Abstract
Background
Enhanced recovery after surgery (ERAS) programs have been reported to decrease complications and shorten hospital stays after lung resections, but their implementation requires time and financial investment with dedicated staff. The aim of this study was to evaluate the clinical and economic outcomes of video-assisted thoracoscopic surgery (VATS) anatomical pulmonary resections before and after implementation of an ERAS program.
Methods
The first 50 consecutive patients undergoing VATS lobectomy or segmentectomy for malignancy after implementation of an ERAS program were compared with 50 consecutive patients treated before its introduction. The ERAS protocol included preoperative counseling, reduced preoperative fasting with concomitant carbohydrate loading, avoidance of premedication, standardized surgery, anesthesia and postoperative analgesia, early removal of chest tube, nutrition and mobilization. Length of stay, readmissions and cardio-pulmonary complications within 30 days were compared. Total costs were collected for each patient and a cost-minimization analysis integrating ERAS-specific costs was performed.
Results
Both groups were similar in terms of demographics and surgical characteristics. The ERAS group had significantly shorter postoperative length of stay (median: 4 vs. 7 days, P<0.0001), decreased pulmonary complications (16% vs. 38%; P=0.01) and decreased overall post-operative complications (24% vs. 48%, P=0.03). One patient in each group was readmitted and there was no 30-day mortality. ERAS-specific costs were calculated at €729 per patient including the clinical nurse and database costs. Average total hospitalization costs were significantly lower in ERAS group (€15,945 vs. €20,360, P<0.0001), mainly due to lower costs during the post-operative period (€7,449 vs. €11,454, P<0.0001) in comparison with the intra-operative period (€8,496 vs. €8,906, P=0.303). Cost-minimization analysis showed a mean saving in the ERAS group of €3,686 per patient.
Conclusions
An ERAS program for VATS anatomical lung resection is cost-effective and is associated with a lower complication rate and a shorter postoperative hospitalization.
Keywords: Enhanced recovery, thoracoscopy, segmentectomy, lobectomy, lung cancer
Introduction
Despite the various progresses observed in the field of chest surgery performed for malignancies in recent years, anatomical lung resections combined with a mediastinal lymph node dissection remain an invasive and traumatic procedure especially in elderly and friable patients affected by lung cancer. Although video-assisted thoracoscopic surgery (VATS) is now the preferred approach for early stage lung cancer, this type of surgery still induces important side effects and surgical stress and is associated with considerable postoperative, mainly cardio-pulmonary morbidity. Moreover, VATS anatomical resections are well-standardized procedures with respect to anesthesia and surgery as well as the postoperative management including chest drainage and analgesia. We hypothesized that an enhanced recovery after surgery (ERAS) program could help to reduce undesirable side effects related to lung cancer surgery leading to a shorter hospital stay and reduced treatment costs. Reports dealing with ERAS programs in thoracic surgery are relatively sparse in the literature (1).
ERAS is a multimodal approach, which combines various procedures from patient’s initial referral through to discharge (2-4). Based on the best available clinical evidence for each step, the goal of ERAS programs is to minimize surgical stress, reduce complications, shorten hospital stay, increase quality of life during hospitalization and thus decrease overall costs related to surgery. Initially reported for colorectal surgery (3), ERAS programs have been shown to successfully improve clinical outcomes in other surgical specialties despite some financial and operational investments as well as maintenance costs (4-7). Key elements to their successful implementation are the setting up of a suitable team, including a clinical nurse dedicated to the project, educational support and implementation of a database to record all relevant items. In the more specific case of thoracic surgery, despite the adoption of minimally invasive approaches and general standardization of the perioperative phase, clinical outcomes after implementation of an ERAS program are currently limited and results controversial (8-19). This is probably due to the variable programs’ contents and designs of the published studies. The aim of this study was to assess the clinical and economic outcomes of the implementation of an ERAS program in cancer patients undergoing anatomical pulmonary resection by VATS.
