Abstract
BACKGROUND:
Urogenital recurrent infections represent a global medical issue in the world, affecting millions of women because of dramatic shifts in bacterial composition and concentrations in response to numerous endogenous and exogenous factors. Urogenital microbiota forms a mutually beneficial relationship with their host and has a major impact on health and disease.
AIM:
This study aimed to compare probiotic therapy versus placebo in Oxidative Stress Values (OSVs) and histological features in urogenital infections in female patients.
METHODS:
Forty (n = 40) patients diagnosed with recurrent urogenital infections were recruited to be treated as test group (n = 20), receiving Probiotics, and a control group (n = 20), receiving looking similar placebo, both for 90 days. Both the groups were assessed for total oxidant capacity (d-ROMs test) and biological antioxidant potential as iron-reducing activity (BAP test) at baseline, after 1 and 3 months. Histological changes on inner vaginal mucosa were also investigated, during the entire study.
RESULTS:
d-ROM assay clearly showed that the values of the test group were significantly different, thus leading the general health conditions from a state of high oxidative stress to low oxidative stress levels. Increasing of BAP values were more significant, and clinically relevant, in probiotic test group over time.
CONCLUSION:
Our pilot study gave interesting and promising elements to confirm the safety and effectiveness of oral probiotics in preventing/reducing the recurrent urogenital infections by an overall modification of inner vaginal microbiota.
Keywords: Probiotics, Urogenital Tract Infections, Vaginal infections, Vaginal microbiota, Nutraceutics
Introduction
Urogenital infections are among the most common worldwide human infectious diseases [1]. Annually it is estimated that one billion women around the world suffer from non-sexually transmitted urogenital infections, including bacterial vaginosis, yeast vaginitis, and urinary tract infection (UTI). Although most patients respond to antimicrobial treatment, the recurrence rate is high and associated with side effects [2], with costs to health care providers amounting to over $6 billion annually worldwide. [3] UTI can lead to pelvic inflammatory disease, infertility, ectopic pregnancy, premature labour, low birth weight babies, chronic pain, and increased vulnerability to human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs). The urogenital system is an amalgamation of the urinary tract and reproductive system. Because both systems are open to the external environment, they are susceptible to infections. Some infections are introduced from outside, whereas others result from imbalances in the microbiota of the urogenital tract.
Urinary Tract Infections are typically caused by bacteria that normally live in the colon and rectum. Once bacteria are introduced into the urethra, they multiply and travel up to the bladder. E. coli is usually the most prevalent organism responsible for UTIs and accounts for 80–85% of the total isolates, with Staphylococcus saprophyticus being the cause in 5–10% [4] [5]. Other bacterial causing UTIs include Klebsiella, Proteus, Pseudomonas, Enterococcus, Enterobacter spp. Etc. Women are especially more prone to developing UTIs due to anatomical factors that allow quick bacterial access to the bladder, poor hygiene; sexual intercourse and use of contraceptive are also contributory factors. Other complications caused by UTIs are a bladder infection (cystitis), urethra infection (urethritis), kidney infection (pyelonephritis) and ureter (ureteritis) [6] [7] The main clinical and biological aspects reported in UTIs are briefly listed in Table 1.
Table 1.
