Abstract
Objective
This study aimed to develop a nomogram that predicts ongoing pregnancy after in vitro fertilization and embryo transfer (IVF-ET) using patient age and serum hormonal markers.
Methods
A total of 284 IVF-ET cycles were retrospectively analyzed. At 14 days post-oocyte pick-up (OPU), the serum human chorionic gonadotropin (HCG) and progesterone levels were measured. The main predicted outcome was ongoing pregnancy.
Results
Patient age and serum of HCG and progesterone levels at 14 days post-OPU were good predictors of ongoing pregnancy. The cut-off value and area under the curve (AUC) (95% confidence interval) were 36.5 years and 0.666 (0.599–0.733), respectively, for patient age; 67.8 mIU/mL and 0.969 (0.951–0.987), respectively, for serum HCG level; and 29.8 ng/mL and 0.883 (0.840–0.925), respectively, for serum progesterone level. When the prediction model was constructed using these three parameters, the addition of serum progesterone level to the prediction model did not increase its overall predictability. Furthermore, a high linear co-relationship was found between serum HCG and progesterone levels. Therefore, we developed a new nomogram using patient age and HCG serum level only. The AUC of the newly developed nomogram for predicting ongoing pregnancy after IVF-ET cycles using patient age and serum HCG level was as high as 0.975.
Conclusion
We showed that ongoing pregnancy may be predicted using only patient age and HCG serum level. Our nomogram could help clinicians and patients predict ongoing pregnancy after IVF-ET if the serum JCG level was ≥5 IU/L at 14 days post-OPU.
Keywords: Nomograms, Human chorionic gonadotropin, Progesterone, Pregnancy, In vitro fertilization
Introduction
During in vitro fertilization and embryo transfer (IVF-ET) cycles, clinicians and infertile couples are eager to know pregnancy outcomes as accurately and quickly as possible [1]. In most infertility clinics, the initial establishment of pregnancy after ET is assessed by measurement of the serum level of human chorionic gonadotropin (HCG). A single serum HCG assay after ET is predictive of pregnancy outcomes and assists in the planning of subsequent follow-up [2,3,4]. These studies demonstrated that a single HCG measurement after embryo transfer could predict a viable pregnancy with good sensitivity and specificity. However, by simply measuring HCG, it is difficult to distinguish viable pregnancy from biochemical pregnancy, miscarriage, and ectopic pregnancy [5].
Nomograms are considered useful for physicians during their decision-making processes [6]. In the past, only the cut-off value could be presented. On the other hand, using a nomogram, clinicians can determine the numerical value of probability according to each result. In our previous study, we reported for the first time a nomogram for predicting ongoing pregnancy after IVF-ET using patient age and serum HCG and progesterone levels at 14 days after oocyte pick-up (OPU) [7]. We established our nomogram using a modeling dataset that consisted of 103 infertile women who underwent IVF-ET cycles between April 2011 and December 2012. However, the number of women included in our previous study to develop the nomogram was relatively small. Moreover, we also understood that in many infertility centers, the serum level of progesterone at 14 days post-OPU is not routinely measured. Therefore, the nomogram we previously developed was not widely adopted.
Hence, to reinforce our nomogram, we attempted another analysis of more patients while reevaluating the necessity of serum progesterone measurements for predicting ongoing pregnancy. The main aim of this study was to develop a more accurate and convenient nomogram for predicting ongoing pregnancy after IVF-ET using patient age and serum biomarkers.
Materials and methods
1. Study subjects and design
We analyzed a database of clinical and laboratory information of 284 women who underwent IVF-ET between April 2011 and April 2016. The basic IVF protocol of the present study was similar to that of our previous study [7]. All participants received progesterone (50 mg/day intramuscularly; Watson Pharmaceuticals, Morristown, NJ, USA) from the day of OPU to the 6th or 7th week of gestation as luteal support. In all cases, embryos were transferred on day 3. Serum HCG and progesterone levels were measured at the time of the pregnancy test (exactly 14 days post-OPU), and pregnancy outcomes were followed. This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (B-1707-408-101).
