Abstract
Foot and Mouth Disease (FMD) is endemic in Nepal and causes substantial economic losses in the livestock industry. The goal of this study was to perform an epidemiological analysis of FMD outbreaks reported to the Veterinary Epidemiology Center, Tripureshwor, Nepal during 2010–2015, in order to strengthen the National FMD Control Program. These current data were considered in the context of historical data on FMD virus (FMDV) serotypes detected in the country between 1965 and 2015. During 2010–2015, a total of 1333 livestock holdings reported FMD outbreaks in Nepal. On average, 71.2 animals were affected in each outbreak, with a case fatality rate of 3.6%. FMD was reported throughout the country, and the proportion of affected holdings was not significantly among eco‐zones, regions, or species. The Hill eco‐zone had the highest number of holdings affected (782), followed by Mountain (304), and Terai (247). When analysed by the developmental region, the Western (381) and Central (368) Developmental Regions had the highest numbers of holdings affected. Cattle were the most frequently affected species (39%), followed by buffalo (33%), and goats (19%). FMD occurred throughout the year, with peaks in winter (December/January) and in the pre‐monsoon period (April/May). Between 1965 and 2015 FMDV serotype O had the highest prevalence (81%), followed by Asia‐1 (11%), A (6%), and C (2%). Serotype C was not detected after 1996, and only serotype O was reported after 2011. These descriptive analyses provide critical landmarks to establish baselines, and document early progress of the ongoing Progressive Control Pathway of FMD (PCP‐FMD) which could be useful in Nepal and other South Asian nations.
Keywords: analysis, Epidemiological, FMD, Nepal, Outbreak, Serotypes
Introduction
Foot‐and‐mouth disease (FMD), caused by FMD virus (FMDV; Aphthovirus, Picornaviridae), is a highly contagious and economically important disease of cloven‐hoofed domestic livestock and wildlife species worldwide (Knowles & Samuel 2003; Grubman & Baxt 2004). Acute infection is characterized by fever, ptyalism, lameness, and vesicles and erosions in the mouth, on the feet, and teats (Arzt et al. 2011a; Arzt et al. 2011b). Mortality is generally low in adult animals. However, morbidity is high, and the disease results in substantial economic losses due to decreased milk yields and growth rates, as well as imposed trade restrictions (Yang et al. 1999; Bouma et al. 2003; Junker et al. 2009; Knight‐Jones & Rushton 2013).
Seven immunologically distinct serotypes of FMDV have been described (O, A, C, Asia‐1, and Southern African Territories [SAT] 1–3). Virus strains are further grouped into seven distinct geographical regions, or pools, which represent independently circulating and evolving FMDV genotypes/lineages (Rweyemamu et al. 2008; Di Nardo et al. 2011; Brito et al. 2017). Nepal is included in FMDV pool 2, in which serotypes O, A, and Asia‐1 are endemic. Serotype C was present in Nepal historically (Ferris et al. 1992), but has not been detected since 1996 (DLS 2015). Outbreaks of FMD occur in all parts of the country irrespective of altitude and climate. However, higher incidence has previously been described during the monsoon and post‐monsoon periods (Ferris et al. 1992; VEC 2015). Additionally, movement of livestock within the country as well as legal and illegal cross‐border trade with India are believed to be important factors contributing to the high frequency of FMD outbreaks in Nepal (Ferris et al. 1992).
Nepal has a population of 28.8 million people, and is situated between China and India (Fig. 1). Three eco‐zones (Mountain, Hill, and Terai) divide the country from north to south, and the climate ranges from alpine in the Mountain region, through temperate in the Hill region, to subtropical in the Terai region. The country is further divided into five developmental regions from east to west, each containing all three eco‐zones. The five developmental regions are further divided into a total of 75 districts (Fig. 1). Livestock density is highest in the Terai eco‐zone, and in the Eastern and Central Developmental Regions (Acharya 2015). Approximately 65% of the population is involved in agriculture, of which livestock rearing plays an integral role (MOAD 2014a, MOLD 2016). The main species of livestock in the country are cattle, buffalo, sheep, goats, yaks and pigs, however, there are relatively few pigs, and yaks are restricted to very high altitudes in northern regions (Ferris et al. 1992). Major challenges for livestock development in Nepal include the high prevalence of endemic diseases, lack of proper nutrition and veterinary care, and poor herd management leading to low production rates (Giri & Parshin 2010). In 2015, FMD accounted for 22.6% of the total reported disease outbreaks and 3.7% of the deaths caused by the major infectious and parasitic diseases in Nepal (VEC 2016a,b). Economic losses due to FMD in terms of reduced milk yields and meat production were estimated to be 66 million US dollars per year (Gongal 2002). However, actual economic losses could be much higher if the reduction in breeding efficiency, costs of veterinary care, and reduction in draught power of animals were accounted for.