UTIs Common Features
| • A strong, persistent urge to urinate. |
| • A burning sensation when urinating. |
| • Passing frequent, small amounts of urine. |
| • Urine that appears cloudy. |
| • Urine that appears red, a sign of blood in the urine. |
| • Strong-smelling urine. |
| • Pelvic pain. |
Recent studies have emphasised the importance of a healthy, lactobacilli dominated microbiota not only to prevent sexually transmitted diseases and preterm labour [8] but also to maintain the quality of life of women [9] [10]. The depletion of lactobacilli organisms in women susceptible to urinary and vaginal infections raised the question of whether artificial supplementation of lactobacilli could lower infection rates [8] [9] [10]. Nevertheless, drug resistance to commonly used antibiotics (e.g., trimethoprim/sulfamethoxazole) is increasing among uro-pathogens and patients are trying continuous alternative natural remedies such as cranberry juice, which appears to contain antiadhesive compounds that are active against uro-pathogens and can help prevent UTIs. Preventive therapies for UTIs are currently almost completely dependent on the use of antibiotics. In real terms, no true prophylaxis exists: current therapy involves long-term, low-dose antibiotic treatment, which involves the active killing of bacteria that enter the bladder. To develop a non-chemotherapeutic means to restore and maintain a healthy urogenital tract, probiotic therapy using lactobacilli has been considered, and there is evidence to indicate that certain strains can be effective when inserted directly into the vagina or when ascending from the rectum after oral ingestion [11] [12] [13]. Two strains, Lactobacillus rhamnosus and Lactobacillus fermentum, appear to be particularly adept at the latter [11] [12] [13] [14]. Repeated intake of probiotics could be important not only in women subjected to recurrent urogenital infections but also for all the healthy women to prevent severe infections and superinfections of their vaginal mucosa; in fact, the urogenital environment is often altered, and lactobacilli are decreased, thereby increasing the risk of locally acute infection [13]. Probiotics have been demonstrated to be able to protect against UTIs, maintaining the vaginal microbiome in proper balance. Researches in this field have shown that daily administration of Lactobacillus rhamnosus and Lactobacillus fermentum, specifically, can positively improve vaginal flora.
The aim of this pilot small-sized study is to investigate, in a 90days long clinical trial, whether daily administration of probiotics replacing lactobacilli (Hyperbiotics PRO-Women) can influence the vaginal microbiota.
Material and Methods
The present study was multicentric, entirely carried out in Italian clinics and healthcare buildings. All patients involved in this study were requested to read, understand and sign an informed consent. The study was conducted in compliance with the “Ethical principles for medical research involving human subjects” of Helsinki Declaration. Patients received a verbal description of the clinical protocol to be followed in this proposal of a clinical study.
Subjects recruited
A group of 51 patients were randomly recruited, after a careful preliminary screening for inclusion and exclusion criteria (Figure 1): patients receiving antibiotic therapy were excluded from the study.
Figure 1.
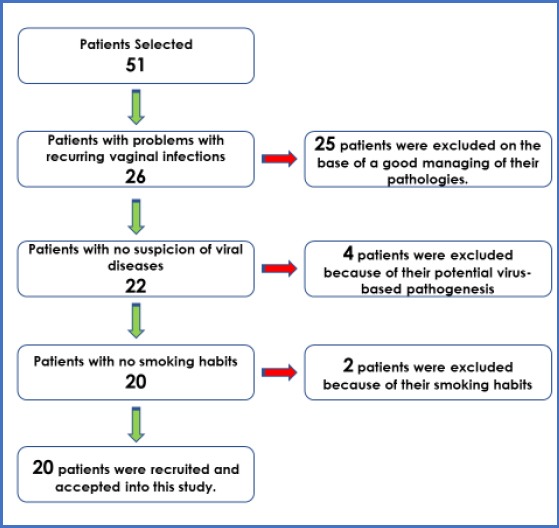
Patient recruitment and selection
Urine samples were analysed in private microbiological labs to assess the pathogens underlying the infections. A positive result was mainly found with specific pathogens, like E Coli (found in 44/51 patients) and Candida spp (found in 37/51 patients). Inclusion criteria were recurrent UTIs in patients reporting dysuria, frequency, urgency, and abdominal/flank pain with or without fever. Forty (n = 40) patients diagnosed with recurrent urogenital infections were recruited in this study. Half of them has been treated as test group (n = 20), receiving Probiotics investigated in this study, and a a control group (n = 20), receiving tablets looking similar to probiotics: tablets were administered for 90 days.
