Abstract
Tick‐borne relapsing fever (TBRF) caused by the bacteria Borrelia, is poorly documented in veterinary medicine. Given the widespread presence of the soft tick vectors – Ornithodoros and the recently discovered hard tick vectors, as well as their close association with animal hosts, it is highly likely that infection occurs, but is rarely reported to be of veterinary importance. Sporadic reports of canine infection, some being fatal through to probable cause of abortion in horses have been published. Some of these pathogens exist in regions where there are limited diagnostic facilities, hence, they are likely to be missed and their impact on productivity may be unquantified. Here we review available literatures on cases of TBRF in domestic and wild animals in order to show their potential veterinary medical impact. Future efforts using field and laboratory surveys are needed to determine pathogenesis, vector competence and distribution in animals, their impact on animal health and productivity as well as to prevent further spill to the human population, where it is already a public health problem in some parts of the world.
Keywords: spirochaetes, Borrelia, Ornithodoros, Argasid, ticks
Introduction
Borrelia species are tick‐borne, Gram‐negative, spiral‐shaped bacteria that causes several diseases across the world, grouped into Lyme borreliosis (LB) and tick‐borne relapsing fever (TBRF) (Socolovschi et al. 2009). Tick‐borne relapsing fever (TBRF) is a bacterial febrile illness caused by the spirochaete Borrelia (Vial et al. 2006). Tick‐borne relapsing fever is endemic and an important public health problem in some parts of the world. In Western Africa, the incidence of human tick‐borne relapsing fever (TBRF) is high, accounting for about 13% of febrile illnesses (Parola et al. 2011). In endemic regions of East Africa, TBRF borreliosis is one of the highest ranked causes of mortality among children (Talbert et al. 1998).
The organisms are transmitted either via saliva or in excreted coxal fluid of Ornithodoros soft ticks or some hard ticks including Ixodes and Rhipicephalus (Boophilus) species during feeding. These ticks are widely distributed in sub‐Saharan Africa, Asia, the Americas and some parts of Europe (Cutler 2006; Sato et al. 2014; Lopez et al. 2016). Ornithodoros ticks often reside within cracks and crevices of animal dwellings, feeding indiscriminately on many different kinds of animals, including humans (Breitschwerdt et al. 1994). In North America, TBRF agents are principally: Borrelia hermsii, Borrelia turicatae and Borrelia parkeri transmitted by Ornithodoros hermsi, Ornithodoros turicata and Ornithodoros parkeri respectively (Felsenfeld 1971; Dworkin et al. 2008; Lopez et al. 2016). In South America, O. brasiliensis is a potential vector of TBRF caused by Borrelia brasiliensis in the Southern Brazilian highlands (Martins et al. 2011). Ornithodorus talaje which are potential vectors of Borrelia mazzottii are also prevalent in South American countries including: Ecuador, Colombia, Venezuela, Argentina and Brazil (Guglielmone et al. 2006). Borrelia persica also referred to as the Persian relapsing fever and transmitted by Ornithodorus tholozani ticks, is the causative agent of TBRF in Central Asia and Middle Eastern countries including Iran, Israel, Egypt, Syria, Pakistan and Uzbekistan (Rebaudet & Parola 2006; Cutler et al. 2009; Elbir et al. 2014; Baneth et al. 2016). Borrelia hispanica has been reported in Spain, Portugal, Cyprus, Greece and North Africa. It has been isolated in the soft ticks, Ornithodorus erraticus (Rebaudet & Parola 2006). Borrelia caucasica, another agent of TBRF is present in Caucasus and Iraq and transmitted by Ornithodorus asperus (Rebaudet & Parola 2006). In East Africa, Ornithodorus moubata tick complex are known vector of Borrelia duttonii (Fukunaga et al. 2001; Mitani et al. 2004). Other Borrelia species documented in humans from different parts of Africa include: B. crocidurae common in Western Africa and transmitted by Ornithodorus sonrai with rodents and insectivores as reservoir host (Schwan et al. 2012). Others within Africa are B. hispanica transmitted by O. erraticus and small mammals as reservoir host (Trape et al. 2013). Borrelia anserina is the causative agent of avian spirochaetosis, with a worldwide distribution. It belongs to the relapsing fever (RF) group transmitted by Argas ticks and is not currently reported in man (Aslam et al. 2015).
