Abstract
Introduction
Varenicline doubles cessation over nicotine replacement therapy (NRT) patch for “normal,” but not “slow,” nicotine metabolizers, as assessed by the nicotine metabolite ratio (NMR). Metabolism-informed care (MIC) could improve outcomes by matching normal metabolizers with non-nicotine medication (e.g., varenicline) and slow metabolizers with NRT patch.
Methods
We conducted a feasibility randomized controlled trial of MIC versus guideline based care (GBC) among 81 outpatient adult daily smokers with medical comorbidity. Participants reported perceptions of MIC, underwent blood draw for NMR, and received expert cessation counseling. For MIC participants, medication selection was informed by NMR result (normal (≥0.31) vs. slow (< 0.31)). The primary outcome was MIC feasibility, reflected by attitudes toward MIC and by match rates between NMR and medication. Secondary endpoints (cessation confidence, medication use, smoking status) were assessed over 6 months to inform future studies.
Results
Participants were median age 53 years, 46% female, 28% black, and ~90% endorsed MIC. Despite high varenicline prescription rates (~60%) in both arms, NMR-medication matching was higher in MIC (84%) versus GBC (58%) participants (p=0.02); unadjusted odds ratio (OR) 3.67, 95% confidence interval [1.33, 11.00; p-value=0.02]. Secondary endpoints were similar at 1, 3, and 6 months.
Conclusions
MIC, an NMR-based precision approach to smoking cessation, was acceptable to 90% of smokers and improved NMR-medication match rates more than 3-fold compared to GBC, even with generally high use of varenicline. These data support the feasibility of MIC, which could maximize efficacy of smoking cessation medication while minimizing side effects and cost.
Implications
Among treatment-seeking daily smokers with medical comorbidity, most viewed metabolism-informed care (MIC), guided by the nicotine metabolism ratio (NMR), favorably, and were willing to accept MIC-guided medication. Compared to GBC participants (58%), more MIC participants (84%) were prescribed NMR-matched medication (i.e., normal metabolizers received varenicline; slow metabolizers received NRT patch). MIC increased the odds of optimized matching between NMR and medication more than 3-fold over GBC. Because the number needed to treat (NNT) to help one normal metabolizer quit smoking is only 4.9 for varenicline versus 26 for patch, broad implementation of MIC will improve drug efficacy in normal metabolizers as well as minimize side effects in slow metabolizers.
Introduction
Smoking is the leading preventable cause of disease and death in the world.1 In 2015, 15% of US adults, approximately 40 million people, smoked cigarettes and 11% were daily smokers.2 Although 70% of adult smokers see a healthcare provider annually, only one third of those attempting to quit use proven pharmacotherapy such as varenicline or nicotine replacement therapy (NRT).3,4 Additionally, even with use of proven behavior therapy and FDA-approved medications, less than 25% of smokers, on average, are abstinent for 6 months or more.5
The rate of nicotine metabolism predicts nicotine dependence, cessation success, and outcomes with pharmacotherapy.6–13 Nicotine is rapidly metabolized in the liver, primarily by the cytochrome P450 enzyme CYP2A6,14 to cotinine, which is further metabolized to 3-hydroxycotinine (3-HC) by CYP2A6.15 The ratio of 3-HC to cotinine, known as the nicotine metabolite ratio (NMR), reflects CYP2A6 activity and the rate of nicotine metabolism among regular smokers.6 In a 2015 landmark randomized controlled trial (RCT),13 varenicline nearly doubled end of treatment cessation rates over NRT patch among “normal” metabolizers as assessed by NMR. Among normal metabolizers, the number needed to treat (NNT) was 4.9 for varenicline and 26 for NRT patch. By contrast, quit rates were equal in “slow” metabolizers, who also reported more side effects with varenicline compared to placebo (NNT 8.1 for varenicline, 10.3 for NRT patch). Bupropion could be a viable non-nicotine alternative for normal metabolizers who are unable or unwilling to take varenicline.12
These findings provide a rationale for metabolism-informed care (MIC), an approach that matches normal metabolizers with non-nicotine based therapy (preferably varenicline) and slow nicotine metabolizers with NRT patch. By thus increasing the match rates between NMR and pharmacotherapy, MIC could maximize the efficacy for normal metabolizers while minimizing side effects for slow metabolizers. Several key knowledge gaps remain regarding the feasibility of employing MIC for smoking cessation in a clinical population. First, smokers’ perceptions and attitudes about MIC are unknown, and thus it is unclear whether they would be willing to undergo extra testing for NMR (i.e., blood draw) and whether they would follow through with the NMR-informed medication recommendation. If smokers accept the precision intervention, then this should be reflected in high match rates between NMR and medication, such that most normal metabolizers accept varenicline or bupropion, while most slow metabolizers accept NRT patch. We hypothesized that MIC would be accepted by most smokers, and that MIC participants would exhibit higher NMR-medication match rates than GBC participants.
