Abstract
Persistent mating‐induced endometritis (PMIE) is a significant cause of mare infertility hence its treatment would advance the management of susceptible mares. Preimplantation factor (PIF) is secreted by viable embryos, including human, mouse and cattle, and is essential for maternal immune‐tolerance without immune‐suppression by modulating inflammation. This preliminary study aimed to test whether PIF exerts inflammatory‐modulating properties upon equine endometrium challenged with Escherichia coli‐derived lipopolysaccharide (LPS) using endometrial explant culture. Follicular (n = 3), luteal (n = 4), anoestrous (n = 4) and transitional (n = 4) stage endometrial explants were established and cultured in triplicate in either serum‐free medium alone (control) or medium with; 50 or 100 nmol/L synthetic PIF (sPIF); 3 μg/mL LPS; LPS and 50 or 100 nmol/L sPIF; or scrambled PIF (PIFscr; same amino acid composition arranged in a different order). Media samples were collected at 24 and 72 h, representing acute and chronic inflammatory response. Radioimmunoassay determined Prostaglandin F2α (PGF 2α) as an inflammatory marker. The only significant observation was the abrogation of PGF 2α response to LPS challenge by 100 nmol/L PIF for follicular stage tissue, 24 h after treatment. Further studies are therefore, warranted to realise PIF potential in managing PMIE.
Keywords: equine endometritis, inflammation, treatment, preimplantation factor
Introduction
In mares, the acute but transient, mating‐induced uterine inflammation (MIE) observed after insemination is a ubiquitous innate immune response to semen and/or bacteria (Allen & Pycock 1989; Katila 2001; LeBlanc 2010). Bacteria are frequently recovered from the uterus after mating; particularly, Streptococcus zooepidemicus and Escherichia coli (Hughes & Loy 1975; Wingfield Digby & Ricketts 1982) and bacterial antigens stimulate an innate immune response (Christoffersen & Troedsson 2017). However, in up to 40% of mares, inflammation persists (persistent mating‐induced endometritis; PMIE) causing embryonic loss (Pycock & Newcombe 1996). In practice, treatment may only commence after PMIE is established (LeBlanc 2010), therefore there is interest in agents which may modulate the innate immune response before inflammation becomes established. Steroids, Mycobacterium cell wall extract and N‐acetylcysteine have shown potential (Fumuso et al. 2007; Bucca et al. 2008; Gores‐Lindholm et al. 2013; Melkus et al. 2013); however, with each there are important drawbacks (LeBlanc 2010; Gores‐Lindholm et al. 2013; Woodward et al. 2015). Therefore, a need exists to discover novel anti‐inflammatories which may complement the innate immune response.
Preimplantation factor (PIF) is a protein secreted exclusively by viable embryos shortly after fertilisation, inducing maternal acceptance through immune tolerance without maternal immune‐suppression (Barnea 2004; Paidas et al. 2010; Stamatkin et al. 2011). PIF exerts its immune function on the maternal system directly on endometrial and immune cells; PIF up‐regulates human decidual cell genes including interleukin 6 (IL‐6) and IL‐1β and changes expression at the protein level (Paidas et al. 2010). Due to its immune‐modulatory function, PIF is therefore a potential novel anti‐inflammatory.
An optimised and validated tissue explant culture system using equine endometrium provides an exemplar for bacteria‐induced MIE (Nash et al. 2008). Explants respond to E. coli‐derived lipopolysaccharide (LPS) challenge by secreting the inflammatory marker prostaglandin F2α (PGF2α) similarly to the whole animal and is integral to physiological reproductive function as well as inflammation (Nash et al. 2008, 2010).
The aim of this preliminary study was to determine the effect of synthetic PIF (sPIF) on the innate immune response to LPS challenge using equine endometrial explants collected from mares at different stages of the oestrous cycle, using PGF2α as the inflammatory marker.
Materials and methods
All experiments used uteri from mares sent to slaughter at a commercial abattoir for reasons unrelated to the study. Therefore, ethics approval was not required.
