Abstract
Background
Higher-protein (HP) diets are advocated for several reasons, including mitigation of sarcopenia, but their effects on kidney function are unclear.
Objective
This meta-analysis was conducted to determine the effect of HP intakes on kidney function in healthy adults.
Methods
We conducted a systematic review and meta-analysis of trials comparing HP (≥1.5 g/kg body weight or ≥20% energy intake or ≥100 g protein/d) with normal- or lower-protein (NLP; ≥5% less energy intake from protein/d compared with HP group) intakes on kidney function. Medline and EMBASE databases were searched. Randomized controlled trials comparing the effects of HP with NLP (>4 d duration) intakes on glomerular filtration rate (GFR) in adults without kidney disease were included.
Results
A total of 2144 abstracts were reviewed, with 40 articles selected for full-text review; 28 of these were analyzed and included data from 1358 participants. Data were analyzed using random-effects meta-analysis (RevMan 5; The Cochrane Collaboration), meta-regression (STATA; StataCorp), and dose-response analysis (Prism; GraphPad). Analyses were conducted using postintervention (post) GFR and the change in GFR from preintervention to post. The post-only comparison showed a trivial effect for GFR to be higher after HP intakes [standardized mean difference (SMD): 0.19; 95% CI: 0.07, 0.31; P = 0.002]. The change in GFR did not differ between interventions (SMD: 0.11; 95% CI: −0.05, 0.27; P = 0.16). There was a linear relation between protein intake and GFR in the post-only comparison (r = 0.332, P = 0.03), but not between protein intake and the change in GFR (r = 0.184, P = 0.33). The main limitation of the current analysis is the unclear risk of selection bias of the included trials.
Conclusions
Postintervention GFR comparisons indicate that HP diets result in higher GFRs; however, when changes in GFR were compared, dietary protein had no effect. Our analysis indicates that HP intakes do not adversely influence kidney function on GFR in healthy adults.
Keywords: glomerular filtration rate, GFR, renal failure, chronic kidney disease, protein, meta-analysis
Introduction
Higher-protein (HP) intakes [>1.0–1.2 g · kg body weight (BW)−1 · d−1] promote greater muscle hypertrophy during periods of resistance training (1), and not only increase the absolute amount of weight lost (2) but preserve lean body mass during weight loss (2, 3). HP intake (compared with the RDA) has also been advocated for older persons to preserve skeletal muscle mass loss due to sarcopenia (4, 5). Hypothesized mechanisms underpinning greater exercise-induced changes in lean mass with protein supplementation in resistance training include greater and more regular stimulation of muscle protein synthesis (6), and the same may be true in the setting of weight loss (2, 3). HP intake during weight loss may also increase satiety, resulting in lower daily energy intake (7); and protein ingestion has an increased thermic effect, resulting in greater daily energy expenditure (8). Given the prevalence of overweight, obesity, and global aging, the preservation of muscle mass via consumption of an HP diet may be advantageous.
Despite the proposed benefits of consuming an HP diet to preserve muscle mass or promote muscle hypertrophy, HP diets are often discouraged because of potential negative effects on kidney function, particularly glomerular filtration rate (GFR). Broadly stated, the thesis that protein affects kidney function is that with persistent consumption of an HP diet the increased renal solute load (as urea) is an antecedent step leading to compensatory hyperfiltration, which then leads to glomerular damage and eventual kidney damage and failure (9). In individuals with compromised kidney function [i.e., chronic kidney disease (CKD)], where there is a reduction in the ability of nephrons to filter solutes such as urea, a lower-protein diet (0.6–0.8 g · kg BW−1 · d−1) can improve the metabolic abnormalities associated with CKD (10) and delay mortality (11). More recent investigations have, however, found no benefit, and potential harm, in consuming a lower-protein diet, even in those with CKD; and recent guidelines recommend aiming for 0.8 g protein · kg BW−1 · d−1 and not consuming >1.3 g · kg BW−1 · d−1 (12). However, it is on the basis of the aforementioned hypothesis that some argue that increased protein intake in healthy individuals without CKD will lead to kidney damage. Such a hypothesis persists despite no evidential link between increased protein intake and a causal role in kidney damage in healthy individuals having been established. In fact, a causal role for protein in the decline in kidney function is dismissed in guidelines for protein intake (13).
Schwingshackl and Hoffmann (14) conducted a systematic review and meta-analysis of the effects of HP compared with normal- or lower-protein (NLP) intake on kidney function in people without CKD. The analysis included acute studies (as short as 1 d in duration) and found that HP diets induced an increase in GFR, serum urea, and urinary calcium excretion compared with NLP diets. However, in their analysis, the authors compared only postintervention (post) GFR between groups rather than the absolute change in GFR in response to the dietary treatments. Given that GFR may have differed between groups at study entry, conducting an analysis only on post values may have influenced the findings of the meta-analysis. Furthermore, no attempt was made to examine whether there is a dose-response effect of protein intake on GFR. The purpose of the current systematic review, meta-analysis, and meta-regression was to examine whether GFR increases to a greater extent after an HP diet as determined by change from baseline as compared with an NLP diet in individuals without CKD.
Methods
Search strategy and study identification
A systematic search of published studies was conducted in Medline and EMBASE from inception inclusive to 3 April 2017. Search terms included a combination of keywords and subject headings as appropriate for dietary proteins, amino acids (essential and nonessential), protein-restricted diet, vegetarian diet, fish protein, vegetable protein, milk, yolk, eggs, soy protein, protein metabolism, nitrogen metabolism, high protein diet, low protein diet, glomerular filtration rate, inulin clearance, kidney circulation, renal circulation, kidney function, kidney circulation, proteinuria, albuminuria, creatinine, inulin, and hemoglobinuria (Supplemental Methods 1). Searches were limited to clinical trials by applying the maximizing sensitivity McMaster Health Information Research Unit filters for Medline and EMBASE (15, 16). Searches were further limited to humans by excluding studies that used mice and rats because limiting the search to humans was insufficient. Last, searches were limited to the English language and duplicates were removed. MCD and AS reviewed the search strategies, abstracts, and full-text articles and abstracted all data. We also reviewed the reference lists of previous systematic reviews and included relevant trials.
Study inclusion and exclusion criteria
Studies were included if they were randomized controlled trials (RCTs) that studied the effect of HP intake with moderate (greater than the RDA but lower than the HP intake) or lower-protein (RDA or less) intake on GFR in adults aged ≥18 y. Studies were included if they enrolled participants who were healthy, obese, had type 2 diabetes, and/or had hypertension. Studies were excluded if they enrolled participants with type 1 diabetes, CKD, or any other pre-existing indicators of kidney impairment (i.e., proteinuria, previous stone formation) or if subjects had recently undergone any surgical procedures. To be included, protein had to be consumed orally but could be in any form (food, powder, oral pill). Furthermore, the HP diet had to meet ≥1 of the following criteria: 1) ≥1.5 g protein ⋅ kg BW−1 ⋅ d−1, 2) ≥20% of total caloric intake coming from protein, or 3) ≥100 g protein/d. In addition, the NLP diet had to provide ≥5% less, as a percentage of total daily energy intake, protein than the HP diet (17). To avoid acute transient changes in kidney function, which we viewed as irrelevant for ascertaining the impact on long-term kidney function, studies were excluded if the intervention was ≤3 d or if the study investigated the acute effects of only one meal or protein load. All of the following calculations or estimations of GFR were permitted: creatinine clearance, isotope clearance, inulin clearance, iothalamate clearance, iohexol clearance, sinistrin clearance, Cockgroft-Gault calculations, modification of diet in renal disease calculations, and chronic kidney disease epidemiology collaboration calculations. Finally, studies were excluded if they were only provided in abstract form.
Data extraction and data syntheses
Study characteristics and data were extracted to RevMan 5 (Review Manager, version 5.3; The Cochrane Collaboration, 2015). Where necessary and possible, all subject baseline data and outcome measures were converted to the same units. If data were missing, then the authors were contacted or values were extracted from published tables and graphs. When data were missing and values could not be inferred from the publication, these studies were excluded from the respective comparison or analysis.
For the post-only analysis, post GFR values and SDs from parallel-group trials were directly input into RevMan. To account for within-participant differences in the crossover trials in the post-only analysis we used the generic inverse-variance method in RevMan. To input data using this method we first calculated the standardized mean difference (SMD) between the post values and the pooled SD for the HP and NLP groups.
In the preintervention (pre)/post change analysis, only 4 studies used a crossover design and not enough data were available to account for within-participant differences; thus, data from crossover studies were included as if they were parallel-group analyses, and mean differences and change SDs (SDΔ) were calculated and input. Mean differences for each group within a study (HP and NLP) were calculated as follows:
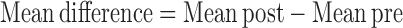 |
(1) |
SDΔ was input from reported values where possible. When SDΔ was not reported and raw data were not available, SDΔ was calculated as follows:
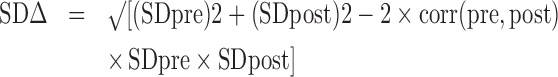 |
(2) |
where corr(pre, post) is the correlation between pre- and post-values across participants. This was calculated from studies that reported SDΔ and/or raw data for a given outcome and applied across trials as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (18). If a study reported mean differences and SDs for 2 different populations (due to age or another demographic characteristic), then they were treated as separate studies in the data analysis.
Meta-analyses
Because of differences in the presentation of data between studies (i.e., reporting GFR after the intervention only compared with reporting GFR both before and after the intervention), 2 meta-analyses were performed on GFR, the outcome of interest. Not all studies reported GFR values before starting the dietary intervention, and thus one meta-analysis evaluated the difference in GFR between groups after the dietary intervention (post-only). However, given the variability in GFR within the population, it may be more appropriate to assess the effect of a dietary intervention on kidney function by comparing the change in GFR induced by the dietary interventions. Thus, where possible, the other meta-analysis compared the change in GFR from baseline induced by each diet (pre/post change). In the post-only analysis, we conducted 3 separate analyses, one on the crossover studies using generic inverse-variance data, one on the parallel-group studies using continuous data, and one on the combined data from crossover and parallel-group trials using generic inverse-variance data. In addition, because a lack of a washout period could influence the overall findings of a study, we conducted a sensitivity analysis where we removed the crossover trials that did not include a washout period between arms. Furthermore, because crossover trials were included as if they were parallel-group trials in the pre/post change analysis, we conducted a sensitivity analysis by including only the parallel-group studies to ensure that the inclusion of crossover studies did not influence the overall results. Effect sizes were categorized as trivial, small, moderate, large, very large, nearly perfect, or perfect (SMD = 0.0, 0.2, 0.6, 1.2, 2.0, 4.0, or infinite) as defined by Hopkins (19), which are based on Cohen's thresholds but align better with Cohen's thresholds for correlation coefficients (20).