Theoretically, MIC could also offer smokers a reason to be more confident about quitting smoking. For example, if normal metabolizers knew that varenicline could double their quit rates over NRT patch, they may be more likely to use the medication and to quit smoking. Similarly, if slow metabolizers knew they could avoid potential side effects of varenicline and do just as well on NRT patch, then this knowledge could also support adherence and cessation efforts.
In this context, we determined the feasibility of MIC by assessing smokers’ attitudes toward a precision-guided approach to cessation and quantifying the extent to which MIC improves match rates between NMR and pharmacotherapy as compared to GBC. Secondary outcomes for which the study was not powered included self-reported confidence to quit smoking, use of medications, and 7-day point-prevalence abstinence at 1, 3, and 6 months (with biochemical validation at 6 months). Biochemically validated abstinence was defined as end-expired CO <5 parts per million (ppm).16 Self-reported use of medication was assessed at 1 and 3 months.
Methods
Enrollment and Randomization
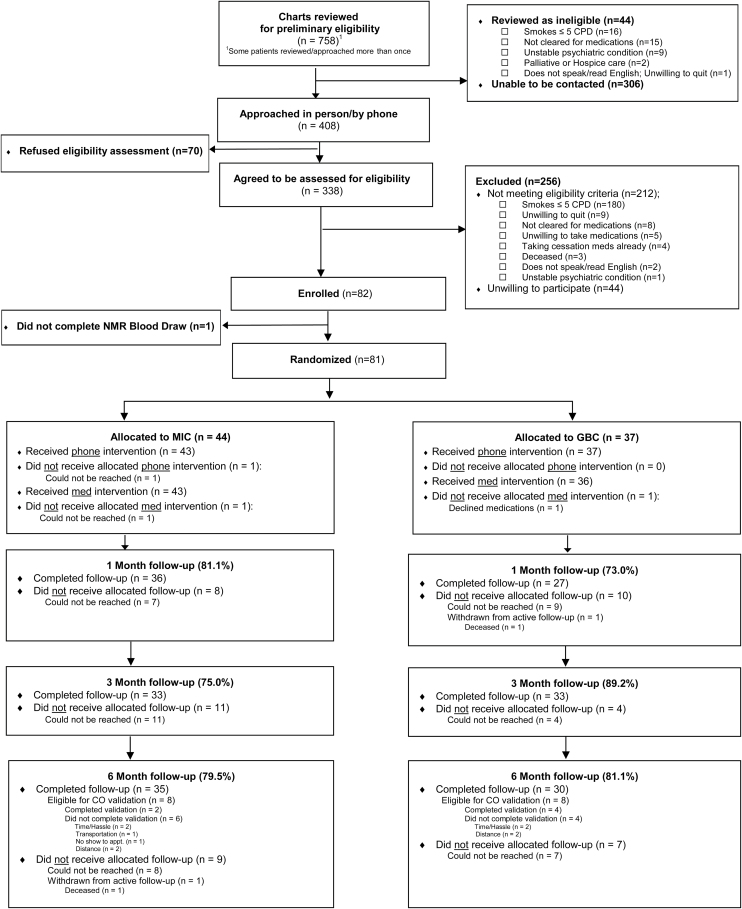
The study protocol was approved by the Vanderbilt University Medical Center (VUMC) Institutional Review Board, and enrollment was conducted between May 18, 2016 and September 27, 2016. This study was registered with ClinicalTrials.gov (identifier NCT03227679). Participants were recruited from multiple venues across VUMC, including outpatient Cardiology and Inflammatory Bowel Disease (IBD) clinics and the Vanderbilt Clinical Trials Center (CTC), which focused on patients who had recently attended a clinic visit for cardiovascular care. Selection of cardiovascular and IBD patients was based on the desire to recruit smokers with medical comorbidity, who were considered to be the most representative of future MIC recipients in clinical practice. Participants enrolled directly from Cardiology and IBD clinics were approached in person by study staff for eligibility screening and consent. Enrollment through the CTC consisted of telephone-based engagement/screening of smokers who recently attended a cardiovascular clinic visit as identified in the electronic health (EHR), with subsequent in-person consent/enrollment at the dedicated CTC facility on the VUMC campus. Eligible participants were adult (≥18 years), daily smokers (≥5 cigarettes/day) who were medically appropriate to receive at least 2 FDA-approved smoking cessation medications (nicotine patch and either varenicline or bupropion). Participants had to be willing to accept a medication prescription for one FDA-approved medication for which they were eligible. Smokers were ineligible if they had serious or unstable psychiatric disease (schizophrenia, psychosis, active suicidal ideation, or psychiatric hospitalization/change