2. Hormone assays
Serum HCG levels were measured using a chemiluminescent microparticle immunoassay (Architect i2000; Abbott Laboratories, Abbott Park, IL, USA) that detects nicked and non-nicked HCG and a free beta subunit. The serum HCG levels were 0–15,000 mIU/mL. The samples with a serum HCG level >15,000 mIU/mL were diluted using normal saline and multiplied by the dilution factor. The intra- and inter-assay coefficients of variation (CVs) were 1.2–4.7% and 1.6–4.9%, respectively. The serum progesterone level was measured using an electrochemiluminescence immunoassay (Elecsys Progesterone II; Hitachi High-technologies, Tokyo, Japan). The serum progesterone levels were 0.03–60 ng/mL. The samples with a serum progesterone level >60 ng/mL were diluted (1:10) using Elecsys Diluent Estradiol/Progesterone and multiplied by the dilution factor. The intra- and inter-assay CVs were 1.5–2.7% and 4.1–5.5%, respectively.
3. Pregnancy outcomes
The classifications of pregnancy outcomes were as follows: biochemical pregnancy, defined as an HCG level >5 IU/L at the time of pregnancy test without any clinical signs of pregnancy; clinical pregnancy, defined as the presence of a gestational sac with or without a fetal heartbeat on ultrasound examination; and clinical abortion, defined as pregnancy failure after visual confirmation of the intrauterine gestational sac. Ongoing pregnancy was defined as progression beyond 12 weeks' gestation.
4. Statistical analysis and nomogram construction
All statistical analyses were performed using SPSS version 18 (SPSS, Chicago, IL, USA) and R software version 2.14.2 (Vienna, Austria; http://www.rproject.org). Statistical significance was set at P-values <0.05. A one-way analysis of variance was used to compare continuous variables between groups. Univariate and multiple logistic regression analyses were performed to assess the association between ongoing pregnancy and age and serum HCG and progesterone levels, as well as to assess the joint effect of the variables. We fitted the restricted cubic splines to each continuous variable to help relax the assumption of a linear association between the variable and predicted outcomes. Pearson's correlation coefficient was used to measure the strength of the association between the two variables. To assess the predictive accuracy and estimate the sensitivity and specificity of each variable and combination of variables, receiver operating characteristic curves were generated. We used McFadden R2 and Hosmer-Lemeshow tests to compare the degree of fitness for each model and determine the best model. In general, a McFadden R2 of 0.5 represents a high degree of fitness.
Results
The basic clinical characteristics of our participants are shown in Table 1. The mean age was 36.3±4.4 years (range, 24–46 years), while the mean number of transferred embryos was 2.0±0.8. Rates of clinical pregnancy per ET and ongoing pregnancy per ET were 29.9% and 23.6%, respectively.
Table 1. Demographic variables for cohort of patients used to construct nomogram predicting ongoing pregnancy.
| Variables | Values | |
|---|---|---|
| Age of women (yr) | 36.3±4.4 | |
| Age of husband (yr) | 39.3±5.0 | |
| BMI (kg/m2) | 22.4±3.4 | |
| Type of infertility | ||
| Primary infertility | 80.3% (228/284) | |
| Secondary infertility | 19.7% (56/284) | |
| No. of living child | 0.3±0.7 | |
| Basal serum AMH (ng/mL) | 2.6±2.4 | |
| Basal serum FSH (IU/L) | 6.7±3.5 | |
| No. of retrieved total oocytes | 6.0±4.6 | |
| No. of transferred embryos | 2.0±0.8 | |
| Clinical pregnancy per embryo transfer | 29.9% (85/284) | |
| Ongoing pregnancy (>12 weeks GA) per embryo transfer | 23.6% (67/284) | |
Values are presented as mean±standard deviation.
BMI, body mass index; AMH, anti-Müllerian hormone; FSH, follicle stimulating hormone; GA, gestational age.
As shown in Table 2, the initial serum HCG titer was elevated in 97 women. Among them, 12 ultimately had a chemical pregnancy, while 18 experienced a clinical abortion. The mean patient age in the ongoing pregnancy group was significantly lower than those of other groups. The serum HCG and progesterone levels in the clinical pregnancy group were significantly higher than those in the chemical pregnancy group.
Table 2. Demographic variables according to pregnancy outcomes.
| Variables | Non-pregnancy (n=187) | Chemical pregnancy (n=12) | Clinical abortion (n=18) | Ongoing pregnancy (n=67) | P value |
|---|---|---|---|---|---|
| Age (yr) | 36 (33–41) | 37 (34–38.8) | 37 (35–38.8) | 34 (33–36) | 0.001 |
| Progesterone (ng/mL) | 21.9 (16.6–28.5) | 27.4 (13.8–29.5) | 63.0 (44.3–81.1) | 60.0 (35–97.2) | <0.001 |
| HCG (IU/L) | 1.2 (1.2–1.2) | 28.9 (11.4–61.1) | 146 (102.9–201.2) | 234.2 (122.2–321.2) | <0.001 |
Values are presented as median (interquatile range).