Methods
An ERAS protocol for VATS anatomical pulmonary resection was developed and implemented at the University Hospital of Lausanne. The dedicated ERAS team was composed of surgeons, anesthesiologists, nutritionists and a specifically ERAS designated study nurse. ERAS was initiated in September 2016 by establishing internal guidelines, care maps and a specific patient logbook during the first 6 months. In parallel, nurses and medical staff members received specific instructions concerning the ERAS program. Prospective ERAS patient enrollment started in April 2017. The control group consisted of consecutive patients undergoing VATS anatomical lung resection between June 2016 and March 2017.
Patient selection and study design
The first 50 consecutive patients undergoing VATS lobectomy or segmentectomy for malignancy after implementation of ERAS program were compared with a retrospective group of 50 consecutive patients treated in the year before its introduction. For both groups, patients older than 18 years undergoing elective VATS anatomical pulmonary resections (segmentectomy or lobectomy) for malignant diseases (primary lung cancer or metastases) were included. Exclusion criteria consisted for both groups of benign lesions, conversion thoracotomy, and age less than 18 years or emergency operations. All cases of both treatment groups were discussed preoperatively in an interdisciplinary tumor board and had to comply with current oncological and functional guidelines. For both groups, VATS was performed under general anesthesia with lung exclusion by double lumen intubation. Surgical anatomical resection was performed or supervised by an attending surgeon (M Gonzalez, T Krueger, HB Ris) using a three-port anterior approach with individual dissection and division of all broncho-vascular structures. In addition, for primary lung cancer, a formal mediastinal lymph node dissection was undertaken in all patients. All three surgeons had a prior experience of >100 anatomical VATS resections. All patients were extubated in the operative room and transferred to intermediate care depending on their preexisting comorbidity profile. Patients of the control group were retrospectively identified from our prospective database and included operations performed between June 2016 and March 2017. ERAS data collection started in April 2017 and continued until November 2017. The Local Ethics Committee approved the study and individual consent was waived (No. 2018-00591).
ERAS program
The ERAS protocol is summarized in Table 1 and is divided into a preoperative, a perioperative and a postoperative phase and consists of preoperative counseling by a dedicated clinical nurse (V Doucet), reduced preoperative fasting and carbohydrate loading, avoidance of premedication, standardized surgery, anesthesia and postoperative analgesia, and early postoperative nutrition, mobilization and removal of chest drainage. All patients were followed on a daily base by the attending surgeon and the clinical nurse and were discharged if postoperative pain was well controlled by oral analgesia and ambulation independency was achieved. All patients were revisited by their surgeon in the outpatient department 10 days after discharge and were contacted by the study nurse at day 30 postoperatively.
Table 1. Guidelines for VATS anatomical lung resections before and after ERAS implementation.
| Variables | Controls | ERAS |
|---|---|---|
| Preoperative consultation | No standardized information | Standardized information |
| ERAS education | No | Information by clinical nurse: standardized preoperative education protocol; information booklet with daily goals; smoking cessation; nutritional advice; preoperative incentive spirometer instruction |
| Carbohydrate drink | No | 2 h before operation; post-operative carbohydrate drinks (3 times/day) |
| Premedication | Yes | No |
| Antibiotics | Induction | Induction |
| Anesthesia | Epidural catheter or intercostal; halogenated anesthetics gases/propofol; opioids; paracetamol | Intercostal blocks; halogenated anesthetics propofol; opioids; dexmedetomidine; ketamine; NSAID; paracetamol |
| Crystalloid overload | No | No |
| Intraoperative warming | Yes | Yes |
| VTE prophylaxis | LMWH | LMWH |
| Urinary catheter | Only if epidural catheter | No |
| Chest drainage | 2 chest tubes, digital chest drainage or water seal, suction −20 cm; drain removed <250 mL/24 h and no air flow | 1 chest tube connected to a digital chest drainage system, suction −20 cm; drain removed <400 mL/24 h and no air flow |
| Postoperative iv fluids | No | No |
| Opiate analgesia | Paracetamol; fixed doses Morphine s.c.; tramadol (chest tube removal) | Paracetamol; NSAID; morphine PCA; tramadol (chest tube removal) |
| Feeding | Early | Early |
| Targeted PONV | Not standardized | Standardized; Ondesantran; dexamethasone 21-phosphate disodium |
| Mobilization within 24 h | Yes | Yes |
VATS, video-assisted thoracoscopic surgery; ERAS, enhanced recovery after surgery; NSAID, nonsteroidal anti-inflammatory drugs; VTE, venous thromboembolism; PONV, postoperative nausea and vomiting; PCA, patient-controlled analgesia; LMWH, low-molecular-weight heparin.