Strips of endometrium were collected from 17 slaughtered mares post‐mortem (aged 10.3 years ±1.25) at follicular (n = 3), luteal (n = 6), anoestrous (n = 4) and transition (n = 4) stages of cycle, assessed as previously described (Nash et al. 2008). Uteri were harvested from mares presented at a commercial abattoir for reasons unrelated to this study. Uteri were placed on ice and transported to the laboratory. The uterine body was opened with a scalpel and the endometrium dissected from underlying myometrium using sterile scissors and forceps. Strips were chopped to 1 mm3 and washed in HBSS (Hank's buffered salt solution). Sterile lens tissue‐covered aluminium mesh platforms were placed into each well of a six‐well plate (Corning Costar). A quantity of 150 mg endometrial tissue was weighed onto each platform and 4.25 mL of supplemented William's medium (Sigma) added as previously described (Nash et al. 2008). Tissues were incubated for 24 h in a humidified atmosphere at 37°C and 5% carbon dioxide (CO2). After 24 h, the medium was replaced with fresh, growth factor‐free medium alone (control) or treatment medium (designated time point 0 h). Explants from each horse were cultured in triplicate for each treatment. Treatments were: medium alone (control); 50 nmol/L sPIF; 100 nmol/L sPIF; 3.0 μg/mL LPS from E. coli strain 0111:B4 (Source Bioscience); 3.0 μg/mL LPS with 50 or 100 nmol/L sPIF; 100 nmol/L scrambled PIF (PIFscr; same amino acid composition arranged in a different order, routinely used as a control measure in other published work in this field (Barnea et al. 2012; Roussev et al. 2013). Culture supernatants were collected 24 and 72 h after treatment, representing the acute and persistent inflammatory periods respectively. The sPIF concentrations chosen were those found to affect human endometrium in culture (Paidas et al. 2010). Culture supernatants were radio immunoassayed for PGF2α.
Serum progesterone concentrations were measured from blood collected at exsanguination was measured using a solid phase ELISA kit (Immunodiagnostic Systems) to retrospectively confirm the stage of oestrous cycle as previously described (Nash et al. 2008). Briefly, coupled with morphological assessment of the ovarian and cervical characteristics, stage of cycle was defined where progesterone concentrations were: <1 ng/mL, transition and anoestrous stage; <2 ng/mL, follicular stage; >2 ng/mL luteal stage.
PGF2α secretion by treated explants was expressed as percentage change from control (±SEM) to account for individual mare variation. Data were square root transformed to achieve normality. A univariate general linear model was used to compare PGF2α secretion between control and treated explants and Dunnett's post hoc test was used in SPSS v22. Significance was attributed when P < 0.05.
Results
Synthetic sPIF alone and PIFscr did not stimulate PGF2α secretion by explants from any stage of the oestrous cycle after 24 or 72 h. After 24 h, LPS treated explants secreted significantly more PGF2α than control tissue (follicular and luteal stages, P < 0.001; Figs 1a and 2a). The inflammatory response to LPS by follicular stage explants was abrogated in the presence of 100 nmol/L sPIF at 24 h (PGF2α concentrations were not significantly different to the control; Fig. 1a). There was a significant difference between PGF2α secreted by explants treated with LPS alone compared to LPS and 50 nmol/L PIF (P < 0.05; Fig. 1a). There was no effect of any treatment on explants collected from follicular or luteal mares after 72 h (Figs 1b and 2b), or after 24 or 72 h for transition or anoestrous explants (data not shown).
Figure 1.