Heterogeneity and risk-of-bias assessment
All of the extracted data were assessed for heterogeneity and publication bias. Heterogeneity was tested by using chi-square and I2 tests. Significance was set as P < 0.05 for the chi-square test. I2 values of 30–60%, 50–90%, and 75–100% were taken to indicate moderate, substantial, and considerable heterogeneity, respectively. In the presence of heterogeneity, random-effects meta-analysis was used. Publication bias was assessed with visual inspection of funnel plots. Last, when funnel plot asymmetry existed in the presence of heterogeneity, then the results from both fixed- and random-effects models were compared to verify that the results of the random-effects meta-analysis were not greater than those of the fixed-effects model (21).
Risk of bias was assessed by using domain-based evaluation as described by the Cochrane Collaboration (18). Studies were not included if they reported >2 high-risk domains. Furthermore, sensitivity analyses were performed to determine if the inclusion of trials with >3 unclear risk domains or 2 high-risk domains influenced the results.
Assessment of the certainty in the evidence
We conducted an evaluation of the certainty in the evidence using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach. Certainty in the evidence means to what extent the estimates in treatment effect are correct or close to the truth (22). In GRADE, when assessing certainty, RCTs start as high; however, serious or very serious issues across 5 domains may reduce the certainty of: 1) risk of bias, 2) inconsistency, 3) imprecision, 4) indirectness, and 5) publication bias (23). We conducted this assessment for all included outcomes across studies.
Subgroup analyses
Given the potential for a carryover effect from the previous treatment with crossover trials, subgroup analysis was conducted to determine whether trial type influenced the overall outcome. We also examined whether measuring GFR after the intervention only, compared with determining the change induced by the intervention, influenced the overall results. To do so we compared the effect size from the post-only comparison with that of the pre/post change comparison in only those studies that reported GFR before and after the dietary intervention. Because different populations and interventions were used, subgroup analysis was also conducted to determine the effects of an HP diet on GFR: during energy balance or energy restriction, in participants with type 2 diabetes, on the basis of intervention length, and whether carbohydrate or fat intake was manipulated. Given the potential for changes in protein intake to influence serum creatinine concentrations (24, 25), subgroup analysis was performed on the basis of measurement type (i.e., true measurements of clearance compared with estimations of GFR from serum creatinine concentrations). Given that various methods with different units were used to assess glomerular function, SMDs were analyzed with the use of fixed- or random-effects meta-analyses. Forest plots were generated for each outcome to show study-specific effect sizes and their corresponding 95% CIs as well as the overall pooled effect. Means ± SDs are reported.
Meta-regression
We used meta-regressions to probe several other variables that may influence GFR. Five covariates were chosen a priori to be included in the meta-regression. Continuous variables were differences in protein intake between groups (grams per kilogram of BW per day), trial length (weeks), and age. Categorical variables were whether or not participants had type 2 diabetes and whether the study was conducted under conditions of energy restriction or energy balance. The covariates were meta-regressed individually and together in random-effects meta-regression model using Stata (Stata Statistical Software, release 12, 2011; StataCorp LP). The random-effects meta-regression used residual restricted maximum likelihood to measure between-study variance (τ2) with a Knapp-Hartung modification as recommended (26). Additional covariates were identified and individually analyzed post hoc to further examine the unexplained variance of the effect of HP intake on GFR. Continuous variables were as follows: weight, energy intake, difference in protein intake as a percentage of total energy intake, and difference in protein intake (grams per day). Whether the study design was a parallel-group or crossover RCT was included as a categorical variable.
Dose-response analysis
To determine if there was a dose-response effect of protein consumption on GFR we conducted a linear regression analysis on the effect of daily protein intake on the change in GFR and the post-only GFR response. Linear and biphasic regressions were performed using GraphPad Prism (version 6; GraphPad Software, Inc.) to determine models of best fit as previously described (27, 28). Significance was set at P < 0.05, and the breakpoint analysis is presented as means (95% CIs).
Results
Study and subject characteristics
The Medline search yielded a total of 1326 articles and the EMBASE search yielded a total of 818 articles after all duplicates were removed within each database. A total of 2144 abstracts were examined, and 40 studies were selected for full-text review on the basis of the inclusion and exclusion criteria. In addition, reviewing the reference lists of previous systematic reviews and included trials identified a further 7 articles. Five studies were excluded because they were either only published in abstract form or the full text could not be obtained. Fourteen studies were excluded upon review of the full text, which yielded a remaining total of 28 studies that were included in the analyses. The study inclusion and exclusion process was conducted by 2 investigators (MCD and AS) and is shown in Figure 1.
FIGURE 1.
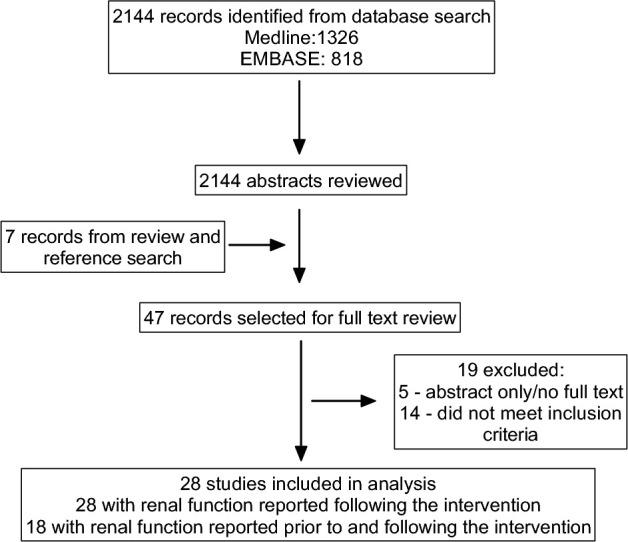
PRISMA flow diagram showing flow of studies through the systematic review process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Articles reporting on 28 trials from 2144 articles were eligible for inclusion on the basis of the inclusion and exclusion criteria. The detailed progression of study inclusion and exclusion is shown in Figure 1. The included studies were published between 1975 and 2016. Fourteen of the studies were parallel-group RCTs and 14 were crossover RCTs. Of the crossover trials, 8 reported including a washout period ranging from 1 to 8 wk (29–36), whereas the remaining 6 studies did not report whether a washout period was included in the protocol (37–42). Although the GFR values after the dietary intervention were reported by all 28 studies, only 18 trials provided both pre- and post-GFR values (33, 34, 37, 38, 43–56).
The 28 trials represent data from 1358 subjects ranging in age from 23 to 72 y (mean ± SD: 49 ± 15 y). The studies recruited normal-weight, overweight, and obese participants, with the weights of participants ranging from 64.5 to 106.0 kg (92.4 ± 11.9 kg) and BMI (in kg/m2) from 21.2 to 36.1 (31.1 ± 4.5), respectively. Two of the studies were carried out to determine if there was a differential kidney response to protein feeding dependent on age, and data were reported for younger and older adults separately; thus, the results were treated as 2 different studies for accurate data input (35, 36). There were 3 crossover trials that involved 3 arms (31, 33, 39). In the study by Chu et al. (39), 3 different amounts of protein intake were tested (5.6, 75, and 150 g/d). In this situation, the 150-g/d intake was used as the HP group and the 75-g/d intake was used as the NLP group because this more adequately reflects the normal protein intake of the population, whereas an intake of 5.6 g/d does not fall within the acceptable macronutrient distribution range and thus would not be an appropriate comparison. In the study by Juraschek et al. (33), the 3 different diets were HP (25% of energy intake; used as the HP group); high-fat, regular protein (37% fat, 15% protein); and high-carbohydrate, regular protein (58% carbohydrate, 15% protein). In this situation, the data from the high-carbohydrate, regular-protein group were used as the NLP comparison because this would more accurately reflect a habitual diet of the North American population. In the study by Gross et al. (31), the 3 diets were as follows: usual-protein intake (1.43 g/kg BW), a chicken diet in which the red meat from the usual diet was replaced with chicken (1.35 g/kg BW), and a low-protein diet (0.66 g/kg BW). In this situation, the greatest difference between low-protein and HP intakes was chosen, and thus the data from the usual diet and low-protein diet were compared. Furthermore, only data for subjects with normo-albuminuria were used in the analysis (31). Individual study characteristics and overall summary characteristics pertaining to participant sex, diabetic status, dietary characteristics, and GFR measurement type can be found in Tables 1 and 2.
TABLE 1.
