Abstract
BACKGROUND:
Paclitaxel-induced peripheral neuropathy is the most important side effect limiting the use of this medication.
AIM:
This study aimed to compare the effects of omega-3 and vitamin E on the incidence of peripheral neuropathy in patients receiving Taxol.
METHODS:
In this clinical trial, 63 patients who were a candidate for receiving taxol, were enrolled based on inclusion and exclusion criteria. In group O, patients received 640 mg omega-3 three times a day, and group E, received 300 mg vitamin E two times a day. Patients took the supplements up to three months after the onset of Taxol. Group P received placebo for a similar period. All patients referred to a neurologist for electrophysiological evaluation before the onset of chemotherapy and at months 1 and 3. The presence of neuropathy and its progression was recorded by the neurologist.
RESULTS:
Neurological examination in this study indicated that 6 patients (28.6%) in Group O, 7 patients (33.3%) in group E, and 15 patients (71.4%) in placebo group started peripheral neuropathy. There was a significant difference between intervention groups and the placebo group (p = 0.0001) and no significant difference between intervention groups (p = 0.751).
CONCLUSION:
Our data suggested that vitamin E and omega-3 may significantly reduce the incidence of Paclitaxel-induced peripheral neuropathy. Routine administration of such supplements that have no special side effect for patients under chemotherapy may greatly enhance their quality of life.
Keywords: Neuropathy, Omega 3, Paclitaxel, Vitamin E
Introduction
Taxol is one of the taxane-derived chemotherapeutic agents used to treat solid tumours such as breast, ovary, lung and Kaposi’s sarcoma. The source of this drug is a plant called Taxus brevifolia, a native of North America. Taxol is anti microtubular agent that causes polymerisation of the tubulin, and it also leads to the formation of stable microtubules without dynamic instability [1] [2]. Peripheral neuropathy is likely to be the major side effect of Taxol. The exact pathology of this complication is still not well known. Due to the accumulation of microtubules in axons and Schwann cells, Taxol causes an axonal sensory peripheral neuropathy. This drug, even in more severe cases, may also cause fibre demyelination [1]. It usually takes three weeks to develop toxic, toxic signs of Taxol, which affects the sensory nervous system more than the autonomic nervous system (ANS). The most common symptoms of taxol due to neuropathy include anaesthesia, paresthesia and burning pain in the gloves. The onset of symptoms often occurs in the hands and feet simultaneously, and some patients also complain of discomfort [3] [4].
Omega-3 fatty acids, such as EPA and DHA, are unsaturated fatty acids present in phospholipid membranes of cells, including central and peripheral nervous system cells [5]. They have very beneficial effects in some psychiatric and neurodegenerative diseases. They play a decisive role in the biophysical properties of neuronal membranes and modulate the signal transduction by effects on ion channels and receptor function [6]. In addition, the production of anti-inflammatory cytokines that cause neuropathy is reduced by these fatty acids, especially DHA [5] [6]. DHA-linked myelogenesis has been proven in recent studies [7]. Vitamin E is widely used to prevent diseases caused by oxidative stress. Its vital effects on neuronal function have been indicated in many diseases of vitamin E deficiency and central and peripheral nervous system manifestations. Studies have shown that neuropathic effects of cisplatin may have neuroprotective effects [8] [9]. Another study found that vitamin E was effectively and safely protected against neuropathy in cancer patients treated with taxol [10]. Most side effects of chemotherapy are eliminated after treatment discontinuation, but peripheral neuropathy may be somewhat reversible or irreversible in some patients, which can have adverse effects on the patient’s quality of life. Therefore, this study aimed to compare the effect of omega-3 and vitamin E on the incidence of peripheral neuropathy in taxol recipients.
Material and Methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and was approved by Arak University of Medical Sciences Institutional Review Board (Protocol number IR.ARAKMU.REC.2015.219; November 23, 2015).
