Abstract
Objective
There is concern regarding the safety of delayed cord clamping (DCC) in babies born at less than 34 weeks' gestation. Therefore, the primary objective of this study was to compare the rates of hyperbilirubinemia and polycythemia during initial 7 days in infants born at less than 34 weeks' gestation and randomized to receive DCC by 120 seconds or early cord clamping (ECC) within less than 30 seconds.
Methods
One hundred pregnant women were randomly subjected to DCC or ECC at the time of birth in a tertiary referral hospital setting. Blood samples were obtained from each newborn at 48 hours and 7 days for hematocrit measurement. Serum bilirubin levels were estimated once the infant had clinically significant jaundice or at 72 hours. For the statistical analysis, the χ2 test, student's t-test, or Wilcoxon rank sum test was used.
Results
The hematocrit was significantly higher in the DCC group than in the ECC group (P<0.001). None of the babies had polycythemia. Mean total serum bilirubin level was 6.6 mg/dL in the DCC group and 8.7 mg/dL in the ECC group (P<0.001). There was no increased risk of hyperbilirubinemia in the DCC group.
Conclusion
DCC benefits preterm neonates with no significant adverse effects.
Keywords: Preterm, Neonates, Polycythemia, Hyperbilirubinemia, Clamping
Introduction
The traditional practice of early cord clamping (ECC) has started to give way to delayed cord clamping (DCC) due to the several benefits to the newborn infant, both during the immediate neonatal period and in the long term, especially reducing the incidence of anemia in early infancy [1,2,3,4].
A systematic review in term babies (n=3,911) showed no significant difference in neonatal morbidity or mortality rates between DCC and ECC groups, but there was a significant increase in hemoglobin concentration in the former versus the latter. However, more babies in the DCC group than in the ECC group developed jaundice and required phototherapy [5].
Several studies have demonstrated the safety and efficacy of DCC in term neonates, but concerns have been raised about its safety in preterm infants, especially those born at less than 34 weeks' gestation. A systematic review of preterm babies showed that fewer babies in the DCC group then the ECC group required transfusion for anemia. However, the peak bilirubin concentration was higher in the DCC group than in the ECC group (n=320 infants; mean difference, 15.01 mmol/L; 95% confidence interval, 5.62–24.40) [6]. The studies included in this systematic review were mainly performed in western countries.
DCC is beneficial for term and preterm babies, and the suggested beneficial effects include lower incidences of respiratory distress, need for respiratory support, intra-ventricular hemorrhage, need for blood transfusion, and anemia during infancy [7]. However, concern persists about the risk of polycythemia, hyperbilirubinemia, and hypothermia in preterm babies and also whether there are any other harmful effects [5,8]. To the best of our knowledge, few studies were performed in Indian settings [9,10]; hence, the present study was designed to assess the safety of DCC in preterm infants born at less than 34 weeks' gestation in an Indian setting.
Materials and methods
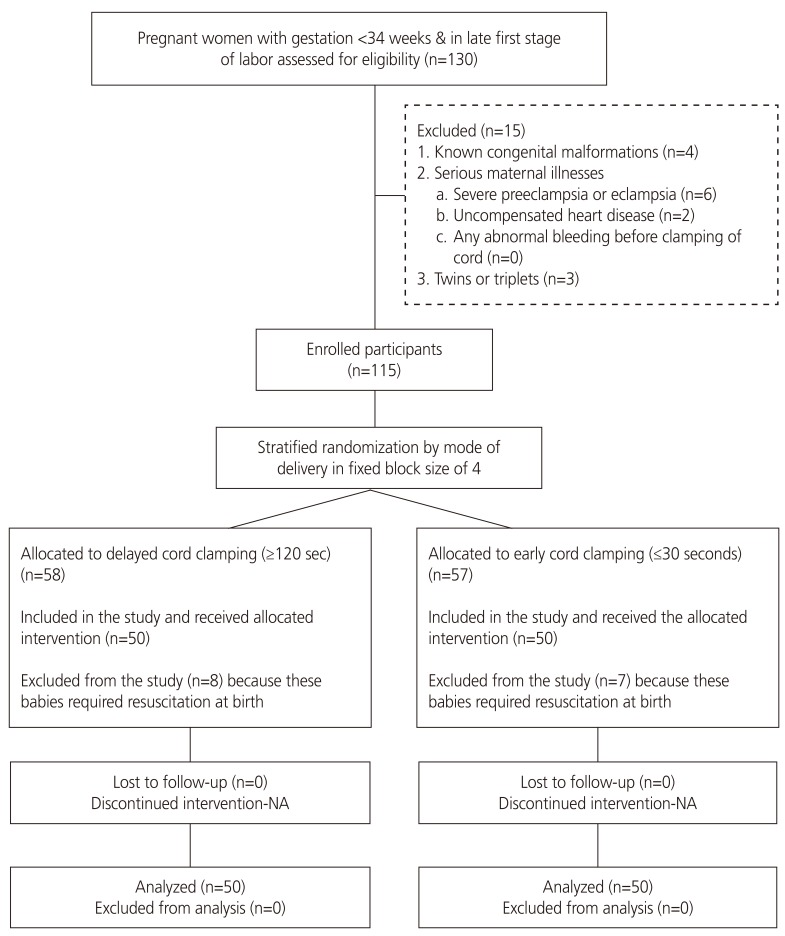