Study and subject characteristics of studies included in the meta-analysis of whether higher-protein intakes affect kidney function in healthy people1
| Sample size | GFR3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (ref) | Study design | Diet groups | Total,2n | Women, % | n/Group | Diabetic (yes/no) | Mean age, y | Mean BMI, kg/m2 | Study duration, wk | Dietary protein sources | Daily protein intake | Diet type | GFR method | Pre | Post |
| Antonio et al. (37) | X-over | HP | 12 | 0 | 12 | No | 26 | 26.8 | 8 | Usual dietary protein + whey protein | 3.3 g/kg | EB, ad libitum | eGFR (Eq not specified, mL · min−1 · 1.73 m−2 | 96 ± 20 | 101 ± 18 |
| NLP | Usual dietary protein | 2.6 g/kg | 96 ± 20 | 102 ± 18 | |||||||||||
| Antonio et al. (38) | X-over | HP | 14 | 0 | 14 | No | 26 | n.d. | 24 | Usual dietary protein + whey protein | 3.3 g/kg | EB, ad libitum | eGFR (Eq not specified, mL · min−1 · 1.73 m−2) | 95 ± 19 | 98 ± 16 |
| NLP | Usual dietary protein | 2.5 g/kg | 95 ± 19 | 101 ± 17 | |||||||||||
| Bergstrom et al. (29) | X-over | HP | 8 | 50 | 8 | No | 26 | n.d. | 1 | Dairy, meat, fish | 2 g/kg | EB, isocaloric | Inulin clearance (mL/min) | n.d. | 113 ± 12 |
| NLP | EAA tablets | 0.3 g/kg | n.d. | 100 ± 14 | |||||||||||
| Brinkworth et al. (44) | PG | HP | 29 | 72 | 14 | No | 52 | 34.0 | 68 wk : 12 wk ER; 56 wk EB | Dairy, meat, poultry | 30% | ER/EB, isocaloric | Creatinine clearance (mL/min) | 100 ± 44 | 124 ± 40 |
| NLP | 15 | Meat, poultry | 15% | 102 ± 27 | 113 ± 30 | ||||||||||
| Brinkworth et al. (43) | PG | HP | 68 | 63 | 33 | No | 51 | 33.5 | 52 | Dairy, poultry, meat, fish, nuts | 35% | ER, isocaloric | eGFR (MDRD, mL · min−1 · 1.73 m−2) | 90 ± 17 | 91 ± 18 |
| NLP | 35 | Dairy, poultry, meat, nuts, beans/lentils | 24% | 84 ± 14 | 84 ± 12 | ||||||||||
| Chu et al. (39) | X-over | HP | 6 | 0 | 6 | No | 25 | 21.2 | 2 | Meat, poultry | 150 g | EB, isocaloric | Creatinine clearance (mL/min) | n.d. | 122 ± 15 |
| NLP | Meat, poultry | 75 g | n.d. | 105 ± 14 | |||||||||||
| Frank et al. (30) | X-over | HP | 24 | 0 | 24 | No | 24 | 22.3 | 1 | Plant and animal sources including dairy | 2.4 g/kg | EB, ad libitum | Sinistrin clearance (mL/min) | n.d. | 141 ± 8 |
| NLP | Plant and animal sources including dairy | 1.2 g/kg | n.d. | 125 ± 5 | |||||||||||
| Friedman et al. (45) | PG | HP | 307 | 68 | 153 | No | 46 | 36.1 | 104 | Animal sources according to Dr. Atkins’ New Diet Revolution | Unlimited | ER, ad libitum | Creatinine clearance (mL/min) | 135 ± 35 | 139 ± 35 |
| NLP | 154 | n.d. | 15% | ER, kcal Rx | 133 ± 42 | 130 ± 42 | |||||||||
| Gross et al. (31) | X-over | HP | 15 | 27 | 15 | Yes | 57 | 24.8 | 4 | Usual diet except chicken replaces beef | 1.2–1.5 g/kg | EB, ad libitum | 51Cr-EDTA clearance (mL/min) | n.d. | 101 ± 23 |
| NLP | Vegetable and milk protein only | 0.5–0.8 g/kg | n.d. | 94 ± 20 | |||||||||||
| Hegsted and Linkswiler (32) | X-over | HP | 6 | 100 | 6 | No | 23–28 | n.d. | 9 wk with one diet; 2 wk with the other diet in random order | Beef, casein, egg white, wheat gluten | 123 g | EB, isocaloric | Creatinine clearance (mL/min) | n.d. | 103 ± 5 |
| NLP | Beef | 46 g | 91 ± 3 | ||||||||||||
| Jenkins et al. (40) | X-over | HP | 20 | 25 | 20 | No | 56 | 26.0 | 4 | Vegetable protein | 27.4% | EB, isocaloric | Creatinine clearance (mL/min) | n.d. | 110 ± 31 |
| NLP | Vegetable protein | 15.6% | n.d. | 104 ± 36 | |||||||||||
| Johnston et al. (46) | PG | HP | 16 | 90 | 9 | No | 19–54 | 28.9 | 6 | Poultry, dairy, beans | 31.5% | ER, isocaloric | Creatinine clearance (mL · min−1 · m−2) | 104 ± 25 | 85 ± 25 |
| NLP | 7 | Poultry, dairy, beans | 15% | 82 ± 17 | 85 ± 22 | ||||||||||
| Juraschek et al. (33) | X-over | HP | 156 | 45 | 156 | No | 54 | 30.2 | 6 | Plant and animal protein | 25% | EB, isocaloric | eGFR (CKD epi, cystatin C, mL · min−1 · 1.73 m−2) | 92 ± 16 | 96 ± 8 |
| NLP | Plant and animal protein | 15% | 92 ± 16 | 92 ± 10 | |||||||||||
| Kerstetter et al. (34) | X-over | HP | 7 | 100 | 7 | No | 26 | 23.0 | 1 | Poultry, fish, meat, dairy | 2.1 g/kg | EB, isocaloric | eGFR (Eq not specified, mL/min) | 104 ± 13 | 116 ± 22 |
| NLP | Meat, dairy | 0.7 g/kg | 98 ± 12 | 102 ± 10 | |||||||||||
| Kim and Linkswiler (41) | X-over | HP | 6 | 0 | 6 | No | 21–29 | n.d | 1.5 | Beef, casein, wheat gluten, vegetable sources | 142 g | EB, isocaloric | Creatinine clearance (mL/min) | n.d. | 116 ± 7 |
| NLP | Beef, vegetable sources | 47 g | n.d. | 105 ± 10 | |||||||||||
| Larsen et al. (47) | PG | HP | 99 | 52 | 53 | Yes | 59 | 27–40 | 52 wk : 12 wk ER; 36 wk EB | Lean meat, chicken, fish | 30% | ER/EB, isocaloric | eGFR (Eq not specified, mL · min−1 · 1.73 m−2) | 70 ± 12 | 73 ± 18 |
| NLP | 46 | Lean meat, chicken, fish | 15% | 73 ± 15 | 76 ± 18 | ||||||||||
| Leidy et al. (48) | PG | HP | 46 | 100 | 21 | No | 50 | 30.6 | 12 | 40% of protein from pork; other sources n.d. | 30% | ER, isocaloric | eGFR (MDRD, mL · min−1 · 1.73 m−2) | 86 ± 9 | 84 ± 9 |
| NLP | 25 | 13% of protein from milk; other sources n.d. | 15% | 74 ± 14 | 78 ± 10 | ||||||||||
| Longland et al. (49) | PG | HP | 40 | 0 | 20 | No | 23 | 29.7 | 4 | Animal, dairy, whey | 2.4 g/kg | ER, isocaloric | eGFR (CKD epi, mL · min−1 · 1.73 m−2) | 109 ± 9 | 114 ± 11 |
| NLP | 20 | Animal, dairy | 1.2 g/kg | 114 ± 11 | 117 ± 11 | ||||||||||
| Luger et al. (50) | PG | HP | 42 | 55 | 21 | Yes | 62 | 33.3 | 12 | Soy-based foods, dairy, fish, poultry | 30% | EB, isocaloric | eGFR (MDRD, mL · min−1 · 1.73 m−2) | 71 ± 15 | 74 ± 14 |
| NLP | 21 | n.d. | 15% | 66 ± 15 | 69 ± 19 | ||||||||||
| Luscombe-Marsh et al. (51) | PG | HP | 57 | 56 | 27 | No | 50 | 34.0 | 16-12 wk ER; 4 wk EB | Lean meat, poultry, low-fat dairy | 40% | ER/EB, isocaloric | Creatinine clearance (mL/min) | 121 ± 37 | 141 ± 45 |
| NLP | 30 | Lean meat, poultry, higher-fat milk, nuts | 20% | 117 ± 52 | 124 ± 60 | ||||||||||
| Noakes et al. (52) | PG | HP | 98 | 100 | 50 | No | 49 | 32.0 | 12 | Low-fat dairy, lean meat, poultry, fish | 34% | ER, isocaloric | Creatinine clearance (mL/min) | 82 ± 23 | 77 ± 20 |
| NLP | 48 | Low-fat milk, lean meat, poultry, fish | 17% | 82 ± 23 | 73 ± 21 | ||||||||||
| Roughead et al. (42) | X-over | HP | 15 | 100 | 15 | No | 60 | 26.5 | 8 | Meat, poultry | 20% | EB, isocaloric | Creatinine clearance (mL/min) | n.d. | 83 ± 11 |
| NLP | Vegetable | 12% | n.d. | 73 ± 11 | |||||||||||
| Skov et al. (53) | PG | HP | 50 | 76 | 25 | No | 40 | 30.4 | 24 | Dairy, meat, poultry, fish, offal | 25% | ER, ad libitum | 51Cr-EDTA clearance (mL/min) | 106 ± 15 | 111 ± 18 |
| NLP | 25 | Dairy, meat, poultry, fish, offal | 12% | 114 ± 19 | 105 ± 16 | ||||||||||
| Tay et al. (54) | PG | HP | 115 | 43 | 58 | Yes | 58 | 34.6 | 52 | Poultry, meat, fish, dairy | 28% | ER, isocaloric | eGFR (CKD epi, mL · min−1 · 1.73 m−2) | 96 ± 12 | 92 ± 12 |
| NLP | 57 | Poultry, meat, fish, dairy | 17% | 92 ± 12 | 90 ± 12 | ||||||||||
| Teunissen-Beekman et al. (55) | PG | HP | 48 | 30 | 24 | No | 55 | 29.0 | 4 | Protein isolate supplement (pea, soy, egg white, milk) | 1.5 g/kg | EB, ad libitum | Inulin clearance (mL/min) | 130 ± 25 | 127 ± 25 |
| NLP | 24 | Isocaloric maltodextrin | 1.0 g/kg | 137 ± 26 | 134 ± 26 | ||||||||||
| Wagner et al. (35): young | X-over | HP | 12 | 67 | 12 | No | 31 | 25.1 | 1 | Meat, dairy, egg white | 2 g/kg | EB, isocaloric | eGFR (MDRD, mL · min−1 · 1.73 m−2) | n.d. | 95 ± 11 |
| NLP | Meat, dairy, egg white | 0.5 g/kg | n.d. | 92 ± 10 | |||||||||||
| Wagner et al. (35): old | X-over | HP | 10 | 70 | 10 | No | 60 | 25.8 | 1 | Meat, dairy, egg white | 2 g/kg | EB, isocaloric | eGFR (MDRD, mL · min−1 · 1.73 m−2) | n.d. | 77 ± 9 |
| NLP | Meat, dairy, egg white | 0.5 g/kg | n.d. | 69 ± 10 | |||||||||||
| Walrand et al. (36): young | X-over | HP | 10 | 50 | 10 | No | 24 | 23.3 | 1.5 | n.d. | 2.1 g/kg | EB, isocaloric | Iothalamate clearance (mL · min−1 · SA−2) | n.d. | 128 ± 6 |
| NLP | n.d. | 1.0 g/kg | n.d. | 106 ± 4 | |||||||||||
| Walrand et al. (36): old | X-over | HP | 9 | 44 | 9 | No | 70 | 27.2 | 1.5 | n.d. | 2.1 g/kg | EB, isocaloric | Iothalamate clearance (mL · min−1 · SA−1) | n.d. | 74 ± 6 |
| NLP | n.d. | 1.0 g/kg | n.d. | 81 ± 7 | |||||||||||
| Wycherly et al. (56) | PG | HP | 64 | 0 | 32 | No | 51 | 33.0 | 52 | Low-fat dairy, meat, fish | 35% | ER, isocaloric | Creatinine clearance (mL · min−1 · 1.73 m−2) | 106 ± 25 | 110 ± 40 |
| NLP | 32 | Low-fat dairy, meat, fish | 17% | 103 ± 23 | 101 ± 27 | ||||||||||
1CKD epi, Chronic Kidney Disease Epidemiology Collaboration equation; EAA, essential amino acid; EB, energy balance; eGFR, estimated glomerular filtration rate; Eq, equation; ER, energy restriction; GFR, glomerular filtration rate; HP, high-protein; MDRD, Modification of Diet in Renal Disease equation; n.d., not described/determined; NLP, normal- or low-protein; PG, parallel-group study design; Post, postintervention; Pre, pre-intervention; ref, reference; SA, surface area; X-over, crossover study design.