A separate category of TBRF Borrelia are those transmitted by hard ticks such as Ixodid, Rhipicephalus and Amblyomma ticks, hereafter referred to as hard‐bodied tick‐borne relapsing fever (hTBRF) Borreliae. New species of Borrelia (B. miyamotoi) transmitted by hard‐bodied (Ixodid) tick species that transmit the Lyme borreliosis have been recently reported to cause relapsing fever in some humans in North America, Asian region of Russia, Europe and Japan (Platonov et al. 2011; Sato et al. 2014; Krause et al., 2015). Borrelia theileri is also a member of the hTBRF that causes spirochaetosis in cattle, sheep and goats (McCoy et al. 2014). In addition, there are other hard ticks such as Rhipicephalus and Amblyomma spp that have shown competence to serve as vectors of the relapsing fever Borrelia. For example, a study carried in Nigeria to determine pathogen prevalence in ticks reported 0.4% prevalence for Borrelia species in questing Rhipicephalus evertsi ticks (the vector of bovine borreliosis). Although, sequence obtained from the 16S rRNA gene in that study from Nigeria for Borrelia species identification was unsuccessful, it showed 99% nucleotide homology to B. burgdorferi sensu lato (the Lyme disease pathogen), thus may possibly belong to a presently unclassified Borrelia species (Reye et al. 2012). The ability of hard ticks to transmit relapsing fever Borrelia has further expanded the potential geographical range of relapsing fever Borrelia group and stimulated much current research interest.
Many animals are potential reservoirs and final hosts for TBRF infection (Piccione et al. 2016). Natural vertebrate reservoirs of relapsing Borrelia include wild birds, rodents, chipmunks, squirrels, rabbits, owls and lizards (Breitschwerdt et al. 1994; Hamer et al. 2012). Both rodents and birds have been confirmed to act as reservoir hosts of B. miyamotoi (Wagemakers et al. 2017). A study carried out in Japan reported seabirds as potential reservoir hosts for relapsing fever because Borrelia spp closely related to B. turicatae was isolated from seabird tick vectors (Takano et al. 2009); and Carios ticks from bat in the USA (Schwan et al. 2009). Although TBRF is most likely under recognized and under diagnosed in veterinary medicine (Piccione et al. 2016), naturally occurring spirochaete infections have been detected in the blood of a variety of mammals, including squirrel monkeys, opossums, and armadillos, calves and horses (Dunn & Clark 1933; Lopez et al. 2016). The impact that these Borrelia spirochaetes have on the health of wild and domestic animals is largely understudied compared to the disease in humans (Schwan et al. 2005). This review compiles a comprehensive information of TBRF in various animal species around the world with the aim of identifying why it is an important differential in managing febrile illnesses in veterinary practice. In addition, we report the likelihood of its emergence in areas previously thought to be free of the disease especially with increasing close interaction between man and animals either from their domestication, encroachment on bushes, gaming and ranching.
Tick‐borne relapsing fever in dogs and cats
Tick‐borne relapsing fever is a potentially fatal infection in pet animals such as dogs and cats (Baneth et al. 2016). Dogs are most likely to be fed upon by Borrelia‐infected Ornithodoros ticks when they sleep in tick‐infested cabins or while foraging in excavated, or underground burrows or caves (Kelly et al. 2014). Other likely sources of infection to dogs and cats are animal cages or cardboard boxes used to transport animals from suburban areas to cities for sale. These cages may harbour minute infected larval ticks and/or early stage nymphs that might be transferred with them thus serving as source of infections to pet animals and humans (Shirani et al. 2016). In 1939, Brumpt and Brumpt demonstrated that a 3‐week‐old dog was susceptible to infection with B. turicatae when fed upon by 10 relapsing fever‐infected O. turicata ticks (Brumpt & Brumpt 1939). More recently there has been confirmed reports of TBRF in canines from United States (Whitney et al. 2007; Kelly et al. 2014; Piccione et al. 2016). In addition, recent case reports from Israel and Iran in dogs with clinical manifestation resembling TBRF were confirmed using molecular methods to be infected with B. persica (Baneth et al. 2016; Shirani et al. 2016). More recently, there have also been confirmed reports of Borrelia persica as the cause of relapsing fever in cats (Schwarzer et al. 2015; Baneth et al. 2016). Infection of dogs and cats can, however, be complicated with co‐infection with other haemoparasites such as Babesia (Baneth et al. 2016). In the United State, B. turicatae, B. hermsii were the predominant cause of TBRF in dogs, these species of Borrelia are also the most important affecting humans in those areas (Kelly et al. 2014; Piccione et al. 2016). There are currently no reports of other species of relapsing fever Borrelia (B. crocidurae, B. duttonii and B. hispanica) causing pathogenic illnesses in dogs in Africa, where there are endemic foci of infection, and are pathogenic to human. This may warrant specific studies to determine the presence or absence of the disease in dogs because of the close interaction of dog with human and the reports of tick vectors in human dwellings where dogs are also resident.
