Abstract
Introduction
Three-quarters of homeless people smoke cigarettes. Financial incentives for smoking abstinence have appeared promising in nonexperimental studies of homeless smokers, but randomized controlled trial (RCT) data are lacking.
Methods
We conducted a pilot RCT of financial incentives for homeless smokers. Incentive arm participants (N = 25) could earn escalating $15–$35 rewards for brief smoking abstinence (exhaled carbon monoxide <8 parts per million) assessed 14 times over 8 weeks. Control arm participants (N = 25) were given $10 at each assessment regardless of abstinence. All participants were offered nicotine patches and counseling. The primary outcome was a repeated measure of brief smoking abstinence across 14 assessments. The secondary outcome was brief abstinence at 8 weeks. Exploratory outcomes were self-reported 1-day and 7-day abstinence from (1) any cigarette and (2) any puff of a cigarette. Other outcomes included 24-hour quit attempts, nicotine patch use, counseling attendance, and changes in alcohol and drug use.
Results
Compared to control, incentive arm participants were more likely to achieve brief abstinence overall (odds ratio 7.28, 95% confidence interval 2.89 to 18.3) and at 8 weeks (48% vs. 8%, p = .004). Similar effects were seen for 1-day abstinence, but 7-day puff abstinence was negligible in both arms. Incentive arm participants made more quit attempts (p = .03). Nicotine patch use and counseling attendance were not significantly different between the groups. Alcohol and drug use did not change significantly in either group.
Conclusions
Among homeless smokers, financial incentives increased brief smoking abstinence and quit attempts without worsening substance use. This approach merits further development focused on promoting sustained abstinence.
Registration
ClinicalTrials.gov (NCT02565381)
Implications
Smoking is common among homeless people, and conventional tobacco treatment strategies have yielded modest results in this population. This pilot RCT suggests that financial incentives may be a safe way to promote brief smoking abstinence and quit attempts in this vulnerable group of smokers. However, further development is necessary to translate this approach into real-world settings and to promote sustained periods of smoking abstinence.
Introduction
Three-quarters of homeless adults smoke cigarettes,1 contributing to 3- to 5-fold higher rates of tobacco-attributable mortality compared to the general population.2 Homeless smokers want to quit smoking,3 but the proportion who are able to do so is one-fifth the US national average.1
Prior randomized controlled trials (RCTs) focusing on homeless smokers have tested combinations of conventional behavioral and pharmacologic treatments with generally modest quit rates of 9–17% at 6 months that did not differ significantly from control conditions.4,5 The persistence of smoking among homeless people and its resistance to standard treatment approaches may relate to the heavy burden of comorbid conditions1 and competing priorities6 in this population. These challenges contribute to a present-focused perspective in which the immediate rewards of continuing to smoke outweigh the more distant and less tangible benefits of quitting. Financial incentives for smoking cessation target this imbalance by providing immediate and tangible rewards for abstinence. This approach is one form of contingency management, a treatment paradigm that leverages operant conditioning7 and behavioral economics8 to promote changes in substance use through the systematic application of reinforcement for abstinence, such as monetary rewards.9,10
Financial incentives for smoking cessation have shown efficacy in vulnerable populations, including low-income smokers,11–13 pregnant smokers,14,15 and smokers with schizophrenia16,17 or substance use disorders.18,19 Although less tested in homeless smokers, financial incentives have appeared promising in 2 small nonexperimental studies, generating reported abstinence rates of 30–50% at 4 weeks.20,21 To date, there have been no published RCTs to confirm these findings. To address this gap, we conducted a pilot RCT to assess the effect of financial incentives for smoking abstinence among homeless smokers in Boston, Massachusetts. Our principal goal was to determine whether smoking in this vulnerable population is sensitive to financial rewards for brief abstinence delivered using a contingency management framework.
Methods
We conducted a three-arm, parallel group, nonblinded, 8-week pilot RCT that tested two separate smoking cessation interventions, (1) financial incentives for smoking abstinence and (2) text messaging to support smoking abstinence against (3) a shared control condition consisting of counseling and nicotine replacement therapy. We originally designed a two-arm trial testing financial incentives against control treatment. However, prior to commencement, we received additional funding to add a third arm comparing a separate text messaging intervention to the same control condition. We prespecified a plan to analyze the financial incentives and text messaging interventions separately because of the pilot nature of the trial and the differing rationales for each treatment approach. This article compares the effect of the financial incentives intervention to the control condition, which was the primary focus of the trial.
Participants and Setting
The study protocol was approved by the Partners Human Research Committee and registered with ClinicalTrials.gov (NCT02565381) prior to the accrual of participants. All study procedures occurred between October 2015 and June 2016 at Boston Health Care for the Homeless Program (BHCHP) headquarters. The study design was informed by 2 focus groups with homeless smokers in addition to interactive presentations involving BHCHP medical leadership and the BHCHP Consumer Advisory Board, a group of homeless-experienced patients who participate in the governance of the program.22
We recruited participants via in-person advertisement in the BHCHP lobby, flyers posted in BHCHP clinics, and referrals from BHCHP clinicians. Inclusion criteria were (1) age ≥18 years, (2) lifetime smoking of ≥100 cigarettes23 with current smoking of ≥5 cigarettes/day, verified by an exhaled carbon monoxide (CO) level of ≥8 parts per million (ppm),24 (3) readiness to quit smoking within the next month, (4) current homelessness, and (5) self-reported English proficiency. We defined current homelessness as usually staying in an emergency shelter, transitional shelter, abandoned building, place of business, car or other vehicle, church or mission, hotel or motel, or anywhere outside during the past 7 days, or if currently in a residential treatment program, in the 7 days prior to program entry. Additionally, individuals were considered currently homeless if they usually stayed in somebody else’s place in the past 7 days because of not having their own place to stay. This definition is generally concordant with the US federal definition of homelessness25 and identical to the definition that we and others have used in prior studies.26–30 Exclusion criteria were (1) current pregnancy, (2) past month use of any smoking cessation medication, (3) prior serious adverse reaction to the nicotine patch, (4) myocardial infarction or undiagnosed chest pain in the past 2 weeks, and (5) inability to read a sentence written at a Flesch-Kincaid fourth-grade level. Participants were not excluded because of active substance use or mental illness.