2Subject numbers are reported for the number of subjects included in the renal function assessment within each study and may be less than the total subject number for the study.
3Values are means ± SDs.
TABLE 2.
Summary of study characteristics of studies included in the meta-analysis of whether higher-protein intakes affect kidney function in healthy people1
| Study characteristic | Study references | Notes |
|---|---|---|
| Study design | ||
| Parallel group | (43–56) | |
| Crossover | (29–42) | |
| Includes washout | (29–36) | |
| No washout | (37–42) | |
| Type 2 diabetes | (31, 47, 50, 54) | |
| Sex | ||
| Men | (30, 37, 39, 41, 49, 56) | |
| Women | (32, 34, 42, 48, 52) | |
| Both | (29, 31, 33, 35, 36, 40, 43–47, 50, 51, 53–55) | |
| Diet | ||
| Isocaloric | ||
| Modified CHO | (33–35, 40, 42, 44, 47, 48, 50, 52, 56) | |
| Modified fat | (36, 49, 51) | |
| Modified both | (29, 30, 32, 41, 43, 45, 46, 54) | |
| Ad libitum | ||
| Modified CHO | (31, 53, 55) | |
| Food provided | ||
| All | (29, 32–36, 39–42, 46, 49) | |
| Some | (37, 38) | Protein powder provided to HP group |
| (48, 51, 52, 54) | 30–60% of total energy provided | |
| (56) | 60% provided during weeks 1–12 | |
| Dietary advice only | (30, 31, 43–45, 47, 50, 55) | Includes counselling, detailed menus, diet tools (i.e., food scales) |
| Major sources of protein provided | ||
| Yes | (29–35, 37–44, 46–49, 51–56) | |
| (45, 50) | For HP only | |
| No | (36) | |
| Type of protein | ||
| Animal + vegetable | (29–39, 41–56) | |
| Non-animal | (40) | |
| Energy status | ||
| Energy balance | (29–42, 50, 55) | |
| Energy restriction | (43, 45, 46, 48, 49, 52–54, 56) | |
| Both | (44, 47, 51) | |
| Diet adherence measured | ||
| Yes | (30–40, 43, 44, 46–53, 55, 56) | Includes participant diet logs and urinary output measures |
| No | (29, 32, 41, 42, 45) | |
| Study completion rates | ||
| All completed | (29, 32, 37, 39, 41, 49, 53, 54) | |
| Dropouts reported | (30, 31, 33, 35, 36, 38, 42–48, 50–52, 55, 56) | Drop-out rates similar between groups |
| Text implies all completed | (34, 40) | |
| GFR measurement type | ||
| Isotope clearance | (31, 36, 53) | 125I-Iothalamate, 99mtechnetium-DTPA, 51chromium-EDTA |
| Creatinine clearance | (32, 39–42, 44–46, 51, 52, 56) | |
| Inulin/sinistrin clearance | (29, 30, 55) | |
| eGFR | (33–35, 37, 38, 43, 47–50, 54) | |
1CHO, carbohydrate; DTPA, diethylenetriaminepentacetate; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; HP, high-protein.
Diet protocol characteristics
The dietary interventions ranged from 4 d to 104 wk. In 25 of the 28 trials participants underwent an HP or an NLP diet for the length of the intervention. In the remaining 3 crossover trials, participants underwent 3 different diet interventions as detailed above (31, 33, 39). Protein intakes in the NLP and HP groups differed substantially between trials at 1.81 ± 0.60 g · kg BW−1 · d−1 for the HP group and 0.93 ± 0.51 g · kg BW−1 · d−1 for the NLP group. Sixteen trials were designed for study participants to be in weight maintenance (29–42, 50, 55), 9 trials were designed to induce weight loss (43, 45, 46, 48, 49, 52–54, 56), and 3 trials involved both weight-loss and weight-maintenance phases (44, 47, 51). Of the studies involving both energy-restriction and energy-balance phases, 2 trials reported GFR data after both phases of the diet (44, 47). In this situation, the overall effect of HP compared with NLP intake across the full study was input into the main analysis and results from each phase of the trial (energy restriction or energy balance) were included in the subgroup analyses. The other trial that involved both energy restriction and balance only reported data at baseline and the end of the study (51), and thus was not included in subgroup analysis for energy intake. Data for dietary protein intake were provided in various formats, but all of the HP diets met the inclusion requirements for dietary intervention.
Publication bias, heterogeneity, and risk of bias
Funnel plots were visually inspected for publication bias. Little-to-moderate asymmetry was observed for the post-only, but not the pre/post, change; SMDs indicated the potential for publication bias. The chi-square test indicated significant heterogeneity for the pre/post change analysis (30.26; P = 0.02), but not the post-only analysis (15.8; P = 0.98). Furthermore, the I2 test (pre/post change: I2 = 44%, post-only: I2 = 0%) indicated that there was moderate heterogeneity between trials included in the pre/post change analysis. This significant heterogeneity may have arisen because of substantial differences between trials pertaining to diet type (i.e., caloric restriction compared with energy balance), intervention length, mode of GFR measurement, and subject characteristics (BMI, age, pre-existing health conditions).
No trials were removed owing to risk of bias (Supplemental Table 1). Unclear risk domains predominated for random-sequence generation, allocation concealment, and selective reporting due to inadequate details provided in the methodologies of the trials. Two trials (34, 40) reported >3 unclear risk domains. Sensitivity analysis showed that removal of these trials from the analysis did not influence the overall result in either the pre/post change or post-only meta-analysis.
Certainty in the evidence
The summary of findings from the GRADE assessment is shown in Table 3. When applying the GRADE approach, we identified serious issues of risk of bias or limitations in study design, specifically related to poor reporting in the domains of random-sequence generation and allocation concealment. In addition, we also identified serious issues of inconsistency given the unexplained heterogeneity and serious issues of imprecision. The quality of the evidence across outcomes was low to very low.
TABLE 3.
Summary of findings from the GRADE assessment of studies included in the meta-analysis of whether higher-protein intakes affect kidney function in healthy people1
| Anticipated absolute effects2 | ||||
|---|---|---|---|---|
| Outcomes | Risk with low protein | Risk with high protein, SMD (95% CI) | Participants, n (studies, n) | Quality of the evidence (GRADE)3 |
| Pre/post change | — | 0.11 higher (−0.05 lower to 0.27 higher) | 1547 (19 RCTs) | ⊕ ◯ ◯ ◯Very low4,5 |
| Post-GFR | — | 0.19 higher (0.07 higher to 0.32 higher) | 1688 (31 RCTs) | ⊕ ⊕ ◯ ◯Low4 |
1GFR, glomerular filtration rate; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation; RCT, randomized controlled trial; SMD, standardized mean difference.
2The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
3GRADE Working Group grades of evidence are as follows—high quality: we are very confident that the true effect lies close to that of the estimate of the effect; moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
4Most studies had serious issues of reporting and were classified as unclear for the domains of random-sequence generation and allocation concealment.
5Unexplained heterogeneity (P = 0.02, I2 = 44%). The 95% CI includes a negligible reduction, no difference, and a moderate increase in GFR (−0.05 to 0.27).
Meta-analysis
Overall effect of HP compared with NLP diets on GFR
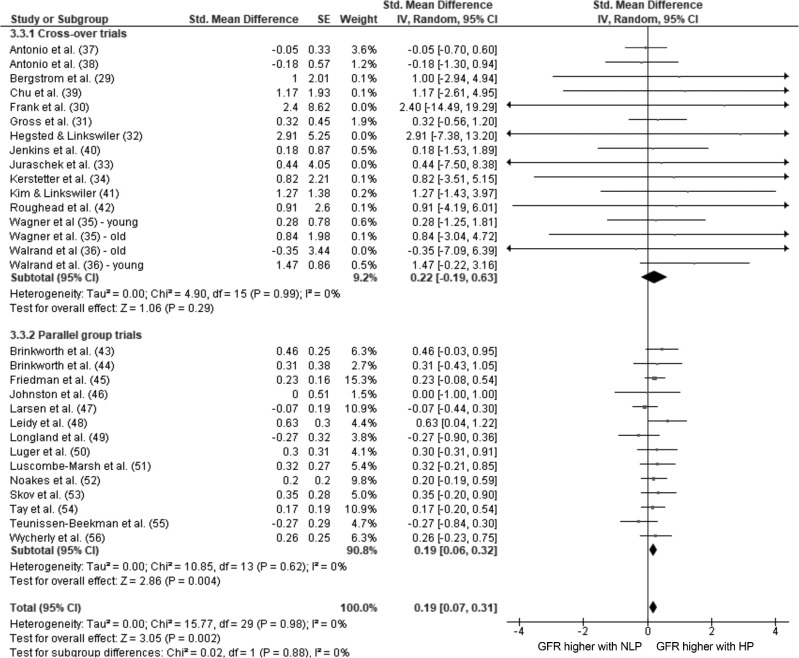
The pooled estimates of effect size (95% CIs) for the post-only and pre/post change analyses are shown in Figures 2 and 3. Overall, there was a trivial effect of consuming an HP diet compared with an NLP diet on GFR in the combined post-only analysis using the generic inverse-variance method (P = 0.002), whereas there was no effect in the pre/post change analysis using continuous data and the inverse-variance method (P = 0.16).
FIGURE 2.
Forest plot of a random-effects meta-analysis on renal function after HP compared with NLP intake in healthy people. Values are standardized mean differences (95% CIs). The shaded circles represent the point estimate for each individual trial, and the horizontal line extending from each circle represents the upper and lower limits of the 95% CI. The size of the shaded circle indicates the relative weight of the trial in the meta-analysis. The diamonds represent the overall standardized mean difference of the trials. GFR, glomerular filtration rate; HP, high-protein; IV, inverse variance; NLP, normal- or low-protein; Std., standardized.