Figure 1.
Map of Nepal showing Development Regions, Districts and Eco‐zones.
Nepal is a member of the World Trade Organization (WTO), and the endemic presence of FMD in the country constitutes a barrier to international trade of livestock and animal products (Thakuri 2012). Nepal's National FMD Control Program was established in 2012, initially targeting the Eastern and Far Western Development Regions and eventually expanding to cover the entire country (Acharya 2015). The main goal of this current study was to perform an epidemiological analysis of FMD outbreaks that were reported in the country during 2010–2015. Additionally, historical data on FMDV serotypes detected in the country between 1965 and 2015 was analysed. Results of this study will help to inform FMD control programmes in Nepal and other South Asian nations, including prioritizations for vaccine deployment and consideration of improvements to border control, and outbreak response strategies.
Materials and methods
FMDV reports (2010–2015)
Monthly epidemiological reports on the current, ongoing FMD situation were collected by the Veterinary Epidemiology Center and Directorate of Animal Health under the Department of Livestock services of Nepal Government from the district livestock service offices (DLSO) in each of the 75 districts for the period of 2010 to 2015. The collected data consisted of the number of holdings reporting FMD by species, including the number of animals affected and dead per holding, as well as the number of animals that were vaccinated per district per month. One species on one farm was considered one holding of that species. The district livestock service offices were the primary source of the data analysed herein. Diagnosis of FMD at the district level is generally made based upon clinical presentation and assessment by a district veterinarian. However, samples are often sent to the National FMD and TADs Laboratory for laboratory confirmation of the clinical diagnosis as well as serotype determination.
FMDV serotype data (1965–2015)
The FMDV serotypes reported in Nepal from 1965 to 2015 were analysed. Serotype determination was performed by the National FMD and TADs Laboratory using an indirect sandwich antigen‐ELISA (Ferris et al. 1992; OIE 2012) which was performed according to the manufacturers’ instructions.
Data analysis
One affected holding was defined as one report of FMD in one species on one farm. The mean case count was calculated as the number of animals affected per outbreak. The case fatality rate was calculated as the number of dead animals divided by the number of animals affected per outbreak. The chi‐squared test was used to test for significant differences in the frequency of FMD‐affected holdings among years. Additionally, the total number of holdings by species in each district in 2011/2012 was retrieved from statistical information prepared by the Ministry of Agricultural Development (CBS 2013; MOAD 2014b). This was the most recent year for which data was available, and the distribution of livestock was not expected to change significantly during the study. The distribution of FMD‐affected holdings was compared to the distribution of all holdings of FMDV‐susceptible species (cattle, buffalo, sheep, goats, swine, yak) by eco‐zone, region, and species using the Chi‐squared test. A two‐tailed ANOVA test was used to test for significant differences in the case fatality rate within years, eco‐zones, regions, and animal species. The distribution of FMDV serotypes O, A, C, and Asia‐1 in samples tested at the National FMD and TADs Laboratory was determined by year. Data analysis was performed using Microsoft Excel 2013, SPSS 16 (SPSS, Inc., Chicago, IL, USA), and R (R Core Team 2016).
Results
Spatial and temporal distribution of FMD (2010–2015)
A total of 1333 holdings reported FMD in Nepal during 2010–2015, with an average of 222 (95% CI 125, 320) holdings affected per year. The number of holdings affected varied significantly (χ 2 = 194, df = 5, P < 0.0001) across years, and ranged from 99 in 2012 to 338 in 2014 (Fig. 2a). The mean case count ranged from 29.3 in 2012 to 92.8 in 2014, with an average of 64.9 (Fig. 2b). The average case fatality rate ranged from 1.4% in 2010 to 12.4% in 2012, with an average of 3.6% (Fig. 2b), and significant differences between years (F = 18.37, P < 0.0001).
Figure 2.