This randomized controlled trial was conducted in the Department of Obstetrics and Gynecology of a tertiary-level teaching hospital in India between January and December 2013. Pregnant women whose pregnancies had reached less than 34 weeks' gestation and were in the late first stage of labor were enrolled. The exclusion criteria included any known congenital malformations, serious maternal illnesses (severe preeclampsia or eclampsia, uncompensated heart disease, any abnormal bleeding before cord clamping), twins or triplets, and babies requiring immediate resuscitation at birth. The gestational period was assessed using the date of last menstrual period (LMP). If LMP was unknown, an early pregnancy ultrasound was used to calculate the gestational period. Written informed consent was provided by each eligible participant.
An uninvolved statistician generated 2 randomization sequences — e1 for vaginal and 1 for cesarean deliveries — in variable 4 by 6 block sizes. The random sequence was kept in serially numbered, sealed, and opaque envelopes. The investigator opened the envelope containing the random allocation sequence after enrollment of the woman just prior to the baby's birth (Fig. 1).
Fig. 1. Participant flow through the study.
NA, not applicable.
In the DCC group, the infant was dried and wrapped in a dry sterile towel and kept on the mother's abdomen after birth. The umbilical cord was clamped after 120 seconds. The investigator ensured that the baby was safe and secure. In the ECC group, after the delivery, the cord was cut as soon as possible within 30 seconds of birth. The infants were managed in the neonatal intensive care unit (NICU) as per standard protocols.
The primary outcomes were serum total bilirubin (STB) level at 72±12 hours and hematocrit level at 48 hours and 7 days after birth. The infants were monitored every twelve hours until seven days after birth for the development of outcomes. STB was routinely measured by photospectrometry at 72±12 hours or anytime if significant clinical jaundice was noted. Hematocrit was routinely measured at 48 hours and 7 days after birth. The nursing staff in the NICU who withdrew the blood samples as well as the laboratory technician who performed STB and hematocrit were blinded to the type of intervention performed.
Hyperbilirubinemia was defined according to American Academy of Pediatrics criteria [11]. Polycythemia was defined as a venous hematocrit ≥65% [12]. Secondary outcomes included need for resuscitation; axillary temperature at 5 and 30 minutes of life; incidence of major morbidities such as respiratory distress syndrome (RDS), sepsis, intraventricular hemorrhage, hypoperfusion requiring fluid bolus, and/or vasopressors; and need for blood transfusion. NICU and hospital stays were calculated. Morbidities were defined according to the standard definitions.
The incidence of hyperbilirubinemia in babies subjected to ECC was approximately 30%. To assume that the risk of these outcomes would not be higher than 36% in babies subjected to DCC, the required sample size was 1958 in 2 groups for a power of 80% and alpha value of 5%. However, since this was a single-center study, a convenient sample size of 100 was used and 50 subjects were recruited in each group.