FIGURE 3.
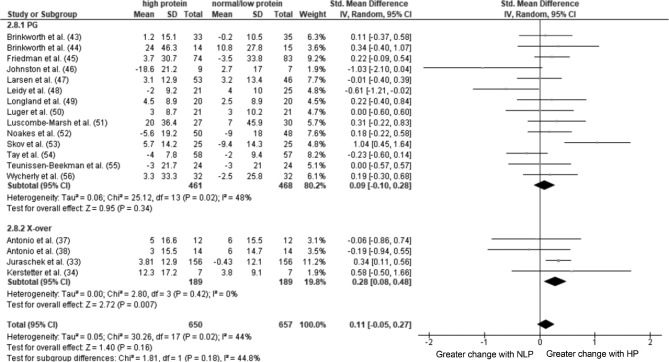
Forest plot of a random-effects meta-analysis on the change in renal function induced by HP compared with NLP intake in healthy people. Values are standardized mean differences (95% CIs). The shaded circles represent the point estimate for each individual trial, and the horizontal line extending from each circle represents the upper and lower limits of the 95% CI. The size of the shaded circle indicates the relative weight of the trial in the meta-analysis. The diamonds represent the overall standardized mean difference of the trials. HP, high-protein; IV, inverse variance; NLP, normal- or low-protein; PG, parallel-group; Std., standardized: X-over, crossover.
Effect of trial type on the effect of HP compared with NLP diets on GFR
Given the potential confounding influence of the previous intervention influencing the secondary intervention with crossover trials, we conducted a subgroup analysis on the basis of intervention type. In the post-only comparison, there was a trivial effect for HP intake to result in a higher GFR than NLP intake for parallel-group trials when analyzed using either continuous data and the inverse-variance method or generic inverse-variance methods (P = 0.004; Figure 2). There was no effect of HP consumption on post GFR in the crossover trials (P = 0.29) and no differences between subgroups (P = 0.88; Figure 2). When only parallel-group trials were included in the pre/post change analysis, the results of the full analysis were maintained (P = 0.34; Figure 3). Sensitivity analysis showed that removing the crossover studies that did not include a washout period did not influence the overall result in either the post-only or pre/post change analyses.
Effect of post-only compared with pre/post change on GFR
There was a small, but significant effect of HP consumption on GFR when post-only values from studies that reported GFR before and after the intervention were analyzed (SMD: 0.20; 96% CI: 0.07, 0.33; P = 0.003); however, when the change induced by the dietary intervention in these same studies was analyzed, the effect size was not significant (SMD: 0.10; 95% CI: −0.09, 0.30; P = 0.3), with no difference between the subgroups (P = 0.44). This analysis included crossover trials; however, the results were not affected when the analysis was conducted without the crossover trials.
Effect of GFR measurement type on the overall effect of HP compared with NLP diets on GFR
There was no difference in the effect size of HP compared with NLP diets on GFR dependent on whether a study determined kidney function using measurements of solute clearance or estimated GFR (eGFR) from serum creatinine concentrations for pre/post change (clearance measures—SMD: 0.17; 95% CI: −0.02, 0.35; P = 0.07; eGFR—SMD: 0.09; 95% CI: −0.15, 0.34; P = 0.45; between-subgroup comparison, P = 0.63). In the post-only analysis, although there were no differences between subgroups (P = 0.58); however, there was a small but statistically significant effect of HP intake when studies used clearance methods (SMD: 0.22; 95% CI: 0.06, 0.39; P = 0.008), but not eGFR (SMD: 0.16; 95% CI: 0.03, 0.34; P = 0.09).
Effects of energy intake during HP compared with NLP diets on GFR
Different results were found when a subanalysis was performed on studies that involved energy restriction compared with those that involved energy balance. For this comparison, the study by Luscombe-Marsh et al. (51) was excluded as detailed above and the specific results pertaining to the energy-restriction and energy-balance portions of Larsen et al. (47) and Brinkworth et al. (44) were used. For the post-only analysis there was no difference between HP and NLP consumption on GFR when studies were conducted in energy balance (SMD: 0.06; 95% CI: −0.2, 0.33; P = 0.64); however, there was a small effect for post GFR to be higher after HP intake when the study was conducted in energy deficit (SMD: 0.20; 95% CI: 0.04, 0.35; P = 0.1). For the pre/post change analysis, there was a trivial effect for the change induced by HP intakes to be greater when the study was conducted in energy balance (SMD: 0.18; 95% CI: 0.02, 0.34; P = 0.03). The effect of HP intakes on the change in GFR during energy balance was mainly due to the effects seen in the first 8 wk of consuming an HP diet (SMD: 0.30; 95% CI: 0.10, 0.51; P = 0.004), with longer-term HP consumption not influencing GFR any differently than NLP consumption (intervention length of 8–24 wk—SMD: −0.07; 95% CI: −0.48, 0.33; P = 0.73; intervention length >24 wk—SMD: 0.00; 95% CI: −0.35, 0.34; P = 0.99). When the studies were conducted during energy restriction, the change induced by HP intakes was not different from that with NLP intakes (SMD: 0.09; 95% CI: −0.15, 0.34; P = 0.46).
Effects of health status on GFR during HP and NLP diets
A subanalysis was conducted in participants with type 2 diabetes, given their elevated risk for kidney disease (57). In both the pre/post change and post-only analysis there was no difference in GFR between conditions of an HP- and NLP-containing diet (pre/post change—SMD: −0.11; 95% CI: −0.35, 0.14; P = 0.40; post-only—SMD: 0.11; 95% CI: −0.13, 0.34; P = 0.37).
Effects of intervention length on GFR during HP and NLP diets
A subgroup analysis was conducted on trials with durations of <8 wk, 8–24 wk, and >24 wk. The post-only analysis showed that HP intakes influenced post GFR to a greater extent after longer duration interventions (intervention length of 8–24 wk—SMD: 0.27; 95% CI: 0.06, 0.48; P = 0.01; >24 wk—SMD: 0.20; 95% CI: 0.03, 0.37; P = 0.02), with no differences in the effect of diet on GFR between HP and NLP intakes with interventions of <8 wk (SMD: −0.06; 95% CI: −0.39, 0.27; P = 0.72) and no differences between subgroups (P = 0.78). When weight-loss studies were removed from this analysis there was no influence of intervention length on post GFR between HP and NLP groups (<8 wk—SMD: 0.02; 95% CI: −0.39, 0.43; P = 0.93; 8–24 wk—SMD: 0.10; 95% CI: −0.31, 0.51; P = 0.62). In the pre/post change comparison, there was no influence of intervention length on the change in GFR between HP and NLP groups (intervention length of <8 wk—SMD: 0.14; 95% CI: −0.22, 0.5; P = 0.45; 8–24 wk—SMD: 0.11; 95% CI: −0.26, 0.48; P = 0.55; >24 wk—SMD: 0.07; 95% CI: −0.10, 0.24; P = 0.43). Removal of the crossover trials from the pre/post change analysis did not influence the results.
Effect of varying fat or carbohydrate intake during HP diets on GFR
To increase the proportion of energy intake from protein there needs to be a reduction in either carbohydrate or fat intake if diets are to remain isocaloric. Whether a study chose to modify carbohydrate or fat intake did not influence the results (fat—post-only SMD: 0.19; 95% CI: −0.39, 0.77; P = 0.52; pre/post change SMD: 0.27; 95% CI: −0.13, 0.67; P = 0.18; carbohydrate—post-only SMD: 0.18; 95% CI: 0.01, 0.35; P = 0.06; pre/post change SMD: 0.13; 95% CI: −0.08, 0.34; P = 0.22; both—post-only SMD: 0.24; 95% CI: 0.02, 0.41; P = 0.02; pre/post change SMD: 0.06; 95% CI: −0.26, 0.37; P = 0.72).
Meta-regression
The results from the full-model meta-regressions are presented in Table 4. In the pre/post change meta-regression, when all covariates were combined, differences in protein intake, trial length, age, diabetic status, and energy intake status did not explain any of the variance (τ2 = 0.12) in the changes in GFR (P = 0.93). Univariate meta-regressions on changes in GFR in the pre/post change analysis are also presented in Table 4. Differences in protein intake (P = 0.42), trial length (P = 0.84), age (P = 0.59), diabetic status (P = 0.13), and whether the study was conducted in energy restriction or energy balance (P = 0.71) did not explain any of the heterogeneity of the effect of protein intake on GFR. None of the additional covariates examined [weight (P = 0.44), energy intake (P = 0.54), difference in protein intake as a percentage of total energy intake (P = 0.89), absolute difference in protein intake (P = 0.49), or study design (parallel-group compared with crossover RCT, P = 0.61)] explained any of the variance in the change in GFR (data not shown). Removal of the crossover trials from the meta-regression did not influence the results.
TABLE 4.
Meta-regression analyses of studies included in the meta-analysis of whether higher-protein intakes affect kidney function (GFR) in healthy people1
| Model | n 2 | Coefficient (95% CI) | τ2 | Adjusted R2, % | I 2, % | P |
|---|---|---|---|---|---|---|
| Pre/post change analysis3 | ||||||
| No covariates | 18 | 0.11 (−0.05, 0.27) | 0.05 | 44 | 0.16 | |
| Univariate | ||||||
| Difference in protein intake, g · kg−1 · d−1 | 16 | 0.32 (−0.51, 1.16) | 0.06 | 5 | 44 | 0.42 |
| Trial length, wk | 18 | 0.00 (−0.01, 0.01) | 0.06 | −27 | 49 | 0.84 |
| Age, y | 18 | −0.01 (−0.02, 0.01) | 0.05 | −4 | 48 | 0.59 |
| Diabetic, yes/no | 18 | −0.31 (−0.73, 0.11) | 0.02 | 57 | 37 | 0.13 |
| Energy restriction, yes/no | 18 | 0.06 (−0.37, 0.26) | 0.07 | −10 | 51 | 0.71 |
| All covariates | 16 | 0.12 | −67 | 56 | 0.93 | |
| Difference in protein intake, g · kg−1 · d−1 | 16 | 0.15 (−1.63, 1.92) | 1.00 | |||
| Trial length, wk | 16 | 0.00 (−0.02, 0.02) | 1.00 | |||
| Age, y | 16 | −0.00 (−0.04, 0.04) | 1.00 | |||
| Diabetic, yes/no | 16 | −0.30 (−1.11, 0.51) | 0.84 | |||
| Energy restriction, yes/no | 16 | 0.04 (−0.42, 0.50) | 1.00 | |||
| Post-only analysis4 | ||||||
| No covariates | 30 | 0.19 (0.07, 0.32) | 0.00 | 0 | 0.002 | |
| Univariate | ||||||
| Difference in protein intake, g · kg−1 · d−1 | 28 | −0.13 (−0.71, 0.45) | 0.00 | 0 | 0 | 0.66 |
| Trial length, wk | 30 | −0.00 (−0.00, 0.01) | 0.00 | 0 | 0 | 0.55 |
| Age, y | 30 | 0.00 (−0.01, 0.02) | 0.00 | 0 | 0 | 0.57 |
| Diabetic, yes/no | 30 | −0.10 (−0.39, 0.19) | 0.00 | 0 | 0 | 0.48 |
| Energy restriction, yes/no | 30 | −0.04 (−0.26, 0.19) | 0.00 | 0 | 0 | 0.75 |
| All covariates | 28 | 0.00 | 100 | 0 | 0.46 | |
| Difference in protein intake, g · kg−1 · d−1 | 28 | 0.00 (−0.87, 0.87) | 1.00 | |||
| Trial length, wk | 28 | 0.01 (−0.00, 0.01) | 0.73 | |||
| Age, y | 28 | 0.01 (−0.01, 0.03) | 0.88 | |||
| Diabetic, yes/no | 28 | −0.31 (−0.71, 0.08) | 0.38 | |||
| Energy restriction, yes/no | 28 | −0.06 (−0.29, 0.18) | 0.99 | |||
1GFR, glomerular filtration rate.