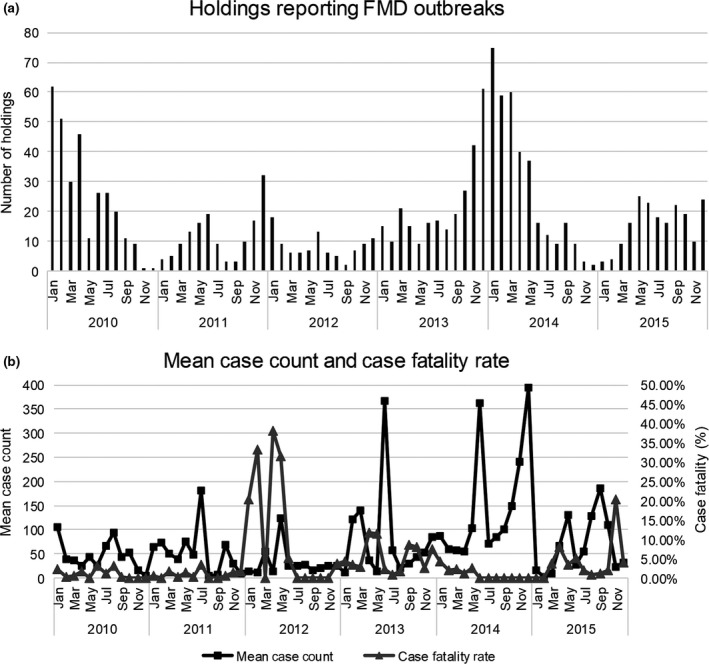
Seasonal trend of FMD in Nepal (2010–2015). (a) Number of holdings reporting FMD by month. (b) Mean case count and case fatality rate by month.
Holdings reported FMD throughout the year, however higher numbers of holdings affected were reported during the winter months, with a smaller peak during the summer (Fig. 2). The mean case count varied throughout the year, and tended to be higher during the summer. Although the case fatality rate varied significantly across years in the study, there was no significant seasonal trend (P = 0.078).
The greatest number of affected holdings was reported in the Hill eco‐zone (782), followed by Mountain (304) and Terai (247) (Table 1). However, the proportion of affected holdings was not significantly different among eco‐zones (χ 2 = 6, df = 4, P = 0.20). The mean case count per outbreak during the study was similar among the three eco‐zones. The average case fatality rate was similar for the Mountain (2.1%) and Terai (2.0%) eco‐zones, however the rate was significantly higher in the Hill eco‐zone (4.6%, F = 8.28, P = 0.0003).
Table 1.
Distribution of FMD in Nepal by eco‐zone (2010–2015)
| Eco‐zone | Total holdings | Holdings affected (% of Total) | Mean case count (95% CI) | Case fatality rate (95% CI) |
|---|---|---|---|---|
| Mountain | 655089 | 304 (0.046%) | 88.6 (54.3, 122.8) | 2.1% (1.0, 3.1) |
| Hill | 3446202 | 782 (0.023%) | 66.3 (58.2, 74.3) | 4.6% (3.7, 5.6) |
| Terai | 2747789 | 247 (0.009%) | 65.6 (51.4, 79.9) | 2.0% (1.1, 2.9) |
The Western developmental region reported the highest number of holdings affected (381), followed by Central (368), Mid‐western (259), Far‐western (237), and Eastern developmental regions (88), however the Far‐western region had the highest proportion of holdings affected (Table 2). Overall, the proportion of affected holdings was not significantly different among regions (χ 2 = 20, df = 16, P = 0.22). Interestingly, the mean case count was similar among all regions except the Central region, which had a lower mean case count. The average case fatality rate was significantly lower in the Far‐Western region (F = 2.94, P = 0.02) (Table 2).
Table 2.
Distribution of FMD in Nepal by development region (2010–2015)
| Region | Total holdings | Holdings affected (% of Total) | Mean case count (95% CI) | Case fatality rate (95% CI) |
|---|---|---|---|---|
| Eastern | 1788924 | 88 (0.005%) | 84.2 (53.0, 115.5) | 3.2% (1.1, 5.3) |
| Central | 1824854 | 368 (0.020%) | 44.2 (36.1, 52.3) | 4.7% (3.5, 6.0) |
| Western | 1374901 | 381 (0.028%) | 80.9 (53.0, 108.8) | 3.0% (2.2, 3.8) |
| Mid‐Western | 1085627 | 259 (0.024%) | 82.0 (62.6, 101.5) | 4.4% (2.2, 6.7) |
| Far‐Western | 774774 | 237 (0.031%) | 81.1 (71.8, 90.4) | 1.9% (1.4, 2.3) |
FMD outbreaks were reported from 71 out of 75 districts of Nepal from 2010 to 2015. The highest number of holdings affected was reported from Dolakha (72), followed by Achham (62), Tanahu (56), and Kathmandu (51). Twelve other districts reported more than 30 holdings affected (Kapilvastu (49), Mugu (40), Kaski (39), Jumla (38), Doti (36), Parbat (34), Syangja (34), Darchula (34), Bhaktapur (33), Baitadi (33), Dhadhing (32), Kavre (31)), and four districts reported no FMD‐affected holdings (Taplejung, Solukhumbu, Parsa, Kailali). The remaining districts each reported <30 holdings affected during 2010–2015 (Fig. 3).