Clinical presentation in dogs although nonspecific includes pyrexia, possible lethargy, anorexia, neurological signs (ataxia, tail tucking and cranial nerve deficits) (Piccione et al. 2016). Other common signs reported in dogs are fever, ambulation or postural defects (arched back, lameness), anorexia/weight loss and ocular lesions such as uveitis, corneal oedema (Breitschwerdt et al. 1994; Kelly et al. 2014). There is also report of persistent hind limb weakness and pain reported by Piccione et al. (2016), as well as lameness in the left rear leg with swollen stifle joint reported post treatment (Breitschwerdt et al. 1994). Haematological abnormalities in infected dogs include microcytic, normo‐chromic anaemia, slight poikilocytosis and severe thrombocytopenia (Breitschwerdt et al. 1994). The clinical signs in cats is similar to those reported in dogs and include mainly anorexia, lethargy, pale mucous membrane as a result of anaemia, some with icterus and haematology showing thrombocytopenia (Baneth et al. 2016).
Tick‐borne relapsing fever in birds
Borrelia anserina is the causative agent of avian spirochaetosis. It is transmitted by the soft tick, Argas and manifests in birds as fever, ruffled feather, inappetence and greenish diarrhoea (Ataliba et al. 2007). An experimental study to determine the clinical manifestation of avian spirochaetosis due to B. anserina in Sudan, reported clinical signs including pyrexia, dullness, ruffled feather, weight loss, drop in egg production and paleness of comb and wattles (Nasri et al. 2010). Avian spirochaetosis presents a potential economic problem in places like Africa, where poultry are an important source of protein. A study carried out in Ethiopia, Argas persicus ticks were found to carry B. anserina in 7.5% of A. persicus tick pools (Cutler et al. 2012). In Nigeria, clinical cases of avian spirochaetosis have been reported (Sa'idu et al. 1995). Domestic chicken was shown to be a naturally suitable host for Borrelia with an infection rate of 11% reported in Tanzania, where B. duttonii is a common human infection (McCall et al. 2007). In North America, Schwan et al. (2007) successfully produced spirochaetaemia in chicken by inoculating B. hermsii subcutaneously (Schwan et al. 2007).
Tick‐borne relapsing fever in cattle
Borrelia theileri transmitted by hard‐bodied ticks including Rhipicephalus (Boophilus) is the causative agent of bovine borreliosis. The disease has also been reported from cattle, sheep and horses from several countries in Africa, South America, Europe and Australia (Uilenberg et al. 1988; Bishop 1994; McCoy et al. 2014). It is often associated with babesiosis. The clinical signs observed include fever, haemoglobinuria, loss of appetite, diarrhoea, pale mucous membranes, enlarged superficial lymph nodes and rough hair coats (Sharma et al. 2000). However, the author in that study could not determine if the clinical was as a result of co‐infection of Borrelia and Babesia. Clinically infection is usually benign hence underreported especially if there is mixed infection.
Tick‐borne relapsing fever in pigs
Although no clinical case has been reported so far, ticks that are vectors of Borrelia have been reported in pigpens ((McCall et al. 2007). Relapsing fever Borrelia that shared great homology with B. duttonii has been reported in domestic pigs with a prevalence rate of 8.9% in Tanzania (McCall et al. 2007). In Europe, O. erraticus, the soft tick vector of human B. hispanica is usually found in traditionally raised pig herd that are allowed to forage in non‐intensive systems (Palma et al. 2011). The role of pigs as potential reservoir hosts of the disease as well as clinical importance would warrant further studies.
Tick‐borne relapsing fever in horses
There was a previous report of equine abortion due to TBRF spirochaete (B. parkeri) for which the mare might have been an incidental host for an infected tick (Walker et al. 2002). A past case–control study reported that TBRF Borrelia species in Africa is associated with high perinatal mortality of about 436/1000 births and abortion in pregnant humans which may be as a result of trans‐placental transmission of the pathogen (Jongen et al. 1997). Similar pathogenesis on the abortifacient ability of TBRF in humans might explain the equine abortion reported in that case report. Further epidemiological studies bearing in mind TBRF as a probable cause of equine abortion in endemic areas is suggested.
Tick‐borne relapsing fever in wildlife
Relapsing fever Borreliae has been isolated from wild animals in areas where human outbreaks were reported and tick vectors present. Wild birds have been reported to maintain and move ticks and Borrelia pathogens by serving as pathogen reservoir hosts (Hamer et al. 2012; Wagemakers et al. 2017). Borrelia miyamotoi has been reported in wild turkey (Meleagris gallopavo) with a very high prevalence of 58% (Scott et al. 2010). In a study carried out in North America to characterize Borrelia species from argasid bat tick, Carios kelleyi, the isolates although genetically distinct but were closely related to B. turicatae and B. parkeri species of tick‐borne relapsing fever spirochaetes (Gill et al. 2008). Furthermore, Borrelia hermsii has also been detected in blood of mule deer during surveillance (Nieto et al. 2012); and was isolated from chipmunks (Tamias umbrinus), during human disease outbreak investigation of relapsing fever in United States (Trevejo et al. 1998; Christensen et al. 2015).