2Number of studies in the analysis,
3Pre- to post-change analysis is reported for studies that provided GFR values both pre- and post, allowing for determination of the effect of the nutritional intervention on the change in GFR.
4Post-only analysis is reported for all included trials and is determined on the basis of the post-intervention GFR only.
In the post-only meta-regression there was no unexplained variance (τ2); and thus, unsurprisingly, when all covariates were combined, differences in protein intake, trial length, age, diabetic status, and energy intake status did not explain any of the variance in the change in GFR (P = 0.46; Table 4). Univariate meta-regressions on post GFR also found that none of the covariates explained any of the variability in GFR (Table 4). Furthermore, none of the additional covariates examined [body weight (P = 0.93), study design (P = 0.74), energy intake (P = 0.40), difference in protein intake relative to percentage of total energy intake (P = 0.16), or absolute difference in protein intake (P = 0.42)] explained any of the variability in GFR.
Dose-response analysis
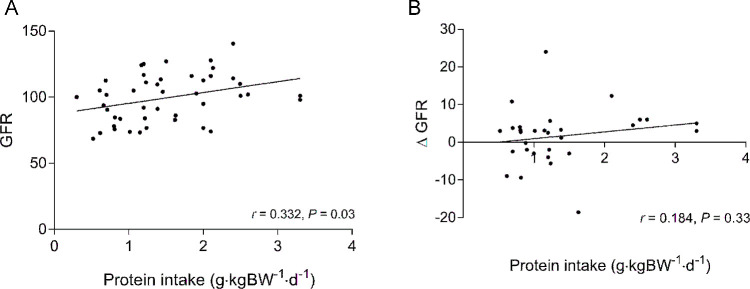
Given the large variability in the protein doses used within each study, we examined whether there was a dose-response effect of protein on GFR after the intervention or whether the change in GFR in response to the intervention was influenced by protein dose. Linear regression analysis indicated that there was a dose-response effect of protein dose on GFR after the intervention (Figure 4A); however, protein dose did not explain the change in GFR in response to the intervention (Figure 4B). Biphasic regression analysis was not significant (P = 0.38), indicating that the linear model best fit the data.
FIGURE 4.
Linear regression analysis showing the dose-response effect between increasing protein intake and post eGFR (r = 0.332, P = 0.03) (A) and the change in GFR in response to the intervention (r = 0.184, P = 0.33) (B) in healthy people. GFR and ΔGFR were reported as mL/min or as mL · (min · 1.73 m2)−1 depending on whether studies used clearance or eGFR measurements. BW, body weight; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
Discussion
We conducted a systematic review and meta-analysis to determine the effects of consuming an HP (1.81 ± 0.60 g · kg BW−1 · d−1) compared with an NLP (0.93 ± 0.51 g · kg BW−1 · d−1) diet on kidney function in individuals without CKD. Similar to the results of a previous meta-analysis (14), we report that there was a trivial effect for GFR to be greater in the HP group compared with the NLP group when GFR was examined using only post data. However, and in contrast to the post-only analysis, the novel finding of this meta-analysis is that when the change in GFR from baseline was compared, there was no difference between the HP and NLP groups. Furthermore, when we directly compared the effect sizes of post-only with pre/post change data in the 18 studies that included these data, the effect size was significant when the post-only values were compared, but not when the pre/post change values were compared. These discordant findings highlight the need to include pre/post change analyses as well as post-only analyses when examining the effect of a nutritional intervention on a health outcome. Our subgroup analyses further confirmed this need because different results were found depending on whether post-only or pre/post change data were analyzed. The post-only comparison found a small effect of consuming an HP diet when studies were conducted in energy restriction but not in energy balance. On the other hand, the pre/post change analysis found a trivial effect of consuming an HP diet when studies were conducted in energy balance but not in energy restriction. Furthermore, although the post-only analysis found that intervention length and method of GFR measurement influenced whether GFR was higher after the intervention with HP intakes, the pre/post change analysis did not. Similarly, we found a dose-response effect for protein intake on the post GFR; however, protein dose was not related to the change in GFR in response to the intervention.
One of the main reasons for conducting this meta-analysis was to examine whether the results obtained from comparing post GFRs were similar to those obtained when the change in GFR induced by the intervention were compared. Convention stipulates that it is appropriate to conduct meta-analysis on post-only values from, for example, pharmaceutical trials in which participants enter the trial with no exposure to the drug before the intervention. However, participants entering a nutritional intervention will have had varying exposures to different macronutrients (i.e., protein), which may influence the baseline GFR as well as the direction of change in the GFR response upon commencement of the intervention. Proper randomization would minimize baseline differences between groups; however, differences in baseline mean GFR between groups of >5% were observed in 6 of the 19 studies that reported baseline GFR values (34, 43, 46, 48, 50, 53), suggesting that, despite randomization, groups were different at study entry. This is supported by the findings of the GRADE assessment pertaining to the unclear risk of bias of the included trials. Here we show that when only the studies that reported GFR before and after the intervention were included, the post-only analysis found that HP intakes resulted in higher post GFR, whereas the pre/post change analysis was not significant (SMD: 0.24 compared with 0.12). These findings suggest that the post-only analysis exaggerated an effect of an HP diet on GFR. Similarly, differing results were found for post-only compared with pre/post change when studies were conducted in energy balance and energy deficit. Furthermore, although neither were significantly different, the direction of effect differed between post-only and pre/post change analyses when only studies involving individuals with type 2 diabetes were included. Given the interindividual variability in GFR within a healthy population (58, 59), simply comparing the GFR between HP and NLP groups after the intervention and inferring that the differences are the result of the intervention may lead to inaccurate interpretations. Although it is unclear which method is the best to use when conducting a meta-analysis, these findings do bring into question whether we can rely exclusively on the results from post-only analyses because the current analysis showed differences in the magnitude and direction of results depending on whether post-only or pre/post change values were analyzed. Future nutrition studies should include measurements of GFR (or any outcome of interest) both before and after the intervention to allow for determination of both post-only and pre/post change effects.
HP or protein-supplemented diets are known to induce greater muscle hypertrophy during resistance training (1) and to preserve muscle mass (2, 3) and promote fat loss (2) during energy restriction. Despite these well-known benefits there may be reluctance to recommend HP intakes due to an ensuing increase in GFR, which some have argued leads to glomerular damage and eventual kidney failure (9). The link between glomerular hyperfiltration and kidney dysfunction was originally proposed by Brenner et al. (9), who suggested that the increase in GFR was compensatory in response to nephron loss. Indeed, in response to kidney injury resulting in nephron loss, glomerular hypertension has been shown to precede progressive kidney damage in animal models (60, 61). However, in these situations, the increase in GFR occurred at the single-nephron level, whereas in humans in response to protein-feeding or other stimuli, such as pregnancy or nephrectomy, glomerular hyperfiltration occurs at the whole-kidney level as a result of increased kidney blood flow (62, 63). In fact, the capacity to increase GFR in response to protein feeding, known as kidney functional reserve, is a normal adaptive function of the kidney to increase solute clearance in response to an increase in solute load (i.e., nitrogen load). Importantly, this adaptive response does not represent a risk factor for the development of CKD (62, 64). Indeed, GFR increases by ∼65% during pregnancy (65) but does not increase the risk of kidney disease (66). Furthermore, despite significant hypertrophy and hyperfiltration in the remaining kidney after nephrectomy, kidney function remains normal over a prolonged period (>20 y) (67, 68). A recent trial found that after kidney transplant there was a negative correlation between protein intake and mortality and graft failure in normal-weight individuals with an eGFR >45 mL ⋅ min−1 ⋅ 1.73 m−2 (69), suggesting a protective effect of protein intake on kidney health. Moreover, despite numerous studies that found that an HP diet increases GFR (30, 33, 49, 53, 70, 71), to date there is no evidence linking HP intake to kidney disease in healthy individuals or those at risk of kidney disease due to pre-existing conditions such as obesity, hypertension, or dyslipidemia (62). Furthermore, animal studies have shown that markers of renal damage do not differ in obese rats fed a high-mixed-protein diet for 12 wk as compared with those fed a lower-protein diet, despite an increase in kidney size (72). In addition, monocyte chemoattractant protein 1 (MCP-1) concentration, a known mediator of kidney disease susceptibility (73), was lower in the high-mixed-protein group (72). These animal data again highlight that increased kidney size and GFR are not linked to kidney damage and disease. Together, our meta-analysis and other lines of evidence provide no evidence that the increase in GFR in response to an increase in blood solute load increases the risk of CKD.
Previous trials have found that HP intake increases GFR (30, 33, 49, 53, 70, 71). A critical consideration, however, is what this finding implies because it alone is not evidence that the risk of CKD is modified. With increasing age there is a progressive decline in GFR, ranging from 4 to 8 mL/min per decade (74), and therefore what is considered to be a normal GFR changes over the life span. Of the studies included in this meta-analysis, only 2 studies (45, 51) reported mean GFR values above what would be considered to be within the normal range for the age of the subjects tested (74).