Enrollment and Randomization
We used a multistep enrollment process to ensure that participants sufficiently understood the study and were committed to participating. In the first step, we conducted in-person screening of interested individuals, which included measurement of exhaled CO to verify their smoking status. To allow ample time to consider enrollment, eligible individuals were given a paper copy of the consent form and asked to return on any subsequent weekday. In the second step, those who returned for enrollment underwent repeat measurement of exhaled CO to confirm their smoking status. For those who remained eligible, study staff reviewed the consent form using visual aids and offered to read the text aloud. Participants were required to correctly answer 7 basic knowledge questions to confirm their understanding of the study before providing written informed consent. Enrolled participants were then asked to return on the subsequent Friday for randomization. In the third step, those who returned were randomized 1:1:1 to the financial incentives arm, text messaging arm (not discussed further), or control arm. The allocation sequence was computer-generated in random permuted blocks of 3, 6, 9, 12, or 15 and concealed from study staff. Randomized participants were given a mobile phone with a prepaid 2-month voice and text plan to facilitate communication and follow-up.
Baseline Measures
Participants completed a baseline survey upon enrollment. Sociodemographic measures included age, sex, and self-reported race and ethnicity. We asked participants to rate their general health status. We used the Addiction Severity Index (ASI)—5th Edition,31 which has been validated in homeless populations,32–34 to generate composite scores (range 0–1) for past month alcohol use, drug use, and psychiatric symptom severity. For descriptive purposes, we used ASI cut points from the National Survey of Homeless Assistance Providers and Clients to define past month alcohol use problem, drug use problem, and psychiatric problem.35 We assessed nicotine dependence with the Fagerstrom test of nicotine dependence (FTND; range 0–10).36 We asked participants about previous smoking cessation attempts and assessed their current confidence to quit and perceived importance of quitting using 10-point scales.
Assessment Procedure
Following randomization, all participants were asked to make 14 assessment visits over 8 weeks, including 3 per week (Monday, Wednesday, and Friday) during Weeks 1–2, 2 per week (Monday and Friday) during Weeks 3–4, and 1 per week (Friday) during Weeks 5–8. At each visit, study staff measured participants’ exhaled CO levels using a Micro+ Smokerlyzer CO monitor (Bedfont Scientific). All assessment visits occurred between 1 and 4 pm to ensure time-of-day consistency in CO measurement. To enhance retention, participants in both arms were unconditionally offered nominal items (e.g., coffee, socks, and snacks) at each assessment visit and were given a round-trip public transportation ticket to facilitate attendance of the subsequent visit.
Control Condition
Participants assigned to the control arm were offered 8 weeks of nicotine patch therapy and weekly in-person counseling.
Nicotine Patch Therapy
Nicotine patches were distributed in 1-week allotments at no cost to participants. Individuals who smoked ≥10 cigarettes per day were started on 21-mg/day patches and tapered to 14-mg/day at Week 6. Individuals who smoked <10 cigarettes per day were maintained on 14-mg/day patches throughout the 8-week study.
In-Person Counseling
Participants were offered 15 minutes of in-person counseling each week according to a protocol developed by our study team in collaboration with a certified Master Tobacco Treatment Specialist (TTS). The counseling protocol was structured around the American Lung Association “Freedom from Smoking” program theme of addressing the “3-link chain” of tobacco addiction: physical, mental, and social.37 Counseling sessions were tailored to the unique circumstance of homeless people and incorporated elements of motivational interviewing and cognitive–behavioral therapy. Participants were encouraged to set a quit date during the baseline counseling session and at each subsequent counseling session if nonabstinent. To prepare for the counseling role, the study counselor completed a 9-module online training course on basic skills for working with smokers, 6 hours of case-based didactics conducted by a certified Master TTS, 6 hours of observing a certified TTS counsel smokers, and 3 hours of observing a clinician interact with homeless patients.
Financial Incentives Condition
Participants assigned to the financial incentives arm received nicotine patch therapy and in-person counseling in a fashion identical to the control arm. Additionally, participants in this arm could receive escalating financial rewards at each assessment visit for brief smoking abstinence, defined as an exhaled CO <8 ppm.24 Consistent with the principles of contingency management,9,10 financial rewards were independent of self-report and based exclusively on the exhaled CO criterion for abstinence. An 8 ppm cut point for defining smoking abstinence is consistent with expert guidelines,24 identical to that used in a nonexperimental study of financial incentives for homeless smokers20 and more conservative than the 10 ppm cut point used in the largest smoking cessation RCT for homeless smokers.4 Although recent evidence has suggested lower CO cut points for defining abstinence,38 we used 8 ppm to allow for homeless individuals’ potentially high level of ambient CO exposure due to frequent contact with other smokers,39 poorly-ventilated living conditions, and time spent in close proximity to urban sources of air pollution.