However, there was a recent discovery of a relapsing fever spirochaete causing fatal borreliosis in an infected juvenile female bat (Pipistrellus species) in the United Kingdom. A PCR‐based analysis targeting the flaB and glpQ gene fragments showed that the causative agent had close similarities with the relapsing fever Borrelia previously reported in Africa – a cluster containing B. recurrentis, B. duttonii and B. crocidurae (Evans et al. 2009). Also, suspected fatal borreliosis in Northern spotted owl has been reported in United States, the Borelia organisms isolated had 99.6% similarities with B. hermsii which is one of the agents of human borreliosis in United States (Fischer et al. 2009). The Northern spotted owls prey on small rodents (dusky‐footed and bushy‐tailed woodrats, northern flying squirrels and red tree voles) which are documented reservoir of relapsing fever Borrelia and might have been the source of infection (Thomas et al. 2002). Other sources of infection such as through consumption of brains of TBRF infected animals by wild animals have been documented in experimental models (Horrenberger 1955). A relapsing fever Borrelia was also isolated from the endangered African penguin, Spheniscus demersus. In that study, one bird was believed to have died of borreliosis based on gross, microscopic lesions and analysis of partial flaB gene sequences – which is specific to relapsing fever Borrelia (Yabsley et al. 2012).
Although most of the studies in wildlife documented their importance as reservoir hosts, the few fatal cases due to borreliosis such as reported in owl, penguin and bat shows that TBRF may be an important disease of wildlife species that might be missed or underreported. Efforts should be made to understand the disease epidemiology in these group of animals as they may be important reservoir hosts infecting human and domestic animal population.
Diagnosis and treatment of tick‐borne relapsing fever
Diagnosis of TBRF in human is made by microscopic examination of Giemsa‐Wright stained blood smears during acute spirochaetaemia (Lopez et al. 2016), and has been widely carried out to detect the presence of the spirochaetes in animal blood samples (Baneth et al. 2016). Serological assays to detect Borrelia immunogenic protein A (BipA) and glycerophosphodiester phosphodiesterase (GlpQ) antigen have also been carried out and have shown usefulness in differentiating RF Borrelia and Lyme disease group, although caution should be taken in interpreting results as current or past infection (Schwan et al. 1996; Lopez et al. 2013). PCR‐based analysis of specific gene regions such as 16S rRNA, flaB and glpQ genes fragments has been effective in diagnoses and speciation of RF Borrelia (Schwan et al. 2005; Piccione et al. 2016). Although there are no specific diagnostic methods for TBRF in animals, these methods listed above have been employed in confirming suspected animal cases or during surveillance studies. Examination of Wright‐Giemsa stained blood smears has been shown to provide a rapid diagnosis of TBRF in dogs as only relapsing fever Borrelia causes spirochaetaemia that is detectable in blood smear (Breitschwerdt et al. 1994; Piccione et al. 2016). Other spirochaetes that are of veterinary importance include, Leptospira spp, Brachyspira spp. and Borrelia burgdorferi sensu lato (agent of Lyme disease), and they should be considered as differentials in animals presenting with similar clinical signs (Piccione et al. 2016). Molecular‐based methods have also been useful in confirming the species of relapsing fever Borrelia in past studies involving animals (Schwan et al. 2007; Evans et al. 2009; Takano et al. 2009).
Drugs used in treatment of human TBRF have shown usefulness in treating canine TBRF. There are very few information on specific treatment of TBRF in other species of animals. Doxycycline administered orally at the dose of 7.5 mg/kg for 6 weeks (Piccione et al. 2016) or doxycycline (200 mg q 12 h) and amoxicillin (400 mg q 12 h) for 14 days (Kelly et al. 2014) has been successfully used to treat TBRF in dogs. Tetracycline administered at the dose of 1 g every 8 h orally for 2 weeks, and triple antibiotic‐corticosteroid ophthalmic ointment every 6 h has also been successfully used in treating infected dogs (Breitschwerdt et al. 1994). Rapid recovery has been reported in cats treated with amoxicillin/clavulanic acid combination or with doxycycline and others treated with a combination of amoxicillin/clavulanic acid with long‐acting injectable tetracycline (Baneth et al. 2016). In case of avian spirochaetosis, clinically sick birds have been successfully treated with procaine penicillin, whereas those infected with A. persicus ticks were dusted with organophosphorus compound (Asuntol [coumaphos]) or carbamate (Kartzimet 20). Although Jarisch–Herxheimer reaction has been documented in humans following antibiotic treatment for RF borreliosis, so far there is no evidence of such reactions in animals (Baneth et al. 2016). However, this may be due to lack of adequate documentation of the disease in veterinary medicine, clinicians should therefore monitor closely animals being treated for RF borreliosis. It is also recommended that the poultry housing be treated for tick infestation (Sa'idu et al. 1995).
