurons from the GHSR‐deficient cultures than from the WT cultures (>14 DIV, Fig. 1 B) and GHSR overexpression decreased CaV current levels to the same extent in both conditions. To confirm that the reduction of CaV current was due to GHSR ligand‐independent activity, we overexpressed a mutant version of GHSR, GHSRA204E, which lacks constitutive activity but signals upon ghrelin binding (Pantel et al. 2006; Inoue et al. 2011). We found that CaV current levels in neurons overexpressing GHSRA204E are not different from the WT condition (Fig. 1 B). Hence, our approach dismisses a possible effect of a ligand mediating this CaV current impairment. Altogether our results indicate that GHSR expression reduces CaV current levels in hippocampal neurons and that this effect is related to its constitutive activity.
Figure 1. GHSR constitutive activity affects native I CaV levels in hippocampal cultured neurons.
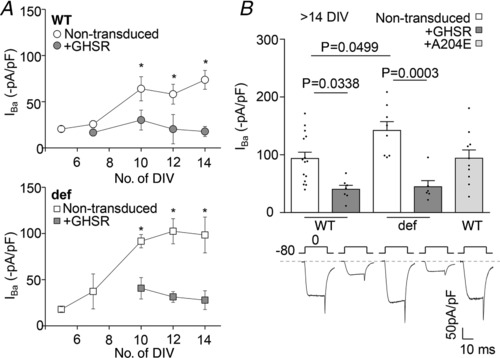
A, average I Ba levels from 5–14 DIV wild‐type (WT) and GHSR‐deficient (def) hippocampal neurons transduced with lentiviral plasmids encoding GHSR (+GHSR) or GHSRA204E (+A204E) or not transduced. Statistical significance evaluated by Mann–Whitney test (n = 122, 3–12 per mean data point). B, representative traces and average I Ba levels from mature (>14 DIV) hippocampal neurons in the same conditions as in A. Statistical significance evaluated by ANOVA and Tukey's post hoc test.
Since CaV channels couple depolarization to neurotransmitter release, we explored the effect of GHSR expression on CaV‐dependent synaptic activity. We measured the amplitude of EPSCs and IPSCs evoked by field stimulation in mature hippocampal cultures from WT and GHSR‐deficient mice. We found that EPSC amplitude was unaffected by GHSR or GHSRA204E overexpression in WT and GHSR‐deficient cultures (Fig. 2 A). In contrast, IPSC amplitude was significantly reduced by GHSR expression in WT and GHSR‐deficient cultures (Fig. 2 B). Additionally, in WT cultures, IPSC amplitude was unaffected by overexpression of GHSRA204E. These results indicate that GHSR expression exerts selective inhibitory effect on GABAergic neurotransmission. Next, we reasoned that CaV channels were likely involved in the GHSR‐dependent GABAergic neurotransmission impairment since GHSR constitutive activity reduces CaV currents (Figs. 1 and 2; Lopez Soto, 2015). We used different approaches to identify the specific targets of GHSR on inhibitory neurotransmission in hippocampal cultures. We estimated release probability, a presynaptic activity feature, in GHSR‐deficient and WT hippocampal cultures overexpressing or not overexpressing GHSR by measuring the ratio of two postsynaptic currents elicited by two consecutive pulses with different time intervals. We found that excitatory paired pulse ratios (PPR) were similar among GHSR‐deficient, WT and WT overexpressing GHSR cultures at any inter‐pulse time assayed (between 20 and 200 ms; Fig. 3 A). In contrast, inhibitory PPR was decreased in GHSR‐deficient cultures in comparison with cultures overexpressing GHSR at inter‐pulse intervals shorter than 50 ms (Fig. 3 B). This change could be attributed to the decrease in presynaptic CaV2 channel density and, in consequence, to more synaptic vesicles remaining available for release by the second pulse when GHSR is expressed. Notably, this PPR difference between GHSR‐deficient and GHSR‐expressing cultures disappeared at longer inter‐pulse intervals (Fig. 3 B), as expected since a longer time between pulses would allow synaptic vesicle pool replenishment in the GHSR‐deficient condition. We further characterized the calcium sensitivity of neurotransmitter release in hippocampal GHSR‐expressing or GHSR‐deficient cultures. We recorded EPSC and IPSC at five different external calcium concentrations ranging from 0.5 to 10 mm in WT and GHSR‐deficient cultures. While we found no differences in EPSC curves (Fig. 4 A), we observed that the y‐intercept of the IPSC size versus external calcium concentration curve in GHSR‐deficient neurons was significantly shifted from about −0.5 to −0.8 nA (Fig. 4 B). This suggests that the number of presynaptic CaV available for coupling the depolarization to synaptic vesicle release is smaller when GHSR is expressed in GABAergic neurons. Moreover, the IPSC versus calcium concentration curve slopes in WT and GHSR‐deficient cultures were the same (pooled slope = 0.501558, Fig. 4 B) indicating that the intrinsic ability of presynaptic CaV channels to trigger neurotransmission is unchanged by GHSR expression. Additionally, we assayed the effect of GHSR expression on CaV‐independent neurotransmitter release by two experimental paradigms. First we tested the frequency of excitatory and inhibitory miniature spontaneous events (mEPSC and mIPSC) in the presence of 100 μm of cadmium chloride, a specific CaV blocker, and found no differences among conditions (Fig. 5). We also found no differences in the current mobilized by neurotransmitter release (GABA or glutamate) evoked by a hyperosmotic solution (500 mm sucrose, Fig. 6). Taken together our results show that GHSR constitutive activity reduces presynaptic CaV density, decreasing probability of GABA release, while glutamate release is not affected, indicating a presynaptic mechanism exclusive for inhibitory neurotransmission impairment.
Figure 2. GHSR constitutive activity impairs CaV‐dependent GABAergic transmission while fails to modify glutamatergic transmission.