Data were entered on a predesigned proforma at birth and at 48 hours, 72 hours, and 7 days after birth. The data were entered into Microsoft Office Access (Microsoft Corp., Redmond, WA, USA). The statistical analysis was done using Stata 11.2 (StataCorp, College Station, TX, USA). Categorical data were compared by the χ2 test or Fisher's exact test. Continuous data were compared by Student's t-test for normally distributed data or the Wilcoxon rank sum test for non-normally distributed data.
Approval for conducting the study was obtained from the institutional ethics committee (registration number: ECR/329/Inst/DL/2013) before commencement of the trial. The trial was registered at the Clinical Trials Registry – India (CTRI) of the Indian Council of Medical Research before its commencement (CTRI number: CTRI/2013/04/003529 [registered on 04/04/2013]; CTRI website: http://ctri.nic.in REF/2013/01/004476).
Results
The study subjects' baseline demographic and clinical characteristics are shown in Table 1. The mean gestational age was comparable in the 2 groups. Most of the women were primigravida or second gravidas. Premature rupture of the membranes was the most common associated morbidity. Most of the women went into spontaneous labor in both groups. Fetal distress and placenta previa were the most common indications for cesarean section. Babies in the DCC group were significantly heavier than those in the ECC group. None of the babies in the DCC group were small for gestational age (SGA), whereas 8% of the babies were SGA in the ECC group.
Table 1. Patients' baseline characteristics.
| Variables | DCC group (n=50) | ECC group (n=50) | P-value | ||
|---|---|---|---|---|---|
| Maternal | |||||
| Period of gestation (wk) | 32.3±1.1 | 32.4±1.0 | 0.88 | ||
| Pregnancy order | 0.63 | ||||
| 1 | 20 (40) | 25 (50) | |||
| 2 | 20 (40) | 17 (34) | |||
| 3 | 9 (18) | 6 (12) | |||
| 4 or more | 1 (2) | 2 (4) | |||
| Associated morbidities | |||||
| Gestational-hypertension | 4 (8) | 2 (4) | 0.40 | ||
| Preterm premature rupture of membranes | 18 (34) | 17 (34) | 0.83 | ||
| Oligohydramnios | 0 | 1 (2) | 1.00 | ||
| Fetal distress | 4 (8) | 4 (8) | 1.00 | ||
| Spontaneous onset of labor | 49 (98) | 48 (96) | 0.56 | ||
| Caesarean | 8 (16) | 9 (18) | 0.79 | ||
| Indications of caesarean section | 0.75 | ||||
| Fetal distress | 4 (8) | 4 (8) | |||
| Placenta previa | 3 (6) | 2 (4) | |||
| Others | 1 (2) | 3 (6) | |||
| Neonatal | |||||
| Male gender | 28 (56) | 28 (56) | 1.00 | ||
| Birth weight (g) | 1,818±282 | 1,679±373 | 0.04 | ||
| SGA | 0 | 4 (8) | 0.04 | ||
| Apgar score | |||||
| 1 min | 9 (9–9) | 9 (9–9) | |||
| 5 min | 9 (9–9) | 9 (9–9) | |||
Values are presented as mean±standard deviation, median (interquartile range) or number (%).
DCC, delayed cord clamping; ECC, early cord clamping; SD, standard deviation; SGA, small for gestational age; IQR, interquartile range.