Data collection
For each patient of the ERAS and the control group, prospective data collection was performed. Patient demographics, comorbidity, cardiac evaluation and pulmonary function testing, tumor stage and histology, operative characteristics and clinical outcome during hospitalization and up to 30 days after discharge, including length of stay, readmissions, reoperations and cardio-pulmonary complications were evaluated. Postoperative complications were categorized according the Clavien-Dindo classification, adapted for thoracic surgery while considering grade I–II as minor and grade III–IV as major complications, and grade V as 30-day postoperative mortality (20). Compliance to individual ERAS items was assessed and monitored by the dedicated study nurse. The average compliance rate was defined as the number of protocol items observed divided by the total number of items.
Costs analysis
The overall costs per patient were evaluated in a detailed manner by the hospital administration, which was blinded to the treatment groups. They were split into two categories (intraoperative and pre-/postoperative costs) and were expressed in euros (€) considering an exchange rate of 1€ =1.17 CHF. Intraoperative costs included consumables used during surgery and costs related to anesthesia and operating room (OR) exploitation, based on the duration of OR occupation. Costs related to anesthesia included the salary costs of the medical staff and nursery (counted per minute and based on the duration of anesthesia) as well as anesthesia-related consumables. Preoperative and postoperative expenditures included costs related to intensive care unit and/or intermediate care unit stay (costs per day), medical and nursing care and physiotherapy on the ward, drugs, blood transfusion and testing, costs related to laboratory tests, radiological and pathological exams, housing, administration and other ancillary costs such as social work and occupational therapy. Costs related to ward nursing care were based on a list of care actions required for each patient (Project Research in Nursing) (21). Housing costs were accrued per hospital day whereas administrative costs were accrued per admission.
Costs related to the implementation of ERAS were composed of individual variables emerging from ERAS database (€100 per patient), patient carbohydrate drinks and post-operative drinks (€25 per patient) and patient logbooks (€4 per patient), as well as from fixed costs related to a 6 months’ salary on a 60% part-time basis of the dedicated ERAS study nurse of €30,000. Specific ERAS costs were then calculated by distributing the fixed costs among the 50 patients and adding the individual variable costs and estimated at a per-patient cost of €729.
Cost-minimization analysis
A cost-minimization analysis was undertaken to estimate the cost saving per patient related to ERAS in the context of societal debates on general healthcare costs. It was obtained by calculating the difference between the average costs per patient generated in the ERAS and the control group, respectively. These differences were used to calculate an average per patient saving for each group. The ERAS-specific costs were subtracted from the average per patient saving in the ERAS group.
Sensitivity analysis
Fixed costs are independent of the number of patients. When they are allocated on a per-patient basis (fixed costs divided by number of patients), the result varies widely with the number of patients treated within the study period. Therefore, a sensitivity analysis of the costs of ERAS implementation was carried out. The number of patients treated within the study period was varied (±50 per cent) in order to assess its impact on the cost-minimization results.
Statistical analysis
Continuous variables were compared using two-tailed Mann-Whitney or Student’s t-test, as appropriate. Categorical variables were analyzed by means of Fisher’s exact or chi square test. Despite an imbalance in the distribution of cost data, the arithmetic mean was considered as the most informative measure from the decision-maker’s perspective. The non-parametric bootstrap test was used for the cost analysis and P<0.05 was considered statistically significant. All analyses were performed using SPSS® 20 (IBM, Armonk, New York, USA).