Participants were not informed about the half-life of CO or the duration of abstinence required to achieve a negative result. If asked, study staff provided a scripted response recommending complete abstinence. Participants were also informed that any smoked substance (e.g., cannabis) could produce a positive CO result and should be avoided.
Reward values for exhaled CO levels <8 ppm started at $15 and increased in $5 increments with each successive abstinent measurement,40,41 up to a maximum of $35. Nonabstinence or nonattendance resulted in no payment and reset the subsequent payment to the starting value of $15.40,41 The maximum amount that participants could earn for smoking abstinence was $440.
Payment Procedure
Control arm participants received $10 for attending an assessment visit and providing an exhaled CO sample, regardless of abstinence. Participants in the financial incentives arm were paid at assessment visits according to the reward schedule described above only if their exhaled CO level was <8 ppm. All payments were made in real time through a debit card system hosted by CT Payer (www.ctpayer.com), a secure web-based platform that facilitates Health Insurance Portability and Accountability Act-compliant clinical trial payments onto reloadable MasterCard debit cards issued to each participant at enrollment. Transferred funds were available immediately for use at any retail outlet accepting MasterCard.
Outcomes
Primary and Secondary Outcomes
The primary outcome was a repeated measure of brief smoking abstinence, defined as an exhaled CO <8 ppm24 and measured at each of the 14 study visits. The secondary outcome was brief smoking abstinence at the end of treatment (8 weeks), defined as an exhaled CO <8 ppm24 at the 14th study visit.
Exploratory Smoking Outcomes
The prespecified analysis plan did not include self-report in the outcome measures because of the potential for differential misreporting in the setting of abstinence-contingent rewards. Specifically, we were uncertain about the degree to which incentive arm participants would accurately report abstinence because of their socioeconomic vulnerability and the potential for mistrust of researchers. Nevertheless, to facilitate exploratory analyses examining whether financial incentives produce sustained versus merely intermittent abstinence, we collected data from each participant on self-reported time since last smoking all or part of a cigarette and time since last puff of a cigarette, each assessed after incentive payments were made. We used this information to create the following composite abstinence metrics for each time point:
1) Past 1-day cigarette abstinence: CO <8 ppm and last cigarette ≥1 day ago;
2) Past 1-day puff abstinence: CO <8 ppm and last puff ≥1 day ago;
3) Past 7-day cigarette abstinence: CO <8 ppm and last cigarette ≥7 days ago;
4) Past 7-day puff abstinence: CO <8 ppm and last puff ≥7 days ago.
Other Outcomes
Additional outcomes included assessment visit attendance, counseling visit attendance, nicotine patch use (assessed by self-report each week), past-month 24-hour quit attempts (assessed at 4 and 8 weeks), and changes in alcohol and drug use (based on ASI scores at baseline and 8 weeks).
Analysis
Primary Outcome
We used repeated-measures logistic regression with generalized estimating equations (GEE) to compute the overall effect of financial incentives on brief smoking abstinence across the 14 measurements. Our principal analyses included only treatment effect in the GEE model. In a multivariable sensitivity analysis, we adjusted for age, sex, race/ethnicity, nicotine dependence score, and baseline alcohol use, drug use, and psychiatric symptom severity scores.
Secondary Outcome
We used the Fisher exact test to compare the proportion in each study arm who achieved brief abstinence at 8 weeks.
Exploratory Smoking Outcomes
We used repeated-measures logistic regression with GEE to assess the differences between study arms for each of the exploratory smoking outcomes. For past 1-day abstinence, we used data from all 14 time points. For past 7-day abstinence, we used data only from the 8 Friday visits to avoid overlapping reference periods in the repeated-measures analysis.
Other Outcomes
We used the Wilcoxon rank-sum test to compare assessment visit attendance and counseling visit attendance between arms. We used repeated-measures linear regression with GEE to compare the mean number of quit attempts per month and the mean days of nicotine patch use per week between arms and to assess within-groups changes in ASI alcohol and drug use scores over 8 weeks and between-groups differences in these changes.
Missing Data Approach
We followed the convention of assuming that participants who missed a study visit were nonabstinent. In sensitivity analyses involving the primary outcome, we considered three alternative approaches to missing abstinence data: (1) excluding missing values from the analysis, (2) carrying forward the last nonmissing observation, and (3) using multiple imputation to impute missing smoking status42,43 based on nonmissing data.
All analyses were based on the intention-to-treat principle and used a 2-sided significance level of 0.05. We used SAS software, version 9.4 (SAS Institute, Cary, North Carolina) to conduct the analyses.