Figure 3.
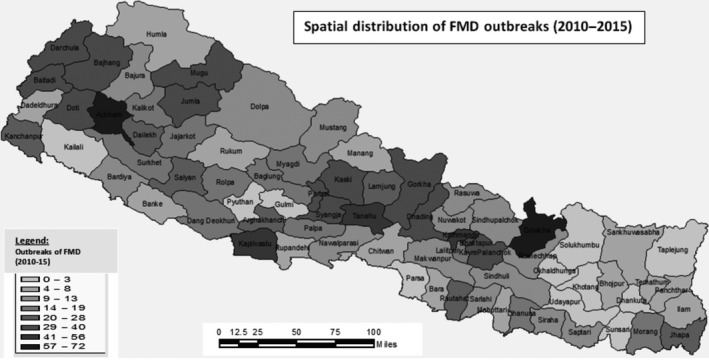
Distribution of FMD in Nepal by district (2010–2015).
Species‐specific distribution of FMD (2010–2015)
The highest number of holdings affected raised cattle (522, 39.2%), followed by buffalo (444, 33.3%), goats (249, 18.7%), swine (65, 4.9%), sheep (50, 3.8%), and yak (3, 0.2%) (Table 3). The proportion of affected holdings was not significantly different among species over the entire study (χ 2 = 30, df = 25, P = 0.22) or by year (χ 2 = 25.74, df = 25, P = 0.422). The mean case count was highest in goats and sheep, and lowest in yak. However, the average case fatality rate was similar for all species (F = 1.86, P = 0.099) (Table 3).
Table 3.
Distribution of FMD outbreaks among susceptible species in Nepal 2010–2015
| Species | Total holdings | Holdings affected (% of Total) | Mean case count (95% CI) | Case fatality rate (95% CI) |
|---|---|---|---|---|
| Buffalo | 1668820 | 444 (0.027%) | 49.9 (41.9, 57.9) | 2.6% (1.7, 3.6) |
| Cattle | 2280542 | 522 (0.023%) | 75.3 (62.1, 88.4) | 3.4% (2.4, 4.5) |
| Goats | 2352453 | 249 (0.011%) | 108.4 (69.6, 147.2) | 5.1% (3.4, 6.8) |
| Sheep | 96245 | 50 (0.052%) | 101.4 (54.6, 148.1) | 3.5% (1.2, 5.8) |
| Swine | 444785 | 65 (0.015%) | 22.2 (15.4, 28.9) | 5.45% (2.6, 8.1) |
| Yak | 6235 | 3 (0.048%) | 13.7 (0, 33.0) | 0 |
Vaccination of animals against FMD (2010–2015)
A quadrivalent vaccine (serotypes O, A, C, Asia‐1) was introduced in 2010. Approximately 39 000 FMD vaccine doses were administered in Nepal in 2010, increasing yearly to approximately 533 000 in 2015 (Fig. 4). Vaccines were administered as part of the official vaccination campaign and in response to FMD outbreaks (ring vaccination). Of the total vaccines administered during 2010–2015, the highest proportion were administered to cattle (64%), followed by buffalo (26%), goats (7%), sheep (2%), and pigs (1%). Additionally, 675 yaks were vaccinated against FMD in 2015.
Figure 4.
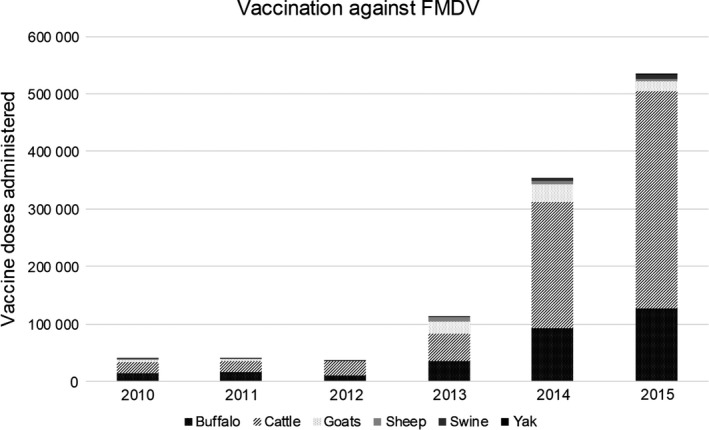
Total number of FMDV vaccine doses administered in Nepal by species (2010–2015).