Conclusions
Although a number of studies have been carried out on hTBRF such as B. theileri in animals and world widely distributed B. anserina, majority of studies on other soft ticks TBRF in animals have are very few – summarized in Fig. 1. Some of these are case reports majorly in dogs. There are no reports of either suspect or confirmation of soft ticks TBRF in domestic animal population from most parts of the world. However, there are several reports of the TBRF in humans perhaps because it is a public health problem. With the existence of tick vectors in most places in Africa and across the world, TBRF is a likely animal health problem, should be investigated for, and included as differential in febrile case management that have accompanying similar clinical symptoms. In addition, further studies on the epidemiology of TBRF in domestic and indeed wildlife is suggested because of human co‐habitation with animals and their associated tick vectors could pose further public health risk.
Figure 1.
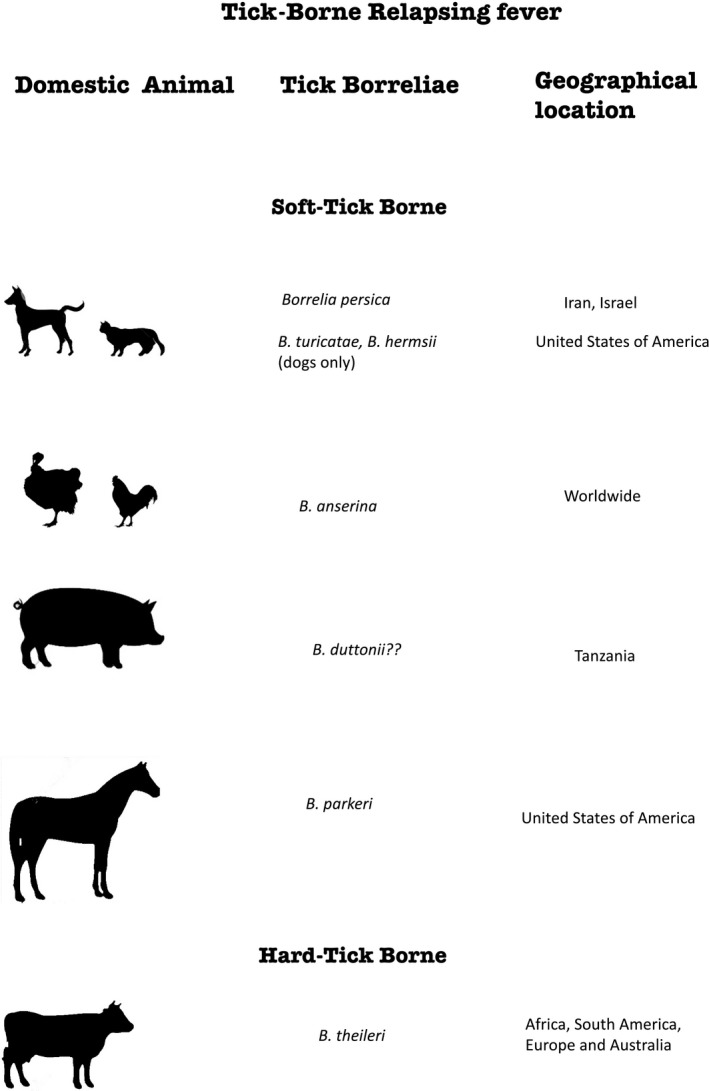
Tick‐borne relapsing fever borreliae of domestic animals and their geographical locations.
Conflict of interest
None to declare.
Ethical statement
No ethical approval was required as this is a review article with no original research data.
Acknowledgement
The author would like to acknowledge Prof Sally J. Cutler for providing mentorship and for correcting the manuscript.