Results
Screening, Enrollment, and Randomization
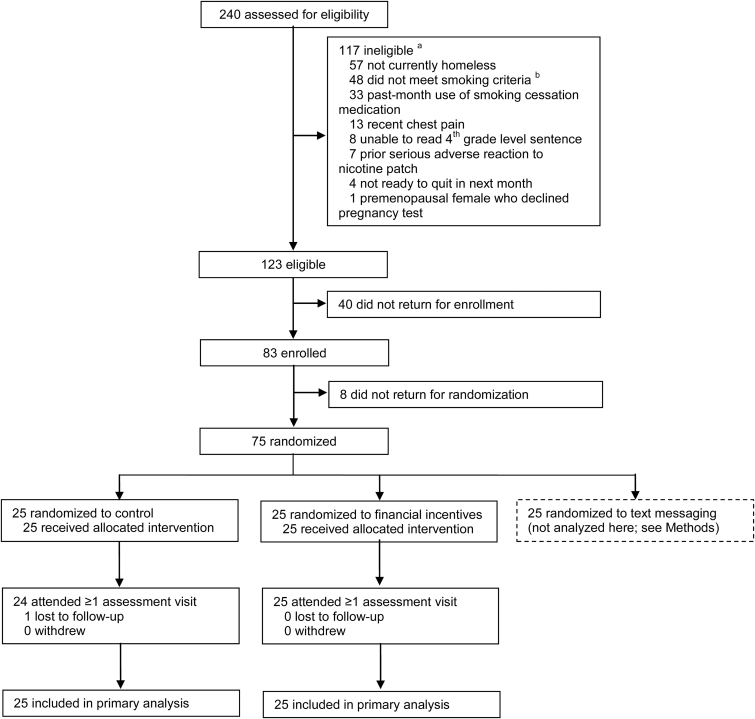
Of 123 eligible individuals (Figure 1), 83 (67.5%) enrolled and completed the baseline assessment. Eight enrollees (9.6%) did not return for randomization. The remaining 75 were randomized to one of the three study conditions. Participants who did not return for randomization reported higher confidence to quit than randomized participants (p = .01) but did not differ in other baseline characteristics. The remainder of the results focus on the 50 participants assigned to the control arm (N = 25) or incentives arm (N = 25).
Figure 1.
CONSORT diagram. aSum of exclusion reasons totals greater than 117 because individuals could be ineligible for more than one reason. bSmoking inclusion criteria were: 1) lifetime smoking of ≥100 cigarettes, 2) current daily smoking of ≥5 cigarettes per day, and 3) exhaled carbon monoxide level of ≥8 parts per million on 2 separate occasions.
Baseline Characteristics
Participants smoked an average of 15.9 cigarettes per day and had previously attempted to quit smoking a median of 2 times (Table 1). Forty-four percent rated their health as fair or poor, and considerable proportions met criteria for current alcohol use problem (16.0%), current drug use problem (59.2%), and current psychiatric problem (54.0%).
Table 1.
Characteristics of randomized participants, overall and by study arm
| All n = 50 |
Control n = 25 |
Incentives n = 25 |
|
|---|---|---|---|
| Sociodemographic | |||
| Age, years, mean (SD) | 46.6 (9.1) | 45.1 (9.6) | 48.1 (8.5) |
| Female, n (%) | 26 (52.0) | 14 (56.0) | 12 (48.0) |
| Race/ethnicity, n (%) | |||
| White, non-Hispanic | 21 (42.0) | 10 (40.0) | 11 (44.0) |
| Black, non-Hispanic | 16 (32.0) | 9 (36.0) | 7 (28.0) |
| Hispanic | 11 (22.0) | 5 (20.0) | 6 (24.0) |
| Other | 2 (4.0) | 1 (4.0) | 1 (4.0) |
| Health | |||
| Fair or poor health, n (%) | 22 (44.0) | 13 (52.0) | 9 (36.0) |
| Alcohol problem, past month, n (%) | 8 (16.0) | 3 (12.0) | 5 (20.0) |
| Drank to intoxication, past month, n (%) | 11 (22.0) | 6 (24.0) | 5 (20.0) |
| Drug problem, past month, n (%) | 29 (59.2) | 14 (58.3) | 15 (60.0) |
| Heroin use, past month, n (%) | 7 (14.0) | 4 (16.0) | 3 (12.0) |
| Methadone use, past month, n (%) | 13 (26.0) | 8 (32.0) | 5 (20.0) |
| Other opiate/painkiller use, past month, n (%) | 14 (28.0) | 5 (20.0) | 9 (36.0) |
| Cocaine/crack use, past month, n (%) | 6 (12.0) | 3 (12.0) | 3 (12.0) |
| Cannabis use, past month, n (%) | 18 (36.0) | 10 (40.0) | 8 (32.0) |
| Psychiatric problem, past month, n (%) | 27 (54.0) | 15 (60.0) | 12 (48.0) |
| Serious depression, past month, n (%) | 22 (44.0) | 12 (48.0) | 10 (40.0) |
| Serious anxiety, past month, n (%) | 31 (62.0) | 15 (60.0) | 16 (64.0) |
| Trouble concentrating, past month, n (%) | 22 (44.0) | 14 (56.0) | 8 (32.0) |
| Smoking | |||
| Cigarettes per day, mean (SD) | 15.9 (7.1) | 16.2 (6.3) | 15.6 (8.0) |
| Nicotine dependence (0–10), mean (SD) | 5.1 (1.8) | 5.1 (1.8) | 5.1 (1.9) |
| Past quit attempts, median (IQR) | 2 (1–5) | 2 (1–4) | 2 (2–5) |
| Quitting importance (1–10), mean (SD) | 8.7 (1.8) | 8.8 (1.5) | 8.5 (2.1) |
| Quitting confidence (1–10), mean (SD) | 6.6 (2.3) | 6.8 (2.2) | 6.4 (2.5) |
Assessment Visit Attendance
Of 14 assessment visits, participants attended a median of 10.5 visits (interquartile range [IQR] 7–13), with no significant difference between the groups (p = .73). Ninety-eight percent of participants attended at least one assessment visit, and 78% attended at least half of the assessment visits.
