Abstract
Background
Lower rates of smoking cessation are a major reason for the higher prevalence of smoking among socioeconomically disadvantaged adults. Because barriers to quitting are both more numerous and severe, socioeconomically disadvantaged smokers may benefit from more intensive intervention. We sought to determine whether a smoking cessation intervention delivered by public housing residents trained as Tobacco Treatment Advocates (TTAs) could increase utilization of cessation resources and increase abstinence.
Methods
We conducted a group-randomized trial among Boston public housing residents who were interested in quitting smoking. Participants at control sites received standard cessation materials and a one-time visit from a TTA who provided basic counseling and information about cessation resources. Participants at intervention sites were eligible for multiple visits by a TTA who employed motivational interviewing, cessation counseling, and navigation to encourage smokers to utilize cessation treatment (Smokers’ Quitline and clinic-based programs). Utilization and 7-day and 30-day point prevalence abstinence were assessed at 12 months. Self-reported abstinence was biochemically verified.
Results
Intervention participants (n = 121) were more likely than control participants (n = 129) to both utilize treatment programs (adjusted odds ratio [aOR]: 2.15; 95% confidence interval [CI]: 0.93–4.91) and 7-day and 30-day point prevalence abstinence (aOR: 2.60 (1.72–3.94); 2.98 (1.56–5.68), respectively). Mediation analysis indicated that the higher level of utilization did not explain the intervention effect.
Conclusions
An intervention delivered by peer health advocates was able to increase utilization of treatment programs and smoking abstinence among public housing residents. Future studies of similar types of interventions should identify the key mechanisms responsible for success.
Implications
In order to narrow the large and growing socioeconomic disparity in smoking rates, more effective cessation interventions are needed for low-income smokers. Individual culturally-relevant coaching provided in smokers’ residences may help overcome the heightened barriers to cessation experienced by this group of smokers. In this study among smokers residing in public housing, an intervention delivered by peer health advocates trained in motivational interviewing, basic smoking cessation skills, and client navigation significantly increased abstinence at 12 months. Future research should address whether these findings are replicable in other settings both within and outside of public housing.
Introduction
Cigarette smoking is the leading cause of preventable disease and death in the United States and accounts for more than 480 000 deaths every year.1 The prevalence of smoking is substantially higher among socioeconomically disadvantaged adults, and the gap has widened between 2005 and 2015 whether measured by education, income, or health insurance status.2 Although higher rates of smoking initiation play a role, the lower rate of smoking cessation among socioeconomically disadvantaged smokers is a major reason for the widening gulf.3 Smoking cessation rates are two-thirds lower among those with lower educational attainment compared to those with higher educational attainment.4
A recent review of the literature on smoking among socioeconomically disadvantaged adults highlighted a number of factors that contribute to the disparity in quit rates including, among others, greater stress; lower motivation, self-efficacy, and social support; and lower levels of utilization and completion of evidence-based cessation treatments.5 Because of these barriers, socioeconomically disadvantaged smokers may particularly benefit from more intensive intervention to motivate a quit attempt; assist in initiating and maintaining engagement in evidence-based treatment, such as medication and behavioral counseling; and provide social support.
We therefore conducted a smoking cessation intervention among smokers living in public housing in Boston, delivered by public housing residents who were trained as tobacco treatment advocates (TTAs). Compared with smokers receiving a single standard cessation counseling visit, we hypothesized that smokers receiving a tailored intervention that included multiple visits from a peer TTA who utilized motivational interviewing and provided assistance in connecting to quitlines and clinic-based cessation programs and obtaining nicotine replacement therapy (NRT) would have higher rates of both utilization of quitlines and clinic-based cessation programs and of smoking abstinence.
Methods
Study Setting
Approximately 25 000 residents reside in Boston public housing developments (PHDs), managed by the Boston Housing Authority (BHA). The study was conducted in the 26 PHDs that are categorized as primarily family housing developments. Two of the PHDs were used as pilot sites; because some PHDs were small and situated in close proximity to one another, the remaining 24 PHDs were combined into 22 groups.
Design
We conducted a two-parallel-groups stratified cluster-randomized trial. Although the intervention was targeted to individuals, randomization was implemented at the level of the PHD to reduce the risk of contamination resulting from interactions between participants assigned to the intervention and control conditions. PHDs were divided into two strata based on median number of living units, and treatment group was assigned by a random number generator within each stratum to achieve a relatively equal balance of residents in each arm. Participants were evaluated at study entry and at 3-, 7- and 12-month follow-up. The Boston University Medical Center Institutional Review Board approved the study, and a Data and Safety Monitoring Board provided oversight of procedures involving human subjects. The study was registered with ClinicalTrials.gov (NCT01651611).