In this clinical trial study, 63 patients aged 30-70 years who received taxol from Ayatollah Khansari Hospital in Arak city were included in the study. Inclusion criteria included all patients with a solid tumour (lung, breast, ovaries, etc.), patients with a good liver and kidney status that received the first taxol treatment.
Exclusion criteria include patients with previous history of chemotherapy, the presence of neuropathy due to diabetes, AIDS, alcohol abuse, thyroid dysfunction and hereditary neuropathy, the use of any dietary supplement (fish oil, vitamins and minerals) at least three months before entering the study, smoking and drugs use, low back pain and the use of other chemotherapy drugs.
Blood samples were taken from patients for liver and kidney function, and BUN, creatinine, ALT and AST values were measured. Patient’s history of treatment was also fully completed. Then, the patients were randomly divided into three equal groups according to the number table. In the first group (group O), patients received omega-3 capsules of 640 mg three times a day while receiving taxol (11), the second group (group E) received vitamin E supplements at a dose 300 mg twice daily (10). The duration of use of omega-3 and vitamin E was determined from the time of receipt of taxol to three months after its discontinuation. In the third group (group P), patients also used placebo capsules for the same period.
Before chemotherapy and one and three months later, all patients were examined by a neurologist who is responsible for the clinical and electrophysiological evaluation of the patients. Then the presence of neuropathy was recorded by the specialist. The person who registered the information was not aware of the patient’s group, and the patients were not aware of their position in the relevant groups considering the medical ethics conditions.
After completing the checklist, data were entered into the SPSS software version 20. The analytical analysis was performed with appropriate methods such as independent T-test or nonparametric methods to test the difference in mean in different groups. Paired t-test was used to compare the quantitative parameters at the beginning of the study and after 4 months of treatment, while the qualitative data were analysed using the chi-square test.
Results
In this study, 69 patients with different types of cancers were studied. Six patients were excluded from the study due to discontinuation of the drug. Finally, 63 patients were evaluated in three groups. In the omega-3 group, 15 women (71.4%) and 6 men (28.6%) were present. In the vitamin E group, 16 women (76.2%) and 5 men (23.8%) were admitted, while 15 (71.4%) and 6 men (28.6%) were presented in the control group. Data analysis using chi-square test depicted that there was no significant difference between the three groups regarding gender (p = 0.886), (Figure 1).
Figure 1.

Frequency of sex in three groups
The mean age of patients in the group O was 51.5 ± 9.2 years, followed by 50.9 ± 10.4 years in the group E and 52.2 ± 10.1 years in the group P. The t-test revealed no significant difference between the age of three groups (p = 0.664), (Figure 2).
Figure 2.
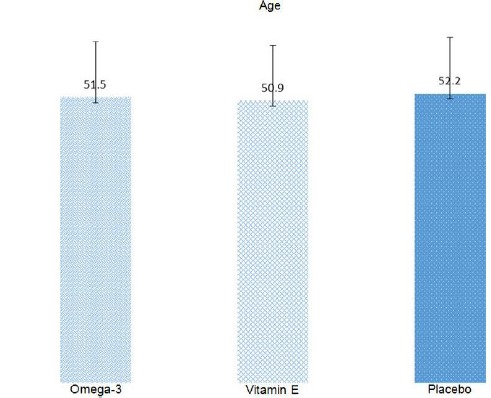
The mean age (± standard deviation) of patients in three groups of malignancy
The mean age (± standard deviation) of patients was shown in Figure 2. Accordingly, 17 patients (27.0%) suffered from various types of lung cancer, followed by patients with breast cancer (57.1%, 36 individuals) and ovarian cancer (15.9%, 10 patients).
In the omega-3 group, 11 patients (52.4%) had breast cancer, followed by lung cancer (28.6%, 6 cases) and ovarian cancer (19%, 4 cases).
In the vitamin E group, 12 (57.1%) had breast cancer, followed by lung cancer (23.8%, 5 cases) and ovarian cancer (19%, 4 cases). In the placebo group, 13 (61.9%) had breast cancer, followed by lung cancer (28.6%, 6 cases) and ovarian cancer (9.5%, 2 cases), (Figure 3, and Table 1). Chi-square test exhibited no significant difference in the type of malignancy between the groups (p = 0.448).