Both the groups were assessed for total oxidant capacity (d-ROMs test) and biological antioxidant potential as iron-reducing activity (BAP test) at baseline, after 1 and 3 months. Histological changes on inner vaginal mucosa were also investigated, during the entire study. The investigated probiotics were administered as oral tablets, thrice a day for the first month, and one tablet per day for the remaining two months (a total of 90 days was considered enough to get significative results). Variations in tablets administration, in patients’ compliance and other variations of protocol, were also recorded and reported in the final results.
Oxidative Stress Assay
The technical procedure for the evaluation of oxidative stress has been previously reported [15]. Oxidative stress assessment was performed using an integrated analytical system composed of a photometer and a mini-centrifuge (FRAS4, H&D S.R.L., Parma, Italy). Samples of whole capillary blood were taken by a finger puncture in a heparinized tube and immediately centrifuged; a small amount of samples plasma (10 μL) was thereafter tested for total oxidant capacity (d-ROMs test) and biological antioxidant potential as iron-reducing activity (BAP test) (Diacron International S.R.L., Grosseto, Italy). The d-ROMs test is based on the ability of a plasma sample to oxidise the N,N-Diethyl-p-phenylenediamine to its radical cation; the reaction is monitored photometrically at 37°C at 505 nm, and the results are expressed as Carratelli Units (CARR U, ΔAbs5050 nm/min). The oxidant capacity of plasma against N,N-Diethyl-p-phenylenediamine is mainly due to hydroperoxides, with the contribution of ferroxidase activity of ceruloplasmin and myeloperoxidase. Normal Values of d-ROMs Test range between 250 and 300 CARR Units. The BAP test is based on the ability of a plasma sample to reduce the iron of a coloured complex containing ferric ions to its ferrous derivative, not coloured; the reaction is monitored photometrically at 37°C at 505 nm, and the results are expressed in mol/L of reduced iron (using ascorbic acid as standard reference). Such biological antioxidant potential is mainly due to vitamin C, uric acid, bilirubin, albumin and tocopherols hydroperoxides. Normal values of BAP test are assessed over 2200 μM. BAP test values over 2200 μM was detected on the same machine, by using the same lot of kits; all tests were performed by the same operator in each of the sampling points, and the analytical instrumentation was calibrated before the analytical session using plasma with known values of d-ROMs and BAP test. To further check the reliability of the analysis mean, it was performed the same tests on samples of whole capillary blood taken from 5 healthy volunteers (recruited from the research staff) as a control. For each patient, a sample of capillary blood was taken up and subjected to BAP-test (normal values >2200 μM) and d-ROMs test (normal values between 250 and 300 U CARR). After 30 days (T1) and after 90 days (T2) the same assay was performed for Test-group and Control-group, as well as for healthy patients [16].
Microbiological assays
Patients of both groups were subjected to sampling in the inner part of their vaginal mucosa, for Gram staining examination. A Gram-staining system, proven to be effective in assessing the physiology of healthy vaginal flora was performed [15] [16]. Sealed packages containing the swabs, slides and transport medium for each subject were labelled and distributed by physicians to patients of each group. During the examination with a speculum, three cotton-tipped swabs of upper vaginal secretions were taken up. A first swab was rolled against a glass slide, which was sprayed immediately with cytological fixative. A second swab was placed in a modified Amies clear transport medium, and the third swab was rolled onto a glass slide which was air-dried. The air-dried slide was delivered to the laboratory within 8 hours. All slides were heat-fixed and then Gram-stained. The Gram-stained slides were prepared by expert laboratory technicians. Each of two technicians independently assessed the quality (clarity and preservation of Gram-negative and positive organisms) of each slide. The same examinations were carried out at intervals T1 and T2.
Probiotics
The probiotics used in this study are made with 6 targeted probiotic strains (L. plantarum, L. fermentum, L. acidophilus, L. reuteri, L. rhanmnosus, B. bifidum). Each probiotic tablet contains more than 5 billion Colony Forming Units. The probiotics used in this study have been created and produced to support digestive, urinary, and immune function, by combining the efficacy of cranberry extract with the clinically studied effects of naturally occurring D-Mannose. D-Mannose actively works to prevent undesirable recurrent pathologies, like bacterial infections, located in the urinary tract.