Participants
Participants were current smokers aged 18–79 who spoke English or Spanish and either planned to quit smoking in the next month or were thinking about quitting in next 6 months, with no plans to leave public housing in the next 12 months. Participants who had abstained from tobacco for the past 7 days, reported current use of pharmacological treatment for smoking cessation, were currently engaged with a smokers’ quitline or other clinic-based cessation program, and/or had major cognitive or psychiatric impairment were not eligible to participate. Participants in each arm who completed at least one TTA visit were considered enrolled in the study and eligible for follow-up.
Recruitment and Data Collection
Study staff met with property managers and tenant leaders (members of tenant task forces and/or individuals widely regarded as informal leaders) at each PHD before initiating recruitment at that site. Two trained public housing residents who operated independently of the TTAs conducted recruitment and data collection. Training was administered by study staff (TE, JD) and consisted of human subject research ethics, with a major focus on confidentiality and informed consent; detailed review of the study protocol, consent form, and questionnaire; use of the CO monitor; and personal safety. The major recruitment strategy consisted of door-knocking and flyer distribution at each site; additional techniques included organization of community meetings, attendance at PHD events such as summer fairs, notices in BHA newsletters and enclosed with the rent statement, and a toll-free study phone number. Other participants learned about the study through word-of-mouth. Interested smokers provided informed consent and were administered a brief screening questionnaire.
After the study was underway, BHA unexpectedly announced its intention in 2010 to adopt a smoke-free policy that would prohibit lit tobacco use in all indoor common areas and residents’ living quarters; the policy took effect simultaneously at all PHDs in September 2012. The great majority of residents were aware of the upcoming policy; BHA also provided residents with the option of attending smoking cessation groups, though utilization was very low. Recruitment and data collection for our study was ongoing throughout the period before and after implementation of the policy. Our study was neither designed nor powered to assess the impact of the policy change, and we were not able to make any modifications other than to clarify with participants that our study was independent of BHA’s effort. In the data analysis, we did adjust for calendar date of study enrollment in relation to the smoke-free policy adoption to account for potential confounding.
Questionnaires were administered in English or Spanish at baseline and at 3, 7, and 12 months after enrollment; participants received $25, $30, $35, and $50, respectively, for completion of the questionnaires. Recruitment commenced in November 2011, and follow-up was completed in June 2014.
Interventions
Control Group
Participants at control PHDs received written materials with general tips for quitting smoking, using NRT, and information on the Smokers’ Quitline and local clinic-based smoking cessation programs, plus a single session of approximately 45 minutes with a control TTA. The control TTA provided basic smoking cessation information, including benefits of quitting, importance of support, medication and counseling options, and strategies to prevent relapse. For those participants ready to make a quit attempt, the control TTA helped develop a quit plan.
Intervention Group
The intervention was based on previously developed smoking cessation protocols for integrating Motivational Interviewing with cognitive-behavioral strategies for smoking cessation by one of the co-investigators (BB).6,7 The intervention also included patient navigation to connect participants with community resources for quitting smoking (in particular, the Massachusetts Smokers Quitline and clinic-based smoking cessation programs). The TTAs assisted in making connections to these resources by contacting the Quitline together with the participant or by sending a referral, with the Quitline then initiating contact, and also by finding transportation-accessible health centers that offered smoking cessation programs. They also helped participants obtain NRT at no or minimal cost, generally by doctors’ prescription covered through the Medicaid program. Participants in the intervention arm were eligible to receive up to nine home visits from a TTA over a 6-month span.
The TTAs met with participants in their home. Visits used motivational interviewing communication skills (open-ended questions, reflections, summaries). Visit 1 focused on building rapport, exploring participants’ smoking habits and motivations for quitting, and preliminary goal setting. Visit 2 focused on strategies to maximize social support, including discussion of aspects of participants’ social networks that might facilitate or impede a successful quit attempt. During subsequent visits, participants could select from a menu of topics, including risks of continuing to smoke, building confidence to quit smoking, concerns about gaining weight, curbing cravings, managing stress and mood, quitting for good, and secondhand smoke.
Once participants were motivated to make a quit attempt, they completed a written quit plan in which they set a quit date and identified smoking triggers and coping strategies, steps to procure pharmacological treatment for cessation, and people to help support their attempt. At this point, the TTA switched focus to motivating the participant to connect with cessation treatment resources. Participants were given the choice to continue to meet with the TTA or solely utilize the community resources. The great majority selected the former option.
TTA Selection, Training, and Treatment Fidelity
Four TTAs—three of whom were current residents of public housing—were selected to provide gender (3 female/1 male), racial/ethnic (3 Latino/1 African American), and linguistic (2 English/1 Spanish/1 bilingual Spanish-English) diversity. Three TTAs worked with participants only in the intervention arm, while the fourth focused only on control participants.