Figure 3.

Malignancy in three groups
Table 1.
Demographic and baseline variables in three groups
| Omega-3 | Vitamin E | Placebo | P-value | |
|---|---|---|---|---|
| Gender (female) | (4.71%) 15 | (2.76%) 16 | (4.71%) 15 | 0.861 |
| Age (year) | 2.9±5.51 | 4.10±9.50 | 1.10±2.52 | 0.664 |
| Body mass index (kg / m2) | 7.5±3.24 | 2.6±8.24 | 4.6±2.25 | 0.780 |
| The type of malignancy | ||||
| Lung cancer | (6.28%) 6 | (8.23%) 5 | (6.28%) 6 | |
| Breast cancer | (4.52%) 11 | (1.57%) 12 | (9.61%) 13 | 0.482 |
| Ovarian cancer | (0.19) 4 | (0.19) 4 | (5.9%) 2 |
Table 2.
Overall incidence and severity of peripheral neuropathy in patients in three groups
| Without neuropathy | Mild neuropathy | Medium Neuropathy | Severe neuropathy | |
|---|---|---|---|---|
| Omega-3 | (4.71%) 15 | (19%) 4 | (8.4%) 1 | (8.4%) 1 |
| Vitamin E | (7.66%) 14 | (3.14%) 3 | (3.14%) 3 | (8.4%) 1 |
| Placebo | (6.28%) 6 | (3.33) 7 | (19%) 4 | (19%) 4 |
There was no significant difference between the groups receiving the drug and the placebo group (p = 0.0001). However, the difference between the two groups of omega 3 and vitamin E was not significant (p = 0.751). Figure 4 shows the incidence and severity of peripheral neuropathy among the patients.
Figure 4.

The incidence and severity of peripheral neuropathy in the three groups
In the present study, the peripheral nerves were examined electrophysiologically. To evaluate motor neurons, two ulnar and peroneal nerves were evaluated, and the sural and ulnar nerves were assessed for sensory function.
In these studies, motor delay (DL), amplitude (Amp), conduction velocity (CV) was measured. Electrophysiological variables of motor neurons and sensory nerves were indicated in Table 3 and 4.
Table 3.
Electrophysiological variables of motor neurons during study period
| Group | Before chemotherapy | One month later | Three months later | P-value | |
|---|---|---|---|---|---|
| Ulnar nerve | |||||
| DL (ms) | Omega-3 | 84.2 | 94.2 | 17.3 | 0.720 |
| Vitamin E | 81.2 | 98.2 | 16.3 | ||
| Placebo | 77.2 | 14.3 | 26.3 | ||
| CV (m/s) | Omega-3 | 12.49 | 34.49 | 21.50 | 0.431 |
| Vitamin E | 14.49 | 37.49 | 28.50 | ||
| Placebo | 09.49 | 42.49 | 11.50 | ||
| Amp (mV) | Omega-3 | 11.6 | 01.7 | 55.7 | 0.446 |
| Vitamin E | 05.6 | 97.6 | 49.7 | ||
| Placebo | 14.6 | 01.7 | 87.7 | ||
| Peroneal nerve | |||||
| DL (ms) | Omega-3 | 31.6 | 43.6 | 57.6 | 0.375 |
| Vitamin E | 39.6 | 46.6 | 58.6 | ||
| Placebo | 32.6 | 47.6 | 68.6 | ||
| CV (m/s) | Omega-3 | 35.44 | 84.44 | 51.45 | 0.694 |
| Vitamin E | 32.44 | 87.44 | 32.45 | ||
| Placebo | 24.44 | 80.44 | 11.45 | ||
| Amp (mV) | Omega-3 | 16.5 | 31.5 | 71.5 | 0.091 |
| Vitamin E | 24.5 | 35.5 | 80.5 | ||
| Placebo | 28.5 | 37.5 | 66.5 | ||
Table 4.