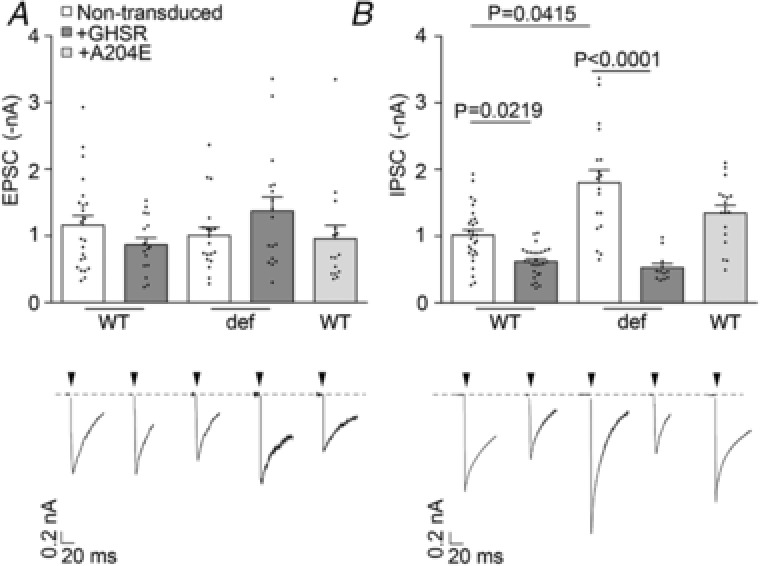
EPSC (A) and IPSC (B) evoked by electrical stimuli (indicated by arrowheads). Representative traces and average values from mature (>14 DIV) wild‐type (WT) and GHSR‐deficient (def) hippocampal neurons transduced with lentiviral plasmids encoding GHSR (+GHSR) or GHSRA204E (+A204E) or not transduced. Statistical significance evaluated by Kruskal–Wallis ANOVA and Dunn's post hoc test.
Figure 5. CaV‐independent mEPSCs and mIPSC are unaffected by GHSR constitutive activity.
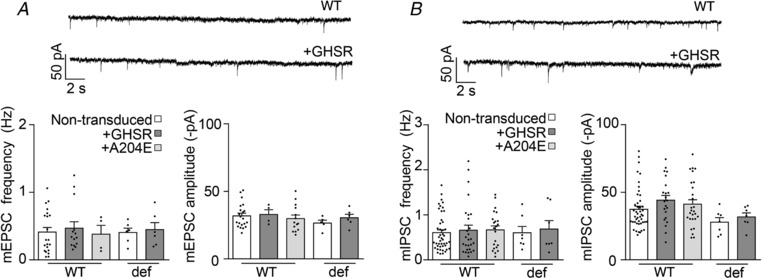
mEPSC (A) and mIPSC (B) average amplitude and frequency values and representative traces from mature (>14 DIV) wild‐type (WT) and GHSR‐deficient (def) hippocampal neurons transduced with lentiviral plasmids encoding GHSR (+GHSR) and GHSRA204E (+A204E) or not transduced. Statistical significance evaluated by Kruskal–Wallis ANOVA and Dunn's post hoc test. Both mEPSC and mIPSC were recorded in the presence of 1 μm TTX and the CaV blocker 100 μm Cd2+.
Figure 6. Hyperosmotic sucrose response is unaffected by GHSR expression.
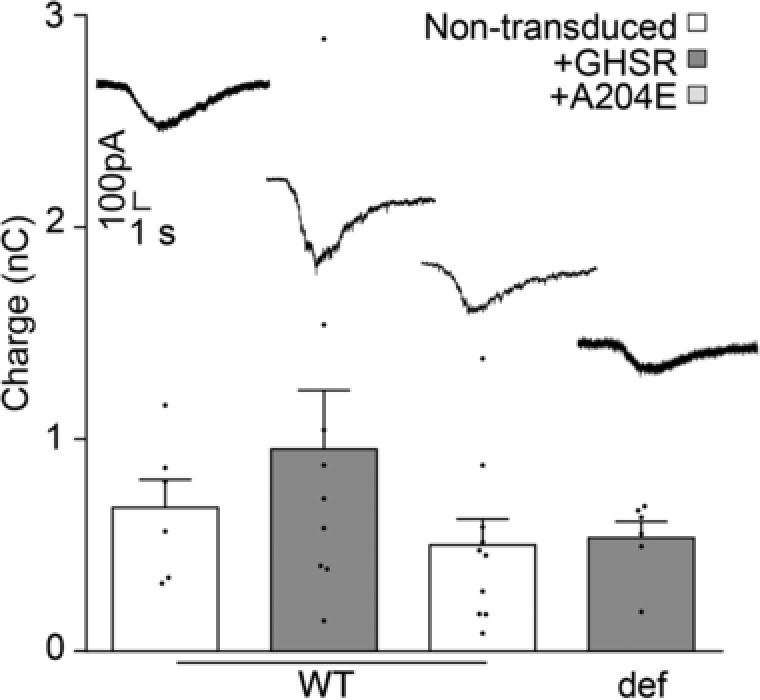
Representative traces and average mobilized charge values from mature (>14 DIV) wild‐type (WT) and GHSR deficient (def) hippocampal neurons transduced with lentiviral plasmids encoding GHSR (+GHSR) and GHSRA204E (+A204E) or not transduced (non‐transduced) in response to 0.5 m sucrose stimulation in the presence of 1 μm TTX and 100 μm Cd2+. Statistical significance evaluated by Kruskal–Wallis ANOVA and Dunn's post hoc test.