The project director (TE) provided tailored training in human subject research and good research practice to all TTAs, and an experienced tobacco treatment specialist provided an in-person version of an online course titled “Basic Skills for Working with Smokers” offered by the University of Massachusetts. The intervention TTAs also received training in motivational interviewing and in the protocol by a certified trainer (BB) over 3.5 days. TTAs practiced the protocol with several pilot participants until they attained proficiency on the Motivational Interviewing Treatment Integrity Scale.8
All sessions throughout the study were audiotaped. The project director (TE) reviewed all tapes for adherence to protocol, and the motivational interviewing trainer (BB) also reviewed a sample. Recordings of intervention sessions were also reviewed with TTAs during weekly supervision. TTAs were required to complete booster sessions and targeted skill exercises if they were judged to be non-adherent in motivational interviewing skills or in protocol delivery.
Measures
The primary outcomes were point prevalence abstinence in the last 7 days and 30 days at 12-month follow-up. An exhaled carbon monoxide (CO) sample was obtained from all participants who reported not smoking for the past 7 days. A CO level <8 ppm was considered consistent with smoking abstinence.9 Participants who self-reported being abstinent but with values above this level were reclassified as currently smoking. The secondary outcome was self-reported utilization of the Massachusetts Smokers’ Quitline or local clinic-based programs.
Covariates measured at baseline included age, language, race/ethnicity (black non-Hispanic, Hispanic, and white non-Hispanic; four participants reported a different race and were included with whites for purposes of analysis), educational attainment, period of enrollment in the study in relation to the effective date of the smoke-free policy (>6 months before, ≤6 months before, and post-policy adoption), cigarettes per day, Fagerstrom Test for Nicotine Dependence (FTND) score, prior quit attempts, prior pharmacological and/or behavioral counseling cessation treatments, motivation to quit (“Readiness to Quit Ladder,” a 10-point Likert scale of motivation with respect to readiness to quit smoking),10 self-efficacy to quit,11 social support (eight items from the Interpersonal Support Evaluation List [ISEL]),12 stress (Perceived Stress Scale),13 Abbreviated Hassles Index,14 overall health status, 10-item Center for Epidemiologic Studies Depression Scale (CESD-10), and self-reported physician diagnosis of depression or anxiety.
Data Analysis
General estimating equation (GEE) logistic regression models were used to calculate odds ratios for the effect of TTA intervention on main and secondary outcomes. We experimented with log-binomial models in order to obtain risk ratios, but the study was not of sufficient size and models did not converge. All models used an exchangeable correlation structure to account for potential similarities between participants in the same public housing site arising from the group-randomized design. SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used for all analyses.
Analyses were performed based both on treatment group assignment and also accounting for number of TTA sessions in the intervention group at the time of follow-up (1, >1). To address potential confounding, a propensity score model that predicted intervention status was constructed using race, enrollment time, health, depression, nicotine dependence, age, gender, cigarettes per day, lifetime quit attempts, previous NRT use, motivation to quit, and self-efficacy to quit.15 Propensity score distributions were compared between intervention and control groups to ensure sufficient overlap and the propensity score values were used in the final outcome models.
Multiple imputation procedures were used for 7-day and 30-day outcomes to account for missing outcome data from nonrespondents. Missing values were imputed separately for each TTA treatment group from data on age, gender, race/ethnicity, enrollment time, health status, depression, nicotine dependence, cigarettes per day, program utilization, NRT use, motivation to quit, and self-efficacy to quit. Missing outcomes were imputed for ten datasets and subsequently analyzed using an exchangeable correlation structure and adjusting for the same propensity scores as the complete case analysis. In addition to multiple imputation, we also conducted an analysis based on a “worst-case” scenario in which all participants with missing outcome data were assumed to still be smoking.
We explored whether effects of the intervention may have been mediated by greater utilization of the Quitline or clinic-based cessation programs, NRT use, motivation to quit, or self-efficacy to quit, utilizing an approach described by Valeri and VanderWeele.16 The method is based on a counterfactual approach where the total effect of group assignment on quit status is divided into indirect and direct effects. The former represents the effect of the intervention on quit status mediated through one of the hypothesized mediators, while the latter represents the effect of the intervention through pathways that do not involve the mediator.
The main benefit of this approach is that it allows determination of both the estimated magnitude and the precision of measurement of the components by examining their estimates and confidence intervals (CIs) directly. An estimate of 1.0 for the indirect effect indicates that all the impact of the intervention operated through mechanisms other than the hypothesized mediator. An estimate that is substantially similar to the marginal total effect indicates that the entire impact of the intervention operated through the hypothesized mediator, while intermediate estimates indicate partial mediation. The mediation analysis was conducted using logistic regression models combined with bootstrapping techniques to estimate standard errors and CIs.