References
- Aslam B., Hussain I., Zahoor M.A., Mahmood M.S. & Rasool M.H. (2015) Prevalence of Borrelia anserina in Argas ticks. Pakistan Journal of Zoolology 47, 1125–1131. [Google Scholar]
- Ataliba A.C., Resende J.S., Yoshinari N. & Labruna M.B. (2007) Isolation and molecular characterization of a Brazilian strain of Borrelia anserina, the agent of fowl spirochaetosis. Research in Veterinary Science 83, 145–149. [DOI] [PubMed] [Google Scholar]
- Baneth G., Nachum‐Biala Y., Halperin T., Hershko Y., Kleinerman G., Anug Y. et al (2016) Borrelia persica infection in dogs and cats: clinical manifestations, clinicopathological findings and genetic characterization. Parasites & Vectors 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.C. (1994) Borrelia theileri infection In: Infectious diseases of livestock with special reference to Southern Africa. (eds Coetzer J.A.W., Thomson G.R. & Tustin R.C.), Oxford University Press: Oxford. [Google Scholar]
- Breitschwerdt E.B., Nicholson W.L., Kiehl A.R., Steers C., Meuten D.J. & Levine J.F. (1994) Natural infections with Borrelia spirochaetes in two dogs from Florida. Journal of Clinical Microbiology 32, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumpt E. & Brumpt L.C. (1939) Identité du spirochete des fièvres récurrentes a tiques des plateaux mexicains et du Spirochaeta turicatae agent de la fièvre recurrente sporadique des Etats‐Unis. Annales De Parasitologie Humaine Et Comparee 17, 287–298. [Google Scholar]
- Christensen J., Fischer R.J., McCoy B.N., Raffel S.J. & Schwan T.G. (2015) Tick borne relapsing fever, Bitterroot Valley, Montana, USA. Emerging Infectious Diseases 21, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.J. (2006) Possibilities for relapsing fever reemergence. Emerging Infectious Diseases 12, 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.J., Abdissa A. & Trape J.F. (2009) New concepts for the old challenge of African relapsing fever borreliosis. Clinical Microbiology and Infection 15, 400–406. [DOI] [PubMed] [Google Scholar]
- Cutler S., Abdissa A., Adamu H., Tolosa T. & Gashaw A. (2012) Borrelia in Ethiopian ticks. Ticks and tick‐borne diseases 3, 14–17. [DOI] [PubMed] [Google Scholar]
- Dunn L.H. & Clark H.C. (1933) Notes on relapsing fever in Panama with special reference to animal hosts. American Journal of Tropical Medicine and Hygiene 13, 201–209. [Google Scholar]
- Dworkin M.S., Schwan T.G., Anderson D.E. & Borchardt S.M. (2008) Tick‐borne relapsing fever. Infectious Disease Clinicsof North America 22, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri E. L., Iman M. & Shigidi M. (2010) Pathology of domestic fowl spirochaetosis in different age groups of chicken experimentally infected with Borrelia anserine . The Sudan Journal of Veterinary Research 25, 23–28. [Google Scholar]
- Elbir H., Larsson P., Normark J., Upreti M., Korenberg E., Larsson C. & Bergström S. (2014) Genome sequence of the Asiatic species Borrelia persica . Genome Announcements 2, e01127–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N.J., Bown K., Timofte D., Simpson V.R. & Birtles R.J. (2009) Fatal borreliosis in bat caused by relapsing fever spirochaete, United Kingdom. Emerging Infectious Diseases 15, 1331–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld O. (1971) Borrelia: strains, vectors, human and animal borreliosis. Warren H Green Inc: St Louis (MO). [Google Scholar]
- Fischer R.J., Johnson T.L., Raffel S.J. & Schwan R.G. (2009) Identical strains of Borrelia hermsii in mammal and bird. Emerging Infectious Diseases 15, 2064–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M., Ushijima Y., Aoki Y. & Talbert A. (2001) Detection of Borrelia duttonii, a tick‐borne relapsing fever agent in central Tanzania, within ticks by flagellin gene‐based nested polymerase chain reaction. Vector Borne and Zoonotic Diseases 1, 331–338. [DOI] [PubMed] [Google Scholar]
- Gill J.S., Ullmann A.J., Loftis A.D., Schwan T.G., Raffel S.J., Schrumpf M.E. & Piesman J. (2008) Novel relapsing fever spirochaete in bat tick. Emerging Infectious Disease 14, 522–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmone A.A., Beati L., Barros‐Battesti D.M., Labruna M.B., Nava S., Venzal J.M. et al (2006) Ticks (Ixodidae) on humans in South America. Experimental and Applied Acarology 40, 83–100. [DOI] [PubMed] [Google Scholar]
- Hamer S.A., Hickling G.J., Keith R., Sidge J.L., Walker E.D. & Tsao J.I. (2012) Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan. USA. Parasites and Vectors 5, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrenberger R. (1955) Transmission expe′rimentale de Spirochaeta hispanica au chien par morsure de rat. Comptes Rendu Societe Biologie 149, 1432–1444. [PubMed] [Google Scholar]