To test if there is a CaV subtype coupling preference to GABA or glutamate release underlying the specific GHSR effect on inhibitory neurotransmission, we characterized the CaV2.1 and CaV2.2 dependency of neurotransmitter release in hippocampal cultures expressing or not expressing GHSR. First, we assayed the effect of ω‐conotoxin‐GVIA, a specific inhibitor of CaV2.2, and ω‐agatoxin‐IVA, a specific inhibitor of CaV2.1, on CaV currents from neurons from mature GHSR‐deficient, WT and WT overexpressing GHSR mature cultures (Fig. 7). We found that the amount of CaV current inhibited by ω‐conotoxin‐GVIA was smaller in WT (∼30%) and GHSR‐overexpressing neurons (∼15%), compared with GHSR‐deficient neurons (∼45%), while the percentages of current inhibited by ω‐agatoxin‐IVA were the same in these conditions in comparison with GHSR‐deficient neurons (∼20%). These data suggest that GHSR constitutive activity impairs mainly CaV2.2 current in hippocampal neurons. Of note, consistent with our results (Fig. 1), we found a smaller total CaV current from WT neurons and GHSR‐overexpressing neurons in comparison with GHSR‐deficient neurons. Based on this result, we hypothesized that there is a CaV2.2 coupling preference to GABA rather than to glutamate release which in turn mediates the GHSR effect on inhibitory neurotransmission. Thus, we analysed the effect of ω‐conotoxin‐GVIA on IPSCs and EPSCs. We found a larger reliance of IPSC (37.7 ± 4.1%, n = 9) rather than of EPSC (21.23 ± 3.3%, n = 7) on CaV2.2 (P = 0.0097, Student's t test) in WT cultures. Additionally, as we previously showed, GHSR overexpression reduced IPSC amplitudes ∼50% (Figs. 2 and 8 A and B). IPSCs in WT cultures overexpressing GHSR were only ∼20% ω‐conotoxin‐GVIA sensitive, while in WT cultures the ω‐conotoxin‐GVIA sensitive IPSCs were ∼40% (Fig. 8 C). Consistent with our hypothesis, IPSC reliance on CaV2.1 of both WT and WT overexpressing GHSR cultures was similar (∼15%, Fig. 8 D). Thus, CaV2.1 dependency of IPSC was significantly smaller than the CaV2.2 dependency in GHSR WT cultures and it remained unchanged when GHSR was overexpressed. Taken together our results suggest that GHSR constitutive activity specifically decreases inhibitory neurotransmission by impairing CaV2.2 currents that support GABA release in hippocampal cultures.
Figure 7. GHSR expression reduces the contribution of CaV2.2 to total CaV currents.
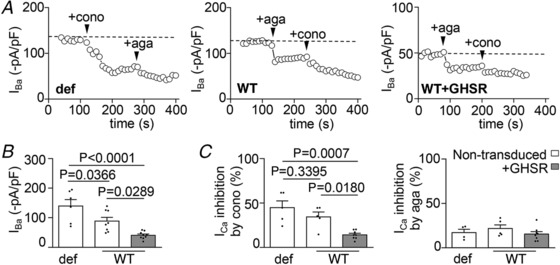
I Ba time courses of application of 1 μm ω‐conotoxin‐GVIA (+cono) and 0.2 μm ω‐agatoxin‐IVA (+aga) (A), averaged basal I Ba levels (B) and average percentage of I Ba inhibition (C) from >12 DIV GHSR‐deficient (def), wild‐type (WT) or wild‐type transduced with lentiviral plasmids encoding GHSR (WT+GHSR) hippocampal neurons. Statistical significance evaluated by Kruskal–Wallis ANOVA and Dunn's post hoc test.
Figure 8. GHSR expression inhibition of GABAergic transmission is related to a higher reliance on CaV2.2.
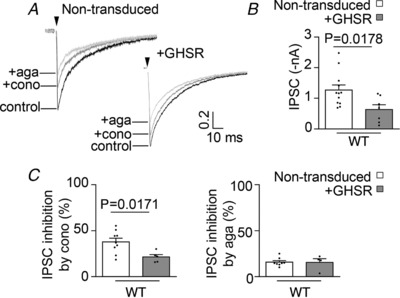
A, IPSC representative traces before (control) and after successive application of 1 μm ω‐conotoxin‐GVIA (+cono) and 0.2 μm ω‐agatoxin‐IVA, B and C, average basal levels (B) and percentage of inhibition (C) from >12 DIV wild‐type (WT) hippocampal neurons and wild‐type hippocampal neurons transduced with lentiviral plasmids encoding GHSR (+GHSR). Statistical significance evaluated by Student's t test.
Finally, to confirm that our results obtained in primary cultures are conserved in intact adult hippocampal tissue, we performed electrophysiological recordings in acute horizontal brain slices from dentate gyrus granule cells and CA3 pyramidal neurons from GHSR‐deficient and WT mice. In each experiment we monitored the intrinsic properties of both neuron types and we found no differences either in the resting membrane potential or in the firing voltage threshold between GHSR‐deficient and WT slices (in mV: V resting WT: −68.50 ± 2.90, GHSR‐deficient: −69.08 ± 4.53 from granule cells; WT: −57.27 ± 4.18, GHSR‐deficient: −57.50 ± 2.50 from CA3 pyramidal cells; V threshold WT: −45.33 ± 2.04, GHSR‐deficient: −41.54 ± .91 from granule cells; WT: −48.75 ± 5.32, GHSR‐deficient: −43.33 ± 1.67 from CA3 pyramidal cells. These values are in accordance with previous data showing that granule cells from the dentate gyrus display a characteristic low input resistance and a hyperpolarized resting membrane potential (Staley et al. 1992; Mongiat et al. 2009). As these cells are also subjected to strong inhibition by local interneurons (Dieni et al. 2013; Temprana et al. 2015), they display low firing frequencies at resting membrane potential (Krueppel et al. 2011).
In this experimental setting, we then recorded the magnitude of intra‐dentate gyrus IPSCs (GC‐IPSCs), using a stimulating electrode placed within the granule cell layer (Fig. 9 A). We found that GC‐IPSCs recorded from WT mice were significantly smaller than those from GHSR‐deficient mice (Fig. 9 B). In the same preparation, we explored if GHSR constitutive activity affects the excitatory output from the dentate gyrus to CA3, by measuring the amplitude of EPSCs in CA3 pyramidal neurons stimulating the granule cell layer (Fig. 9 C). In line with our previous results, we found that GHSR constitutive activity fails to modify excitatory transmission.