in psychiatric medications in prior 3 months) or advanced neurocognitive disease (dementia, severe mental retardation). By contrast, individuals with stable depression were not excluded. Additional exclusion criteria included: inability to reliably receive telephone calls, inability to read and speak English, being pregnant or actively breastfeeding, receiving hospice or palliative care, or complete abstinence from cigarettes for >3 days (which invalidated the NMR results). Participants were assigned to MIC or GBC via a stratified block randomization with block size of 10 and four strata defined by self-reported baseline cigarettes per day (<10/≥10) and venue (Cardiology/IBD) (Figure 1). Randomization was not stratified by NMR status.
Figure 1.
CONSORT Diagram.
NMR Determination
At the time of consent, all participants underwent a blood draw for subsequent measurement of nicotine, cotinine, and 3-HC. VUMC currently contracts with ARUP national reference laboratory (Salt Lake City, Utah) to conduct this clinical assay by quantitative high performance liquid chromatography-tandem mass spectrometry using Clinical Laboratory Improvement Amendments (CLIA)-approved protocols (http://ltd.aruplab.com/tests/pub/0092361), with a limit of detection of 2 ng/ml for all analytes. Results are reported in the EHR within 2–5 days from sample collection and values were entered into a secure database17 by personnel not involved in the study intervention or follow-up assessments. The NMR was calculated automatically as the ratio of 3-HC to cotinine. Following the a priori threshold used by Lerman et al., slow metabolizers were defined by an NMR <0.31 and normal metabolizers by an NMR ≥ 0.31.13
Interventions and Medication Provision
All participants received a telephone-based intervention from a nurse certified tobacco treatment specialist 1–2 weeks after enrollment, during which they received smoking cessation counselling and referral to their state tobacco quitline. For participants in GBC, the nurse tobacco specialist educated participants regarding smoking cessation medications for which they were eligible (as determined during eligibility screening), including efficacy and common side effects, and co-selected medication from those they were medically able to receive. Procedures for MIC were identical to GBC except that for MIC, the nurse tobacco specialist, who was unblinded to treatment arm, recommended NMR-informed choice of pharmacotherapy (i.e., varenicline as a first choice for normal metabolizers and NRT patch for slow metabolizers). MIC participants were made aware of their NMR result and were encouraged to follow the NMR-informed recommendations, but could choose any medication for which they were medically eligible. By contrast, GBC participants were blinded to their NMR results, as was the nurse tobacco specialist for this treatment arm.