Statistical Analysis
All data were collected and statistically evaluated. T0 data were statistically compared with T1 and T2 data, using “paired t-test”.
Results
All recruited patients were compliant to this pilot study: no patients reported adverse reactions, and none dropped out of the study before the conclusion of the experimental stages. Free radical production occurs continuously in the cell during metabolism. These radicals (hydroxyl, superoxide anion, nitric oxide, etc.) are in part toxic to cell and cell membranes; however, they are normally controlled by countervailing biologic mechanisms. Severe oxidative stress produces ROS and induces uncontrolled lipid peroxidation [17]. Urinary tract infection causes oxidative stress, increases lipid peroxidation level, and leads to insufficiency of antioxidant enzymes [18]. Oxidative stress in test and control groups showed significant differences among baseline (T0) and at the end of the study, i.e. 90 Days (T2). The results showed significantly higher levels of oxidative stress at baseline in the test group compared to the control group (Table 3).
Table 2.
Subject Selection Criteria
| Inclusion Criteria |
| a. Caucasian aged between 18-50 years. |
| b. Volunteer to participate in the study. |
| c. Diagnosed with recurrent urogenital infection. |
| Exclusion Criteria |
| a. Any systemic disease like liver, kidney and thyroid. |
| b. Recent surgical treatments. |
| c. Pregnant Women. |
| d. Smoking habits. |
| e. Patients with sexually transmitted viruses onset on vaginal mucosa. |
| f. Patients with frequently recurring complicated vaginitis with a high fever. |
| g. Diseases are leading to influence oxidative stress, like syndromic conditions & rheumatological diseases. |
| h. Subject received antibiotics for the last 3 months and/or during the study |
| i. Subjects received immunosuppressive drugs |
Table 3.
Test group: oxidative stress values
| BAP Test (mmol/L) | d-ROM Test (U CARR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | T0 | T1 | T2 | T0-T1 % Variation | T0-T2 % Variation | T0 | T1 | T2 | T0-T1 % Variation | T0-T2 % Variation |
| Test | 1629 | 2199 | 2365 | 34.99% | 45.18% | 432 | 358 | 322 | -17.12% | -25.46% |
| Control | 1645 | 1905 | 1805 | 15.80% | 9.72% | 440 | 450 | 457 | 2.27% | 3.86% |
The oxidative stress reduction was significantly lower in the test group, compared to the control group. On recruitment (T0), the mean value of d-ROMs in test and control group was 432 and 440 respectively. The first follow up (T1) result in -17.12 % decreases in d-Rom values in the test group as compared to an increase of 2.27% increase in the control group. The second follow (T2) there was further decrease in d-ROM value in the test group, i.e. total reduction of 25.46% from baseline value as compared to control group where d-ROM values further increased and reached to 3.86% enhancement from baseline to second follow up. On recruitment (T0), the mean value of BAP in test and control group was 1629 and 1645 respectively. The first follow up (T1) result in 34.99 % increases in BAP values in the test group as compared to an increase of 15.80% increase in the control group. The second follow (T2) there was further increase in BAP value in the test group, i.e. total improvement of 45.18% from baseline value as compared to control group where BAP values further decreased and hence reached to only 9.72% enhancement from baseline to second follow up. The ANOVA test showed high statistical significance (p < 0.001) between compared data (Figure 2 and 3). This statistically significant difference in test and control group in both d-ROM and BAP values highlights the importance of oral probiotics in improving oxidative stress level in cases of urogenital infections.
Figure 2.
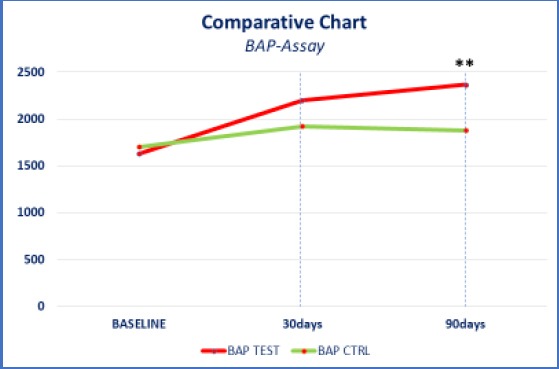
Comparative chart: bap-assay
Figure 3.