Electrophysiological variables of sensory nerves during study period
| Group | Before chemotherapy | One month later | Three months later | P-value | |
|---|---|---|---|---|---|
| Sural nerve | |||||
| DL (ms) | Omega-3 | 31.4 | 94.3 | 87.3 | 0.720 |
| Vitamin E | 27.4 | 98.3 | 86.3 | ||
| Placebo | 28.4 | 14.4 | 68.3 | ||
| CV (m/s) | Omega-3 | 24.40 | 31.40 | 29.41 | 0.341 |
| Vitamin E | 27.40 | 34.40 | 32.41 | ||
| Placebo | 16.40 | 35.40 | 41.41 | ||
| Amp (mV) | Omega-3 | 17.6 | 38.6 | 84.6 | 0.446 |
| Vitamin E | 19.6 | 46.6 | 81.6 | ||
| Placebo | 26.6 | 52.6 | 98.6 | ||
| Ulnar nerve | |||||
| DL (ms) | Omega-3 | 42.2 | 53.2 | 76.2 | 0.375 |
| Vitamin E | 46.2 | 58.2 | 83.2 | ||
| Placebo | 53.2 | 63.2 | 95.2 | ||
| CV (m/s) | Omega-3 | 67.50 | 33.50 | 87.49 | 0.694 |
| Vitamin E | 84.50 | 41.50 | 84.49 | ||
| Placebo | 73.50 | 20.50 | 31.49 | ||
| Amp (mV) | Omega-3 | 59.8 | 16.8 | 64.7 | 0.091 |
| Vitamin E | 67.8 | 14.8 | 54.7 | ||
| Placebo | 64.8 | 02.8 | 13.7 | ||
Discussion
The results of this study clearly showed that the use of omega-3 or vitamin E-d supplements significantly reduced the incidence of peripheral neuropathy compared with placebo. However, there was no significant difference in the rate and severity of neuropathy in the two groups, and omega-3 and vitamin E did not have a significant superiority in this regard. Chemotherapy-induced peripheral neuropathy (CIPN) is a major clinical problem, which causes one of the dose-limiting side effects of some commonly used anti-neoplasms, such as taxol, cisplatin, and Vinca alkaloids.
However, its onset may also affect the unpleasant quality of life in cancer patients, when CIPN is not a dose-limiting complication and causes chronic complications in these patients [12]. Studies show that 60-70% of patients receiving chemotherapy drugs are dose-dependent neurotoxicants [4]. Several studies have been conducted to reduce the incidence and severity of CIPN, but most of these studies have contradictory results. To best of our knowledge, no clinical study has compared the effects of omega-3 fatty acids and vitamin E on the incidence of chemotherapy-induced neuropathy.
On the other hand, until the completion of this study, only a few studies examined the effect of omega-3 on neuropathy. Meanwhile, Weymouth et al. only examined the role of omega-3 polyunsaturated fatty acids on diabetic neuropathy, which showed positive effects of omega-3 [13]. Another study specifically designed to evaluate the role of omega-3 fatty acids in the prevention of taxol-induced peripheral neuropathy [11]. The results of the aforementioned clinical trial suggested that 30% of patients receiving Omega-3 and 59.3% of patients in the control group exhibited peripheral neuropathy. These findings are consistent with our study because only 28.6% of Omega-3-receiving patients had peripheral neuropathy in the present study.
The findings of our study are consistent with previous studies that have examined the efficacy of fatty acids in diabetic neuropathy. These studies have shown that omega-3 fatty acids can reduce the severity of neuropathy in patients with type 2 diabetes [14] [15]. These micronutrients also reduced Na +/K + ATPase activity by reducing the neuronal conduction velocity in the sciatic nerve of the rats [16]. In a few pilot studies, vitamin E has been introduced as a neuroprotective agent [17] [18]. In a clinical study, patients undergoing paclitaxel-based chemotherapy received either chemotherapy with vitamin E [10]. The results of this Phase II clinical trial revealed that vitamin E, effectively and safely, could protect patients with cancer from paclitaxel-induced peripheral nerve damage.