Distribution of FMDV serotypes (1965–2015)
FMDV was detected by antigen‐ELISA in 809 samples submitted to the National FMD and TADs laboratory, Budhanilakantha, from 1965 to 2015 (Table S1). The large majority of viruses were serotype O (81%), however serotypes Asia‐1 (11%), A (6%), and C (2%) were also identified during the period (Fig. 5).
Figure 5.
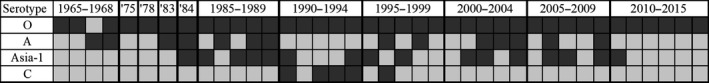
FMDV serotypes detected in Nepal (1965–2015). Dark grey boxes indicate the serotype was detected during the year.
During 1965–1975, serotype O was found in 64% of positive samples. The proportion of serotype O subsequently increased to 74% through the next three decades and reached 97% in the last decade of 2006–2015. Serotypes A and Asia‐1 were detected at lower levels during much of the study period, and serotype C was detected sporadically. Serotype C has not been detected since 1996, and only serotype O has been detected since 2011 (Fig. 5).
Discussion
Foot‐and‐mouth disease is endemic in Nepal, causing substantial economic losses due to decreased production and associated costs for treatment of animals and sanitary measures. During the period examined in the current study (2010–2015), an average of 222 holdings per year reported FMD outbreaks. In contrast, between 2001–2005 an average of 1290 holdings per year reported FMD outbreaks, with subsequent decrease to 456 holdings per year between 2006–2010 (VEC 2016b). Although the comprehensive reasons for this decreasing trend are not definitive, the decreasing cases over time may be due to the increased vaccination against FMD, resulting in fewer outbreaks between 2010 and 2015 compared to the previous decade. During the years 2010–2015, the fewest outbreaks occurred in 2011 and 2012, which may reflect under reporting by DLSOs or the launch of the National FMD Control Program (Acharya 2015). Contrastingly, the greatest number of outbreaks occurred in 2014, concurrent with the identification of a new lineage in the region (O/ME‐SA/PanAsia‐2/KAT‐15) (WRLFMD 2018). It is possible that the increased number of outbreaks in 2014 was associated with suboptimal efficacy of the vaccines in use at that time against this novel lineage. Taken together, these findings highlight potential success of the vaccination campaign and the need for continued surveillance to match vaccines with circulating viruses.
The highest number of livestock holdings in Nepal are located in the Hill eco‐zone, and although the Hill eco‐zone had the highest number of holdings affected, the proportion of holdings affected and the mean case count were similar across eco‐zones. Additionally, 50–75% of livestock in the Mountain eco‐zone and 25–50% in the Hill eco‐zone are raised in a transhumance system, whereas livestock rearing in the Terai eco‐zone is sedentary (Acharya 2015). These differences in animal husbandry likely result in fewer contacts among the sedentary holdings in the Terai eco‐zone, and therefore fewer FMD outbreaks overall in this region despite a higher livestock density. Interestingly, the case fatality rate was significantly higher in the Hill eco‐zone, which may be due to differences in husbandry practices, species or breeds affected among the regions or differences in FMDV strains circulating in the regions. The extent to which the reported mortality was due to primary FMDV‐associated myocarditis or secondary infections was not determined. Myocarditis typically occurs in young animals and is associated with specific viral strains and poorly understood host‐specific factors (Arzt et al. 2011a). The higher fatality in the Hill zone may have been associated with that region's higher quantities of pigs which have been demonstrated to be highly susceptible to myocarditis from certain strains of FMDV (Stenfeldt et al. 2014). Additional factors which might have contributed to differential fatality include climatic influences, environmental stability of secondary pathogens or opportunistic organisms, and access to adequate quality and quantity of feed and water. However, definitive determination of the causality could not be determined by the data contained herein.
The high absolute number of holdings affected in the Western and Central Developmental Regions may be due to the large livestock population and frequent movement of animals in those regions. The Eastern Development Region also has a large livestock population; however, the low number of holdings affected in that region may reflect either the relatively lower transboundary movement of animals in that region or an effective implementation of the FMD control and vaccination programme in that region (Acharya 2015).