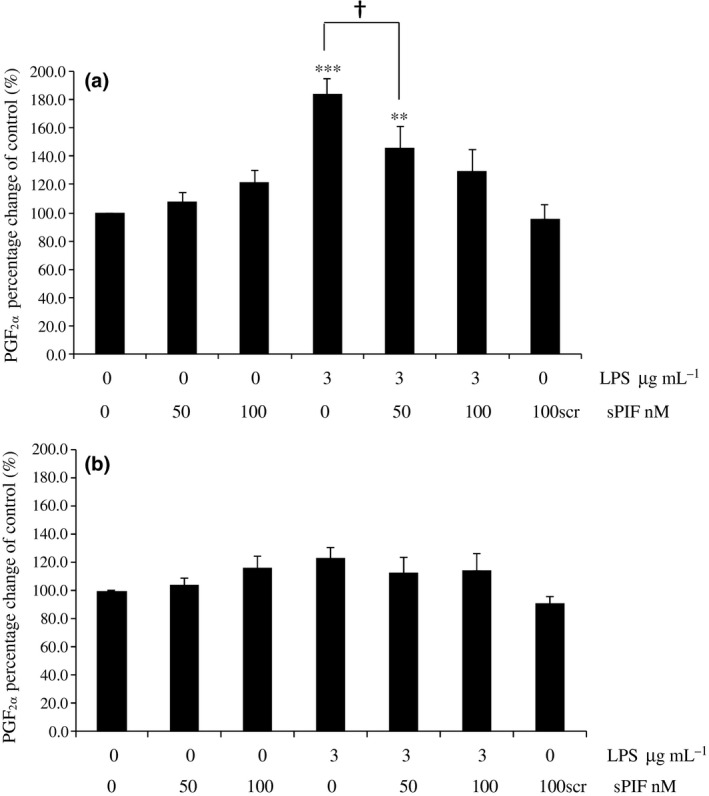
Secretion of PGF 2α by follicular stage equine endometrial explants treated with control (media only), 50 nmol/L PIF, 100 nmol/L sPIF, 3.0 μg/mL LPS, 3.0 μg/mL LPS with 50 or 100 nmol/L PIF or 100 nmol/L scrambled PIF (PIFscr). The PGF 2α concentrations were measured (a) 24 h (acute inflammatory period) or (b) 72 h (chronic inflammatory period) after treatment. Values differ from control, *P < 0.05; **P < 0.01; ***P < 0.001. Values differ between treatments, † P < 0.05.
Figure 2.
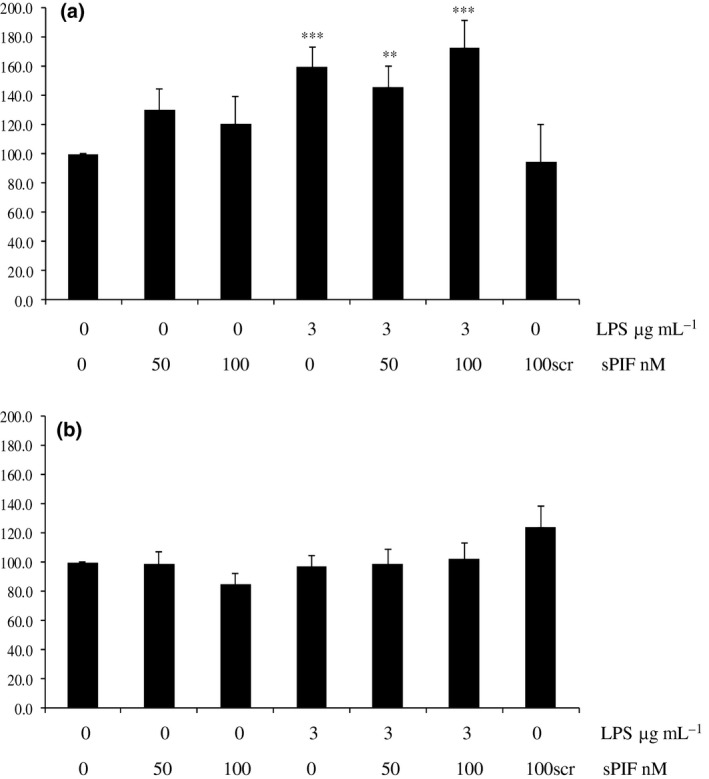
Secretion of PGF 2α by luteal stage equine endometrial explants treated with control (media only), 50 nmol/L PIF, 100 nmol/L sPIF, 3.0 μg/mL LPS, 3.0 μg/mL LPS with 50 or 100 nmol/L PIF or 100 nmol/L scrambled PIF (PIFscr). The PGF 2α concentrations were measured (a) 24 h (acute inflammatory period) or (b) 72 h (chronic inflammatory period) after treatment. Values differ from control, **P < 0.01; ***P < 0.001.
Discussion
This preliminary study demonstrated that 100 nmol/L sPIF abrogated PGF2α in follicular stage explants 24 h after challenge with bacterial LPS. The ability of PIF to abrogate PGF2α during the follicular, but not luteal, stage of the cycle is biologically important as this is the time when mating, and therefore, bacterial challenge occurs. Therefore, PIF may interact with the native steroid milieu of endometrium; endogenous progesterone promotes immune‐suppression but concentrations are low during the follicular stage (Watson et al. 1987). Neither PIF alone (either 50 or 100 nmol/L) nor PIFscr caused an increase of PGF2α compared to controls in explants derived from all stages of the oestrous cycle, demonstrating that PIF itself did not inadvertently cause inflammation, at least in terms of prostaglandin response.