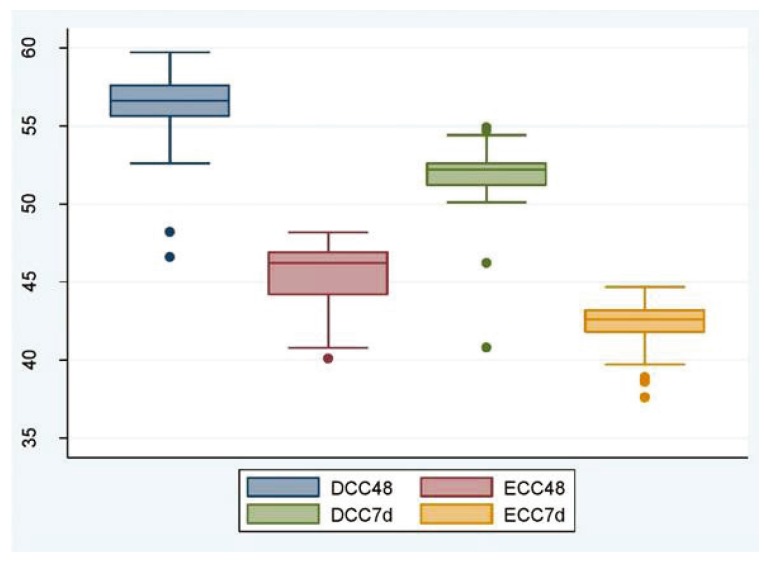
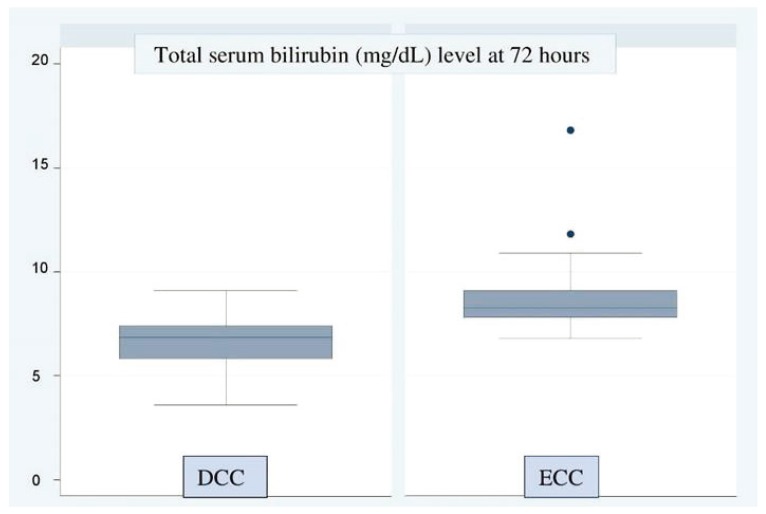
The primary outcome variables are shown in Tables 2 and 3. The mean hematocrit at 48 hours and 7 days was significantly higher in the DCC group than in the ECC group, suggesting that DCC helps increase placental transfusion in preterm infants (Table 2, Fig. 2). However, none of the babies in either group had polycythemia, i.e., hematocrit ≥65%. None of the babies in the DCC group and 1 baby in the ECC group required phototherapy. The baby who required phototherapy was born at a gestational age of 32 weeks, 5 days. Jaundice was diagnosed at 72 hours. The total serum bilirubin level was 16.8 mg/dL. The baby recovered fully and was discharged. Total serum bilirubin levels in the ECC group were surprisingly higher than those in the DCC group (Table 3, Fig. 3).
Table 2. Polycythemia incidence in and hematocrit levels of newborns at 48 hours and 7th day of life.
| Variables | DCC group | ECC group | Difference in mean | P-value | |
|---|---|---|---|---|---|
| Hematocrit (%) | |||||
| At 48 hr | 56.0±2.5 | 45.5±2.0 | 10.5 (9.6–11.5) | <0.001 | |
| At 7 day | 51.6±2.3 | 42.1±1.6 | 9.6 (8.8–10.4) | <0.001 | |
| Polycythemia | 0 | 0 | - | - | |
Values are presented as mean±standard deviation, difference in means (95% confidence interval).
DCC, delayed cord clamping; ECC, early cord clamping.
Table 3. Need for phototherapy and total serum bilirubin level.
| Variables | DCC group | ECC group | Difference in mean | P-value |
|---|---|---|---|---|
| Need for phototherapy | 0 (0) | 1 (2) | - | 0.320 |
| Total serum bilirubin (mg/dL) at 72 hours of birth | 6.6±1.2 | 8.7±1.6 | −2.1 (−2.7 to −1.5) | <0.001 |
Values are presented as mean±standard deviation or number (%), difference in means (95% confidence interval).
DCC, delayed cord clamping; ECC, early cord clamping.
Fig. 2. Hematocrit in newborns at 48 hours and the 7th day of life (Y-axis, hematocrit in % age).
DCC, delayed cord clamping; ECC, early cord clamping.
Fig. 3. Total serum bilirubin levels in newborns at 72 hours (Y-axis, total serum bilirubin levels in mg/dL).
DCC, delayed cord clamping; ECC, early cord clamping.