FMD outbreaks occurred in Nepal throughout the year; however more holdings reported FMD in December and January and also in the pre‐monsoon period (April‐May) than at other times of the year. Increased numbers of holdings affected during December and January may be due to increased movement of animals during the Dashain and Tihar festivals (Ferris et al. 1992). Previous studies in India have also shown FMD outbreaks throughout the year, with a higher prevalence of outbreaks in the winter months (Bhat & Taneja 2001; Hegde et al. 2014). In contrast to the current study, a study in Bangladesh reported the highest incidence of FMD outbreaks during the post‐monsoon season (September‐November), followed by the pre‐monsoon season (March‐May), and winter (December‐February) (Kamaruddin & Pandit 1988). Additionally, the Nepal Veterinary Epidemiology Centre reported large numbers of holdings affected during the monsoon and pre‐monsoon periods during 2001–2010 (VEC 2016a). Such seasonal trends in FMD outbreaks may reflect either trends in movements of animals or differences in environmental stability or transmission of the virus.
In the current study, cattle were the most frequently affected species, followed closely by buffalo. The distribution of affected holdings across livestock species was similar to the distribution reported between 2000 and 2009 (Jha 2012). The high proportion of FMD in cattle holdings in Nepal may be due to cross‐breeding and introduction of exotic cattle breeds, which may lead to an increased susceptibility of cattle to FMD (Kitching 2002; Alexandersen et al. 2003). However, cattle also make up a large proportion of the national livestock herd, and the proportion of holdings affected for each species was similar to the proportion of holdings of each species in the national livestock herd. Although the case fatality rate was similar among species, the mean case count was significantly higher in sheep and goats compared to cattle, buffalo, swine, and yak. This may be due to greater numbers of sheep and goats kept per holding, and associated higher stocking density which facilitates viral transmission through increased inter‐animal contacts.
Serotype O was the most commonly detected serotype between 1965–2015. Although only serotype O has been detected in Nepal since 2011, serotypes A and Asia‐1 have been detected regularly in Nepal previously, and these serotypes continue to circulate in neighboring India and Bangladesh (Nandi et al. 2015; Hegde et al. 2016; Islam et al. 2017). The vaccination campaign may have been effective in limiting FMD outbreaks caused by serotypes A and Asia‐1. Alternatively, the lack of detection of these serotypes may be due to limited sampling of outbreaks. Even with a successful vaccination campaign, serotypes A and Asia‐1 may be reintroduced into Nepal from neighbouring countries, and continued surveillance and matching of vaccine strains to field strains will be important for FMDV control in Nepal.
Genotyping of samples was beyond the scope of this study, however a subset of outbreak samples were sent to the World Reference Laboratory for FMD, and are publically available (http://www.wrlfmd.org/fmd_genotyping/asia/nep.htm). WRLFMD genotyping confirmed the presence of serotype O exclusively in samples from the study period, and the Ind‐2001d lineage has been predominant (WRLFMD 2018).
Conclusion
This descriptive study of FMD in Nepal provides valuable insights to the distribution and concentration of disease in various parts of the country, and documents the efforts towards transition from Stage 1 to Stage 2 of the Progressive Control Pathway for FMD (PCP‐FMD), established by the Food and Agriculture Organization and the World Organisation for Animal Health (OIE). FMD is an important disease of livestock in Nepal and is of substantial economic importance due to production losses and costs associated with sanitary measures. Between 2010 – 2015, FMD occurred in almost all parts of the country and during all seasons. A large majority of the reported outbreaks were caused by FMDV serotype O. Despite the efforts of the National FMD Control Program, which began in 2011/12, FMD remains a major threat to the livestock industry in Nepal. The current study highlights the importance of continued surveillance, genotyping of circulating strains, and FMD vaccine matching. Additionally, control of animal movements, quarantine of affected premises, and proper bio‐security measures will be essential to improve FMD control efforts, with alignment between the Nepalese national programme, the PCP‐FMD, and the standards of the OIE.
Source of funding
The authors are greatly indebted to Office of the Dean, Directorate of Research, Institute of Agriculture and Animal Science, Tribhuvan University, Rampur, Chitwan for providing financial assistance to carry out this research.
Conflict of interest
The authors declare that they have no competing interests.
Ethics statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. The work described herein was performed by the federal staff of the TADS Laboratory, Directorate of Animal Health, and Veterinary Epidemiology Center, Nepal, which approved the study. All infections described herein occurred spontaneously in domestic livestock with no experimentation, inoculation, or treatment of live animals.