HCG, human chorionic gonadotropin.
Patient age, serum HCG level, and serum progesterone level were used as predictive variables, and ongoing pregnancy was used as the dependent variable. The results of a univariate logistic regression demonstrated that all predictive variables were significantly correlated with ongoing pregnancy. The odds ratio (OR) and 95% confidence interval (CI) were 0.86 (0.79–0.92) for age; 1.02 (1.02–1.03) for HCG; and 1.05 (1.03–1.06) for progesterone.
As shown in Table 3, patient age and the serum HCG and progesterone levels at 14 days post-OPU were all good predictors of ongoing pregnancy. The cut-off value and area under the curve (AUC) (95% CI) were 36.5 years and 0.666 (0.599–0.733), respectively, for patient age; 67.8 mIU/mL and 0.969 (0.951–0.987) for serum HCG; and 29.8 ng/mL and 0.883 (0.840–0.925) for serum progesterone. Among the 3 variables, the serum HCG level was the most reliable marker for predicting ongoing pregnancy, a finding that was consistent with our previous study [7].
Table 3. Comparison of performance among prediction models.
| Variables | Cut-off | AUC (95% CI) | Model fitness 1 McFadden R2 | Model fitness 2 Hosmer-Lemeshow Test (P-value) |
|---|---|---|---|---|
| Age (yr) | 36.5 | 0.666 (0.599–0.733) | 0.06476128 | 0.05086 |
| Progesterone (ng/mL) | 29.8 | 0.882 (0.840–0.925) | 0.2720570 | 0.002136 |
| HCG (IU/L) | 67.8 | 0.971 (0.954–0.988) | 0.5869664 | <0.000 |
| Age+progesterone | NA | 0.888 (0.802–0.907) | 0.3364037 | 0.1928 |
| Age+HCG | NA | 0.975 (0.954–0.988) | 0.6076166 | 0.01249 |
| Age+progesterone+HCG | NA | 0.977 (0.954–0.987) | 0.6234894 | <0.000 |
AUC, area under the curve; CI, confidence interval; HCG, human chorionic gonadotropin; NA, not applicable.
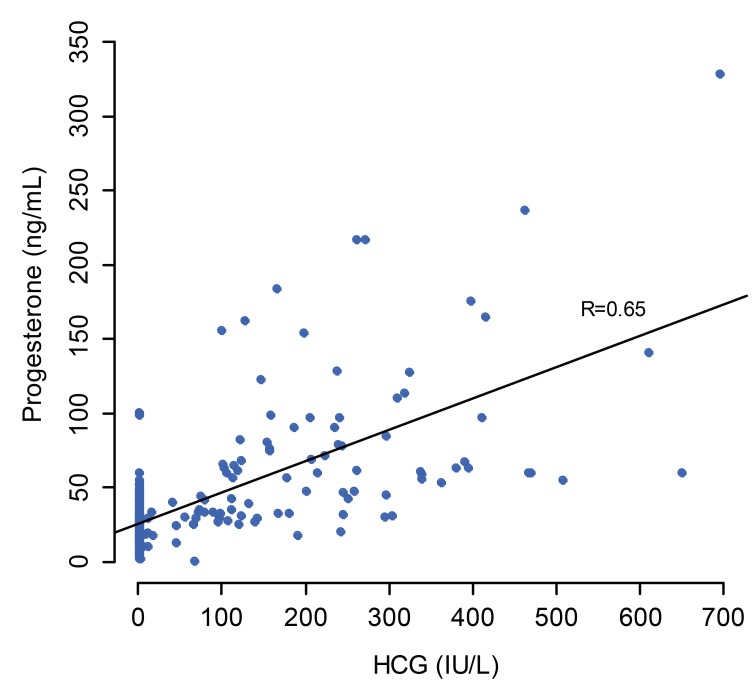
When the prediction model was constructed using all three variables – patient age, serum HCG and progesterone levels — the AUC was 0.975 for patient age and HCG (R2=0.6076166). Addition of serum progesterone level to the prediction model did not increase the overall predictability. Furthermore, a high linear co-relationship (r=0.65) was found between the serum HCG and progesterone levels (Fig. 1). Therefore, it is reasonable to exclude serum progesterone level to develop a new nomogram.
Fig. 1. Linear regression relation between serum human chorionic gonadotropin and progesterone levels.
HCG, human chorionic gonadotropin.