Results
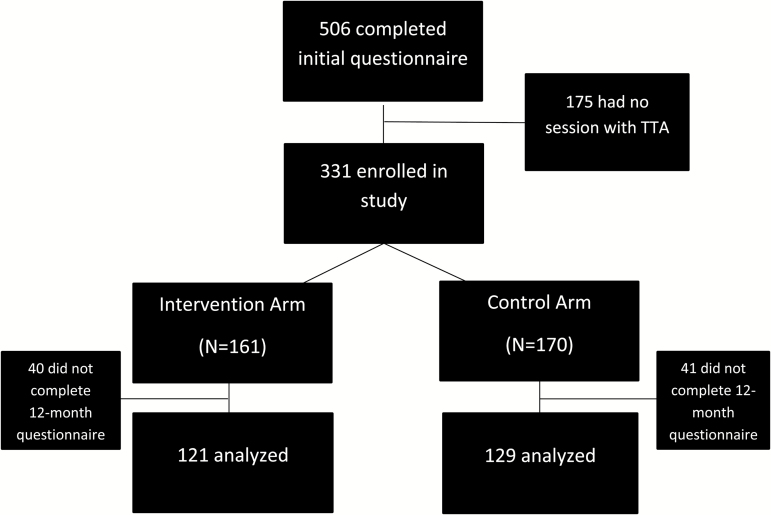
A total of 506 smokers, ranging from 3 to 40 per site, were recruited, of whom 331 completed one visit and were considered enrolled and eligible for follow-up (Figure 1). We restricted primary analyses to participants who completed a 12-month follow-up questionnaire (n = 250; 76%).
Figure 1.
Flow diagram of enrollment and retention.
Demographic, Health and Smoking Characteristics
Characteristics of participants are reported in Table 1. At baseline, participants were mostly female (72%) and greater than age 40 (68%). The gender distribution of study participants was similar to the distribution among adults in family housing developments. The distribution of race/ethnicity was 56% black, 25% Hispanic, and 19% white/Other. Forty-one percent of participants reported they were in fair or poor health, and 39% screened positive for depression according to the CES-D-10. A majority (57%) of participants smoked fewer than 10 cigarettes per day, and most (88%) reported at least one lifetime quit attempt. As expected based on the eligibility criteria, motivation to quit was high (78%). Intervention participants had a median of 3 TTA sessions: 28% had one session, 30% had 2–3 sessions, 26% had 4–5 sessions, and 16% had 6–9 sessions.
Table 1.
Baseline Demographic, Health and Smoking Characteristics Among Participants Who Did and Did Not Complete the 12-Mo Follow-up Questionnairea
| Completed 12-mo questionnaire | Did not complete 12-mo questionnaire | |||||
|---|---|---|---|---|---|---|
| Total (n = 250) | Intervention (n = 121) | Control (n = 129) | Total (n = 81) | Intervention (n = 40) | Control (n = 40) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Age | ||||||
| 18–39 | 78 (31.2) | 48 (39.7) | 30 (23.3) | 28 (34.6) | 17 (42.5) | 11 (26.8) |
| ≥40 | 172 (68.8) | 73 (60.3) | 99 (76.7) | 53 (65.4) | 23 (57.5) | 30 (73.2) |
| Sex | ||||||
| Female | 184 (73.6) | 91 (75.2) | 93 (72.1) | 55 (67.9) | 24 (60.0) | 31 (75.6) |
| Male | 66 (26.4) | 30 (24.8) | 36 (27.9) | 26 (32.1) | 16 (40.0) | 10 (24.4) |
| Race | ||||||
| Hispanic | 54 (21.6) | 28 (23.1) | 26 (20.2) | 30 (37.0) | 14 (35.0) | 16 (39.0) |
| Black | 150 (60.0) | 60 (49.6) | 90 (69.8) | 33 (40.7) | 15 (37.5) | 18 (43.9) |
| White/Other | 46 (18.4) | 33 (27.3) | 13 (10.1) | 18 (22.2) | 11 (27.5) | 7 (17.1) |
| Education | ||||||
| <High school (HS) | 75 (30.0) | 36 (29.8) | 39 (30.2) | 22 (27.2) | 9 (22.5) | 13 (32.5) |
| HS or equivalent | 114 (45.6) | 53 (43.8) | 61 (47.3) | 44 (54.3) | 21 (52.5) | 23 (57.5) |
| Some college | 46 (18.4) | 26 (21.5) | 20 (15.5) | 10 (12.3) | 5 (12.5) | 5 (10.0) |
| College graduate | 15 (6.0) | 6 (5.0) | 9 (7.0) | 5 (6.2) | 5 (12.5) | 0 (0.0) |
| Health | ||||||
| Fair/Poor | 105 (42.0) | 48 (39.7) | 57 (44.2) | 31 (38.3) | 10 (25.0) | 21 (51.2) |
| Excellent/Very Good/Good | 145 (58.0) | 73 (60.3) | 72 (55.8) | 50 (61.7) | 30 (75.0) | 20 (48.8) |
| CES-D-10 depression | ||||||
| Yes | 96 (38.4) | 60 (49.6) 61 | 36 (27.9) | 34 (42.0) | 15 (37.5) | 19 (46.3) |
| No | 154 (61.6) | (50.4) | 93 (72.1) | 47 (58.0) | 25 (62.5) | 22 (53.7) |
| Perceived stress | ||||||
| High | 87 (34.8) | 48 (39.7) | 39 (30.2) | 39 (48.2) | 16 (40.0) | 23 (56.1) |
| Low | 163 (65.2) | 73 (60.3) | 90 (69.8) | 42 (51.9) | 24 (60.0) | 18 (43.9) |
| Cigarettes per day | ||||||
| ≤10 | 142 (56.8) | 64 (52.9) | 78 (60.5) | 47 (58.0) | 23 (57.5) | 24 (58.5) |
| ≥10 | 108 (43.2) | 57 (47.1) | 51 (39.5) | 34 (42.0) | 17 (42.5) | 17 (41.5) |