Kottschade et al. demonstrated that vitamin E did not change the incidence of neuropathy in these patients [19]. In a meta-analysis study published by Eum and colleagues, it has been shown that daily intake of 600-300 mg of vitamin E has significant effects on the reduction of neuropathy induced by chemotherapy [20]. On the other hand, there are studies that violate the positive effects of this vitamin in improving neuropathy. Huang et al., in a meta-analysis study, believed that vitamin E could not reduce the incidence of CIPN [21]. They also said that a large sample size is needed to prove the effectiveness of this vitamin.
In conclusion, taken together, our findings suggest that the use of food supplements such as vitamin E and omega-3s may significantly reduce the paclitaxel-induced neuropathy. The routine use of such supplements, which do not add to certain side effects for patients undergoing chemotherapy, can greatly increase their quality of life.
Acknowledgements
We are thankful to the deputy of research and technology of Arak University of Medical Sciences for their support.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Hagiwara HSY. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11(1):82–5. doi: 10.1007/BF02968008. https://doi.org/10.1007/BF02968008 PMid:14718798. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhry VRE, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Annals of neurology. 1994;35(3):304–11. doi: 10.1002/ana.410350310. https://doi.org/10.1002/ana.410350310 PMid:7907208. [DOI] [PubMed] [Google Scholar]
- 3.Kuroi KSK. Neurotoxicity of taxanes: symptoms and quality of life assessment. Breast Cancer. 2004;11(1):92–9. doi: 10.1007/BF02968010. https://doi.org/10.1007/BF02968010 PMid:14718800. [DOI] [PubMed] [Google Scholar]
- 4.Stillman MCJ. Management of chemotherapy-induced peripheral neuropathy. Current pain and headache reports. 2004;10(4):279–87. doi: 10.1007/s11916-006-0033-z. https://doi.org/10.1007/s11916-006-0033-z. [DOI] [PubMed] [Google Scholar]
- 5.H S. Could n-3 polyunsaturated fatty acids reduce pathological pain by direct actions on the nervous system? Prostaglandins, leukotrienes, and essential fatty acids. 2003;68(3):219–24. doi: 10.1016/s0952-3278(02)00273-9. https://doi.org/10.1016/S0952-3278(02)00273-9. [DOI] [PubMed] [Google Scholar]
- 6.Mazza MPM, Janiri L, Bria P, Mazza S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: an overview. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31(1):12–26. doi: 10.1016/j.pnpbp.2006.07.010. https://doi.org/10.1016/j.pnpbp.2006.07.010 PMid:16938373. [DOI] [PubMed] [Google Scholar]
- 7.Coste TC GA, Vague P, Pieroni G, Raccah D. Neuroprotective effect of docosahexaenoic acid-enriched phospholipids in experimental diabetic neuropathy. Diabetes. 2003;52(10):2578–85. doi: 10.2337/diabetes.52.10.2578. https://doi.org/10.2337/diabetes.52.10.2578 PMid:14514643. [DOI] [PubMed] [Google Scholar]
- 8.Weijl NI HG, Wipkink-Bakker A, Lentjes EG, Berger HM, Cleton FJ, et al. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1999;9(12):1331–7. doi: 10.1023/a:1008407014084. https://doi.org/10.1023/A:1008407014084. [DOI] [PubMed] [Google Scholar]
- 9.Leonetti CBA, Gabellini C, Scarsella M, Maresca V, Flori E, et al. Alpha-tocopherol protects against cisplatin-induced toxicity without interfering with antitumor efficacy. International journal of cancer. 2003;104(2):243–50. doi: 10.1002/ijc.10933. https://doi.org/10.1002/ijc.10933 PMid:12569582. [DOI] [PubMed] [Google Scholar]