Primary and Secondary Outcomes
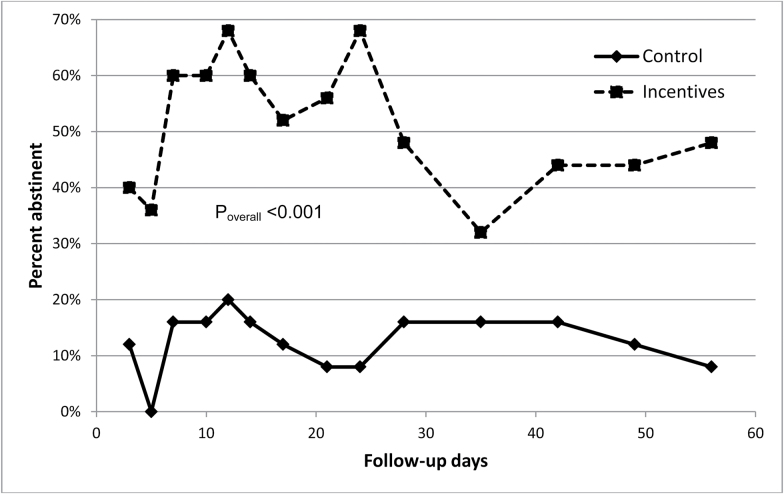
Across the 14 follow-up visits, brief smoking abstinence was substantially higher in the financial incentives arm than in the control arm (ranges 32–68% vs. 0–20%; overall odds ratio [OR] 7.28, 95% confidence interval [CI] 2.89 to 18.3; Figure 2). Multivariable adjustment had minimal impact on the effect estimate (adjusted OR 8.08, 95% CI 3.35 to 19.5). The findings were robust to alternative methods of handling missing smoking status data, including excluding missing values (OR 9.15, 95% CI 3.43 to 24.4), carrying forward the last nonmissing observation (OR 9.27, 95% CI 3.33 to 25.8), and imputing missing values with multiple imputation (OR 4.83, 95% CI 3.40 to 6.85). At the final assessment visit, 48% of financial incentive arm participants and 8% of control arm participants had an exhaled CO <8 ppm (p = .004). On average, financial incentive arm participants earned $180 (SD $138) of $440 possible for smoking abstinence.
Figure 2.
Point-in-time smoking abstinence at the 14 assessment visits, by study group. Note: We defined point-in-time smoking abstinence as an exhaled carbon monoxide (CO) level <8 parts per million. Abstinence percentages shown in this figure are based on the assumption that individuals with missing CO data were nonabstinent. We assessed the overall, time-averaged difference in abstinence between the study groups using repeated-measures logistic regression with generalized estimating equations.
Exploratory Smoking Outcomes
The effect of financial incentives was tempered when exhaled CO measurements were combined with self-reported time of last cigarette or last puff (Table 2). At all time points in both arms, fewer than half of those with CO readings <8 ppm had abstained from smoking a puff in the past day. Nevertheless, incentive arm participants were more likely than control arm participants to achieve past 1-day cigarette abstinence (ranges 20–48% vs. 0–16%; overall OR 4.93, 95% CI 1.72 to 14.1) and past 1-day puff abstinence (ranges 0–24% vs. 0–4%; overall OR 6.68, 95% CI 1.33 to 33.6). Past 7-day cigarette abstinence was generally higher among incentive arm participants, but the difference was not statistically significant (ranges 4–24% vs. 0–12%; overall OR 2.45, 95% CI 0.55–10.8). Past 7-day puff abstinence was negligible and not significantly different between the arms (ranges 0–4% vs. 0–4%; overall OR 2.01, 95% CI 0.13 to 31.0).
Table 2.
Carbon monoxide-verified past 1-day and 7-day smoking abstinencea by study arm
| Past 1-day abstinence | Past 7-day abstinence b | |||||||
|---|---|---|---|---|---|---|---|---|
| All or part of cigarette | Puff of cigarette | All or part of cigarette | Puff of cigarette | |||||
| Study day | Control | Incentives | Control | Incentives | Control | Incentives | Control | Incentives |
| 3 | 8 | 24 | 4 | 12 | – | – | – | – |
| 5 | 0 | 20 | 0 | 12 | – | – | – | – |
| 7 | 8 | 40 | 0 | 24 | 4 | 4 | 0 | 0 |
| 10 | 12 | 28 | 4 | 8 | – | – | – | – |
| 12 | 16 | 20 | 4 | 8 | – | – | – | – |
| 14 | 12 | 32 | 4 | 4 | 12 | 8 | 4 | 0 |
| 17 | 8 | 32 | 0 | 16 | – | – | – | – |
| 21 | 4 | 40 | 0 | 16 | 0 | 16 | 0 | 0 |
| 24 | 4 | 48 | 4 | 20 | – | – | – | – |
| 28 | 8 | 36 | 4 | 20 | 8 | 20 | 0 | 4 |
| 35 | 12 | 20 | 4 | 12 | 8 | 16 | 0 | 4 |
| 42 | 8 | 32 | 0 | 8 | 8 | 8 | 0 | 0 |
| 49 | 8 | 20 | 0 | 0 | 4 | 12 | 0 | 0 |
| 56 | 4 | 28 | 0 | 8 | 4 | 24 | 0 | 0 |
| Effect, Incentives vs. Control | OR 4.93 (95% CI 1.72 to 14.1) |
OR 6.68 (95% CI 1.33 to 33.6) |
OR 2.45 (95% CI 0.55 to 10.8) |
OR 2.01 (95% CI 0.13 to 31.0) |
||||
Abbreviations: CI, confidence interval; OR, odds ratio.