Results
The ERAS and the control group were comparable with respect to age, sex, American Society of Anesthesiologist (ASA) score, body mass index and co-morbidities (Table 2). Tumor histology and stage, and surgical characteristics were also comparable between both groups. However, ERAS patients had a significantly better FEV1 and less tobacco use (P=0.02) than controls. The duration of operation was significantly shorter in the ERAS group (P=0.04).
Table 2. Patients characteristics of patient undergoing VATS anatomical lung resections before and after ERAS implementation.
| Variables | Controls | ERAS | P value |
|---|---|---|---|
| Number of patients | 50 | 50 | – |
| Age median [range] (years) | 68 [51–81] | 64 [44–87] | 0.07 |
| Sex (F/M) | 32/18 | 26/24 | 0.2 |
| BMI (median) (kg/m2) | 23.5 | 24.9 | 0.4 |
| ASA, n [%] | 0.07 | ||
| I | 0 | 0 | |
| II | 24 [48] | 33 [66] | |
| III | 26 [52] | 17 [34] | |
| Co-morbidity, n [%] | |||
| Cardiac diseases | 9 [18] | 8 [16] | 0.4 |
| Hypertension | 27 [54] | 19 [38] | 0.1 |
| Smoking | 45 [90] | 36 [72] | 0.02 |
| COPD | 14 [28] | 13 [26] | 0.8 |
| Diabetes | 7 [14] | 8 [16] | 0.8 |
| Renal failure | 4 [8] | 2 [4] | 0.4 |
| Alcohol abuse | 7 [14] | 10 [20] | 0.4 |
| Previous malignancy | 17 [34] | 17 [34] | 1 |
| FEV1 (%, mean ± SD) | 81±20 | 92±18 | 0.005 |
| DLCO (%, mean ± SD) | 74±21 | 76±17 | 0.7 |
| NSCLC, n [%] | 48 [96] | 44 [88] | – |
| Metastasis, n [%] | 2 [4] | 6 [12] | 0.1 |
| Stage NSCLC, n | 0.6 | ||
| T1 | 27 | 25 | |
| T2 | 15 | 14 | |
| T3 | 6 | 5 | |
| N0 | 42 | 37 | |
| N1 | 3 | 3 | |
| N2 | 3 | 4 | |
| Segmentectomy, n [%] | 20 [40] | 18 [36] | 0.7 |
| Lobectomy, n [%] | 30 [60] | 32 [64] | – |
| Operation time [range] (min) | 150 [55–303] | 134 [75–285] | 0.04 |
ERAS, enhanced recovery after surgery; VATS, video-assisted thoracic surgery; BMI, body mass index; ASA, American Society of Anesthesiologist; COPD, chronic obstructive pulmonary disease; NSCLC, non-small cell lung cancer; SD, standard deviation.
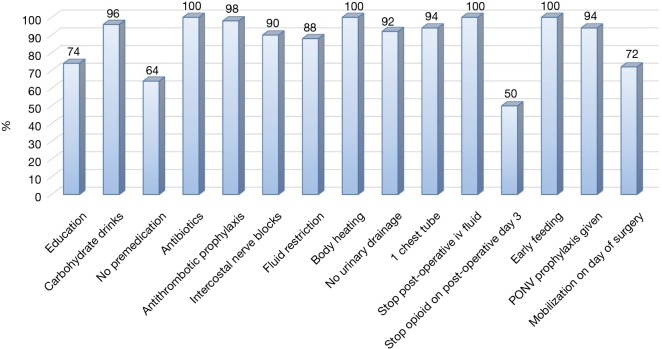
There was no 30-day mortality in both groups and one patient in each group (2%) required a readmission and redo VATS, one for an empyema and one for a persistent pneumothorax (Table 3). The ERAS group had a significantly lower postoperative overall (24% vs. 48%, P=0.03) and pulmonary (20% vs. 42%, P=0.02) complication rate compared to the control group. Likewise revealed the ERAS group a significantly shorter postoperative length of stay (4 vs. 7 days, P<0.0001). In the ERAS group, the average compliance rate was of 87% (Figure 1).