- Jongen V.H., van Roosmalen J., Tiems J., Van Holten J. & Wetsteyn J.C. (1997) Tick‐borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstetricia et Gynecologica Scandinavica 76, 834–838. [DOI] [PubMed] [Google Scholar]
- Kelly A.L., Raffel S.J., Fischer R.J., Bellinghausen M., Stevenson C. & Schwan T.G. (2014) First isolation of the relapsing fever spirochete, Borrelia hermsii, from a domestic dog. Ticks and Tick‐Borne Diseases 5, 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P.J., Fish D., Narasimhan S. & Barbour A.G. (2015) Borrelia miyamotoi infection in nature and in humans. Clinical Microbiology and Infection 21, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.E., Wilder H.K., Boyle W., Drumheller L.B., Thornton J.A., Willeford B. et al (2013) Sequence analysis and serological responses against Borrelia turicatae BipA, a putative species‐specific antigen. PLoS Neglected Tropical Diseases 7, e2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.E., Krishnavahjala A., Garcia M.N. & Bermudez S. (2016) Tick‐borne relapsing fever spirochetes in the Americas. Veterinary Sciences 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins J.R., Doyle R.L., Barros‐Battesti D.M., Onofrio V.C. & Guglielmone A.A. (2011) Occurrence of Ornithodoros brasiliensis Aragas (Acari: Argasidae) in Sao Francisco de Paula, RS, Southern Brazil. Neotropical Entomology 40, 143–144. [DOI] [PubMed] [Google Scholar]
- McCall P.J., Hume J.C.C., Motshegwa K., Pignatelli P., Talbert A. & Kisinza W. (2007) Does tick‐borne relapsing fever have an animal reservoir in East Africa? Vector‐Borne and Zoonotic Diseases 7, 659–666. [DOI] [PubMed] [Google Scholar]
- McCoy B.N., Maïga O. & Schwan T.G. (2014) Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks and Tick‐Borne Diseases 5, 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani H., Talbert A. & Fukunaga M. (2004) New world relapsing fever Borrelia found in Ornithodoros porcinus ticks in Central Tanzania. Microbiology and Immunology 48, 501–505. [DOI] [PubMed] [Google Scholar]
- Nieto N.C., Teglas M.B., Stewart K.M., Wasley T. & Wolff P.L. (2012) Detection of relapsing fever spirochetes (Borrelia hermsii and Borrelia coriaceae) in free‐ranging mule deer (Odocoileus hemionus) from Nevada, United States. Vector Borne and Zoonotic Diseases 12, 99–105. [DOI] [PubMed] [Google Scholar]
- Palma M., Lopes de Carvalho I., Figueiredo M., Amaro F., Boinas F., Cutler S.J. & Nu′ncio M.S. (2011) Borrelia hispanica in Ornithodoros erraticus, Portugal. Clinical Microbiology and Infection 18, 696–701. [DOI] [PubMed] [Google Scholar]
- Parola P., Diatta G., Socolovschi C., Mediannikov O., Tall A., Bassene H. et al (2011).Tick‐borne relapsing fever borreliosis, rural Senegal. Emerging Infectious Diseases 17, 883–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccione J., Levine G.J., Duff C.A., Kuhlman G.M., Scott K.D. & Esteve‐Gassent M.D. (2016) Tick‐borne relapsing fever in dogs. Journal of Veterinary Internal Medicine 30, 1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platonov A.E., Karan L.S., Kolyasnikova N.M., Makhneva N.A., Toporkova M.G., Maleev V.V. & Krause P.J. (2011) Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerging Infectious Diseases 17, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebaudet S. & Parola P. (2006) Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunology and Medical Microbiology 48, 11–15. [DOI] [PubMed] [Google Scholar]
- Reye A.L., Arinola O.G., Hübschen J.M. & Muller C.P. (2012) Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Applied and Environmental Microbiology 78, 2562–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa'idu L., Agbede R.I.S. & Abdu A.P. (1995) Prevalence of avian spirochaetosis in Zaria (1980‐1989). Israel Journal of Veterinary Medicine 50, 39–40. [Google Scholar]