aAll table values are percentages. Individuals with missing carbon monoxide or self-reported smoking data are assumed not to meet the criteria for abstinence.
bOnly data from the 8 Friday assessment visits are included to avoid overlapping 7-day reference periods.
Other Outcomes
Overall, incentive arm participants made about two more 24-hour quit attempts per month than control arm participants (mean difference 2.1, p = .03). Participants reported using a nicotine patch about 4 days per week on average, with no significant difference by arm (mean difference 0.1, p = .80; Supplementary figure). Of eight possible counseling sessions, participants attended a median of one session (IQR 0–2), with no significant difference between groups (p = .43). There was no significant change in alcohol use severity scores (control: −0.02, p = .44; incentives: 0.03, p = .29) or drug use severity scores (control: −0.02, p = .12; incentives: −0.03, p = .15) between baseline and 8 weeks for either arm. Additionally, 8-week changes in alcohol and drug use severity scores did not differ significantly between arms (alcohol: 0.06, p = .20; drug: −0.01, p = .75).
Discussion
In this pilot RCT of homeless smokers in Boston, debit card-issued monetary rewards for smoking abstinence substantially increased brief CO-defined abstinence overall and at 8 weeks. Financial incentives also increased past 1-day abstinence and 24-hour quit attempts but did not result in significantly higher rates of past 7-day smoking abstinence.
These results are concordant with prior nonexperimental studies20,21 in suggesting that financial incentives are a feasible and promising approach to changing smoking behavior in homeless individuals. However, our results depart from these prior studies in finding a very low rate of past week complete smoking abstinence. There are several potential explanations for this. In their nonrandomized study of homeless smokers in Texas, Businelle and colleagues found a 30% prevalence of CO-verified past 7-day puff abstinence at 4 weeks among participants offered financial incentives, but their sample size for this estimate was small (N = 10) and financial rewards were contingent upon self-reporting complete abstinence for 7 days, creating a disincentive for participants to report past week smoking lapses. In their single-arm study of homeless veteran smokers in North Carolina, Carpenter and colleagues reported a 50% prevalence of 7-day abstinence at 4 weeks, but this study involved daily CO monitoring via mobile devices, offered more intensive pharmacotherapy for smoking cessation, and excluded individuals with current substance use.
In contrast, our study sample included a considerable number of participants with active alcohol and drug use problems, which are common among homeless smokers in general.1 The use of financial incentives for smoking cessation in such individuals raises concern about whether these incentives could subsidize or exacerbate other addictions. However, we found no evidence of significant worsening in alcohol or drug use severity in either arm nor did we find significant differences in these changes between arms. This suggests that a debit card payment format may be a safe way to deliver contingent financial rewards for smoking abstinence to homeless smokers with a high burden of comorbid addictions.
By promoting quit attempts and temporary smoking abstinence, the financial incentives approach deployed in our study takes an important step toward harm reduction44 in a vulnerable group of difficult-to-treat smokers. However, producing more sustained periods of abstinence will require modifications to this approach, particularly with respect to abstinence verification. The short half-life of CO generally precludes the detection of smoking more than 1 day before the time of measurement.24 While some financial incentive studies have used daily CO monitoring strategies,16,45 this approach has practical limitations in the setting of homelessness and is less translatable to real-world practice. Mobile devices outfitted with CO monitors and video transmission capabilities may offer a promising work-around21 but are not yet widely available. A lower CO threshold (e.g., 4–6 ppm) for defining abstinence has been used in other financial incentive studies14,46 but only extends the window of smoking detection by about 4–8 hours.24 Alternative methods of biochemically verifying smoking status also have limitations. Nicotine metabolites (e.g., cotinine) have a broader window of detection but are elevated by the use of nicotine replacement products,24 which are the pharmacotherapies preferred by most homeless smokers47 and used in this study. Other biochemical markers of smoking such as anabasine48 currently lack point-of-care testing tools to enable real-time abstinence verification with immediate reward payments, which is an important aspect of contingency management.10 These considerations highlight the need for a more diverse array of methods to verify longer-term smoking abstinence at the point of care.