This systematic review and meta-analysis included trials examining the effect of an HP compared with an NLP diet on GFR. Although not the main focus of the systematic search, thus not included in the meta-analysis, several trials reported on the effect of the dietary interventions on albumin excretion rate. Albumin excretion rate is used as a marker of kidney damage and can be used to detect and stage kidney disease (75), and thus if HP consumption was inducing damage to the kidney you would expect to see an increase in albumin excretion. Of the 8 trials (30, 31, 43, 45, 47, 50, 53, 54) that reported on the effects of an HP compared with an NLP diet on albumin excretion, only 1 trial found that an HP diet increased the albumin excretion rate (30). The other trials found no difference in the change in albumin excretion between HP and NLP diets. These findings once again suggest that consuming an HP diet does not negatively affect kidney function in healthy individuals.
Given the various study populations and study interventions included in this meta-analysis we conducted subgroup analyses to determine whether the overall results of the analysis were upheld in different situations. Of particular interest was the finding in individuals with type 2 diabetes, in whom there was no difference in kidney function with the consumption of an HP diet. This finding was true whether examining post-only or pre/post change in studies ranging in duration from 3 to 52 wk. These findings are in line with a recent meta-analysis that found no benefit to consuming a lower-protein diet in individuals with type 2 diabetes (76) and provide further support that HP diets do not cause a decline in kidney function even in those individuals at higher risk of kidney damage. We acknowledge, however, the small number of studies included in this and the other subgroup analyses, so our findings need to be interpreted with caution.
The current analysis found a dose-dependent effect of increasing protein intake on post GFR. Average protein doses in the included trials were 0.93 ± 0.51 and 1.81 ± 0.60 g · kg BW−1 · d−1 in the NLP and HP groups, respectively. Interestingly, although the dose-response effect of protein on post GFR was significant, there was no relation between protein dose and the change in GFR after the intervention. Although our dose-response relation does not appropriately weight each study on the basis of their sample and effect size as with a meta-regression (see Table 4), we present Figure 4 as a simplified representation that highlights the difficulty in interpreting data from post values because they show that an increasing protein dose does not induce a greater increase in GFR. Furthermore, meta-regression analysis did not find that the difference in protein dose explained any of the unexplained variance in the pre/post change analysis. These findings indicate that the magnitude of the change in GFR in response to differing protein intakes is not related to the magnitude of the difference in protein intakes between the HP and NLP groups. Taken together, the results of the dose-response and meta-regression analyses again bring in question the validity of determining the effect of an intervention on GFR by only comparing the post values.
As with any meta-analysis there are some inherent limitations. A concern when including crossover trials in a meta-analysis is the risk of carryover between arms of the trial (77). Although the potential for carryover with crossover trials exists, including a washout period between arms of the trial mitigates an impact of carryover from occurring (78). In the current analysis, 8 (29–36) of the 14 crossover trials indicated that they included a washout period in their study design. The removal of the 6 crossover trials that did not include a washout period between arms did not influence the overall results of the meta-analysis in either the pre/post change or post-only analyses. The effect size for crossover studies in the post-only analysis was slightly larger than that of the parallel-group trials (SMD: 0.22 compared with 0.19); however, the effect size was not significant for crossover studies, although it was for parallel-group trials, indicating a greater degree of variability in the crossover trials. Because of the risk of carryover in crossover trials, the use of post values, not change from baseline, is preferred when including crossover trials in a meta-analysis (79). The majority of trials that included pre- and post values were parallel-group trials (14 of 18 studies). To ensure that the inclusion of crossover trials in the pre/post change analysis did not influence the overall result, we conducted a subgroup analysis. The removal of the crossover trials in the pre/post change analysis resulted in a slight decrease in effect size (SMD: 0.11 compared with 0.09); however, neither of these analyses were significant. Overall, across all of the analyses included in this meta-analysis, the inclusion of crossover trials did not affect any of the results.
Another limitation of the current analysis was the overall unclear risk of bias of the included trials. Only 4 of the included trials reported the method they used to randomly assign participants into groups (47, 49, 54, 56). Furthermore, only 2 trials (47, 54) provided adequate information to ascertain that group allocation sequence was concealed. These selection bias issues are less of a concern for the crossover trials because all participants completed both arms of the trial; however, crossover trials have their own inherent limitations (i.e., potential for carryover; see discussion above). The overall impact of the unclear risk of selection bias on the results of the analysis is unknown. The results of this analysis highlight that future RCTs investigating the impact of protein intake on kidney function should use strategies to maximize validity and minimize bias using good study design.
In summary, the results of the current meta-analysis suggest a nonexistent or trivial effect of HP consumption on GFR in individuals with normal kidney function. These findings are in line with statements from the WHO (80) and Institute of Medicine (81) on protein intake and kidney function (82). Furthermore, there is no evidential link that shows that HP intake somehow leads to declines in renal function in otherwise healthy persons and, as our analysis indicates, even in populations with greater risk for declines in renal function such as those with type 2 diabetes. Given the proposed advantages of consuming HP diets to promote muscle hypertrophy during resistance training, high-quality weight loss during energy restriction, and maintenance of muscle mass with aging, the finding that an HP diet does not negatively affect kidney function is of relevance.
Supplementary Material
Acknowledgments
We thank Alonso Carrasco-Labra from the Department of Health Research Methods, Evidence, and Impact for conducting the GRADE assessment and R Bryan Rumble, Guideline Specialist at the American Society of Clinical Oncology, for providing advice and expertise in study design. The authors’ responsibilities were as follows—SMP, MCD, KSB, and LB: conceived and designed the study. MCD and AS: carried out the data extraction; MCD, AS, and RWM: carried out the data analysis; MCD, RWM, and SMP: drafted the manuscript; MCD, AS, KSB, LB, RWM, and SMP: contributed to editing and agreed on the final contents of the manuscript; and all authors: read and approved the final manuscript.
Notes
Author disclosures: MCD, AS, KSB, LB, and RWM, no conflicts of interest. SMP reports having received research funding, travel support. and honoraria from the US National Dairy Council, Dairy Farmers of Canada, and the US National Beef Cattlemen's Association.
Supplemental Methods 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: BW, body weight; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation; HP, higher-protein; NLP, normal- or lower-protein; post, postintervention; pre, preintervention; RCT, randomized controlled trial; SD∆, change in SD; SMD, standardized mean difference.
References
- 1. Cermak N, Res P, de Groot L, Saris H, van Loon L. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 2. Wycherley T, Moran L, Clifton P, Noakes M, Brinkworth G. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96(6):1281–98. [DOI] [PubMed] [Google Scholar]
- 3. Josse A, Atkinson S, Tarnopolsky M, Phillips S. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr 2011;141(9):1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft A, Morley J, Phillips S, Sieber C, Stehle P, Teta D et al. Evidence-based recommendation for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 5. Deutz N, Bauer J, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair K et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartman J, Tang J, Wilkinson S, Tarnopolsky M, Lawrence R, Fullerton A, Phillips S. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86(2):373–81. [DOI] [PubMed] [Google Scholar]
- 7. Skov A, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999;23(5):528–36. [DOI] [PubMed] [Google Scholar]
- 8. Westerterp K, Wilson S, Rolland A. Diet-induced thermogenesis measured over 24 h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord 1999;23:287–92. [DOI] [PubMed] [Google Scholar]
- 9. Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease. N Engl J Med 1982;307(11):652–9. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, Klahr S, The Modification of Diet in Renal Disease Study Group Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? J Am Soc Nephrol 1999;10(11):2426–39. [DOI] [PubMed] [Google Scholar]
- 11. Fouque D, Laville M. Low protein diets for chronic kidney disease in nondiabetic adults. Cochrane Database Syst Rev 2009;3:CD001892. [DOI] [PubMed] [Google Scholar]
- 12. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 13. Joint WHO/FAO/UN University Expert Consultation Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser 2007;935:1–265. [PubMed] [Google Scholar]
- 14. Schwingshackl L, Hoffmann G. Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta-analysis. PLoS ONE 2014;9(5):e97656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MEDINFO 2001 : Proceedings of the 10th World Congress on Medical Informatics, Part 1. Volume 84 of Studies in health technology and informatics. Patel VL, Rogers R, Haux R, editors. IOS Press, 2001. [Google Scholar]
- 16. Wilczynski N, Haynes R; Hedges Team Robustness of empirical search strategies for clinical content in MEDLI NE. Proc AMIA Symp 2002:904–8. [PMC free article] [PubMed] [Google Scholar]
- 17. Santesso N, Akl EA, Bianchi M, Mente A, Mustafa R, Heels-Ansdell D, Schunemann HJ. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr 2012;66(7):780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Cochrane Collaboration Cochrane handbook for systematic reviews of interventions. Version 5.1.0. 2011. [cited 2018 Apr 12]. Available from: www.cochrane-handbook.org. [Google Scholar]
- 19. Hopkins W. Effect statistics: a scale of magnitudes for effect statistics. Version current2002. [cited 2018 Apr 12]. Available from: http://www.sportsci.org/resource/stats/effectmag.html. [Google Scholar]
- 20. Cohen J. Statistical power analysis for the behavioural sciences. 1st ed New Work: Academic Press; 1969. [Google Scholar]
- 21. Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 22. Guyatt G, Oxman A, Kunz R, Vist G, Falck-Ytter Y, Schunemann H. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336(7651):995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balshem H, Helfand M, Schunemann H, Oxman A, Kunz R, Brozek J, Vist G, Falck-Ytter Y, Meerpohl J, Norris S et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;54(4):401–6. [DOI] [PubMed] [Google Scholar]