| Fagerstrom Test for Nicotine Dependenceb | ||||||
| ≤4 (low to moderate) | 160 (64.3) | 78 (65.0) | 82 (63.6) | 47 (58.0) | 24 (60.0) | 23 (56.1) |
| ≥5 (moderate to high) | 89 (35.7) | 42 (35.0) | 47 (36.4) | 34 (42.0) | 16 (40.0) | 18 (43.9) |
| NRT use past 12 mo | ||||||
| Yes | 117 (46.8) | 53 (43.8) | 64 (49.6) | 38 (46.9) | 19 (47.5) | 19 (46.3) |
| No | 133 (53.2) | 68 (56.2) | 65 (50.4) | 43 (53.1) | 21 (52.5) | 22 (53.7) |
| Lifetime quit attempts | ||||||
| 0 | 33 (13.2) | 19 (15.7) | 14 (10.9) | 7 (8.6) | 7 (17.5) | 0 (0.0) |
| 1–3 | 133 (53.2) | 61 (50.4) | 72 (55.8) | 40 (49.4) | 17 (42.5) | 23 (56.1) |
| ≥4 | 84 (33.6) | 41 (33.9) | 43 (33.3) | 34 (42.0) | 16 (40.0) | 18 (43.9) |
| Motivation to quit | ||||||
| Low (1–3) | 8 (3.2) | 2 (1.7) | 6 (4.7) | 1 (1.2) | 0 (0.0) | 1 (2.4) |
| Medium (4–6) | 41 (16.4) | 20 (16.5) | 21 (16.3) | 22 (27.2) | 11 (27.5) | 11 (26.8) |
| High (7–10) | 201 (80.4) | 99 (81.8) | 102 (79.1) | 58 (71.6) | 29 (72.5) | 29 (70.7) |
| Enrollment time | ||||||
| Post-policy | 103 (41.2) | 50 (41.3) | 53 (41.1) | 40 (49.4) | 14 (35.0) | 26 (63.4) |
| 0–6 months pre-policy | 90 (36.0) | 49 (40.5) | 41 (31.8) | 32 (39.5) | 20 (50.0) | 12 (29.3) |
| >6 months pre-policy | 57 (22.8) | 22 (18.2) | 35 (27.1) | 9 (11.1) | 6 (15.0) | 3 (7.3) |
aAlthough beliefs and practices differ on statistical significance testing, we have not identified which variables were statistically significantly different between intervention and reference groups or between those who completed and did not complete the 12-mo questionnaire. The main purpose of this table of baseline characteristics, other than a simple description of the sample, is to show which factors are imbalanced and should be considered for adjustment in order to reduce confounding or be considered as possible sources of selection bias. Simulation studies have shown that use of statistical significance does not do an adequate job of identifying these variables, that is, variables that are statistically significant frequently may not be confounders, while the opposite can be true of variables that are not statistically significant.30 In this case, we have used the approach of the propensity score, for which statistical significance is not necessary for inclusion.
bMissing n = 1.
Differences in the distribution of baseline characteristics between intervention and control groups were expected due to the fact that randomization was implemented at the level of the development rather than at the individual level. Most notably, participants in the control group were more likely to be older, black, and have made at least one previous quit attempt, while those in the intervention group were more likely to score positive for depression on the CESD-10 scale (Table 1).
The percentage of participants completing the 12-month questionnaire in the intervention (75%) and control (76%) arms was virtually identical. Hispanic participants were less likely to complete the questionnaire regardless of study arm. Males and those reporting better health status in the intervention arm were less likely to complete a questionnaire, while lower completion rates in the control arm were seen among those enrolled post-policy and with higher levels of perceived stress. Completion rates were lower among participants in the intervention arm who screened negative for depression, but lower in the control arm among those who screened positive for depression (Table 1).