Our financial incentives approach might also be improved by integrating more intensive behavioral strategies to promote the concurrent uptake of evidence-based treatments for smoking cessation, since increasing nicotine patch use has been associated with greater success in quitting among socioeconomically disadvantaged smokers.49 In the current study, nicotine patch use was suboptimal, and counseling participation was low despite efforts to facilitate access to both. Linking financial incentives not only to smoking outcomes but also to smoking treatment itself might further strengthen an incentives-based strategy.50
Limitations
In addition to the limitations highlighted earlier, our study had a small sample size, was short in duration, and did not assess abstinence following the removal of incentives, which was beyond the scope of this pilot work. The study was conducted at a large homeless health care program in a single US city, so the findings may not be generalizable to other settings. Although we had adequate power to detect large differences in abstinence over 14 study visits, we were underpowered to detect smaller effects on abstinence over fewer time points (e.g., 7-day abstinence rates across the 8 Friday visits). While attendance rates were high considering the vulnerable nature of the participants, some outcomes data were missing. However, our findings were robust to 4 different methods of handling missing abstinence data for the primary outcome. Although certain measures used in this study (e.g., ASI scores) have been validated in homeless populations, other measures (e.g. FTND score) have not and contain items (e.g., time to first cigarette) that could be affected by the circumstances of homelessness. Additionally, the optimal exhaled CO cut point for determining abstinence among homeless smokers has not been defined. Our use of 8 ppm was based on the references and rationale described above but departs from lower cut points advocated by other sources.38 Finally, we defined the primary outcome based exclusively on exhaled CO measurement because of anticipated uncertainty about the accuracy of participant reporting. However, we found that participants with exhaled CO levels <8 ppm were willing to disclose recent smoking when the financial rewards were uncoupled from self-report. Therefore, we recommend that future studies of financial incentives for homeless smokers use objective measurements of smoking status to trigger incentive payments but define outcomes according to the more conventional method of bioverified self-report.
Conclusions
Financial incentives, added to nicotine replacement therapy and counseling, increased brief smoking abstinence and quit attempts in homeless cigarette smokers without worsening concurrent substance use. As deployed in this study, our financial incentives approach may facilitate short-term changes in smoking behavior, but its minimal impact on complete past week smoking abstinence suggests a need for modifications to promote more sustained periods of cessation.
Funding
This study was supported by award K23DA034008 (Baggett) from the National Institute on Drug Abuse at the National Institutes of Health, by the Massachusetts General Hospital Department of Medicine Transformative Scholars Program (Baggett), and by award P20GM103644 (Higgins) from the National Institute of General Medical Sciences at the National Institutes of Health. These entities had no role in any aspect of the study. The study content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Massachusetts General Hospital.
Declaration of Interests
Dr. Baggett receives royalty payments from UpToDate for authorship of a topic review on the health care of homeless people in the United States. Dr. Rigotti has a research grant from and has consulted without pay for Pfizer regarding smoking cessation. She receives royalties from UpToDate for authorship of topic reviews on smoking cessation.
Supplementary Material
Acknowledgments
We thank the staff and patients of Boston Health Care for the Homeless Program.
References
- 1. Baggett TP, Rigotti NA. Cigarette smoking and advice to quit in a national sample of homeless adults. Am J Prev Med. 2010;39(2):164–172. [DOI] [PubMed] [Google Scholar]
- 2. Baggett TP, Chang Y, Singer DE et al. . Tobacco-, alcohol-, and drug-attributable deaths and their contribution to mortality disparities in a cohort of homeless adults in Boston. Am J Public Health. 2015;105(6):1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baggett TP, Lebrun-Harris LA, Rigotti NA. Homelessness, cigarette smoking and desire to quit: results from a US national study. Addiction. 2013;108(11):2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okuyemi KS, Goldade K, Whembolua GL et al. . Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction. 2013;108(6):1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okuyemi KS, Thomas JL, Hall S et al. . Smoking cessation in homeless populations: a pilot clinical trial. Nicotine Tob Res. 2006;8(5):689–699. [DOI] [PubMed] [Google Scholar]
- 6. Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skinner BF. The Behavior of Organisms: An Experimental Analysis. New York: D. Appleton-Century; 1938. [Google Scholar]
- 8. Higgins ST. Applying Behavioral Economics to the Challenge of Reducing Cocaine Abuse. In: Chaloupka F, Grossman ME, Bickel WK, Saffer H, eds. The Economic Analysis of Substance Use and Abuse: An Integration of Econometrics and Behavioral Economic Research. Vol 0-262-10047-2 Chicago, IL: University of Chicago Press; 1999:157–186. [Google Scholar]
- 9. Higgins ST, Silverman K. Introduction. In: Higgins ST, Silverman K, Heil SH, eds. Contingency Management in Substance Use Treatment. New York: Guilford; 2008. [Google Scholar]
- 10. Higgins ST, Redner R, White TJ. Contingency Management and the Community Reinforcement Approach. In: Ries RK, Fiellin DA, Miller SC, Saitz R, eds. The ASAM Principles of Addiction Medicine. 5th ed. Philadelphia, PA: Wolters Kluwer; 2014:877–893. [Google Scholar]
- 11. Volpp KG, Gurmankin Levy A, Asch DA et al. . A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev. 2006;15(1):12–18. [DOI] [PubMed] [Google Scholar]
- 12. Kendzor DE, Businelle MS, Poonawalla IB et al. . Financial incentives for abstinence among socioeconomically disadvantaged individuals in smoking cessation treatment. Am J Public Health. 2015;105(6):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Etter JF, Schmid F. Effects of Large Financial Incentives for Long-Term Smoking Cessation: A Randomized Trial. J Am Coll Cardiol. 2016;68(8):777–785. [DOI] [PubMed] [Google Scholar]