Contributions
Study Design: Ganesh Adhikari, Krishna Prasad Acharya, Mukul Upadhyay
Statistical Analysis: Miranda R. Betram, Carolina Stenfeldt, Jonathan Arzt
Draft Manuscript preparation: Ganesh Adhikari, Krishna Prasad Acharya, Rabin Raut, Krishna Kaphle, Tanka Khanal
Manuscript revision and Approval: Krishna Prasad Acharya, Miranda R. Betram, Carolina Stenfeldt, Jonathan Arzt
Supporting information
Table S1. Serotypes of FMD virus detected in Nepal (1965‐2015)
Acknowledgements
The authors are thankful to all the staffs of FMD Laboratory, Budhanilkantha TADS Laboratory, Budhanilkantha, Directorate of Animal Health and Veterinary Epidemiology Center, Tripureshwor for their direct and indirect help. Thanks are also due to Dr. Shyam Bahadur Raut, Dr. Luna Gongal and Mr. Giri Prasad Adhikari for necessary help. Dr. Bertram was a recipient of a Plum Island Animal Disease Center Research Participation Program fellowship administered by Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA).
References
- Acharya K.R. (2015) FMD and farming practices in Nepal. Australian Cattle Veterinarians and Australian Sheep Veterinarians Annual Conference, Hobart, Australia.
- Alexandersen S., Zhang Z., Donaldson A.I. & Garland A.J.M. (2003) The pathogenesis and diagnosis of foot‐and‐mouth disease. Journal of Comparative Pathology 129(1), 1–36. [DOI] [PubMed] [Google Scholar]
- Arzt J., Baxt B., Grubman M.J., Jackson T., Juleff N., Rhyan J. et al (2011a) The pathogenesis of foot‐and‐mouth disease II: viral pathways in swine, small ruminants, and wildlife; myotropism, chronic syndromes, and molecular virus‐host interactions. Transboundary and Emerging Diseases 58(4), 305–326. [DOI] [PubMed] [Google Scholar]
- Arzt J., Juleff N., Zhang Z. & Rodriguez L.L. (2011b) The pathogenesis of foot‐and‐mouth disease I: viral pathways in cattle. Transboundary and Emerging Diseases 58(4), 291–304. [DOI] [PubMed] [Google Scholar]
- Bhat P.N. & Taneja V.K. (2001) Foot and mouth disease: Is it an international concern now? Indian Dairyman 5(5), 53. [Google Scholar]
- Bouma A., Elbers A.R.W., Dekker A., de Koeijer A., Bartels C., Vellema P. et al (2003) The foot‐and‐mouth disease epidemic in The netherlands in 2001. Preventive Veterinary Medicine 57(3), 155–166. [DOI] [PubMed] [Google Scholar]
- Brito B.P., Rodriguez L.L., Hammond J.M., Pinto J. & Perez A.M. (2017) Review of the global distribution of foot‐and‐mouth disease virus from 2007 to 2014. Transboundary and Emerging Diseases 64(2), 316–332. [DOI] [PubMed] [Google Scholar]
- CBS (2013) Statistical year book of Nepal‐2013. Retrieved from http://cbs.gov.np/publications/statisticalyearbook_2013 [Google Scholar]
- Core Team R. (2016) R: A Language And Environment For Statistical Computing. Austria, R Foundation for Statistical Computing; Vienna. [Google Scholar]
- Di Nardo A., Knowles N.J. & Paton D.J. (2011) Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub‐Saharan Africa, the Middle East, and Southeast Asia. Revue Scientifique et Technique de L'OIE 30(1), 63–85. [DOI] [PubMed] [Google Scholar]
- DLS (2015) Technical Report, FMD and TADS Laboratory, Nepal. Retrieved Aug 29, 2017, from http://ahd.gov.np/uploads/files/9033546251.pdf.