Utilization and Abstinence
Intervention participants reported using the Smokers Quitline or clinic-based programs about twice as frequently as control participants (29.8% vs. 14.7%; adjusted odds ratio [aOR]: 2.40, 95% CI: 1.14–5.05) (Table 2). This relationship held for those participants who had ≥2 TTA sessions (aOR: 2.80, 95% CI: 1.07–7.29) but not for those with only one TTA session (aOR: 0.78, 95% CI: 0.23–2.20).
Table 2.
Utilization of Cessation Programs and 7-Day and 30-Day Point Prevalence Abstinence by Study Arm and Number of TTA Sessionsa
| Intervention (%) (n = 121) | Control (%) (n = 129) | cOR (95% CI)b | aOR (95% CI)c | aOR (95% CI)c 1 TTA session (n = 34) | aOR (95% CI)c >1 TTA session (n = 87) | |
|---|---|---|---|---|---|---|
| Utilization | 29.8 | 14.7 | 2.40 (1.14–5.05) | 2.14 (0.93–4.91) | 0.78 (0.23–2.20) | 2.80 (1.07–7.29) |
| Abstinence | ||||||
| 7-day | 16.5 | 10.1 | 1.90 (1.35–2.68) | 2.60 (1.72–3.94) | 2.05 (0.93–4.51) | 2.66 (1.71–4.16) |
| 30-day | 14.9 | 7.8 | 2.29 (1.29–4.04) | 2.98 (1.56–5.68) | 2.78 (1.30–5.95) | 3.06 (1.44–6.51) |
TTA = Tobacco Treatment Advocate.
aThe control group served as the reference category for all analyses.
bcOR: crude odds ratio; CI: confidence interval.
caOR: adjusted odds ratio, using propensity score (see Methods).
Five participants who reported being abstinent for 7 days had CO values ≥8 ppm and were reclassified as smoking (13.2%), with no difference in reclassification rates between intervention (13.0%) and control (13.3%) arms. The unadjusted incidence of biochemically-verified 7-day abstinence was 16.5% in the intervention group and 10.1% in the control group, and 14.9% and 7.8%, respectively, for 30-day abstinence (Table 2). Adjustment for confounding strengthened the effect of the intervention for both 7-day (aOR: 2.60, 95% CI: 1.72–3.94) and 30-day (aOR: 2.98, 95% CI: 1.56–5.68) abstinence. Among all the confounders, adjustment for race/ethnicity and nicotine dependence had the greatest impact on strengthening the association.
Estimates based on multiple imputation were slightly attenuated (7-day aOR 2.22, 95% CI: 1.09–4.50; 30-day aOR: 2.59, 95% CI: 1.18–5.69). Estimates based on the assumption that all participants without outcome data were currently smoking were very similar (7-day aOR 2.09, 95% CI: 1.10–3.97; 30-day aOR: 2.24, 95% CI: 1.00–5.03). The intervention’s impact on 7-day abstinence was similar among participants with one (aOR: 2.05, 95% CI: 0.93–4.51) or greater than one (aOR: 2.66, 95% CI: 1.71–4.16) TTA session. The same pattern was seen for 30-day abstinence.
Analysis of potential mediation indicated that the effect of the intervention was not mediated through use of cessation services, use of NRT, or motivation to quit (Table 3). Only the estimate of the indirect effect for self-efficacy to quit (OR: 1.42, 95% CI: 0.73–2.76) suggested that the effect of the intervention may have partly operated through this mechanism.
Table 3.
Natural Direct and Indirect Effects of the Intervention on 30-Day Point Prevalence Abstinence at 12-Months
| OR (95% CI)a | |||
|---|---|---|---|
| Mediator | Natural direct effect | Natural indirect effect | |
| Quitline or local clinic program use | Crude | 2.39 (1.04–5.47) | 0.89 (0.79–1.00) |
| Mediated* | 2.53 (1.01–6.33) | 0.90 (0.80–1.02) | |
| NRT use | Crude | 2.18 (0.96–4.96) | 0.96 (0.88–1.05) |
| Mediated* | 2.36 (0.94–5.95) | 0.98 (0.90–1.06) | |
| Motivation to quit | Crude | 2.40 (0.94–6.11) | 0.78 (0.35–1.75) |
| Mediated* | 2.70 (0.93–7.83) | 0.85 (0.36–1.98) | |
| Self-efficacy to quit | Crude | 3.70 (0.94–14.67) | 0.82 (0.45–1.49) |
| Mediated* | 2.41 (0.47–12.41) | 1.42 (0.73–2.76) | |
CI = confidence interval; NRT = nicotine replacement therapy; OR = odds ratio.
aAdjusted for baseline measures of race, age, gender, enrollment time, health, depression, cigarettes per day, nicotine dependence, lifetime quit attempts, previous NRT use, motivation to quit, and self-efficacy to quit using propensity score methods
*Mediated analyses include the hypothesized mediator in the analysis.