A treatment of LPS combined with PIFscr as a further control was not included in this study. A PGF2α response greater than the concentrations observed for the LPS treatment alone was unlikely to be secreted in response to an LPS‐PIFscr combination without the addition of arachidonic acid to the medium. Arachidonic acid is a substrate for prostaglandin synthesis and has been shown in work by colleagues to be necessary to add to culture medium when PGF2α secretion may otherwise reach a plateaux and so maximal concentrations not achieved (Herath et al. 2006). The present study was confident that the LPS concentrations used would not exceed this plateaux based on previous optimisation studies and yet would still enable an effect of LPS and in turn its abrogation to be observed (Nash et al. 2008), and so arachidonic acid was not necessary to achieve the aim of this study. Furthermore, to maintain explants in a manner that was as close to the whole animal as possible, avoiding further medium supplementation was desirable.
It is interesting that 100 nmol/L sPIF was effective at 24 h and not 50 nmol/L. Approximately 100 nmol/L PIF is the physiological concentration measured during normal pregnancy in women (Duzyj et al. 2010). sPIF of either concentration was not effective at 72 h. The medium was not replaced after initial treatment, so PIF activity may have been effective up to 24 h but not maintained thereafter. PIF stability is unconfirmed in tissue culture. However, there was also no PGF2α response to LPS alone at the chronic, 72 h time point following challenge, which disagrees with Nash et al. (2008) where the model was reported as functional in response to LPS up to 72 h. Additionally, LPS induced inflammation was evident at 24 h only in follicular stage explants, disagreeing with Nash et al. (2008) who reported an effect only in transition mares. The disparity between studies may be partly explained by the possibility that the response may have finished by 72 h and that improvements in laboratory practice since the model was first validated and optimised have generated data in the present study that are more representative of the whole animal response (Nash et al. 2008). Nonetheless, PIF has demonstrated some anti‐PGF2α properties, but its wider anti‐inflammatory potential to manage PMIE in horses requires further study.
Source of funding
Jennifer Paddison was supported by an Aberystwyth University IBERS Studentship. IBERS receives strategic funding from BBSRC.
Conflict of interest
The authors declare they have no competing interests.
Ethical statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as endometrial tissue was harvested from animals sent to commercial slaughter, for reasons unrelated to this study.
Contributions
DMN participated in study design, data analysis and wrote the manuscript. JP conducted laboratory work. MCGD contributed to writing the manuscript. ERB instigated the study.
Ackowledgements
The authors thank Prof. Claire Wathes and Dr Zhangrui Cheng (The Royal Veterinary College, UK) for kind donation of the PGF2α antisera.
References
- Allen W.E. & Pycock J.F. (1989) Current views on the pathogenesis of bacterial endometritis in mares. Veterinary Record 125, 298–301. [DOI] [PubMed] [Google Scholar]
- Barnea E.R. (2004) Insight into early pregnancy events: the emerging role of the embryo. American Journal of Reproductive Immunology 51, 319–322. [DOI] [PubMed] [Google Scholar]
- Barnea E.R., Kirk D., Ramu S., Rivnay B., Roussev R. & Paidas M.J. (2012) PreImplantation Factor (PIF) orchestrates systemic antiinflammatory response by immune cells: effect on peripheral blood mononuclear cells. American Journal of Obstetrics and Gynecology 207, 313 e311‐313 e311. doi:S0002‐9378(12)00754‐5 [pii]. 10.1016/j.ajog.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Bucca S., Carli A., Buckley T., Dolci G. & Fogarty U. (2008) The use of dexamethasone administered to mares at breeding time in the modulation of persistent mating induced endometritis. Theriogenology 70, 1093–1100. [DOI] [PubMed] [Google Scholar]
- Christoffersen M. & Troedsson M. (2017) Inflammation and fertility in the mare. Reproduction in Domestic Animals 52(Suppl 3), 14–20. 10.1111/rda.13013. [DOI] [PubMed] [Google Scholar]