Figure 9. GHSR constitutive activity modulates synaptic transmission in hippocampal slices.
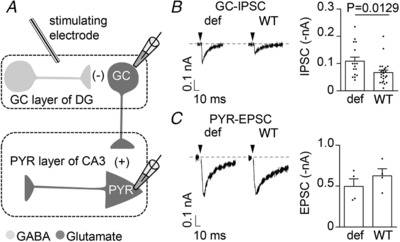
A, diagram of the DG‐CA3 circuit showing positions of extracellular stimulating and recording electrodes on the granule cell layer of the dentate gyrus (GC layer of DG) and pyramidal cell layer of CA3 (PYR layer of CA3). B, representative traces and average intra‐granule cell layer IPSCs (GC‐IPSCs) from wild‐type (WT) and GHSR‐deficient (def) mice. C, representative traces and average EPSCs from WT and GHSR‐deficient (def) pyramidal cell layer (PYR‐EPSCs) mice. Statistical significance evaluated by Mann–Whitney test.
Altogether, these last experiments indicate that the presence of GHSR in the dentate gyrus is capable of modulating the inhibitory transmission in intact hippocampal circuitry.
Discussion
Ghrelin‐induced GHSR activity controls neuronal function through multiple mechanisms. Postsynaptic mechanisms have been widely studied: GHSR controls transcriptional levels, enhances firing rates through Kv7 channel inhibition, and increases postsynaptic receptor membrane density and spine synapse number (Diano et al. 2006; Shi et al. 2013; Ribeiro et al. 2014). Despite the fact that many GPCRs act on neuronal circuits by regulating neurotransmitter release (Catterall & Few, 2008), information about GHSR impact on neurotransmission at the presynaptic level remains scarce (Cowley et al. 2003; Ribeiro et al. 2014; Lopez Soto, 2015; Cabral et al. 2016). In this regard, we have found that GABA release from hypothalamic explants is reduced when mice are exposed to fasting conditions, in which GHSR mRNA levels are increased, indicating that GHSR constitutive activity reduces GABAergic tone in the hypothalamus (Lopez Soto, 2015). Here we show that GHSR constitutive activity impairs inhibitory neurotransmission in hippocampal neurons in culture. Our data support a presynaptic effect mediated mainly by CaV2.2 current impairment that specifically affects GABA release. This study and our previous work add information to the several reports assaying ghrelin's effect in neurons (Cowley et al. 2003; Moran & Gao, 2006; Sleeman & Spanswick, 2014; Cabral, et al. 2015; Hsu et al. 2016) contributing to the understanding of the physiology of this GPCR, which has the highest constitutive activity known (Holst & Schwartz, 2004; Mear et al. 2013).
CaV2 channels are fundamental for neurotransmission, as they allow calcium influx into the presynaptic terminal which triggers neurotransmitter exocytosis (Dunlap et al. 1995). Therefore, control of CaV2 activity is essential for modulating neuronal processes (Catterall & Few, 2008), relying on mechanisms such as changes in CaV membrane distribution or density (Davidova, 2014; Ferron, 2014; Nakamura et al. 2015; Schneider et al. 2015), or changes in CaV activation and deactivation kinetics (Burgoyne & Weiss, 2001; Catterall & Few, 2008). Many GPCRs modulate CaV2 activity. We have previously shown that ghrelin‐induced GHSR activation acutely modulates CaV2.1 and CaV2.2 currents and neurotransmitter release. Moreover, we found that GHSR constitutive activity can also impair CaV currents in a chronic, Gi/o‐ and CaVβ‐subunit‐dependent and voltage‐independent manner (Lopez Soto, 2015; Mustafá et al. 2017). The impairment of CaV currents by GHSR constitutive activity is related to a decrease in CaV channel density at the plasma membrane together with a concomitant increase in CaV channel density at the endoplasmic reticulum and Golgi apparatus. Hence, we postulate that GHSR constitutive signalling reduces CaV channel forward trafficking resulting in a reduction in the CaV channel surface density and current. Since this chronic effect relies on GHSR sustained signalling, it would be independent from GHSR subcellular location. On the other hand, we expect that this effect would be more relevant during periods of active CaV2 channel trafficking such as synaptogenesis, development and adult neurogenesis (Bergami et al. 2015; Nakamura et al. 2015). In this context, we reasoned that primary neuronal culture was an adequate model to uncover the functional effect of GHSR constitutive activity at the presynaptic terminals. This model system is widely used to study the properties of synaptic transmission at the cellular level as it constitutes a preparation with a controlled environment which allows recapitulation of the properties of neuronal cells in vivo (Basarsky et al. 1994; Matteoli et al. 1995; Verderio et al. 1999). In this experimental setting we found that GHSR‐deficient neurons displayed an augmented level of total CaV current, and overexpressing GHSR avoided the CaV current increase over days in culture (Fig. 1). As expected, this effect at the synaptic level only modulates CaV‐dependent neurotransmission. We found no effect of GHSR expression on mEPSC and mIPSC or on GABA and glutamate released by application of hyperosmotic solution. Though we expected GHSR to be expressed on different neuronal subtypes (especially in conditions with receptor overexpression), GHSR constitutive activity specifically affects the CaV‐dependent release of GABA (Fig. 2).