Discussion
Determining smoking cessation interventions that are efficacious for socioeconomically disadvantaged smokers is an integral component of addressing the increasing socioeconomic disparity in quit rates.4,17 A number of authors have described a range of barriers to smoking cessation that are particularly acute among socioeconomically disadvantaged smokers.18–22 We designed this study from the perspective that a higher level of engagement may be warranted to address these additional barriers. Furthermore, the literature showing that peer community health advocates can be effective and cost-effective,23 as well as our own experience with peer health advocates in public housing,24 suggested that public housing residents could be successfully trained as TTAs, and that the peer character of the interaction between TTAs and recipients could result in a more effective intervention.
The results of the study were largely supportive of our a priori hypotheses. Smokers in the intervention arm were more than twice as likely to utilize the Smokers’ Quitline or clinic-based smoking cessation programs. More importantly, although the absolute abstinence rate was modest,25 intervention participants were more than twice as likely as control participants to report 7-day and 30-day smoking abstinence at 12 months post-enrollment. In interpreting these results, it is also noteworthy that the participants in the control arm were provided a 45-minute in-person session, a substantially greater level of assistance than would normally have been available.
Surprisingly, the results also indicated that even one intervention session with a TTA increased the likelihood of abstinence. Although caution is warranted in interpreting this finding, it does suggest the possibility that the impact of the intervention may have been due not only to the additional number of visits but also to the nature of the sessions. The intervention focused on three main components: motivating smoking cessation through using MI, cognitive-behavioral strategies and skills for quitting smoking, and patient navigation. While it is not possible to tease out which of these components were most effective, it is perhaps more useful to view these as a comprehensive package that helps address the particular needs and concerns of socioeconomically disadvantaged smokers. More intensive and tailored treatments that go beyond traditional evidence-based approaches may be needed for these smokers to prevent smoking treatment failure.26
The lack of mediation by utilization of the Massachusetts Quitline or local clinic-based programs, despite the fact that the intervention increased utilization of these programs, further supports the idea that TTA engagement with intervention participants may have been important in the success of the intervention. Interestingly, the mediation analysis suggests that increased self-efficacy may have been part of the causal pathway. Confidence in achieving abstinence might be attributable, at least in part, to engagement with a TTA, rather than via contact with cessation programs. However, any such conclusion is preliminary at best, and additional investigation is needed into the mechanisms leading to greater abstinence.
Very few smoking cessation intervention studies have been conducted within the public housing setting. Okuyemi found no impact of a randomized trial of nicotine gum and motivational interviewing for smoking cessation in public housing sites in Kansas City, Missouri in which five sessions were provided by masters’-level counselors.27 Andrews et al conducted a study among 103 African American women in Georgia.28 The 6-month intervention consisted of nicotine patch plus 8 group counseling sessions led by a nurse tobacco treatment specialist supplemented by weekly additional contact with resident health advocates. The intervention resulted in a 7-day abstinence rate of 39.2% (vs. 11.5% in the control group) when assessed at 6 months. However, in a second, larger study in Georgia and North Carolina, despite using essentially the same intervention, abstinence rates were much lower among both intervention and control groups and did not differ between the groups.29 The authors cite both differences in the amount of time spent engaging with communities prior to the study and the readiness of community residents to engage in the study as possible reasons for the conflicting results. The results of these studies suggest that the broader context in which the study is conducted may have an important impact on success, and illustrates that there is much still to be learned about the characteristics of cessation interventions necessary to decrease smoking among residents of public housing and, by extension, socioeconomically disadvantaged smokers in general.
Some important limitations should be noted. First, 76% of participants completed the 12-month questionnaire, and the potential for bias based on nonresponse exists. The fact that retention was the same in both study arms mitigates this concern to some extent. The similarity of the results based on complete-case and multiple imputation analysis further suggests that the extent of bias was limited. Second, the study was aimed at smokers who were at least thinking about quitting smoking, and further excluded from analysis those who completed the baseline questionnaire but never had a single session with a TTA (n = 175/506). We adopted this approach (a form of the run-in period used in clinical trials to identify and exclude potential participants who are unlikely to adhere to an intervention) because we expected that the number of who dropped out of the study prior to the intervention would be substantial. We attribute the relatively large number who did not complete a first visit to the unstable nature of the desire to make a quit attempt and other issues that may have arisen in residents’ complicated lives. As a result, our study had greater internal validity and was better able to test the efficacy of the intervention, but at the cost of broader generalizability.
Conclusions
This study demonstrated that public housing residents can effectively deliver an intervention that employs motivational interviewing and other community health worker skills, and that the intervention was able to both meaningfully increase utilization of already-existing smoking cessation programs and to increase abstinence at 12-month follow-up. Future research should address whether these findings are replicable in other settings both within and outside of public housing. A peer TTA model for smoking cessation may substantially increase successful outcomes, yet a better understanding is required of the intervention components that promote success. A critical future challenge will be to determine how peer-led cessation interventions might be readied for implementation on a larger scale, while retaining essential components.