Participant Assessments and Follow-up
At enrollment (prior to NMR determination), all participants completed an in-person baseline questionnaire assessing attitudes toward using biological information to inform care of smoking (e.g., “I approve of using tests to determine how my body breaks down nicotine to help me quit smoking.”; “The development of blood tests to help match patients with the drugs that might work best for them is a positive medical progress.”), willingness to participate in precision tobacco treatment research, confidence in ability to quit smoking,18 and smoking behavior (cigarettes per day and time to first cigarette years of smoking to calculate the Heaviness of Smoking Index).19 Participants underwent baseline screening for depression (PHQ-2)20 and hazardous alcohol use (AUDIT-C),21 but were not excluded based on results of these surveys. Participants also completed a baseline assessment of physical, behavioral, and neuropsychiatric symptoms. Follow-up assessments were conducted by telephone at 1, 3, and 6 months by study personnel blinded to treatment arm assignment, and included self-reported confidence to quit smoking/maintain abstinence, medication use in the past 7 days, 7-day point-prevalence abstinence, perceived helpfulness of care received during the study, and a symptom survey. Subjects self-reporting abstinence were invited for biochemical validation by measurement of end-expired carbon monoxide (CO), where abstinence was defined as end-expired CO <5 parts per million (ppm).16 Clinically significant side effects to medication were defined as new or worsening physical complaints (e.g., palpitations, nausea) self-reported as severe, behavioral/neuropsychiatric symptoms (e.g., hostility, agitation) reported as ≥ moderate, or any report of suicidal/homicidal ideation, hospitalization, death, or life-threatening medical diagnosis (e.g., malignancy, myocardial infarction).
Statistical Analysis
Summary statistics were calculated as median with lower and upper quartile for continuous variables, and as percentages for categorical data. Between-group comparisons were performed using Fisher’s Exact test for categorical data and the Kruskal–Wallis rank sum test as appropriate. We used a logistic regression to model the ratio of odds of achieving a pharmacotherapy-NMR match for MIC versus GBC. We did not additionally control for NMR status, which would not be expected to have a main effect on the outcome of pharmacotherapy-NMR matching. Due to sample size, we elected not to test for an interaction between NMR status and treatment arm; p values of <0.05 were considered statistically significant. Analyses were performed using R version 3.1.1.
Results
A total of 81 participants were randomized (Figure 1), including 14 from Cardiology outpatient clinics, 31 from outpatient IBD clinics, and 36 through the CTC. Participants were a median age of 53 years [IQR 44–61 years], 44% female, 28% black, with 44% reporting a high school education or less. Household income ranged widely: <$15K (17%), $15K–$35K (26%), >$35K–$75K (30%), >$75K (12%). Half of participants had a high Heaviness of Smoking Index (HSI ≥ 4), 12% screened positive for depression (PHQ-2 ≥ 3), and 17% screened positive for problem drinking (Audit-C ≥3 (women), ≥4 (men)) (Table 1). Approximately 60% of participants received varenicline, with similar rates of use in both arms.
Table 1.
Baseline characteristics of enrolled participants
| Variable | Total (N = 81) | Treatment Arm | ||
|---|---|---|---|---|
| Metabolism informed care (N = 44) | Guideline based care (N = 37) | |||
| Age (years) | ||||
| Median | 53.5 | 53.2 | 54.4 | |
| Interquartile range | (44.5, 60.6) | 45.8, 60.8 | 43.9, 59.6 | |
| Minimum/maximum | 28.0/79.9 | 28.0/71.9 | 28.6/79.9 | |
| Gender | Female | 36 (44.4%) | 20 (45.5%) | 16 (43.2%) |
| Race | White | 55 (67.9%) | 30 (68.2%) | 25 (67.6%) |
| Black | 23 (28.4%) | 12 (27.3%) | 11 (29.7%) | |
| Heaviness of smoking index | Light | 5 (6.2%) | 3 (6.8%) | 2 (5.4%) |
| Moderate | 35 (43.2%) | 21 (47.7%) | 14 (37.8%) | |
| Heavy | 41 (50.6%) | 20 (45.5%) | 21 (56.8%) | |
| Cigarettes per day over last 7 days | ≥10 | 67 (82.7%) | 35 (79.5%) | 32 (86.5%) |
| Income | <$15000 | 14 (17.3%) | 6 (13.6%) | 8 (21.6%) |
| $15000–$35000 | 21 (25.9%) | 12 (27.3%) | 9 (24.3%) | |
| $35000–$75000 | 24 (29.6%) | 16 (36.4%) | 8 (21.6%) | |
| >$75000 | 10 (12.3%) | 6 (13.6%) | 4 (10.8%) | |
| Don’t know/decline | 12 (14.8%) | 4 (9.1%) | 8 (21.6%) | |