- 24. Butani L, Polinsky M, Kaiser B, Baluarte H. Dietary protein intake significantly affects the serum creatinine concentration. Kidney Int 2002;61(5):1907. [DOI] [PubMed] [Google Scholar]
- 25. Jacobsen F, Christensen C, Mogensen C, Andreasen F, Hailskov N. Pronounced increase in serum creatinine concentration after eating cooked meat. Br Med J 1979;1(6170):1049–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004;23:1663–82. [DOI] [PubMed] [Google Scholar]
- 27. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 28. Morton R, Murphy K, McKellar S, Schoenfeld B, Henselmans M, Helms E, Aragon A, Devries M, Banfield L, Krieger J et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med 2017;52(6):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergstrom J, Ahlberg M, Alvestrand A. Influence of protein intake on renal hemodynamics and plasma hormone concentrations in normal subjects. Acta Med Scand 1985;217(2):189–96. [DOI] [PubMed] [Google Scholar]
- 30. Frank H, Graf J, Amann-Gassner U, Bratke R, Daniel H, Heemann W, Hauner H. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. Am J Clin Nutr 2009;90:1509–16. [DOI] [PubMed] [Google Scholar]
- 31. Gross J, Zelmanovitz T, Moulin C, De Mello V, Perassolo M, Leitao C, Hoefel A, Paggi A, Azevedo M. Effect of a chicken-based diet on renal function and lipid profile in patients with type 2 diabetes. Diabetes Care 2002;25:645–51. [DOI] [PubMed] [Google Scholar]
- 32. Hegsted M, Linkswiler H. Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr 1981;111(2):244–51. [DOI] [PubMed] [Google Scholar]
- 33. Juraschek SP, Appel LJ, Anderson CAM, Miller ER III. Effect of a high-protein diet on kidney function in healthy adults: results from the OmniHeart trial. Am J Kidney Dis 2013;61(4):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr 1998;68:859–65. [DOI] [PubMed] [Google Scholar]
- 35. Wagner E, Falciglia G, Amlal H, Levin L, Soleimani M. Short-term exposure to a high-protein diet differentially affects glomerular filtration rate but not acid-base balance in older compared to younger adults. J Am Diet Assoc 2007;107(8):1404–8. [DOI] [PubMed] [Google Scholar]
- 36. Walrand S, Short K, Bigelow M, Sweatt A, Hutson S, Nair K. Functional impact of high protein intake on healthy elderly people. Am J Physiol Endocrinol Metab 2008;295(4):E921–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antonio J, Ellerbroek A, Silver T, Vargas L, Peacock C. The effects of a high protein diet on indices of health and body composition—a crossover trial in resistance-trained men. J Int Soc Sports Nutr 2016;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Antonio J, Ellerbroek A, Silver T, Vargas L, Tamayo A, Buehn R, Peacock CA. A high protein diet has no harmful effects: a one-year cross-over stu dy in resistance-trained males. J Nutr Metab 2016;2016:9104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chu J, Margen S, Costa F. Studies in calcium metabolism. II. Effects of low calcium and variable protein intake on human calcium metabolism. Am J Clin Nutr 1975;28(9):1028–35. [DOI] [PubMed] [Google Scholar]
- 40. Jenkins D, Kendall C, Vidgen E, Augustin L, van Erk M, Geelen A, Parker T, Faulkner D, Vuksan V, Josse R et al. High-protein diets in hyperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. Am J Clin Nutr 2001;74:57–63. [DOI] [PubMed] [Google Scholar]
- 41. Kim Y, Linkswiler H. Effect of level of protein intake on calcium metabolism and on parathyroid and renal function in the adult human male. J Nutr 1979;109(8):1399–404. [DOI] [PubMed] [Google Scholar]
- 42. Roughead Z, Johnson L, Lykken G, Hunt J. Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr 2003;133(4):1020–6. [DOI] [PubMed] [Google Scholar]
- 43. Brinkworth GD, Buckley JD, Noakes M, Clifton P. Renal function following long-term weight loss in individuals with abdominal obesity on a very-low-carbohydrate diet vs high-carbohydrate diet. J Am Diet Assoc 2010;110:633–8. [DOI] [PubMed] [Google Scholar]
- 44. Brinkworth GD, Noakes M, Keogh J, Luscombe N, Wittert GA, Clifton P. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord 2004;28(5):661–70. [DOI] [PubMed] [Google Scholar]
- 45. Friedman A, Ogden L, Foster G, Klein S, Stein R, Miller B, Hill J, Brill C, Bailer B, Rosenbaum D et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin J Am Soc Nephrol 2012;7:1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnston C, Tjonn S, Swan P. High-protein, low-fat diets are effective for weight loss and favorably alter biomarkers in healthy adults. J Nutr 2004;134:586–91. [DOI] [PubMed] [Google Scholar]
- 47. Larsen R, Mann N, Maclean E, Shaw J. The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia 2011;54:731–640. [DOI] [PubMed] [Google Scholar]
- 48. Leidy H, Carnell N, Mattes R, Campbell W. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity 2007;15(2):421–9. [DOI] [PubMed] [Google Scholar]
- 49. Longland T, Oikawa S, Mitchell C, Devries M, Phillips S. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial. Am J Clin Nutr 2016;103:738–46. [DOI] [PubMed] [Google Scholar]
- 50. Luger M, Holstein B, Schindler K, Kruschitz R, Ludvik B. Feasibility and efficacy of an isocaloric high-protein vs. standard diet on insulin requirement, body weight and metabolic parameters in patients with type 2 diabetes on insulin therapy. Exp Clin Endocrinol Diabetes 2013;121:286–94. [DOI] [PubMed] [Google Scholar]
- 51. Luscombe-Marsh ND, Noakes M, Wittert GA, Keogh J, Foster P, Clifton P. Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am J Clin Nutr 2005;81:762–72. [DOI] [PubMed] [Google Scholar]
- 52. Noakes M, Keogh J, Foster P, Clifton P. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr 2005;81:1298–306. [DOI] [PubMed] [Google Scholar]
- 53. Skov A, Toubro S, Bulow J, Krabbe K, Parving H-H, Astrup A. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord 1999;23(11):1170–7. [DOI] [PubMed] [Google Scholar]
- 54. Tay J, Thompson CH, Luscombe-Marsh ND, Noakes M, Buckley JD, Wittert GA, Brinkworth GD. Long-term effects of a very low carbohydrate compared with a high carbohydrate diet on renal function in individuals with type 2 diabetes: a randomized trial. Medicine 2015;94(47):e2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Teunissen-Beekman K, Dopheide J, Geleijnse J, Bakker S, Brink E, de Leeuw P, van Baak M. Effect of increased protein intake on renal acid load and renal hemodynamic responses. Phys Rep 2016;4(5):e12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wycherley T, Brinkworth GD, Clifton P, Noakes M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr Diabetes 2012;2:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kazancioglu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl 2013;3(4):368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brochner-Mortensen J, Rodbro P. Selection of routine method for determination of glomerular filtration rate in adult patients. Scand J Clin Lab Invest 1976;36:35–43. [DOI] [PubMed] [Google Scholar]
- 59. Delanaye P, Cavalier E, Froissart M, Krzesinski J. Reproducibility of GFR measured by chromium-51-EDTA and iohexol. Nephrol Dial Transplant 2008;23(12):4077–8. [DOI] [PubMed] [Google Scholar]
- 60. Hostetter TH, Olson J, Rennke H, Venkatachalam M, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol 1981;241(1):F85–93. [DOI] [PubMed] [Google Scholar]
- 61. Hostetter TH, Troy J, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 1981;19(3):410–5. [DOI] [PubMed] [Google Scholar]
- 62. Martin W, Armstrong L, Rodriguez N. Dietary protein intake and renal function. Nutr Metab 2005;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Helal I, Fick-Brosnahan G, Reed-Gitomer B, Schrier R. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012;8:293–300. [DOI] [PubMed] [Google Scholar]
- 64. Thomas D, Coles G, Williams J. What does the renal reserve mean. Kidney Int 1994;45:411–6. [DOI] [PubMed] [Google Scholar]
- 65. Conrad K. Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Investig 2004;11(7):438–48. [DOI] [PubMed] [Google Scholar]
- 66. Calderone J, Zadshir A, Norris K. A survey of kidney disease and risk-factor information on the World Wide Web. Med Gen Med 2004;6(4):3. [PMC free article] [PubMed] [Google Scholar]
- 67. Higashihara E, Horie S, Takeuchi T, Natahara K, Aso Y. Long-term consequence of nephrectomy. J Urol 1990;143(2):239–43. [DOI] [PubMed] [Google Scholar]
- 68. Regazzoni B, Genton N, Pelet J, Drukker A, Guignard J. Long-term follow up of renal functional reserve capacity after unilateral nephrectomy in childhood. J Urol 1998;160(3 Part I):844–8. [DOI] [PubMed] [Google Scholar]
- 69. Deetman PE, Said MY, Kromhout D, Dullaart RPF, Kootstra-Ros JE, Sanders JSF, Seelen MA, Gans ROB, Navis G, Joosten MM et al. Urinary urea excretion and long-term outcome after renal transplantation. Transplantation 2015;99(5):1009–15. [DOI] [PubMed] [Google Scholar]
- 70. Chan A, Cheng M, Keil L, Myers B. Functional response of healthy and diseased glomeruli to a large, protein-rich meal. J Clin Invest 1988;81:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simon A, Lima P, Almerinda M, Alves V, Bottini P, de Faria J. Renal haemodynamic responses to a chicken or beef meal in normal individuals. Nephrol Dial Transplant 1998;13:2261–4. [DOI] [PubMed] [Google Scholar]
- 72. Devassy J, Wojcik J, Ibrahim N, Zahdradka P, Taylor C, Aukema H. Mixed compared with single-source proteins in high-protein diets affect kidney structure and function differentially in obese fa/fa Zucker rats. Appl Physiol Nutr Metab 2017;42(2):135–41. [DOI] [PubMed] [Google Scholar]
- 73. Murea M, Register T, Divers J, Bowden D, Carr J, Hightower C, Xu J, Smith S, Hruska KL, Langefeld CD et al. Relationships between serum MCP-1 and subclinical kidney disease: African American-Diabetes Heart Study. BMC Nephrol 2012;13:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Poggio E, Rule A, Tanchanco R, Arrigain D, Butler R, Srinivas T, Stephany B, Meyer K, Nurko S, Fatica R et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 2009;75(10):1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Levey AS, Becker C, Inker L. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 2015;313(8):837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rughooputh MS, Zeng R, Yao Y. Protein diet restriction slows chronic kidney disease progression in non-diabetic and in type 1 diabetic patients, but not in type 2 diabetic patients: a meta-analysis of randomized controlled trials using glomerular filtration rate as a surrogate. PLoS One 2016;10(12):e0145505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sibbald B. Understanding controlled trials crossover trials. BMJ 1998;316(7146):1719–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dangelo G, Potvin D, Turgeon J. Carry-over effects in bioequivalence studies. J Biopharm Stat 2001;11(1-2):35–43. [DOI] [PubMed] [Google Scholar]
- 79. Elbourne D, Altman D, Higgins J, Curtin F, Worthington H, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31(1):140–9. [DOI] [PubMed] [Google Scholar]
- 80. WHO; FAO; UN University Protein and amino acid requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation. World Health Organ Tech Rep Ser; 2007;935, p. 224. [PubMed] [Google Scholar]
- 81. Institute of Medicine Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 82. Phillips S, Chevalier S, Leidy H. Protein “requirements” beyond the RDA: implications for optimizing health. Appl Physiol Nutr Metab 2016;41(5):565–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.