If CaV currents are reduced indiscriminately in hippocampal neurons by GHSR constitutive activity, why does this effect target GABA but not glutamate release? We postulate that the preferential reliance of GABA release on CaV2.2 over CaV2.1 channels could underlie this selectivity. Indeed, it is expected that a reduction in the number of presynaptic calcium channels would have a greater effect on neurotransmitter release at synapses that depend mainly on CaV2.2 rather than on CaV2.1, since it has been demonstrated that CaV2.1 channels are more tightly coupled to synaptic vesicles and have a larger open probability than CaV2.2 channels at depolarizing potentials (Stanley, 2015). Furthermore, Cao and Tsien (2010) overexpressed both permeable and impermeable CaV versions in hippocampal neurons in culture and showed that CaV2.1 and CaV2.2 interact differentially with specific binding sites (slots) at the presynaptic terminals, thus regulating the relative contribution of each subtype to neurotransmitter release (Cao & Tsien, 2010). In particular, the number of presynaptic CaV2.1 channels present saturates a particular subgroup of slots, imposing a ceiling on the synaptic efficacy of these CaV channels. Thus, synapses that are mostly governed by CaV2.2 would be more sensitive to changes in the number of available channels compared to CaV2.1‐enriched presynaptic terminals. In addition, excitatory and inhibitory synapses rely differentially on CaV2 subtypes. Previous studies support a differential reliance of GABA and glutamate release on CaV2.2 that could explain the specific effect of GHSR on GABA release that we describe. Several studies also show that upon excitatory synapse maturation there is a switch from CaV2.2 to CaV2.1 coupling to glutamate release (Scholz & Miller, 1995; Pravettoni et al. 2000; Cao & Tsien, 2010). In contrast, GABA release has been shown to depend to a higher extent on CaV2.2 calcium influx upon synapse maturation (Basarsky et al. 1994). Remarkably, overexpression of CaV2.2 in neuronal cultures does not result in an increase in glutamate release, although the CaV currents do increase (Cao & Tsien, 2010). Furthermore, we observed a larger contribution of CaV2.2 to the total current and found that GHSR reduces the relative CaV2.2 contribution to the total CaV currents in culture. This is in agreement with previous observations indicating that CaV2.2 make a greater contribution than CaV2.1 to the total CaV current in hippocampal neuronal cultures (Pravettoni et al. 2000).
In terms of our choice of experimental setting, we consider that primary culture is a suitable model to study a mechanism that interrupts CaV channel trafficking because it recapitulates axonal and dendritic growth as well as synapse formation in a controlled manner. Despite this, we are aware that exploring the impact of GHSR modulation in an intact hippocampal circuit is necessary for a better understanding of its physiological impact. In the hippocampal formation, the dentate gyrus is a key structure for different types of learning in humans, primates and rodents, given that it is the entry point to the trisynaptic glutamatergic circuit (Burgess, 2002; Leutgeb et al. 2005; Bakker et al. 2008). Acting as an information filter, the granule cells of the dentate gyrus display low intrinsic activity (Liu et al. 2012; Ramirez et al. 2013; Denny et al. 2014; Danielson et al. 2016), strongly controlled by local GABAergic innervation (Nitz & McNaughton, 2004; Sambandan et al. 2010; Pernía‐Andrade & Jonas, 2014). In acute hippocampal slices, we found that GHSR‐deficient mice display larger GC‐IPSCs than WT mice (Fig. 9 B), indicating that GHSR constitutive activity can regulate the hippocampal circuit at the dentate gyrus level. To confirm that GHSR fails to affect the excitatory output from the dentate gyrus to CA3, we recorded EPSCs in CA3 stimulating the granule cell layer (Fig. 9 C). We found no differences between GHSR‐deficient and WT slices. This suggests that GHSR in the dentate gyrus affects exclusively GABAergic transmission. The selective effect of GHSR constitutive activity towards CaV2.2‐dependent GABAergic transmission could have important physiological implications for the hippocampal circuit. Further experiments should be conducted to address if the modulation of GC‐IPSCs by GHSR can affect the hippocampal excitatory/inhibitory balance or short‐term plasticity depending solely on GHSR expression levels (like the decrease in inhibitory PPR we observed in primary cultures, Fig. 3). Also, it would be interesting to investigate if this mechanism could support an already described presynaptic variant of long‐term depression in the intact brain. This form of plasticity has been shown for other Gi/o GPCRs that produce a sustained depression after application and washing of agonist (Kim & Kita, 2013). In the case of GHSR, the depression would originate from the sole expression of this receptor in a period of high CaV2 trafficking.
How could neurons tune the effect of this basally active GPCR? The answer could arise from different physiological situations. In the first place, it has been reported that GHSR expression in the hippocampus varies significantly within DIV (Lattuada et al. 2013; Ribeiro et al. 2014). The developmental profile of GHSR in hippocampal cultured neurons analysed by mRNA expression, western blots and immunofluorescence assays shows that GHSR expression levels are regulated during synapse development in vitro, exhibiting a significant increase from 7 to 21 DIV, in agreement with the timing in which we observed a strong increase in CaV currents in GHSR‐deficient neurons. Also, differences in the organism's energy balance caused by fasting can induce changes in GHSR expression levels in the rodent hypothalamus (Kim et al. 2003; Fernandez et al. 2018). On the other hand, GHSR has been described as heterodimerizing with other GPCRs, and this association could modify the basal signalling of GHSR constitutive activity (Kern et al. 2015; Schellekens et al. 2015; Wellman & Abizaid, 2015). Finally, GHSR constitutive activity could be adjusted by putative naturally occurring inverse agonists. We have previously shown that the chronic effect on CaV1 by another constitutively active GPCR, the melanocortin receptor type 4, can be prevented by its natural inverse agonist, agouti‐related peptide (Agosti et al. 2017). Although a specific natural inverse agonist for GHSR has not been discovered to date, many efforts are being made to develop synthetic inverse agonist with potential pharmacological actions (Holst et al. 2006, 2007; Moulin et al. 2007; Damian et al. 2012; Els et al. 2012).
Additional information
Competing interests
The authors declare no competing financial interests
Author contributions
All experiments presented in this work were performed in the Electrophysiology Laboratory of IMBICE. J.R. directed the conception and experimental design and contributed to data interpretation. S.S.R. amplified and purified the clones for the lentiviral vector assembly. V.M.D. performed data acquisition, analysis and interpretation. J.R. and V.M.D. prepared, wrote and revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by grants from the National Agency of Scientific and Technological Promotion of Argentina PICT2013‐1145 and PICT 2015‐3330; and from National University of La Plata X765 to J.R. V.M.D. and J.R. were supported by a National Scientific and Technical Research Council‐Argentina; and S.S.R. was supported by Comisión de Investigaciones Científicas.