| Education | High school or less | 36 (44.5%) | 21 (47.7%) | 15 (40.5%) |
| Vocational | 8 (9.9%) | 4 (9.1%) | 4 (10.8%) | |
| Some college/college graduate | 37 (45.7%) | 19 (43.2%) | 18 (48.6%) | |
| Audit-C | High (≥3 women, ≥4 men) | 14 (17.3%) | 9 (20.5%) | 5 (13.5%) |
| PHQ-2 | High (≥3) | 10 (12.3%) | 6 (13.6%) | 4 (10.8%) |
| Medication prescribed | Varenicline | 45 (58.4%) | 23 (56.1%) | 22 (61.1%) |
| Bupropion | 7 (9.1%) | 2 (4.9%) | 5 (13.9%) | |
| Nicotine patch | 25 (32.5%) | 16 (39%) | 9 (25%) | |
Participants reported high approval of MIC, with ~90% of smokers endorsing (Agree or Strongly Agree) multiple aspects of NMR-guided care. However, at baseline 15% expressed worry about consequences of knowing their nicotine metabolism status in terms of chances of quitting (i.e., possibly more difficult for normal metabolizers), and 11% expressed fear of knowing about their own nicotine metabolism (Table 2). Both worry and fear were associated with smoking fewer (<10) cigarettes per day (p = 0.03 and p = 0.01, respectively). Fear of knowing was associated with high AUDIT-C (p = 0.05). The study sample size was too small to further analyze by NMR status.
Table 2.
Baseline participant perceptions of metabolism-informed care
| Survey question | Response | N (%) Overall (N = 81) |
N (%) Metabolism informed care (N = 44) |
N (%) Guideline based care (N = 37) |
|---|---|---|---|---|
| I approve of using tests to determine how my body breaks down nicotine to help me quit smoking. | Strongly agree/Agree | 79 (97.5%) | 43 (97.8%) | 36 (97.3%) |
| Neither agree nor disagree/ Disagree/Strongly disagree | 2 (2.5%) | 1 (2.3%) | 1 (2.7%) | |
| Don’t know/ decline | 0 (0%) | 0 (0%) | 0 (0%) | |
| The development of blood tests to help match patients with the drugs that might work best for them is a positive medical progress. | Strongly agree/Agree | 74 (91.4%) | 40 (90.9%) | 34 (91.8%) |
| Neither agree nor disagree/ Disagree/Strongly disagree | 3 (3.7%) | 1 (2.3%) | 2 (5.4%) | |
| Don’t know/ decline | 4 (4.9%) | 3 (6.8%) | 1 (2.7%) | |
| If a blood test told me that I might have a more difficult time quitting smoking than some other people I wouldn’t even bother trying to quit | Strongly agree/Agree | 7 (8.6%) | 5 (11.4%) | 2 (5.4%) |
| Neither agree nor disagree/ Disagree/Strongly disagree | 71 (87.7%) | 37 (84.1%) | 34 (91.9%) | |
| Don’t know/ decline | 3 (3.7%) | 2 (4.5%) | 1 (2.7%) | |
| I worry about the consequences of knowing how my body breaks down nicotine for the chances of quitting smoking. | Strongly agree/Agree | 12 (14.8%) | 9 (20.5%) | 3 (8.1%) |
| Neither agree nor disagree/ Disagree/Strongly disagree | 68 (84.0%) | 34 (77.3%) | 34 (91.9) | |
| Don’t know/ decline | 1 (1.2%) | 1 (2.3%) | 0 (0%) | |
| The idea of knowing how my body breaks down nicotine frightens me. | Strongly agree/Agree | 9 (11.1%) | 7 (15.9%) | 2 (5.4%) |
| Neither agree nor disagree/ Disagree/Strongly disagree | 70 (86.4%) | 35 (79.5%) | 35 (94.6%) | |
| Don’t know/ decline | 2 (2.5%) | 2 (4.5%) | 0 (0%) | |
| I would want to know if the way my body breaks down nicotine makes it harder for me to stop smoking compared to some other people | Strongly agree/Agree | 75 (92.6%) | 40 (90.9%) | 35 (94.6%) |
| Neither agree nor disagree/ Disagree/Strongly disagree | 5 (6.2%) | 3 (6.8%) | 2 (5.4%) | |
| Don’t know/ decline | 1 (1.2%) | 1 (2.3%) | 0 (0%) |
Nicotine metabolites were detected in all subjects. The median NMR was 0.36 [0.27–0.59], and Supplementary Figure 1 shows the distribution of NMR values for the entire cohort. Normal metabolizers represented 55% of MIC participants and 73% of GBC participants, respectively. The median NMR for slow metabolizers was 0.22 [0.18–0.27] and for normal metabolizers was 0.54 [0.38–0.82], which is similar to values reported in studies of ambulatory smokers with fewer comorbidities.13 One participant in each group did not receive the allocated medication intervention. Overall, 36/43 MIC participants (84%) who were assigned a medication accepted the prescription that matched their NMR status. By comparison, among GBC participants who were prescribed a medication, just over half (21/36, or 58%) happened to receive (i.e., by chance) smoking cessation medication that matched their NMR. Put another way, 42% of GBC participants were “mismatched” as compared to only 16% in MIC (p = 0.02). The odds of matching were greater among MIC as compared to GBC participants (OR 3.67, 95% confidence interval [1.33, 11.00; p = 0.015]) (Table 3).