Funding
Funding for this study was provided by the National Cancer Institute (1RO1CA141587, 3RO1CA141587-S1).
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all study participants.
Declaration of Interests
None declared.
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014:679 [Google Scholar]
- 2. Jamal A, Homa DM, O’Connor E et al. Current cigarette smoking among adults - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 3. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults-United States, 2000–2015. Morb Mortal Wkly Rep CDC. 2017;65:1458–1464. [DOI] [PubMed] [Google Scholar]
- 4. Zhuang YL, Gamst AC, Cummins SE, Wolfson T, Zhu SH. Comparison of smoking cessation between education groups: findings from 2 US National Surveys over 2 decades. Am J Public Health. 2015;105(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. [DOI] [PubMed] [Google Scholar]
- 6. Borrelli B. Motivational interviewing for smoking cessation. In: Polosa R, Caponnetto P, eds. Advances in Smoking Cessation. London, UK: Future Medicine; 2013. https://www.futuremedicine.com/doi/full/10.2217/ebo.12.416. [Google Scholar]
- 7. Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41(5-6):815–821. [DOI] [PubMed] [Google Scholar]
- 8. Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat. 2005;28(1):19–26. [DOI] [PubMed] [Google Scholar]
- 9. SNRT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. [DOI] [PubMed] [Google Scholar]
- 10. Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. [DOI] [PubMed] [Google Scholar]
- 11. Condiotte MM, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. J Consult Clin Psychol. 1981;49(5):648–658. [DOI] [PubMed] [Google Scholar]
- 12. Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, eds. Social Support: Theory, Research and Applications. NATO ASI Series (D: Behavioural and Social Sciences), vol 24. Dordrecht, Netherlands: Springer; 1985:73–94. [Google Scholar]
- 13. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 14. Romano PS, Bloom J, Syme SL. Smoking, social support, and hassles in an urban African-American community. Am J Public Health. 1991;81(11):1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 16. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bryant J, Bonevski B, Paul C, McElduff P, Attia J. A systematic review and meta-analysis of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups. Addiction. 2011;106(9):1568–1585. [DOI] [PubMed] [Google Scholar]
- 18. Vangeli E, West R. Sociodemographic differences in triggers to quit smoking: findings from a national survey. Tob Control. 2008;17(6):410–415. [DOI] [PubMed] [Google Scholar]
- 19. Graham H, Inskip HM, Francis B, Harman J. Pathways of disadvantage and smoking careers: evidence and policy implications. J Epidemiol Community Health. 2006;60(Suppl 2):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiscock R, Judge K, Bauld L. Social inequalities in quitting smoking: what factors mediate the relationship between socioeconomic position and smoking cessation?J Public Health (Oxf). 2011;33(1):39–47. [DOI] [PubMed] [Google Scholar]
- 21. Businelle MS, Kendzor DE, Reitzel LR et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29(3):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryant J, Bonevski B, Paul C, O’Brien J, Oakes W. Developing cessation interventions for the social and community service setting: a qualitative study of barriers to quitting among disadvantaged Australian smokers. BMC Public Health. 2011;11:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Margellos-Anast H, Gutierrez MA, Whitman S. Improving asthma management among African-American children via a community health worker model: findings from a Chicago-based pilot intervention. J Asthma. 2012;49(4):380–389. [DOI] [PubMed] [Google Scholar]
- 24. Rorie JA, Smith A, Evans T et al. Using resident health advocates to improve public health screening and follow-up among public housing residents, Boston, 2007-2008. Prev Chronic Dis. 2011;8(1):A15. [PMC free article] [PubMed] [Google Scholar]
- 25. Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2013 reviews. Addiction. 2014;109(9):1414–1425. [DOI] [PubMed] [Google Scholar]
- 26. Borrelli B. Smoking cessation: next steps for special populations research and innovative treatments. J Consult Clin Psychol. 2010;78(1):1–12. [DOI] [PubMed] [Google Scholar]
- 27. Okuyemi KS, James AS, Mayo MS et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low-income housing. Health Educ Behav. 2007;34(1):43–54. [DOI] [PubMed] [Google Scholar]
- 28. Andrews JO, Felton G, Ellen Wewers M, Waller J, Tingen M. The effect of a multi-component smoking cessation intervention in African American women residing in public housing. Res Nurs Health. 2007;30(1):45–60. [DOI] [PubMed] [Google Scholar]
- 29. Andrews JO, Mueller M, Dooley M, Newman SD, Magwood GS, Tingen MS. Effect of a smoking cessation intervention for women in subsidized neighborhoods: a randomized controlled trial. Prev Med. 2016;90:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. [DOI] [PubMed] [Google Scholar]