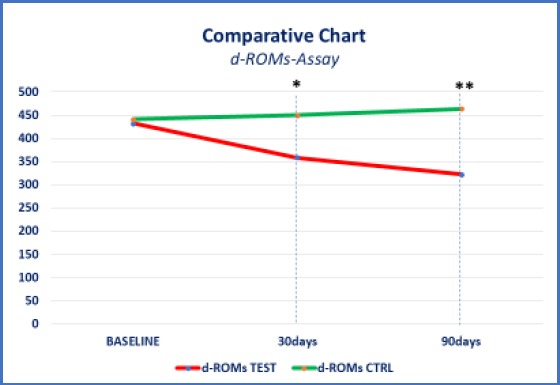
Comparative chart: d-ROM-Assay
Histological examinations also confirmed the positive feedback from vaginal mucosa in test-group. The mature epithelial cells of the surface are returned to be largely flattened and fused, and they show small dense pyknotic nuclei (< 4 μ). The elimination of glycogen by lactobacilli causes an acidic pH that protects against bacterial and fungal infections such as from candida albicans.
Discussion
The vaginal environment has an acidic pH; this condition helps prevent the proliferation of harmful bacteria and maintains lactobacilli levels (beneficial bacteria that protect the environment). Vaginal pH balance can be influenced by some factors such as menstrual flow, antibiotic intake, excessive hygiene, and use of an IUD (intrauterine device), sperm. If the pH changes, there may be an excessive development of anaerobic organisms that replace the normal lactobacilli, causing bacterial vaginosis. Women of reproductive age, between 15 and 44 years, are the category most exposed to risk. In many cases the infection is asymptomatic, but symptoms such as light-coloured vaginal secretions, a strong odour, especially after sexual intercourse, more rarely pain, itching and burning during urination can occur. The causes of bacterial vaginosis are not fully known as their link to sexual activity has not been fully understood. However, an increased incidence of this infection was found in women with many partners. Bacterial vaginosis can be relapsing, and if it is not treated, it can give rise to complications. For example, if it arises during pregnancy, it may increase the risk of spontaneous abortion or premature birth [19].
The application of exogenous organisms, such as lactobacilli, to the host, is termed probiotics, which is broadly defined as a living microorganism administered to promote the health of the host by treating or preventing disease [20]. The results of this study are inconsistent with the previous studies that also advocated the beneficial effects of supplementing oral probiotics for prevention of urogenital infection [12] [20]. Study by Anukam et al. reported in their study an effective 90% improvement of bacterial vaginosis using probiotic lactobacilli [21]. In vivo study concluded by Pascual et al indicated that the probiotic produced both preventative and curative effects on E. coli growth [22]. Studies affirmed that healthy epithelial and mucosal tissues, without any oncological onset, equally in younger and older patients, are the first step towards the protective barrier made by probiotics, that has widely demonstrated to prevent colonisation by pathogenic microorganisms [23] [24]. There is good clinical evidence to show that the intestinal and urogenital microbiota have a central role in maintaining both the health and wellbeing of humans. Furthermore, the use of good bacteria to replace or augment bacterial populations is increasingly achieving scientific acceptance.