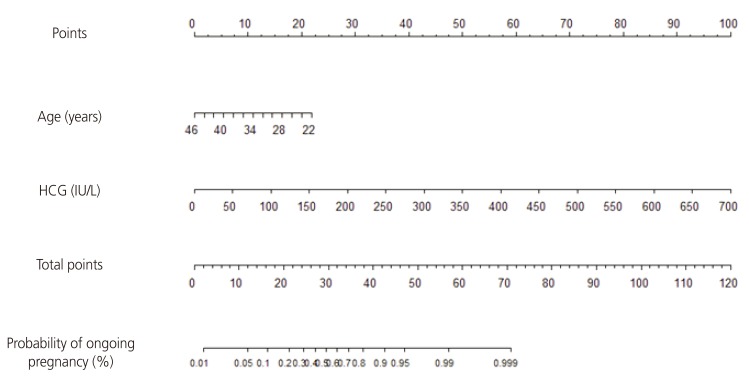
The newly developed nomogram for predicting ongoing pregnancy after IVF-ET cycles using patient age and serum HCG level is demonstrated in Fig. 2. Each point is first assigned by a vertical extension (to top points bar) of patient age and serum HCG level. The total point is obtained by adding up the points identified on the point scale for each variable. The total points projected on the bottom scale indicates the probability of ongoing pregnancy. The nomogram indicates that the probability of ongoing pregnancy increases with increasing serum HCG level; conversely, older age is associated with a reduced chance of ongoing pregnancy. The equation of the nomogram is as follows.
Fig. 2. Nomogram for patient age and serum human chorionic gonadotropin level at 14 days post–oocyte pick-up to predict ongoing pregnancy after in vitro fertilization and embryo transfer treatment.
HCG, human chorionic gonadotropin.
| The probability of ongoing pregnancy = K/(1+K) |
| K=exp(2.06−0.15×age+0.02×HCG) |
Table 4 shows the probability (%) of ongoing pregnancy calculated by our newly reinforced nomogram.
Table 4. Estimates for the probability of ongoing pregnancy (%) according to specific woman's age and serum human chorionic gonadotropin level calculated by our nomogram.
| HCG (mIU/mL) | Woman's age (yr) | ||||
|---|---|---|---|---|---|
| 30 | 35 | 38 | 40 | 42 | |
| 50 | 19.2 | 10.1 | 6.7 | 5.0 | 3.8 |
| 100 | 39.2 | 23.3 | 16.2 | 12.6 | 9.6 |
| 150 | 63.6 | 45.3 | 34.5 | 28.1 | 22.4 |
| 200 | 82.6 | 69.2 | 58.9 | 51.5 | 44.0 |
| 250 | 92.8 | 85.9 | 79.6 | 74.3 | 68.1 |
HCG, human chorionic gonadotropin.
Discussion
In previous studies, HCG level was measured to predict pregnancy outcomes within the range of seven to fourteen days post-embryo transfer [2,3,4]. During early pregnancy, the serum HCG and progesterone levels change rapidly and consistently [8]. Therefore, it is essential to take measurements at a fixed point in time. In the present study, serum HCG and progesterone levels were measured at the time of the pregnancy test (exactly 14 days post-OPU).
It is well known that female age is one of the most important independent factors strongly related to implantation rate [9]. However, most studies conducted to date have only analyzed HCG levels without considering age. The present study demonstrated that mean patient age in the ongoing pregnancy group was significantly lower than those in the other groups. The predictive power for ongoing pregnancy was relatively low compared to serum HCG or progesterone levels, but the predictive power was significant (AUC, 0.666; 95% CI, 0.599–0.733). Therefore, patient age should be considered for predicting pregnancy outcomes.
Progesterone plays a crucial role in maintaining pregnancy by its endocrine and immunological effects [10]. Hence, we initially expected that the serum progesterone level would influence the discrimination of clinical abortion in women with intrauterine pregnancy. However, contrary to our initial expectation, serum progesterone level did not differ between the clinical abortion and ongoing pregnancy groups. When constructing the prediction model, adding serum progesterone level did not improve overall predictability. A recent study [3] also showed that the AUC of HCG only for biochemical pregnancy is not increased by the addition of progesterone measurement (0.936 and 0.937, respectively). Furthermore, the linear relationship between HCG and progesterone was high, suggesting that multi-co-linearity is present in the model. Thus, we decided to exclude serum progesterone level in the new model. Hence, the newly developed nomogram presented in this paper used only patient age and serum HCG level and omits serum progesterone level. We believe that this new nomogram offers greater benefits because fewer variables in a nomogram generally indicate greater convenience. Moreover, with respect to cost, the need for fewer laboratory tests decreases the burden for both physicians and patients.