Table 3. Postoperative complications after VATS anatomical lung resections before and after ERAS implementation.
| Variables | Controls | ERAS | P value |
|---|---|---|---|
| Postoperative mortality, n [%] | 0 | 0 | – |
| Postoperative morbidity, n [%] | 24 [48] | 12 [24] | 0.03 |
| Pulmonary complication, n [%] | 19 [38] | 8 [16] | 0.01 |
| Minor (Clavien I–II), n [%] | 21 [42] | 10 [20] | 0.02 |
| Pneumonia | 9 [18] | 5 [10] | 0.2 |
| Air leak>7 days | 6 [12] | 4 [8] | 0.5 |
| Atelectasis | 4 [8] | 0 | 0.04 |
| Recurrent nerve lesion | 1 [2] | 0 | 0.3 |
| Chylothorax | 1 [2] | 0 | 0.3 |
| AF | 3 [6] | 1 [2] | 0.3 |
| Urinary retention | 1 [2] | 2 [4] | 0.6 |
| Major (Clavien III–IV), n [%] | 4 [8] | 2 [4] | 0.4 |
| Pleural effusion (drainage) | 1 [2] | 0 | 0.3 |
| Pneumothorax (drainage) | 1 [2] | 1 [2] | 1.0 |
| Reoperation at 1 month | 1 [2] | 1 [2] | 1.0 |
| Myocardial infarction | 1 [2] | 0 | 0.3 |
| Chest drainage [range] (days) | 3 [1–15] | 2 [2–15] | 0.06 |
| Readmission, n | 1 | 1 | 1.0 |
| Post-operative length of stay [range] (days) | 7 [2–21] | 4 [1–16] | <0.0001 |
ERAS, enhanced recovery after surgery; VATS, video-assisted thoracic surgery; AF, atrial fibrillation.
Figure 1.
Compliance to components of the ERAS protocol. PONV, postoperative nausea and vomiting; ERAS, enhanced recovery after surgery.
The mean overall hospitalization costs were significantly lower in the ERAS group compared to the control group (€15,945 vs. €20,360, P<0.0001) (Table 4). The intraoperative costs were similar for both groups (€8,496 vs. €8,906, P=0.303) but costs related to the pre- and post-operative period were significantly lower for the ERAS group compared to the control group (€7,449 vs. €11,454, P<0.0001). Therefore, a mean overall saving of €3,686 was observed in favor of the ERAS group (Table 5). As some of the ERAS implementation costs were fixed and were divided by the number of patients (arbitrarily chosen to balance the solidity of the conclusions and the practicality/duration of the study), the per-patient cost was higher (smaller) than if we had studied a larger (smaller) group of patients. To assess this effect, we ran the same calculations and varied the number of patients by ±25 patients (50% of the cohort). The average implementation costs were €1,458 per patient for 25 patients and €486 per patient for 75 patients. The total cost balance remained in favor of the ERAS group, estimated at €2,957and €3,929 for 25 and 75 patients, respectively. It should also be noted that this effect will comparatively increase the savings per patient for all subsequent patients since it will have been amortized on the initial group of 50 patients.