The secondary outcome variables are shown in Table 4. The mean body temperature did not differ between the 2 groups. There was no difference in the incidence of RDS, shock requiring fluid bolus or vasopressors, need for blood transfusion, or the incidence of intra-ventricular hemorrhage between the 2 groups. Only 1 baby in the ECC group had sepsis compared to none in the DCC group. This baby was born at a gestational age of 33 weeks, 1 day. There was no history of prolonged leaking in the mother. The blood culture of the baby was negative. The mean NICU and hospital stays were longer in the ECC group than in the DCC group, but the difference was insignificant.
Table 4. Secondary outcome variables by group.
| Variables | DCC group | ECC group | P-value | |
|---|---|---|---|---|
| Body temperature (°C) of baby | ||||
| At 5 min | 36.9±0.2 | 36.8±0.1 | 0.10 | |
| At 30 min | 37.1±0.2 | 37.0±0.1 | 0.05 | |
| RDS | 0 | 0 | - | |
| Sepsis (%) | 0 | 1 (2) | 0.50 | |
| Hypoperfusion requiring fluid boluses and/or vasopressors | 0 | 0 | - | |
| Need for blood transfusion | 0 | 0 | - | |
| Intraventricular hemorrhage | 0 | 0 | - | |
| NICU stay (day) | 2.5±3.4 | 3.5±3.5 | 0.13 | |
| Hospital stay (day) | 5.2±1.4 | 5.7±1.4 | 0.09 | |
Values are presented as mean±standard deviation.
DCC, delayed cord clamping; ECC, early cord clamping; RDS, respiratory distress syndrome; NICU, neonatal intensive care unit.
Discussion
In our study, delaying umbilical cord clamping by 120 seconds after birth compared to ECC (within 30 seconds of birth) in infants born at <34 weeks' gestation was safe and did not increase the risk of polycythemia, hyperbilirubinemia, or need for phototherapy during the initial 7 days of life. DCC was significantly more effective than ECC at increasing the hematocrit at 48 hours and at 7 days of life. Mean length of NICU stay was higher in the ECC group than in the DCC group, but the difference was insignificant. There was no significant intergroup difference noted in the length of hospital stay.
Our study adds to the growing body of knowledge on the safety of DCC in preterm infants especially from a developing country. Most of the published studies on preterm babies [1,13,14,15,16] except one [17] examined 90-second DCC and found no increase in polycythemia. Our study supports the lack of a risk of polycythemia even at a DCC of 120 seconds. We found no increased risk of hyperbilirubinemia with DCC. The need for phototherapy also did not differ significantly between the 2 groups. Kugelman et al. [4] reported similar results, while Ultee et al. [14] also demonstrated no relationship between DCC and pathological jaundice.
The total serum bilirubin level was higher in the ECC group (8.7±1.6 mg/dL) than in the DCC group (6.6±1.2 mg/dL). This finding was in contrast to that of Rabe et al. [6], who found higher peak bilirubin levels in the DCC group but no significant difference in the need for phototherapy between the 2 groups. Mercer and Skovgaard [15] concluded that the belief that DCC causes hyperbilirubinemia is unsupported by the available research. In our study, higher bilirubin levels in the ECC group than in the DCC group may be a chance factor due to the small number of study subjects.
The significantly higher mean birth weight in the DCC group compared with the ECC group indirectly points to an increase in blood volume due to placental transfusion in the DCC group compared with the ECC group. Kaempf et al. [16] has also reported higher mean birth weights in infants of the DCC group than in those of the ECC group; however, this difference was not significant. This insignificant gain in weight in the study by Kaempf et al. [16] as opposed to the significant gain in weight in our study may be due the fact that the former used a DCC time of up to 45 seconds versus ours of 120 seconds. However, this finding may also be due to other factors such as the low number of study subjects and 8% of the babies in the ECC group being SGA, which might have contributed to the smaller mean birth weight in the ECC than in the DCC.
A study by Vain et al. [18] showed no significant effect of gravity on placental transfusion in term babies. However, the non-inferiority of holding the neonate on the mother's abdomen compared to holding the neonate at the level of the introitus or lower has not been established for preterm infants. Further research is needed to investigate the effect of gravity on placental transfusion in preterm babies. In our study, the neonates were placed on the maternal abdomen to increase obstetric compliance with DCC and enhance maternal–infant bonding.