- Duzyj C.M., Barnea E.R., Li M., Huang S.J., Krikun G. & Paidas M.J. (2010) Preimplantation factor promotes first trimester trophoblast invasion. American Journal of Obstetrics and Gynecology 203, 402 e401‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumuso E.A., Aguilar J., Giguere S., Rivulgo M., Wade J. & Rogan D. (2007) Immune parameters in mares resistant and susceptible to persistent post‐breeding endometritis: effects of immunomodulation. Veterinary Immunology and Immunopathology 118, 30–39. [DOI] [PubMed] [Google Scholar]
- Gores‐Lindholm A.R., LeBlanc M.M., Causey R.C., Hitchborn A., Fayrer‐Hosken R.A., Kruger M. & Ahlschwede S. (2013) Relationships between intrauterine infusion of N‐acetylecysteine, equine endometrial pathology, neutrophil function, post‐breeding therapy and reproductive performance. Theriogenology 80, 218–227. [DOI] [PubMed] [Google Scholar]
- Herath S., Fischer D.P., Werling D., Williams E.J., Lilly S.T., Dobson H. et al (2006) Expression and function of Toll‐like receptor 4 in the endometrial cells of the uterus. Endocrinology 147, 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.P. & Loy R.G. (1975) The relation of infection to infertility in the mare and stallion. Equine Veterinary Journal 7, 155–159. [DOI] [PubMed] [Google Scholar]
- Katila T. (2001) Sperm‐uterine interactions: a review. Animal Reproduction Science 68, 267–272. [DOI] [PubMed] [Google Scholar]
- LeBlanc M.M. (2010) Advances in the diagnosis and treatment of chronic infectious and post‐mating‐induced endometritis in the mare. Reproduction in Domestic Animals 45(Suppl 2), 21–27. [DOI] [PubMed] [Google Scholar]
- Melkus E., Witte T., Walter I., Heuwieser W. & Aurich C. (2013) Investigations on the endometrial response to intrauterine administration of N‐acetylcysteine in oestrous mares. Reproduction in Domestic Animals 48, 591–597. 10.1111/rda.12131. [DOI] [PubMed] [Google Scholar]
- Nash D., Lane E., Herath S. & Sheldon I. (2008) Endometrial explant culture for characterizing equine endometritis. American Journal of Reproductive Immunology 59, 105–117. [DOI] [PubMed] [Google Scholar]
- Nash D.M., Sheldon I.M., Herath S. & Lane E.A. (2010) Markers of the uterine innate immune response of the mare. Animal Reproduction Science 119, 31–39. [DOI] [PubMed] [Google Scholar]
- Paidas M.J., Krikun G., Huang S.J., Jones R., Romano M., Annunziato J. & Barnea E.R. (2010) A genomic and proteomic investigation of the impact of preimplantation factor on human decidual cells. American Journal of Obstetrics and Gynecology 202, 459 e451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock J.F. & Newcombe J. (1996) The relationship between intraluminal fluid, endometritis, and pregnancy rate in the mare. Equine Practice 18, 19–22. [Google Scholar]
- Roussev R.G., Donskoi B.V., Stamatkin C., Ramu S., Chernyshov V.P., Coulam C.B. & Barnea E.R. (2013) Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: implications for recurrent pregnancy loss therapy. Reproductive Biomedicine Online 26, 79–87. doi:S1472‐6483(12)00588‐3 [pii]. 10.1016/j.rbmo.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Stamatkin C.W., Roussev R.G., Stout M., Absalon‐Medina V., Ramu S., Goodman C. et al (2011) PreImplantation Factor (PIF) correlates with early mammalian embryo development‐bovine and murine models. Reproductive Biology and Endocrinology 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E.D., Stokes C.R., David J.S. & Bourne F.J. (1987) Effect of ovarian hormones on promotion of bactericidal activity by uterine secretions of ovariectomized mares. Journal of Reproduction and Fertility 79, 531–537. [DOI] [PubMed] [Google Scholar]
- Wingfield Digby N.J. & Ricketts S.W. (1982) Results of concurrent bacteriological and cytological examinations of the endometrium of mares in routine stud farm practice 1978‐1981. Journal of Reproduction and Fertility Supplement 32, 181–185. [PubMed] [Google Scholar]
- Woodward E.M., Christoffersen M., Horohov D., Squires E.L. & Troedsson M.H. (2015) The effect of treatment with immune modulators on endometrial cytokine expression in mares susceptible to persistent breeding‐induced endometritis. Equine Veterinary Journal 47, 235–239. 10.1111/evj.12266. [DOI] [PubMed] [Google Scholar]