Table 4. Hospitalization costs (€) of VATS anatomical lung resections before and after ERAS implementation.
| Variables | Controls (€) [95% CI]* | ERAS (€) [95% CI]* | Difference (€) [95% CI]* | P value |
|---|---|---|---|---|
| Intraoperative | 8,906 [8,358–8,497] | 8,496 [8,043–9,047] | −410 [−1,240; 365] | 0.303 |
| Material | 3,945 [3,612–4,291] | 3,846 [3,545–4,179] | −99 [−617; 413] | 0.717 |
| Anesthesia | 1,863 [1,734–2,032] | 1,686 [1,592–1,794] | −177 [−363; −1] | 0.039 |
| Operating room | 3,097 [2,889–3,308] | 2,964 [2,794–3,149] | −133 [−426; 141] | 0.272 |
| Pre/postoperative | 11,454 [10,495–13,636] | 7,449 [6,854–8,860] | −4,005 [−6,174; −2,441] | <0.0001 |
| Intermediate care | 2,012 [1,409–2,734] | 727 [475–1,044] | −1,285 [−1,994; −704] | <0.0001 |
| Medical care | 1,677 [1,467–1,927] | 779 [671–903] | −898 [−1,151; −660] | <0.0001 |
| Nursing care | 3,656 [3,032–4,404] | 2,738 [2,229–3,358] | −918 [−1,825; −99] | 0.112 |
| Physiotherapy | 298 [213–392] | 116 [67–175] | −182 [−291; −85] | 0.001 |
| Medication | 341 [255–438] | 440 [331–561] | 99 [−53; 252] | 0.068 |
| Blood analysis | 89 [11–200] | 11 [0–54] | −78 [−175; 3] | 1.000 |
| Laboratory | 358 [258–473] | 198 [153–249] | −160 [−288; −44] | 0.022 |
| Radiology | 474 [370–604] | 320 [264–379] | −154 [−301; −44] | 0.001 |
| Pathology | 1,114 [1,006–1,232] | 1,077 [874–1,305] | −37 [−236; 195] | 0.068 |
| Housing | 1,314 [1,135–1,563] | 956 [841–1,077] | −358 [−635; −134] | 0.080 |
| Others | 124 [101–152] | 89 [94–123] | −35 [−66; −7] | 0.068 |
| Total (€) | 20,360 [19,123–22,935] | 15,945 [15,094–17,546] | −4,415 [−7,030; −2,550] | <0.0001 |
Values are mean (95% CI). *, Bootstrap t-test. ERAS, enhanced recovery after surgery; VATS, video-assisted thoracic surgery; CI, confidence interval.
Table 5. Summary of a per patient cost-minimization analysis in patients undergoing VATS anatomical lung resections before and after ERAS implementation expressed in €.
| Costs (€) | Controls | ERAS | Difference |
|---|---|---|---|
| ERAS-specific costs | 0 | 729 | 729 |
| Intraoperative costs | 8,906 | 8,496 | −410 |
| Pre- and postoperative costs | 11,454 | 7,449 | −4,005 |
| Total | 20,360 | 16,674 | −3,686 |
ERAS, enhanced recovery after surgery; VATS, video-assisted thoracic surgery.
Discussion
Our study suggests that the implementation of an ERAS program for VATS anatomical pulmonary resection may result in improved clinical and economic outcomes. An ERAS program combines various synergetic elements across the entire hospitalization process, from the patient’s first consultation up to its discharge, aiming to minimize the perioperative stress response, reduce complications and decrease postoperative length of stay. Initially conceived for colorectal surgery, ERAS has demonstrated clinical benefits in several other surgical specialties (2,3,7) translating in lower morbidity, shorter hospitalizations and decreased costs (4-6). However, ERAS programs have only recently introduced for thoracic surgery in patients requiring thoracotomy lung resection (13) but the results also suggest improved clinical outcome parameters such as decreased postoperative complications and length of stay (19). Early stage pulmonary cancer is nowadays managed by a minimally invasive approach, which is generally an important key element for ERAS programs (1) but published reports focusing on the impact of ERAS on VATS anatomical lung resections is scant (14).