In conclusion, the role of the human microbiota in urogenital women’s health has been demonstrated to influence the duration, the relapses and the severity of infective episodes. Microbiota has also been related to changes in women’s physiology during pregnancy and some dysmetabolic diseases. The oxidative stress seems to have the role of a biological marker, highlighting such conditions of inflammatory stress that may lead to superinfections or histological changes. Some studies have demonstrated the positive effects of many nutraceuticals and probiotics in regulating the commensal microbiota composition and distribution: these effects reflect biology and physiology of tissues involved, thus preventing and reducing the severity of infections and related inflammatory phenomena. In our pilot study, significant improvements have been reported in test-Group, related to systemic oxidative stress (BAP/d-ROMs), confirming the positive influence of probiotics on preventing/reducing UTIs. Histological findings describing the gram-staining of the vaginal inner mucosa, before and after probiotics treatment, in test-group reported a healthy vaginal microbiota. Our results, in conclusion, in this preliminary study, limited by the small size of involved patients, suggest that a daily probiotic administration should represent a useful tool for improving the women’s overall health, with a specific benefit on UTIs, with no adverse effects. This approach may provide a valuable addition to the current therapeutic options for UTIs in women also by regulating the local mucosal microbiota.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Skerk V, Markotić A. Urogenital infections--antimicrobial treatment. Med Glas (Zenica) 2010;7(1):1–11. [PubMed] [Google Scholar]
- 2.Reid G, Bruce AW. Urogenital infections in women: can probiotics help? Postgrad Med J. 2003;79(934):428–432. doi: 10.1136/pmj.79.934.428. https://doi.org/10.1136/pmj.79.934.428 PMid:12954951 PMCid: PMC1742800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foxman B, Barlow R, D'Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–15. doi: 10.1016/s1047-2797(00)00072-7. https://doi.org/10.1016/S1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 4.Cicinelli E, Ballini A, Marinaccio M, Poliseno A, Coscia MF, Monno R, De Vito D. Microbiological findings in endometrial specimen: our experience. Arch Gynecol Obstet. 2012;285(5):1325–9. doi: 10.1007/s00404-011-2138-9. https://doi.org/10.1007/s00404-011-2138-9 PMid:22113463. [DOI] [PubMed] [Google Scholar]
- 5.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008;16(2):CD005131. doi: 10.1002/14651858.CD005131.pub2. https://doi.org/10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Giudice G, Cutrignelli DA, Sportelli P, Limongelli L, Tempesta A, Gioia GD, Santacroce L, Maiorano E, Favia G. Rhinocerebral Mucormycosis with Orosinusal Involvement: Diagnostic and Surgical Treatment Guidelines. Endocr Metab Immune Disord Drug Targets. 2016;16(4):264–269. doi: 10.2174/1871530316666161223145055. https://doi.org/10.2174/1871530316666161223145055 PMid:28017141. [DOI] [PubMed] [Google Scholar]
- 7.John AS, Mboto CI, Agbo B. A review of the prevalence and predisposing factors responsible for urinary tract infection among adults. Euro J Exp Bio. 2016;6(4):7–11. [Google Scholar]
- 8.Hanson L, VandeVusse L, Jermé M, Abad CL, Safdarv N. Probiotics for Treatment and Prevention of Urogenital Infections in Women: A Systematic Review. J Midwifery Womens Health. 2016;61(3):339–55. doi: 10.1111/jmwh.12472. https://doi.org/10.1111/jmwh.12472 PMid: 27218592. [DOI] [PubMed] [Google Scholar]
- 9.Marrelli M, Tatullo M, Dipalma G, Inchingolo F. Oral infection by Staphylococcus aureus in patients affected by White Sponge Nevus: a description of two cases occurred in the same family. Int J Med Sci. 2012;9(1):47–50. doi: 10.7150/ijms.9.47. https://doi.org/10.7150/ijms.9.47 PMid:22211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32(1):37–41. doi: 10.1111/j.1574-695X.2001.tb00531.x. https://doi.org/10.1111/j.1574-695X.2001.tb00531.x PMid:11750220. [DOI] [PubMed] [Google Scholar]