Acknowledgements
We would like to thank Dr Javier López Soto for his assistance in the writing, editing and proofreading of the manuscript, and Dr Jeffrey Zigman for kindly donating the transgenic GHSR‐deficient mice.
Biography
Valentina Martínez Damonte is a PhD student in the Electrophysiology Laboratory of the Multidisciplinary Institute of Cellular Biology (IMBICE) in La Plata, Buenos Aires. Her current work is focused on voltage‐gated calcium channel modulation and her primary interest is to continue studying the mechanisms that underlie synaptic transmission.

Edited by: Ian Forsythe & Jian Yang
References
- Abizaid A (2011). Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Horm Behav 60, 572–580. [DOI] [PubMed] [Google Scholar]
- Abizaid A & Edwards A (2017). Clarifying the ghrelin system's ability to regulate feeding behaviours despite enigmatic spatial separation of the GHSR and its endogenous ligand. Int J Mol Sci 18, E859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosti F, Cordisco Gonzalez S, Martinez Damonte V, Tolosa M J, Di Siervi N, Schioth HB, Davio C, Perello M & Raingo J (2017). Melanocortin 4 receptor constitutive activity inhibits L‐type voltage‐gated calcium channels in neurons. Neuroscience 346, 102–112. [DOI] [PubMed] [Google Scholar]
- Atcha Z, Chen W‐S, Ong AB, Wong F‐K, Neo A, Browne ER, Witherington J & Pemberton DJ (2009). Cognitive enhancing effects of ghrelin receptor agonists. Psychopharmacology (Berl) 206, 415–427. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M & Stark CEL (2008). Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319, 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, Parpura V & Haydon PG (1994). Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci 14, 6402–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann KK, Schinder AF, Götz M & Berninger B (2015). A critical period for experience‐dependent remodeling of adult‐born neuron connectivity. Neuron 85, 710–717. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR & Jonas P (2006). Patch‐clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc 1, 2075–2081. [DOI] [PubMed] [Google Scholar]
- Burgess N (2002). The hippocampus, space, and viewpoints in episodic memory. Q J Exp Psychol A 55, 1057–1080. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD & Weiss JL (2001). The neuronal calcium sensor family of Ca2+‐binding proteins. Biochem J 353, 1–12. [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Cornejo MP, Fernandez G, De Francesco PN, Garcia‐Romero G, Uriarte M, Zigman JM, Portiansky E, Reynaldo M & Perello M (2017). Circulating ghrelin acts on GABA neurons of the area postrema and mediates gastric emptying in male mice. Endocrinology 158, 1436–1449. [DOI] [PubMed] [Google Scholar]
- Cabral A, De Francesco PN & Perello M (2015). Brain circuits mediating the orexigenic action of peripheral ghrelin: Narrow gates for a vast kingdom. Front Endocrinol 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Fernandez G & Perello M (2013). Analysis of brain nuclei accessible to ghrelin present in the cerebrospinal fluid. Neuroscience 253, 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Portiansky E, Sánchez‐Jaramillo E, Zigman JM & Perello M (2016). Ghrelin activates hypophysiotropic corticotropin‐releasing factor neurons independently of the arcuate nucleus. Psychoneuroendocrinology 67, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Valdivia S, Fernandez G, Reynaldo M & Perello M (2014). Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: critical role of brain accessibility. J Neuroendocrinol 26, 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill SP, Hatchard T, Abizaid A & Holahan MR (2014). An examination of early neural and cognitive alterations in hippocampal‐spatial function of ghrelin receptor‐deficient rats. Behav Brain Res 264, 105–115. [DOI] [PubMed] [Google Scholar]
- Cao YQ & Tsien RW (2010). Different relationship of N‐ and P/Q‐Type Ca2+ channels to channel‐interacting slots in controlling neurotransmission at cultured hippocampal synapses. J Neurosci 30, 4536–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Ghersi M, Schiöth HB & de Barioglio SR (2010). Ghrelin and memory: Differential effects on acquisition and retrieval. Peptides 31, 1190–1193. [DOI] [PubMed] [Google Scholar]
- Catterall WA & Few AP (2008). Calcium channel regulation and presynaptic plasticity. Neuron 59, 882–901. [DOI] [PubMed] [Google Scholar]
- Chen L, Xing T, Wang M, Miao Y, Tang M, Chen J, Li G & Ruan DY (2011). Local infusion of ghrelin enhanced hippocampal synaptic plasticity and spatial memory through activation of phosphoinositide 3‐kinase in the dentate gyrus of adult rats. Eur J Neurosci 33, 266–275. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia‐Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD & Horvath TL (2003). The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Cote DM, Ryabinin AE & Roberto M (2012). Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology 38, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar JN & Isokawa M (2011). Ghrelin‐induced activation of cAMP signal transduction and its negative regulation by endocannabinoids in the hippocampus. Neuropharmacology 60, 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian M, Marie J, Leyris JP, Fehrentz JA, Verdié P, Martinez J, Banères JL & Mary S (2012). High constitutive activity is an intrinsic feature of ghrelin receptor protein: A study with a functional monomeric GHS‐R1a receptor reconstituted in lipid discs. J Biol Chem 287, 3630–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson NB, Kaifosh P, Zaremba JD, Lovett‐Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A & Kheirbek MA (2016). Distinct contribution of adult‐born hippocampal granule cells to context encoding. Neuron 90, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidova D (2014). Bassoon specifically controls presynaptic P/Q‐type Ca2+ channels via RIM‐binding protein. Neuron 82, 181–194. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Clegg DJ & Benoit SC (2011). Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav 103, 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A & Hen R (2014). Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH & Horvath TL (2006). Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 9, 381–388. [DOI] [PubMed] [Google Scholar]