In our study, we compared the outcome of patients undergoing VATS anatomical lung resections for malignant diseases before and after the implementation ERAS with respect to mortality and morbidity, readmission, duration of hospitalization and in-hospital costs. Our results demonstrate a significant reduction in postoperative morbidity, length of hospitalization and costs for ERAS patient in our series. We speculate that the decreased postoperative morbidity and shortened hospitalization constituted the mayor part of the economic benefit related to ERAS. However, the cost savings in ERAS patients were also related to a direct transfer of ERAS patients to the ward while avoiding the intermediate care and to early mobilization of ERAS patients by nurses, leading to a concomitant decrease of physiotherapy cost. Finally, standardized postoperative prescriptions such as blood tests or radiological exams were also accompanied by a cost reduction in ERAS patients. In contrast, the operation-related costs (consumables, anesthesia and OR costs) were similar for ERAS and non-ERAS patients despite the significantly longer operation time in non-ERAS patients.
Brunelli et al. analyzed the outcome of patients treated by VATS lobectomy before and after implementation of an ERAS program and were not able to identify a significant difference in postoperative morbidity or hospitalization in favor of ERAS patients (14). This may be explained by the fact that early mobilization and chest tube removal and standardized pain management has already been applied to their pre-ERAS VATS lobectomy patients, which endorses the importance of those elements in a successful thoracic surgery ERAS program. High-volume thoracic surgery centers tend for a continual integration of novelties in their daily practice, including ERAS elements, which may render the assessment of benefits arising from ERAS difficult (8-10,14).
Another key element of ERAS is a process standardization with established guidelines during hospitalization that has to be applied by all members of the team since, in daily practice, traditional and individualized patient handling may still prevail. The program permitted to harmonize the approach in term of number of chest tubes and the pain management between each surgeon. There was significant decreased use of two chest tubes and epidural catheter in ERAS group. This standardization may serve as a roadmap for daily medical and nursing activities and translates in patient satisfaction and increased compliance as observed in our study. The compliance of the patients to the ERAS program was indeed a predictor of decreased morbidity and length of stay in our and other studies (15). Process standardization and the definition of guidelines were the crucial elements for the successful implementation of our ERAS program. The University Hospital of Lausanne traditionally encourages the introduction of process standardization and establishment of guidelines as well as ERAS programs in various surgical specialties (5,6). This and the engagement of a dedicated clinical nurse greatly facilitated the successful introduction of our ERAS program, accompanied by a rewarding patient compliance.
Regarding the cost-effectiveness of ERAS programs in thoracic surgery, there are only limited data available. Recently, Paci et al. reported on the economic impact induced by the introduction of an ERAS program, which resulted in lower overall costs in ERAS patients but without mentioning the specific, ERAS-related costs (16). The implementation of an ERAS program requires some specific investments such as the engagement of a dedicated clinical nurse and costs related to data management, a logbook system and carbohydrate drinks. In our study, these investments were estimated at €729 per patient. However, they were more than balanced-off by the savings generated by ERAS implementation indicating that our ERAS implementation project was cost-effective. The cost-weight analysis performed in our study enables a detailed insight of all hospitalization costs as well as the costs related to ERAS, duly normalized by the number of included patients and indicated a cost-saving estimated at €3,686 per ERAS patient.
Our study presents several limitations. First, the control group was retrospectively assessed without applying a propensity-matched analysis. The control group groups were not perfectly matched with better pulmonary function and lower ASA score. The difference observed for pulmonary complications may have been affected by mismatch in pre-operative respiratory co-morbidities. However, retrospective data collection for the standard group could have led to incomplete identification of postoperative events and this would have resulted in an under-estimation of pre-ERAS costs, thus further solidifying our conclusions. Second, it is difficult to disentangle the effects of implementation of an ERAS program and the sole effects of standardization. The experience gained during the study could have had an impact on complications and length of hospital stay even in the absence of ERAS protocol. Finally, some costs could not be assessed, like post-operative consultation and also the potential costs transferred from the hospital to the community (such as short-term rehabilitation unit costs, sick leave). We can however safely assume that their grand total would have been small compared to the cost of each additional hospitalization day, or to the costs of surgery itself.
In conclusion, our results indicate that the implementation of an ERAS program for VATS anatomical lung resection results in lesser postoperative complications, a shorter hospitalization and is cost-effective, even during the initial implementation phase.