The limitations of our study include its small number of study subjects on account of it being a single-center study. Additionally, because some of the babies in both groups required immediate resuscitation, they had to be excluded from the study after the pregnant women were enrolled and randomized in the late first stage of labor; as a result, our study may have some bias.
The strengths of our study include that it was a randomized controlled trial. All of the subjects were enrolled and interventions were performed by the principal investigator. The nursing staff in the NICU who drew the blood samples as well as the laboratory technician who performed STB and hematocrit were blinded to the intervention performed. This is the first study in an Indian setting of the benefits and harmful effects of early versus DCC in preterm babies born before 34 weeks' gestation.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Weeks A. Umbilical cord clamping after birth. BMJ. 2007;335:312–313. doi: 10.1136/bmj.39282.440787.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawes GS. Fetal and neonatal physiology: a comparative study of the changes at birth. Chicago: Year Book Medical Publishers; 1968. [Google Scholar]
- 3.Pisacane A. Neonatal prevention of iron deficiency. BMJ. 1996;312:136–137. doi: 10.1136/bmj.312.7024.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kugelman A, Borenstein-Levin L, Riskin A, Chistyakov I, Ohel G, Gonen R, et al. Immediate versus delayed umbilical cord clamping in premature neonates born <35 weeks: a prospective, randomized, controlled study. Am J Perinatol. 2007;24:307–315. doi: 10.1055/s-2007-981434. [DOI] [PubMed] [Google Scholar]
- 5.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;7:CD004074. doi: 10.1002/14651858.CD004074.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;8:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics. 2012;129:e667–72. doi: 10.1542/peds.2011-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage [Internet] Geneva: World Health Organization; c2012. [cited 2014 Jul 30]. Available from: www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548502/en/ [Google Scholar]
- 9.Ranjit T, Nesargi S, Rao PN, Sahoo JP, Ashok C, Chandrakala BS, et al. Effect of early versus delayed cord clamping on hematological status of preterm infants at 6 wk of age. Indian J Pediatr. 2015;82:29–34. doi: 10.1007/s12098-013-1329-8. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Ramji S. Effect of delayed cord clamping on iron stores in infants born to anemic mothers: a randomized controlled trial. Indian Pediatr. 2002;39:130–135. [PubMed] [Google Scholar]
- 11.AAP Subcommittee on Neonatal Hyperbilirubinemia. Neonatal jaundice and kernicterus. Pediatrics. 2001;108:763–765. doi: 10.1542/peds.108.3.763. [DOI] [PubMed] [Google Scholar]
- 12.Alsafadi TR, Hashmi SM, Youssef HA, Suliman AK, Abbas HM, Albaloushi MH. Polycythemia in neonatal intensive care unit, risk factors, symptoms, pattern, and management controversy. J Clin Neonatol. 2014;3:93–98. doi: 10.4103/2249-4847.134683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomised trial. BMJ. 1993;306:172–175. doi: 10.1136/bmj.306.6871.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ultee CA, van der Deure J, Swart J, Lasham C, van Baar AL. Delayed cord clamping in preterm infants delivered at 34–36 weeks' gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F20–F23. doi: 10.1136/adc.2006.100354. [DOI] [PubMed] [Google Scholar]
- 15.Mercer JS, Skovgaard RL. Neonatal transitional physiology: a new paradigm. J Perinat Neonatal Nurs. 2002;15:56–75. doi: 10.1097/00005237-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kaempf JW, Tomlinson MW, Kaempf AJ, Wu Y, Wang L, Tipping N, et al. Delayed umbilical cord clamping in premature neonates. Obstet Gynecol. 2012;120:325–330. doi: 10.1097/AOG.0b013e31825f269f. [DOI] [PubMed] [Google Scholar]
- 17.Strauss RG, Mock DM, Johnson KJ, Cress GA, Burmeister LF, Zimmerman MB, et al. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short-term clinical and laboratory endpoints. Transfusion. 2008;48:658–665. doi: 10.1111/j.1537-2995.2007.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. Lancet. 2014;384:235–240. doi: 10.1016/S0140-6736(14)60197-5. [DOI] [PubMed] [Google Scholar]