- 10.Argyriou AA CE, Koutras A, Iconomou G, Papapetropoulos S, Polychronopoulos P, et al. Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. Journal of pain and symptom management. 2006;32(3):237–44. doi: 10.1016/j.jpainsymman.2006.03.013. https://doi.org/10.1016/j.jpainsymman.2006.03.013 PMid:16939848. [DOI] [PubMed] [Google Scholar]
- 11.Ghoreishi Z EA, Djazayeri A, Djalali M, Golestan B, Ayromlou H, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC cancer. 2012;12:355. doi: 10.1186/1471-2407-12-355. https://doi.org/10.1186/1471-2407-12-355 PMid:22894640 PMCid: PMC3459710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maestri A DPCA, Cundari S, Zanna C, Cortesi E, Crino L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005;91(2):135–8. doi: 10.1177/030089160509100206. PMid: 15948540. [DOI] [PubMed] [Google Scholar]
- 13.Yee PWA, Fletcher EL, Vingrys AJ. A role for omega-3 polyunsaturated fatty acid supplements in diabetic neuropathy. Investigative ophthalmology & visual science. 2010;51(3):1755–64. doi: 10.1167/iovs.09-3792. https://doi.org/10.1167/iovs.09-3792 PMid:19907026. [DOI] [PubMed] [Google Scholar]
- 14.Okuda YMM, Ogawa M, Sone H, Asano M, Asakura Y, et al. Long-term effects of eicosapentaenoic acid on diabetic peripheral neuropathy and serum lipids in patients with type II diabetes mellitus. Journal of diabetes and its complications. 1996;10(5):280–7. doi: 10.1016/1056-8727(95)00081-x. https://doi.org/10.1016/1056-8727(95)00081-X. [DOI] [PubMed] [Google Scholar]
- 15.KA H. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Alternative medicine review: a journal of clinical therapeutic. 2006;11(4):294–329. [PubMed] [Google Scholar]
- 16.Gerbi AMJ, Ansaldi JL, Pierlovisi M, Coste T, Pelissier JF, et al. Fish oil supplementation prevents diabetes-induced nerve conduction velocity and neuroanatomical changes in rats. The Journal of nutrition. 1999;129(1):207–13. doi: 10.1093/jn/129.1.207. https://doi.org/10.1093/jn/129.1.207 PMid:9915901. [DOI] [PubMed] [Google Scholar]
- 17.Bove L PM, Maresca V, Jandolo B, Pace A. A pilot study on the relation between cisplatin neuropathy and vitamin E. Journal of experimental & clinical cancer research. 2001;20(2):277–80. PMid:11484987. [PubMed] [Google Scholar]
- 18.Pace A SA, Picardo M, Maresca V, Pacetti U, Del Monte G, et al. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(5):927–31. doi: 10.1200/JCO.2003.05.139. https://doi.org/10.1200/JCO.2003.05.139 PMid:12610195. [DOI] [PubMed] [Google Scholar]
- 19.Kottschade L SJ, Mazurczak M, Johnson D, Murphy B, Rowland K, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Supportive Care in Cancer. 2011;19(11):1769–77. doi: 10.1007/s00520-010-1018-3. https://doi.org/10.1007/s00520-010-1018-3 PMid:20936417 PMCid: PMC3329941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eum S CH, Chang MJ, Choi HC, Ko YJ, Ahn JS, et al. Protective effects of vitamin E on chemotherapy-induced peripheral neuropathy: a meta-analysis of randomized controlled trials. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2013;83(2):101–11. doi: 10.1024/0300-9831/a000149. https://doi.org/10.1024/0300-9831/a000149 PMid:24491883. [DOI] [PubMed] [Google Scholar]
- 21.Huang H HM, Liu L, Huang L. Vitamin E does not decrease the incidence of chemotherapy-induced peripheral neuropathy: a meta-analysis. Contemp Oncol Pozn. 2016;20(3):237–41. doi: 10.5114/wo.2016.61567. https://doi.org/10.5114/wo.2016.61567. [DOI] [PMC free article] [PubMed] [Google Scholar]