Table 3.
Matching of NMR to pharmacotherapy by treatment arm
| Baseline | Treatment arm by NMR status | ||||
|---|---|---|---|---|---|
| Metabolism-informed Care | Guideline based care | ||||
| (n = 44) | (n = 37) | ||||
| Normal (n = 24) | Slow (n = 20) | Normal (n = 27) | Slow (n = 10) | ||
| Medication prescribed* | Varenicline | 19 (79.2%) | 4 (21.1%) | 14 (52.9%) | 8 (80.0%) |
| Bupropion | 2 (8.3%) | 0 (0.0%) | 5 (18.5%) | 0 (0.0%) | |
| Nicotine Patch | 3 (12.5%) | 15 (75%) | 7 (25.9%) | 2 (20.0%) | |
| NMR-medication matching* | 36/43 (84%) | 21/36 (58%) | |||
*p = 0.02
The study was not powered to assess confidence in quitting, medication adherence, or smoking cessation, and therefore these endpoints were secondary outcomes. Descriptive statistics for these secondary endpoints are presented in Supplementary Table 1. In brief, 6-month follow-up was successfully completed in 65 (80%) participants. Among those completing follow-up at 6 months, the overall self-reported 7-day point-prevalence abstinence rate was 25% and similar between groups (23% for MIC and 27% for GBC). Of the 16 participants self-reporting abstinence at 6-months, 10 agreed to and 6 actually completed end-expired CO measurement. Among these, end-expired CO was <5 ppm in half (3/6), and <10 ppm in all (6/6) participants. At three-months (end of treatment), overall past 7-day use of study medication was 59% and similar in both arms (61% for MIC and 58% for GBC; p = ns). Clinically important medication side effects were uncommon (13% of all participants taking medications; N = 5) and too rare to permit statistical comparisons between treatment groups. Participants in both groups reported high confidence in quitting and high satisfaction with the care throughout the trial.
Discussion
Here we report results of a pilot RCT of metabolism-informed care (MIC), a precision treatment for smoking cessation, in a diverse clinical population of daily smokers in care. Overall, about 9 out of 10 participants endorsed the concept of MIC, including wanting to know the consequences of their NMR for quitting smoking and development of blood tests to help match patients with smoking cessation drugs, and MIC participants demonstrated high willingness to accept the NMR-guided pharmacotherapy. Even with very high rates of varenicline use in both study arms and the preservation of patient autonomy in choice of medication, MIC still produced more than a 3-fold higher odds of matching NMR and medication, with most normal metabolizers receiving varenicline and most slow metabolizers receiving NRT patch.