Acknowledgements
We would like to thank Audrey Roth for the data management and the collection and extraction of the data.
Ethical Statement: The Local Ethics Committee approved the study (No. 2018-00591) and individual consent was waived.
Footnotes
Conflicts of Interest: This work was presented at the 26th Annual Meeting of the European Society of Thoracic Surgery, Ljubljana, Slovenia, 26-29 May 2018.
References
- 1.Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. 10.1093/ejcts/ezv154 [DOI] [PubMed] [Google Scholar]
- 2.Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172-88. 10.1002/bjs.9394 [DOI] [PubMed] [Google Scholar]
- 3.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 4.Lee L, Mata J, Ghitulescu GA, et al. Cost-effectiveness of Enhanced Recovery Versus Conventional Perioperative Management for Colorectal Surgery. Ann Surg 2015;262:1026-33. 10.1097/SLA.0000000000001019 [DOI] [PubMed] [Google Scholar]
- 5.Joliat GR, Labgaa I, Petermann D, et al. Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg 2015;102:1676-83. 10.1002/bjs.9957 [DOI] [PubMed] [Google Scholar]
- 6.Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 2013;100:1108-14. 10.1002/bjs.9184 [DOI] [PubMed] [Google Scholar]
- 7.Senturk JC, Kristo G, Gold J, et al. The Development of Enhanced Recovery After Surgery Across Surgical Specialties. J Laparoendosc Adv Surg Tech A 2017;27:863-70. 10.1089/lap.2017.0317 [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. 10.1067/mtc.2001.114352 [DOI] [PubMed] [Google Scholar]
- 9.Salati M, Brunelli A, Xiume F, et al. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg 2012;41:1083-7; discussion 1087. 10.1093/ejcts/ezr171 [DOI] [PubMed] [Google Scholar]
- 10.Madani A, Fiore JF, Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908; discussion 908-10. 10.1016/j.surg.2015.04.046 [DOI] [PubMed] [Google Scholar]
- 11.Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. 10.1016/j.ejcts.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Das-Neves-Pereira JC, Bagan P, Coimbra-Israel AP, et al. Fast-track rehabilitation for lung cancer lobectomy: a five-year experience. Eur J Cardiothorac Surg 2009;36:383-91; discussion 391-2. 10.1016/j.ejcts.2009.02.020 [DOI] [PubMed] [Google Scholar]
- 13.Fiore JF, Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg 2016;151:708-15.e6. 10.1016/j.jtcvs.2015.09.112 [DOI] [PubMed] [Google Scholar]
- 14.Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [DOI] [PubMed] [Google Scholar]
- 15.Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. 10.1016/j.jtcvs.2017.10.151 [DOI] [PubMed] [Google Scholar]
- 16.Paci P, Madani A, Lee L, et al. Economic Impact of an Enhanced Recovery Pathway for Lung Resection. Ann Thorac Surg 2017;104:950-7. 10.1016/j.athoracsur.2017.05.085 [DOI] [PubMed] [Google Scholar]
- 17.Khandhar SJ, Schatz CL, Collins DT, et al. Thoracic enhanced recovery with ambulation after surgery: a 6-year experience. Eur J Cardiothorac Surg 2018;53:1192-8. 10.1093/ejcts/ezy061 [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Ma H, Chen S. Enhanced recovery after surgery using uniportal video-assisted thoracic surgery for lung cancer: A preliminary study. Thorac Cancer 2018;9:83-7. 10.1111/1759-7714.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Zhou K, Che G, et al. Enhanced recovery programs in lung cancer surgery: systematic review and meta-analysis of randomized controlled trials. Cancer Manag Res 2017;9:657-70. 10.2147/CMAR.S150500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chagnon M, Audette LM, Lebrum L, et al. A patient classification system by level of nursing care requirements. Nurs Res 1978;27:107-12. 10.1097/00006199-197803000-00014 [DOI] [PubMed] [Google Scholar]