- Sato K., Takano A., Konnai S., Nakao M., Ito T., Koyama K. et al (2014) Human infections with Borrelia miyamotoi, Japan. Emerging Infectious Diseases 20, 1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T.G., Schrumpf M.E., Hinnebusch B.J., Anderson D.E. & Konkel M.E. (1996) GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. Journal of Clinical Microbiology 34, 2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T.G., Raffel S.J., Schrumpf M.E., Policastro P.F., Rawlings J.A., Lane R.S. et al (2005) Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick‐borne relapsing fever in Florida. Journal of Clinical Microbiology 43, 3851–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T.G., Raffel S.J., Schrumpf M.E. & Porcella S.F. (2007) Diversity and distribution of Borrelia hermsii . Emerging Infectious Diseases 13, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T.G., Raffel S.J., Schrumpf M.E., Gill J.S. & Piesman J. (2009) Characterization of a novel relapsing fever spirochete in the midgut, coxal fluid, and salivary glands of the bat tick Carios kelleyi . Vector‐Borne and Zoonotic Diseases 9, 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T.G., Anderson J.M., Lopez J.E., Fischer R.J., Raffel S.J., McCoy B.N. et al (2012) Endemic foci of the tick‐borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potential for human infection. PLoS Neglected Tropical Diseases 6, e1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer S., Margos G., Overzier E., Fingerle V., Baneth G. & Straubinger R.K. (2015) Borrelia persica: in vitro cultivation and characterization via conventional PCR and multilocus sequence analysis of two strains isolated from a cat and ticks from Israel. Ticks and Tick‐Borne Diseases 6, 751–757. [DOI] [PubMed] [Google Scholar]
- Scott M.C., Rosen M.E., Hamer S.A., Baker E., Edwards H., Crowder C. et al (2010) High‐prevalence Borrelia miyamotoi in wild turkeys (Meleagris gallopavo) in Tennessee. Journal of Medical Entomology 47, 1238–1242. [DOI] [PubMed] [Google Scholar]
- Sharma S.P., Amanfu W. & Losho T.C. (2000) Bovine borreliosis in Botswana. Onderstepoort Journal of Veterinary Research 67, 221–223. [PubMed] [Google Scholar]
- Shirani D., Rakhshanpoor A., Cutler S.J., Ghazinezhad B. & Naddaf S.R. (2016) A case of canine borreliosis in Iran caused by Borrelia persica . Ticks Tick Borne Diseases 7, 424–426. [DOI] [PubMed] [Google Scholar]
- Socolovschi C., Mediannikov O., Raoult D. & Parola P. (2009) Update on tick‐borne bacterial diseases in Europe. Parasite 16, 259–273. [DOI] [PubMed] [Google Scholar]
- Takano A., Muto M., Sakata A., Ogasawara Y., Ando S., Hanaoka N. et al (2009) Relapsing fever spirochaete in seabird tick, Japan. Emerging Infectious Diseases 15, 1528–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert A., Nyange A. & Molteni F. (1998) Spraying tick infested houses with lambda‐cyhalothrin reduces the incidence of tick‐borne relapsing fever in children under five years old. Transactions of the Royal Society of Tropical Medicine and Hygiene 92, 251–253. [DOI] [PubMed] [Google Scholar]
- Thomas N.J., Bunikis J., Barbour A.G. & Wolcott M.J. (2002) Fatal Spirochetosis due to a relapsing fever‐like Borrelia sp. in a Northern spotted owl. Journal of Wildlife Diseases 38, 187–193. [DOI] [PubMed] [Google Scholar]
- Trape J.‐F., Diatta G., Arnathau C., Bitam I., Sarih M., Belghyti D. et al (2013) The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS ONE 8, e78473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevejo R.T., Schriefer M.E., Gage K.L., Safranek T.J., Orloski K.A., Pape W.J. et al (1998) An interstate outbreak of tick‐borne relapsing fever among vacationers at a Rocky Mountain cabin. American Journal of Tropical Medicine and Hygiene 58, 743–747. [DOI] [PubMed] [Google Scholar]
- Uilenberg G., Hinaidy H.K., Perié N.M. & Feenstra T. (1988) Borrelia infections of ruminants in Europe. Veterinary Quarterly 10, 63–67. [DOI] [PubMed] [Google Scholar]
- Vial L., Diatta G., Tall A., Bael H., Bouganali H., Durand P. et al (2006) Incidence of tick borne relapsing fever in West Africa: longitudinal study. Lancet 368, 37–43. [DOI] [PubMed] [Google Scholar]
- Wagemakers A., Jahfari S., de Wever B., Spanjaard L., Starink M.V., de Vries H.J. et al (2017) Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks and Tick Borne Diseases 8, 370–374. [DOI] [PubMed] [Google Scholar]
- Walker R.L., Read D.H., Hayes D.C. & Nordhausen R.W. (2002) Case report: equine abortion associated with the Borrelia parkeri‐B. turicatae tick‐borne relapsing fever spirochete group. Jounal of Clinical Microbiology 40, 1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney M.S., Schwan T.G., Sultemeier K.B., McDonald P.S. & Brillhart M.N. (2007) Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Veterinary Clinical Pathology 36, 212–216. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Parsons N.J., Horne E.C., Shock B.C. & Purdee M. (2012) Novel relapsing fever Borrelia detected in African penguins (Spheniscus demersus) admitted to two rehabilitation centers in South Africa. Parasitology Research 110, 1125–1130. [DOI] [PubMed] [Google Scholar]