These results, in combination with the recent study demonstrating greater efficacy of varenicline among normal metabolizers,13 have potentially broad implications for tobacco treatment. The landmark EAGLES trial established the safety of varenicline and other FDA-approved smoking cessation medications for diverse smokers, including those with medical and psychiatric illness.22 Because varenicline is more effective than NRT patch (when NMR is not considered), its use is expected to increase. However, varenicline and NRT patch differ greatly in cost and side-effect profiles, and while increased varenicline use may be associated with increased cessation, it is also predicted to result in substantially increased cost and side effects, perhaps reducing overall cost-effectiveness. In this context, there could be significant benefit from precision medicine approaches such as MIC that use patient-specific information to customize prescribing by matching patients with the medications to which they are more likely to respond and/or have fewer side effects. Specifically, the increase in varenicline use could be targeted to the ~2/3 of smokers who are normal metabolizers13 and would derive additional benefit, while varenicline could be avoided in the ~1/3 of smokers who do not derive additional benefit over NRT patch, and who may also experience more side effects with varenicline. Indeed, one of our major findings was dramatically increased concordance between nicotine metabolism status and medication in MIC compared to GBC. This increased concordance was largely driven by the avoidance of varenicline in slow metabolizers. The frequency of medication side effects was low in both groups and there were an insufficient number of events to compare treatment groups.
Nearly half of patients treated with GBC received medications that were not matched with their NMR. Quantifying this discordance illustrates the generic nature of GBC and underscores the need for interventions such as MIC. Further, the 42% mismatch in our study is likely to be a conservative estimate, because the prescription rates of varenicline in GBC were extremely high compared to its relatively low rates of use in clinical care, even in developed countries.23,24 One aspect of MIC that this study was not able to address is whether such a precision approach could actually drive increased prescription rates of varenicline, which is vastly underutilized in large part due to persistent concerns among smokers and providers about potential side effects. These concerns unfortunately persist more than a year after the EAGLES trial was published. It is possible that NMR test results could persuade providers to prescribe varenicline (and persuade smokers to accept the medication) by having a clear biochemical basis for selecting it, especially in light of the dramatically lower NNT for varenicline compared to NRT patch (4.9 vs. 26) and favorable side-effect profile of varenicline in normal metabolizers.13 Our study could not address this question because the medication teaching was conducted by the research nurse tobacco specialist, and the prescriptions were written by the study physicians.
A large majority of participants approved of MIC. However, at baseline a minority of smokers expressed worry and fear regarding receiving NMR results. These results imply that patient education and clinical support for MIC should be augmented to address potential concerns, and highlight the need for future research to assess patient perceptions of NMR-guided care and determine their impact on process measures as well as clinical outcomes. It would be informative for future studies to assess these outcomes both at baseline and after intervention to quantify the effect of MIC on patient attitudes.
This study offers a scalable model for integration of NMR-guided smoking treatment into clinical workflows. The measurement of nicotine metabolites is inexpensive (approximately $25) and widely available as a routine clinical laboratory test. What is not yet clear is how availability of NMR in the electronic health record would actually translate into its use by clinical providers, and to what extent providers would require dedicated tobacco treatment expertise to help interpret and integrate the NMR results into patient care.25 This issue could be probed as part of a future implementation trial.
Strengths of our trial include diverse representation across sociodemographic characteristics and a randomized controlled study design. Additionally, almost two-thirds of participants received varenicline, allowing a “fair” comparison between MIC and the robust GBC intervention that recognized varenicline as first line, in accordance with evidence-based practice.22 Such a robust control condition offers another benefit over a “usual care” comparison in that any demonstrated advantage of MIC over GBC is not simply due to increased varenicline use, but to higher matching of NMR and pharmacotherapy. At the same time, a limitation of the robust GBC comparison is that power was limited to detect a difference in efficacy for smoking cessation, which would require a much larger trial and specifically, a larger population of normal metabolizers, whose quit rates are expected to double with use of varenicline as compared to NRT patch. A future study may require more than two arms to sort out these key nuances. Additionally, although medication use was not a primary study outcome, the self-reported rates of medication use may overestimate true adherence, which would require objective verification such as blood drug levels.