- Dieni CV, Chancey JH & Overstreet‐Wadiche LS (2013). Dynamic functions of GABA signaling during granule cell maturation. Front Neural Circuits 6, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K, Luebke JI & Turner TJ (1995). Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci 18, 89–98. [PubMed] [Google Scholar]
- Els S, Schild E, Petersen PS, Kilian T, Mokrosinski J, Frimurer TM, Chollet C, Schwartz TW, Holst B & Beck‐sickinger AG (2012). An aromatic region to induce a switch between agonism and inverse agonism at the ghrelin receptor. J Med Chem 55, 7437–7449. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Cabral A, Andreoli MF, Labarthe A, M'Kadmi C, Ramos JG, Marie J, Fehrentz J‐A, Epelbaum J, Tolle V & Perello M (2018). Evidence supporting a role for constitutive ghrelin receptor signaling in fasting‐induced hyperphagia in male mice. Endocrinology 159, 1021–1034. [DOI] [PubMed] [Google Scholar]
- Ferron L (2014). Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N‐type calcium channel density. Nat Commun 5, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersi MS, Gabach LA, Buteler F, Vilcaes AA, Schiöth HB, Perez MF & De Barioglio SR (2015). Ghrelin increases memory consolidation through hippocampal mechanisms dependent on glutamate release and NR2B‐subunits of the NMDA receptor. Psychopharmacology (Berl) 232, 1843–1857. [DOI] [PubMed] [Google Scholar]
- Henderson YO, Smith GP & Parent MB (2013). Hippocampal neurons inhibit meal onset. Hippocampus 23, 100–107. [DOI] [PubMed] [Google Scholar]
- Holst B, Cygankiewicz A, Jensen TH, Ankersen M & Schwartz TW (2003). High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol Endocrinol 17, 2201–2210. [DOI] [PubMed] [Google Scholar]
- Holst B, Lang M, Brandt E, Bach A, Howard A, Frimurer TM, Beck‐Sickinger A & Schwartz TW (2006). Ghrelin receptor inverse agonists: identification of an active peptide core and its interaction epitopes on the receptor. Mol Pharmacol 70, 936–946. [DOI] [PubMed] [Google Scholar]
- Holst B, Mokrosinski J, Lang M, Brandt E, Nygaard R, Frimurer TM, Beck‐Sickinger AG & Schwartz TW (2007). Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem 282, 15799–15811. [DOI] [PubMed] [Google Scholar]
- Holst B & Schwartz TW (2004). Constitutive ghrelin receptor activity as a signaling set‐point in appetite regulation. Trends Pharmacol Sci 25, 113–117. [DOI] [PubMed] [Google Scholar]
- Houser CR (2007). Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res 163, 217–233. [DOI] [PubMed] [Google Scholar]
- Hsu TM (2015). Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. Elife 4, e11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TM, Suarez AN & Kanoski SE (2016). Ghrelin: A link between memory and ingestive behavior. Physiol Behav 162, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Kangawa N, Kinouchi A, Sakamoto Y, Kimura C, Horikawa R, Shigematsu Y, Itakura M, Ogata T, Fujieda K; Japan Growth Genome Consortium (2011). Identification and functional analysis of novel human growth hormone secretagogue receptor (GHSR) gene mutations in Japanese subjects with short stature. J Clin Endocrinol Metab 96, E373–E378. [DOI] [PubMed] [Google Scholar]
- Kanoski SE & Grill HJ (2015). Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry 81, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Beynon AL, Hornsby AKE, Bekinschtein P, Bussey TJ, Davies JS & Saksida LM (2015). The orexigenic hormone acyl‐ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology 51, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Mavrikaki M, Ullrich C, Albarran‐Zeckler R, Brantley AF & Smith RG (2015). Hippocampal dopamine/DRD1 signaling dependent on the ghrelin receptor. Cell 163, 1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J & Kita H (2013). Short‐term plasticity shapes activity pattern‐dependent striato‐pallidal synaptic transmission. J Neurophysiol 109, 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M‐S, Yoon C‐Y, Park K‐H, Shin C‐S, Park K‐S, Kim S‐Y, Cho B‐Y & Lee H‐K (2003). Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport 14, 1317–1320. [DOI] [PubMed] [Google Scholar]
- Krueppel R, Remy S & Beck H (2011). Dendritic integration in hippocampal dentate granule cells. Neuron 71, 512–528. [DOI] [PubMed] [Google Scholar]
- Lattuada D, Crotta K, Tonna N, Casnici C, Benfante R, Fornasari D, Bianco F, Longhi R & Marelli O (2013). The expression of GHS‐R in primary neurons is dependent upon maturation stage and regional localization. PLoS One 8, e64183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser M‐B & Moser EI (2005). Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol 15, 738–746. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K & Tonegawa S (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Soto E, Agosti F, Cabral A, Mustafa ER, Damonte VM, Gandini MA, Rodríguez S, Castrogiovanni D, Felix R, Perelló M & Raingo J (2015). Constitutive and ghrelin‐dependent GHSR1a activation impairs CaV2.1 and CaV2.2 currents in hypothalamic neurons. J Gen Physiol 146, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G & Lacaille JC (2003). Interneuron Diversity series: Hippocampal interneuron classifications – Making things as simple as possible, not simpler. Trends Neurosci 26, 564–571. [DOI] [PubMed] [Google Scholar]
- Mani BK, Osborne‐Lawrence S, Mequinion M, Lawrence S, Gautron L, Andrews ZB & Zigman JM (2017). The role of ghrelin‐responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Mol Metab 6, 882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perelló M, Andrews ZB & Zigman JM (2014). Neuroanatomical characterization of a growth hormone secretagogue receptor‐green fluorescent protein reporter mouse. J Comp Neurol 522, 3644–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO & Paulsen O (2007). Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci 30, 343–349. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Verderio C, Krawzeski K, Mundigl O, Coco S, Fumagalli G & De Camilli P (1995). Mechanisms of synaptogenesis in hippocampal neurons in primary culture. J Physiol Paris 89, 51–55. [DOI] [PubMed] [Google Scholar]