- 11.Reid G, Bruce AW. Selection of Lactobacillus strains for urogenital probiotic applications. J Infect Dis. 2001;1(183):S77–S80. doi: 10.1086/318841. https://doi.org/10.1086/318841 PMid:11171021. [DOI] [PubMed] [Google Scholar]
- 12.Reid G, Bruce AW, Taylor M. Influence of three-day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infections. Clin Ther. 1992;14(1):11–6. PMid:1576619. [PubMed] [Google Scholar]
- 13.Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol. 2001;30(1):49–52. doi: 10.1111/j.1574-695X.2001.tb01549.x. https://doi.org/10.1111/j.1574-695X.2001.tb01549.x PMid:11172991. [DOI] [PubMed] [Google Scholar]
- 14.Tatullo M, Marrelli M, Scacco S, Lorusso M, Doria S, Sabatini R, Auteri P, Cagiano R, Inchingolo F. Relationship between oxidative stress and “burning mouth syndrome” in female patients: a scientific hypothesis. Eur Rev Med Pharmacol Sci. 2012;16(9):1218–21. PMid:23047505. [PubMed] [Google Scholar]
- 15.Ballini A, Santacroce L, Cantore S, Bottalico L, Dipalma G, De Vito D, Gargiulo C, Saini R, Inchingolo F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr Metab Immune Disord Drug Targets. 2018 doi: 10.2174/1871530319666181221150352. (in press) PMid:29692270. [DOI] [PubMed] [Google Scholar]
- 16.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardization method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. PMid:1706728 PMCid: PMC269757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatullo M, Simone GM, Tarullo F, Irlandese G, Vito D, Marrelli M, Santacroce L, Cocco T, Ballini A, Scacco S. Antioxidant and Antitumor Activity of a Bioactive Polyphenolic Fraction Isolated from the Brewing Process. Sci Rep. 2016;27(6):36042. doi: 10.1038/srep36042. https://doi.org/10.1038/srep36042 PMid:27786308 PMCid: PMC5081531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belge Kurutas E, Ciragil P, Gul M, Kilinc M. The Effects of Oxidative Stress in Urinary Tract Infection. Mediators Inflamm. 2005;2005(4):242–244. doi: 10.1155/MI.2005.242. https://doi.org/10.1155/MI.2005.242 PMid:16192676 PMCid: PMC1526480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballini A, Cantore S, Fatone L, Montenegro V, De Vito D, Pettini F, Crincoli V, Antelmi A, Romita P, Rapone B, Miniello G, Perillo L, Grassi FR, Foti C. Transmission of non-viral sexually transmitted infections and oral sex. J Sex Med. 2012;9(2):372–84. doi: 10.1111/j.1743-6109.2011.02515.x. https://doi.org/10.1111/j.1743-6109.2011.02515.x PMid:22023797. [DOI] [PubMed] [Google Scholar]
- 20.Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(1):S74–6. doi: 10.1086/318842. https://doi.org/10.1086/318842 PMid:11171020. [DOI] [PubMed] [Google Scholar]
- 21.Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8(12-13):2772–2776. doi: 10.1016/j.micinf.2006.08.008. https://doi.org/10.1016/j.micinf.2006.08.008 PMid:17045832. [DOI] [PubMed] [Google Scholar]
- 22.Pascual L, Ruiz F, Giordano W, Barberis I. Vaginal colonization and activity of the probiotic bacterium Lactobacillus fermentum L23 in a murine model of vaginal tract infection. J Med Microbiol. 2009;59(3):360–364. doi: 10.1099/jmm.0.012583-0. https://doi.org/10.1099/jmm.0.012583-0 PMid:19926731. [DOI] [PubMed] [Google Scholar]
- 23.Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol. 2006;48(3):424–432. doi: 10.1111/j.1574-695X.2006.00162.x. https://doi.org/10.1111/j.1574-695X.2006.00162.x PMid:17059467. [DOI] [PubMed] [Google Scholar]
- 24.Gil NF, Martinez RC, Gomes BC, Nomizo A, De Martinis EC. Vaginal lactobacilli as potential probiotics against Candida spp. Braz J Microbiol. 2010;41(1):6–14. doi: 10.1590/S1517-83822010000100002. https://doi.org/10.1590/S1517-83822010000100002 PMid:24031455 PMCid: PMC3768620. [DOI] [PMC free article] [PubMed] [Google Scholar]