Biochemical validation of self-reported abstinence was obtained at 6 months, but many participants were unable or unwilling to return for the visit, underscoring the challenges of engaging clinical populations, especially those in specialty care at a tertiary care institution. For example, many participants with inflammatory bowel disease lived far away from the medical center, making non-essential travel prohibitively expensive and time consuming. Finally, inclusion of participants from sub-specialty clinics at a single institution could reduce the generalizability of the findings. Although participants enrolled the current study are representative of daily smokers in care in the mid-South, our results may not generalize to healthier populations, such as smokers seen in primary care. The original trial demonstrating greater efficacy of varenicline over NRT patch for cessation in normal metabolizers as assessed by NMR13 was conducted among a healthier group of smokers including those without cardiac disease or psychiatric conditions, suggesting that a population of healthier smokers would be an appropriate target for implementation of precision treatment of smoking. However, that study was not designed to assess whether healthier smokers would be willing to undergo a blood test for NMR and follow the treatment plan suggested by the results. Future research should address ambulatory smokers’ attitudes, beliefs, and acceptance of a precision approach to treatment of smoking.
In summary, MIC, an NMR-based precision smoking treatment, is strongly endorsed by daily smokers and results in improved concordance between nicotine metabolism status and pharmacotherapy. To our knowledge, this is the first study to demonstrate feasibility of an NMR-based approach to smoking cessation in a clinical setting. Future trials are needed to test the effectiveness and implementation of MIC for clinical populations.
Supplementary Material
Supplementary data are available at Nicotine and Tobacco Research online.
Funding
This publication was supported by ViTAL, the Vanderbilt Center for Tobacco, Addiction, and Lifestyle, V-CREATE, The Vanderbilt Center for Clinical Cardiovascular Outcomes Research and Trial Evaluation, and UL1 TR000445 from NCATS/NIH. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Declaration of Interests
Rachel Tyndale has consulted for Apotex on topics unrelated to smoking or NMR. Hilary Tindle is a multiple PI on a cessation study of non-daily smokers (PI: Primack; 5 R01 DA034629 04) and the active study medication (nicotine gum) was donated by the manufacturer.
Supplementary Material
Acknowledgments
Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. We acknowledge the support of a Canada Research Chair in Pharmacogenetics (RFT), the Centre for Addiction and Mental Health and the CAMH Foundation, the Canada Foundation for Innovation (grant numbers 20289 and 16014), and the Ontario Ministry of Research and Innovation.
References
- 1. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. [DOI] [PubMed] [Google Scholar]
- 2. Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–1211. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg MB, Akincigil A, Delnevo CD, Crystal S, Carson JL. Gender and age disparities for smoking-cessation treatment. Am J Prev Med. 2006;30(5):405–412. [DOI] [PubMed] [Google Scholar]
- 4. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–111. [DOI] [PubMed] [Google Scholar]
- 5. Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health (Oxf). 2010;32(1):71–82. [DOI] [PubMed] [Google Scholar]
- 6. Dempsey D, Tutka P, Jacob P 3rd et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. [DOI] [PubMed] [Google Scholar]
- 7. Gambier N, Batt AM, Marie B, Pfister M, Siest G, Visvikis-Siest S. Association of CYP2A6*1B genetic variant with the amount of smoking in French adults from the Stanislas cohort. Pharmacogenomics J. 2005;5(4):271–275. [DOI] [PubMed] [Google Scholar]
- 8. West O, Hajek P, McRobbie H. Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology (Berl). 2011;218(2):313–322. [DOI] [PubMed] [Google Scholar]
- 9. Strasser AA, Benowitz NL, Pinto AG et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20(2):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ho MK, Mwenifumbo JC, Al Koudsi N et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patterson F, Schnoll RA, Wileyto EP et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84(3):320–325. [DOI] [PubMed] [Google Scholar]
- 13. Lerman C, Schnoll RA, Hawk LW Jr et al. ; PGRN-PNAT Research Group Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 15. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob Res. 2013;15(5): 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: a meta-analysis. Psychol Addict Behav. 2009;23(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12 (Suppl):S45–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 21. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–432. [DOI] [PubMed] [Google Scholar]
- 22. Anthenelli RM, Benowitz NL, West R et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
- 23. Hughes JR. An updated algorithm for choosing among smoking cessation treatments. J Subst Abuse Treat. 2013;45(2):215–221. [DOI] [PubMed] [Google Scholar]
- 24. Huang Y, Lewis S, Britton J. Use of varenicline for smoking cessation treatment in UK primary care: an association rule mining analysis. BMC Public Health. 2014;14:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuehn BM. Pilot Programs Seek to Integrate Genomic Data Into Practice. JAMA. 2017;318(5):410–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.