- Mear Y, Enjalbert A & Thirion S (2013). GHS‐R1a constitutive activity and its physiological relevance. Front Neurosci 7, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat LA, Espósito MS, Lombardi G & Schinder AF (2009). Reliable activation of immature neurons in the adult hippocampus. PloS One 4, e5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH & Gao S (2006). Looking for food in all the right places? Cell Metab 3, 233–234. [DOI] [PubMed] [Google Scholar]
- Moulin A, Ryan J, Martinez J & Fehrentz JA (2007). Recent developments in ghrelin receptor ligands. ChemMedChem 2, 1242–1259. [DOI] [PubMed] [Google Scholar]
- Mustafá ER, López Soto EJ, Martínez Damonte V, Rodríguez SS, Lipscombe D & Raingo J (2017). Constitutive activity of the Ghrelin receptor reduces surface expression of voltage‐gated Ca2+ channels in a CaVβ‐dependent manner. J Cell Sci 130, 3907–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Harada H, Digregorio DA, Takahashi T, Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS & Shigemoto R (2015). Nanoscale distribution of presynaptic Ca2+ channels and its impact on vesicular release during article nanoscale distribution of presynaptic Ca2+ channels and its impact on vesicular release during development. Neuron 85, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D & McNaughton B (2004). Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol 91, 863–872. [DOI] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie‐Luton M‐P, Grouselle D, Kerdanet MDe, Kadir A, Epelbaum J, Le Bouc Y & Amselem S (2006). Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 116, 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M & Dickson SL (2015). Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol 27, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang J, Osborne‐Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M & Zigman JM (2010). Ghrelin increases the rewarding value of high fat diet in an orexin‐dependent manner. Biol Psychiatry 67, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernía‐Andrade AJ & Jonas P (2014). Theta‐gamma‐modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron 81, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravettoni E, Bacci A, Coco S, Forbicini P, Matteoli M & Verderio C (2000). Different localizations and functions of L‐type and N‐type calcium channels during development of hippocampal neurons. Dev Biol 227, 581–594. [DOI] [PubMed] [Google Scholar]
- Raingo J, Castiglioni AJ & Lipscombe D (2007). Alternative splicing controls G protein‐dependent inhibition of N‐type calcium channels in nociceptors. Nat Neurosci 10, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin P‐A, Suh J, Pignatelli M, Redondo RL, Ryan TJ & Tonegawa S (2013). Creating a false memory in the hippocampus. Science 341, 387–391. [DOI] [PubMed] [Google Scholar]
- Ribeiro LF, Catarino T, Santos SD, Benoist M, van Leeuwen JF, Esteban JA & Carvalho AL (2014). Ghrelin triggers the synaptic incorporation of AMPA receptors in the hippocampus. Proc Natl Acad Sci U S A 111, E149–E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandan S, Sauer J‐F, Vida I & Bartos M (2010). Associative plasticity at excitatory synapses facilitates recruitment of fast‐spiking interneurons in the dentate gyrus. J Neurosci 30, 11826–11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens H, De Francesco PN, Kandil D, Theeuwes WF, McCarthy T, Van Oeffelen WE, Perelló M, Giblin L, Dinan TG & Cryan JF (2015). Ghrelin's orexigenic effect is modulated via a serotonin 2C receptor interaction. ACS Chem Neurosci 6, 1186–1197. [DOI] [PubMed] [Google Scholar]
- Schneider R, Hosy E, Kohl J, Klueva J, Choquet D, Thomas U, Voigt A & Heine M (2015). Mobility of calcium channels in the presynaptic membrane. Neuron 86, 672–680. [DOI] [PubMed] [Google Scholar]
- Scholz KP & Miller RJ (1995). Developmental changes in presynaptic calcium channels coupled to glutamate release in cultured rat hippocampal neurons. J Neurosci 15, 4612–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Bian X, Qu Z, Ma Z, Zhou Y, Wang K, Jiang H & Xie J (2013). Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat Commun 4, 1435. [DOI] [PubMed] [Google Scholar]
- Sleeman MW & Spanswick DC (2014). Starving for ghrelin. Cell Metab 20, 1–2. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Otis TS & Mody I (1992). Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole‐cell recordings. J Neurophysiol 67, 1346–1358. [DOI] [PubMed] [Google Scholar]
- Stanley EF (2015). PresyNaptic calcium channels: Why is P selected before N? Biophys J 108, 451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM & Schinder AF (2015). Delayed coupling to feedback inhibition during a critical period for the integration of adult‐born granule cells. Neuron 85, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Coco S, Pravettoni E, Bacci A & Matteoli M (1999). Synaptogenesis in hippocampal cultures. Cell Mol Life Sci 55, 1448–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ni J, Dong J, Sun X, Li L & Lv Y (2013). Ghrelin increases hippocampal recombination activating gene 1 expression and spatial memory performance in mice. Neuroreport 24, 712–717. [DOI] [PubMed] [Google Scholar]
- Wellman M & Abizaid A (2015). Growth hormone secretagogue receptor dimers: A new pharmacological target. eNeuro 2, ENEURO.0053‐14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G, Metheny H, Johnson BN & Cohen AS (2017). A comparison of different slicing planes in preservation of major hippocampal pathway fibers in the mouse. Front Neuroanat 11, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW & Currie KPM (2013). Regulation of CaV2 calcium channels by G protein coupled receptors Biochim Biophys Acta 1828, 1629–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y‐H, Bangaru SD, He L, Abele K, Tanabe S, Kozasa T & Yang J (2008). Origin of the voltage dependence of G‐protein regulation of P/Q‐type Ca2+ channels. J Neurosci 28, 14176–14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Liu H, Xiao K, Yu M, Cui L, Zhu Q, Zhao R, Li G‐D & Zhou Y (2014). Ghrelin administration enhances neurogenesis but impairs spatial learning and memory in adult mice. Neuroscience 257, 175–185. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB & Elmquist JK (2006). Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494, 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB & Elmquist JK (2005). Mice lacking ghrelin receptors resist the development of diet‐induced obesity. J Clin Invest 115, 3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]