Only one nomogram to predict pregnancy outcomes after IVF-ET has been published by our research team to date [7]. However, the number of women included in the study was relatively small (n=103). The present study was conducted with large data sets (n=284). The newly developed nomogram presented in this paper showed a much higher accuracy for predicting ongoing pregnancy than our previous version of the nomogram, in which a combination of three variables (patient age, serum HCG level, serum progesterone level) showed an AUC of 0.912 (95% CI, 0.815–1.000), sensitivity of 89.3%, specificity of 80.0%, positive predictive value of 89.3%, and negative predictive value of 80.0%. In the present study, a combination of two variables (patient age and serum HCG titer) showed a higher AUC of 0.975 (95% CI, 0.954–0.988), sensitivity of 98.5%, specificity of 90.8%, positive predictive value of 0.5%, negative predictive value of 23.3%, and the highest McFadden R2 value (0.6076166). We identified an average AUC increase of 0.004 compared with HCG alone.
In conclusion, we developed a novel nomogram that could be used to estimate the individualized probability of ongoing pregnancy using patient age and serum HCG level measured at 14 days post-OPU. This could better help clinicians and patients forecast ongoing pregnancy after IVF-ET if the serum HCG level is ≥5 IU/L at 14 days post-OPU.
Acknowledgements
The authors thank the Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for the statistical analyses. This work was supported by grant (No. A120043) from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Korea.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Turner K, Reynolds-May MF, Zitek EM, Tisdale RL, Carlisle AB, Westphal LM. Stress and anxiety scores in first and repeat IVF cycles: a pilot study. PLoS One. 2013;8:e63743. doi: 10.1371/journal.pone.0063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Shin MS, Yi G, Jee BC, Lee JR, Suh CS, et al. Serum biomarkers for predicting pregnancy outcome in women undergoing IVF: human chorionic gonadotropin, progesterone, and inhibin A level at 11 days post-ET. Clin Exp Reprod Med. 2012;39:28–32. doi: 10.5653/cerm.2012.39.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Zhang R, Jia M, Luo L, Ding C, Li TC, et al. Serum human chorionic gonadotropin measured 7 days following day 3 embryo transfer might predict pregnancy outcome in IVF. Gynecol Endocrinol. 2017;33:62–66. doi: 10.1080/09513590.2016.1203894. [DOI] [PubMed] [Google Scholar]
- 4.Urbancsek J, Hauzman E, Fedorcsák P, Halmos A, Dévényi N, Papp Z. Serum human chorionic gonadotropin measurements may predict pregnancy outcome and multiple gestation after in vitro fertilization. Fertil Steril. 2002;78:540–542. doi: 10.1016/s0015-0282(02)03278-8. [DOI] [PubMed] [Google Scholar]
- 5.van Mello NM, Mol F, Opmeer BC, Ankum WM, Barnhart K, Coomarasamy A, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:603–617. doi: 10.1093/humupd/dms035. [DOI] [PubMed] [Google Scholar]
- 6.Eastham JA, Kattan MW, Scardino PT. Nomograms as predictive models. Semin Urol Oncol. 2002;20:108–115. doi: 10.1053/suro.2002.32936. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Jee BC, Suh CS, Kim SH. Nomogram to predict ongoing pregnancy using age of women and serum biomarkers after in vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol. 2014;172:65–69. doi: 10.1016/j.ejogrb.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Tulchinsky D, Hobel CJ. Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17 α-hydroxyprogesterone in human pregnancy. 3. Early normal pregnancy. Am J Obstet Gynecol. 1973;117:884–893. doi: 10.1016/0002-9378(73)90057-4. [DOI] [PubMed] [Google Scholar]
- 9.van Kooij RJ, Looman CW, Habbema JD, Dorland M, te Velde ER. Age-dependent decrease in embryo implantation rate after in vitro fertilization. Fertil Steril. 1996;66:769–775. doi: 10.1016/s0015-0282(16)58634-8. [DOI] [PubMed] [Google Scholar]
- 10.Arck P, Hansen PJ, Mulac Jericevic B, Piccinni MP, Szekeres-Bartho J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol. 2007;58:268–279. doi: 10.1111/j.1600-0897.2007.00512.x. [DOI] [PubMed] [Google Scholar]