- Ferris N.P., Donaldson A.I., Shrestha R.M. & Kitching R.P. (1992) A review of foot and mouth disease in Nepal. Revue Scientifique et Technique de L'OIE 11(3), 685–698. [DOI] [PubMed] [Google Scholar]
- Giri R. & Parshin P. (2010) Epizootiology of foot and mouth disease in Nepal. International Journal of Infectious Diseases 1(14), 159. [Google Scholar]
- Gongal G.N. (2002) Foot and mouth disease in Nepal. Technical Report Kathmandu, Nepal, National FMD Control Section.1, 19–22. [Google Scholar]
- Grubman M.J. & Baxt B. (2004) Foot‐and‐mouth disease. Clinical Microbiology Reviews 17(2), 465–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R., Gomes A.R., Giridhar P., Kowalli S., Shivashankar B.P., Sudharshana K.J. et al (2014) Epidemiology of foot and mouth disease in Karnataka state, India: a retrospective study. Virus Disease 25(4), 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R., Kowalli S., Nagaraja K., Dharanesha N.K., Seema C.M., Khan T.A. et al (2016) Serosurveillance of foot and mouth disease in Karnataka state, India: a 3 years study. Virus Disease 27(3), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.S., Habib M.A., Saha P.C., Das P.M. & Khan M.A.H.N.A. (2017) Distribution of foot and mouth disease virus serotypes in cattle of Bangladesh. SAARC Journal of Agriculture 15(1), 33–42. [Google Scholar]
- Jha V.C (2012) “Situation of foot and mouth disease and its progressive control initiatives in Nepal”. Retrieved Aug 29, 2017, from http://www.fao.org/docs/eims/upload/299831/an359e00.pdf
- Junker F., Ilicic‐Komorowska J. & van Tongeren F. (2009) Impact of animal disease outbreaks and alternative control practices on agricultural markets and trade: the case of FMD. OECD Food, Agriculture and Fisheries Papers, OECD Publishing. 19 [Google Scholar]
- Kamaruddin K.M. & Pandit K.K. (1988) Pattern of foot and mouth disease virus infection in cattle of Bangladesh. Bangladesh Journal of Veterinary Medicine 5, 54–58. [Google Scholar]
- Kitching R.P. (2002) Clinical variation in foot and mouth disease: cattle. Revue Scientifique et Technique 21(3), 499–504. [DOI] [PubMed] [Google Scholar]
- Knight‐Jones T.J.D. & Rushton J. (2013) The economic impacts of foot and mouth disease – What are they, how big are they and where do they occur? Preventive Veterinary Medicine 112(3–4), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N.J. & Samuel A.R. (2003) Molecular epidemiology of foot‐and‐mouth disease virus. Virus Research 91(1), 65–80. [DOI] [PubMed] [Google Scholar]
- MOAD (2014a) Annual Report, Ministry of Agricultural Development, Government of Nepal. Retrieved Aug 29, 2017, from http://moad.gov.np/public/uploads/2142475980-ADS%20Final%20Report%20-%20as%20of%2023%20september,%202014.pdf
- MOAD (2014b) “Statistical information on Nepalese agriculture 2012/2013”. Retrieved Aug 29, 2017, from http://moad.gov.np/public/uploads/1009021694-YearBook%202013.pdf
- MOLD (2016) Annual Report, Ministry of Livestock Development, Government of Nepal. Retrieved Aug 29, 2017, from http://www.mold.gov.np/downloadfile/Mold_Annual_Report_Inner_mold.pdf
- Nandi S.P., Rahman M.Z., Momtaz S., Sultana M. & Hossain M.A. (2015) Emergence and distribution of foot‐and‐mouth disease virus serotype a and O in Bangladesh. Transboundary and Emerging Diseases 62(3), 328–331. [DOI] [PubMed] [Google Scholar]
- OIE (2012). Foot and Mouth Disease. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Terrestrial Manual). Paris, France, OIE.
- Rweyemamu M., Roeder P., Mackay D., Sumption K., Brownlie J., Leforban Y. et al (2008) Epidemiological patterns of foot‐and‐mouth disease worldwide. Transbound Emerg Dis 55(1), 57–72. [DOI] [PubMed] [Google Scholar]
- Stenfeldt C., Pacheco J.M., Borca M.V., Rodriguez L.L. & Arzt J. (2014) Morphologic and phenotypic characteristics of myocarditis in two pigs infected by foot‐and‐mouth disease virus strains of serotypes O or A. Acta Veterinaria Scandinavica 56(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakuri K. (2012). Status of animal disease outbreak and identification of provisional disease free zone/area. Veterinary Epidemiology Center, Directorate of Animal Health, Kathmandu, Nepal.
- VEC (2015). Annual Epidemiological Bulletin, Veterinary Epidemiology Center, Nepal. Retrieved Aug 29, 2017, from http://epivet.gov.np/uploads/files/6021247917.pdf.
- VEC (2016a). “FMD newsletter quarterly bulletin”. Retrieved Aug 29, 2017, from http://epivet.gov.np/uploads/files/0292208658.pdf.
- VEC (2016b). “Six‐monthly epidemiological bulletin January‐June 2016”. Retrieved Aug 29, 2017, from http://www.epivet.gov.np/uploads/files/4332702382.pdf.
- WRLFMD (2018) “FMDV genotyping Nepal”. Retrieved Apr 27, 2018, from http://www.wrlfmd.org/fmd_genotyping/asia/nep.htm
- Yang P.C., Chu R.M., Chung W.B. & Sung H.T. (1999) Epidemiological characteristics and financial costs of the 1997 foot‐and‐mouth disease epidemic in Taiwan. Veterinary Record 145(25), 731–734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Serotypes of FMD virus detected in Nepal (1965‐2015)