- 14. Heil SH, Higgins ST, Bernstein IM et al. . Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins ST, Bernstein IM, Washio Y et al. . Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105(11):2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tidey JW, O’Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Exp Clin Psychopharmacol. 2002;10(3):241–247. [DOI] [PubMed] [Google Scholar]
- 17. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology (Berl). 2011;217(2):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunn KE, Saulsgiver KA, Sigmon SC. Contingency management for behavior change: applications to promote brief smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2011;19(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigmon SC, Miller ME, Meyer AC et al. . Financial incentives to promote extended smoking abstinence in opioid-maintained patients: a randomized trial. Addiction. 2016;111(5):903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Businelle MS, Kendzor DE, Kesh A et al. . Small financial incentives increase smoking cessation in homeless smokers: a pilot study. Addict Behav. 2014;39(3):717–720. [DOI] [PubMed] [Google Scholar]
- 21. Carpenter VL, Hertzberg JS, Kirby AC et al. . Multicomponent smoking cessation treatment including mobile contingency management in homeless veterans. J Clin Psychiatry. 2015;76(7):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Connell JJ, Oppenheimer SC, Judge CM et al. . The Boston Health Care for the Homeless Program: a public health framework. Am J Public Health. 2010;100(8):1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults–United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 24. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 25. One hundred eleventh Congress of the United States of America. Sec. 1003. Definition of Homelessness. Homeless Emergency Assistance and Rapid Transition to Housing Act of 2009. S. 896, 34–35. January 6, 2009. 2009. [Google Scholar]
- 26. Hwang SW, Colantonio A, Chiu S et al. . The effect of traumatic brain injury on the health of homeless people. CMAJ. 2008;179(8):779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grinman MN, Chiu S, Redelmeier DA et al. . Drug problems among homeless individuals in Toronto, Canada: prevalence, drugs of choice, and relation to health status. BMC Public Health. 2010;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baggett TP, Campbell EG, Chang Y, Magid LM, Rigotti NA. Posttraumatic Stress Symptoms and Their Association With Smoking Outcome Expectancies Among Homeless Smokers in Boston. Nicotine Tob Res. 2016;18(6):1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baggett TP, Campbell EG, Chang Y, Rigotti NA. Other tobacco product and electronic cigarette use among homeless cigarette smokers. Addict Behav. 2016;60:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baggett TP, Rigotti NA, Campbell EG. Cost of Smoking among Homeless Adults. N Engl J Med. 2016;374(7):697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McLellan AT, Kushner H, Metzger D et al. . The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 32. Zanis DA, McLellan AT, Cnaan RA, Randall M. Reliability and validity of the Addiction Severity Index with a homeless sample. J Subst Abuse Treat. 1994;11(6):541–548. [DOI] [PubMed] [Google Scholar]
- 33. Argeriou M, McCarty D, Mulvey K, Daley M. Use of the Addiction Severity Index with homeless substance abusers. J Subst Abuse Treat. 1994;11(4):359–365. [DOI] [PubMed] [Google Scholar]
- 34. Drake RE, McHugo GJ, Biesanz JC. The test-retest reliability of standardized instruments among homeless persons with substance use disorders. J Stud Alcohol. 1995;56(2):161–167. [DOI] [PubMed] [Google Scholar]
- 35. Burt MR, Aron LY, Douglas T et al. . Homelessness: Programs and the People They Serve: Findings of the National Survey of Homeless Assistance Providers and Clients: Technical Report.Washington, DC: U.S. Department of Housing and Urban Development, Office of Policy Development and Research; 1999. [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 37. American Lung Association. Freedom from Smoking Online. http://www.ffsonline.org/. Accessed March 30, 2016. [Google Scholar]
- 38. Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res. 2013;15(5):978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Businelle MS, Cuate EL, Kesh A, Poonawalla IB, Kendzor DE. Comparing homeless smokers to economically disadvantaged domiciled smokers. Am J Public Health. 2013;103Suppl 2:S218–S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roll JM, Shoptaw S. Contingency management: schedule effects. Psychiatry Res. 2006;144(1):91–93. [DOI] [PubMed] [Google Scholar]
- 41. Romanowich P, Lamb RJ. The effects of fixed versus escalating reinforcement schedules on smoking abstinence. J Appl Behav Anal. 2015;48(1):25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine Tob Res. 2009;11(1):77–83. [DOI] [PubMed] [Google Scholar]
- 43. Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. [DOI] [PubMed] [Google Scholar]
- 44. National Institute for Health and Care Excellence (NICE). Smoking: harm reduction. Public health guideline [PH45]. nice.org.uk/guidance/ph45. London: NICE; 2013. [Google Scholar]
- 45. Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2010;18(1):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Higgins ST, Washio Y, Lopez AA et al. . Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med. 2014;68:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nguyen MA, Reitzel LR, Kendzor DE, Businelle MS. Perceived cessation treatment effectiveness, medication preferences, and barriers to quitting among light and moderate/heavy homeless smokers. Drug Alcohol Depend. 2015;153:341–345. [DOI] [PubMed] [Google Scholar]
- 48. Jacob P 3rd, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1668–1673. [PubMed] [Google Scholar]
- 49. Ma P, Kendzor DE, Poonawalla IB, Balis DS, Businelle MS. Daily nicotine patch wear time predicts smoking abstinence in socioeconomically disadvantaged adults: An analysis of ecological momentary assessment data. Drug Alcohol Depend. 2016;169:64–67. [DOI] [PubMed] [Google Scholar]
- 50. Ladapo JA, Prochaska JJ. Paying Smokers to Quit: Does It Work? Should We Do It?J Am Coll Cardiol. 2016;68(8):786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.