Abstract
Background
Currently there are several techniques for endoscopic diagnosis of parenchymal lung abnormalities. Electromagnetic navigation with or without endobronchial ultrasound for diagnosis of the above has been well described. Bronchoscopic Trans Bronchial Access Tool is a novel endoscopic technique that creates a virtual pathway to the lesion and is less limited by location of the airway. The CrossCountryTM Transbronchial Access Tool (CovidienTM, Plymouth, MN, USA) is a Food and Drug Administration (FDA) approved off airway device that utilizes a catheter equipped guide sheath for a trans-parenchymal approach to a distal lesion. Cone beam computer tomography (CBCT) is a real-time onsite extrathoracic navigational modality used in the bronchoscopy suite that allows for an open working channel. All three of the above modalities can have reasonable diagnostic yields when used independently. While utilizing the above tools we frequently found ourselves in situations where one technique was not enough, prompting the use of a combination of modalities to obtain the most efficient and accurate diagnosis. We are reporting the feasibility and safety of utilizing these three modalities in conjunction with one another.
Methods
Patients with peripheral pulmonary nodules on chest computed tomography underwent a navigation bronchoscopy under general anesthesia. CBCT and radial ultrasound was used in every case to confirm navigation to the target lesion. Lesions without definitive airways leading to them were accessed with the transbronchial access tool (TBAT).
Results
Electromagnetic bronchoscopy using CBCT and radial US was performed on 22 patients from April 2016 to September 2016. The TBAT tool was used in 7 patients. The overall diagnostic yield was 77.2% (17 of 22). Diagnostic yield of with use TBAT was 100% (7 of 7). There were no complications. Average case length was 79.95 (range, 50–124) minutes and average fluoroscopy time was 10.39 (1–21.7) minutes.
Conclusions
TBAT is a useful and safe tool when accessing peripheral pulmonary nodules and is used in conjunctions with electromagnetic navigation and CBCT.
Keywords: Lung nodule, lung cancer, bronchoscopy, endobronchial ultrasound, radial ultrasound, electromagnetic ultrasound, cone beam computer tomography (CBCT), trans bronchial access tool
Introduction
Currently there are several techniques for bronchoscopic diagnosis of peripheral lung lesions. Electromagnetic navigation bronchoscopy (ENB) with and without radial endobronchial ultrasound (r-EBUS) has complimented standard bronchoscopy and has demonstrated to be a safe and minimally invasive diagnostic modality (1). The diagnostic yield from this approach has ranged from 55.7% to 80% and the highest yield occurs with lesions with an air-bronchus sign and size >2 cm (2-5). Endoscopic sampling of lesions that are more distant from the bronchus or that have no connection with an airway has been extremely challenging and likely accounts for a significant portion of the variable success of ENB in literature. The Transbronchial Access Tool (TBAT) is a novel endoscopic technique that creates a virtual pathway to the lesion and is less limited by location of the airway. The CrossCountryTM Transbronchial Access Tool (CovidienTM, Plymouth, MN, USA) is a Food and Drug Administration (FDA) approved TBAT device that utilizes a catheter equipped guide sheath for a trans-parenchymal approach to a distal lesion. Preliminary studies have shown this to be a safe option with difficult to reach lesion (6-8). Cone beam computer tomography (CBCT) is a real-time onsite extrathoracic navigational modality that is used in conjunction with bronchoscopy and has provided improvement in diagnostic yield with radiation exposure equivalent to one-time low dose screening CT (9,10). All three of the above modalities have reasonable diagnostic yields when used independently. While utilizing the above tools there are often situations where one technique will not suffice, prompting the use of multiple modalities to obtain the most efficient and accurate diagnosis. Little is known, however, about the complication rate and diagnostic yield when ENB, TBAT and CBCT are used together. Ours is a retrospective review of 22 cases in which CBCT, ENB and r-EBUS were utilized with or without TBAT and set out to determine the safety and diagnostic yield of the combined modalities in the evaluation of the peripheral nodule (PN) or mass.
Methods
Study design and patients
We identified patients who had undergone a bronchoscopy with ENB and CBCT guided biopsies with or without utilization of TBAT to evaluate pulmonary lesions between April 2016 and September 2016 in a single large community teaching facility. Permission to access this information was approved by the MedStar Health Research Institute Institutional Review Board (MHRIRB 2016-189).
All patients had been referred for suspected lung cancer and underwent ENB, r-EBUS and CBCT guided biopsy under general anesthesia. Consent was obtained in accordance with institutional policy. Images from a non-contrast CT were imported into the SuperDimension software (Medtronic; Inc., Minneapolis, MN, USA) and the lesions were identified and marked per software protocol. In all instances the operating physician staged the mediastinum using linear endobronchial ultrasound.
CBCT scan
The cone beam CT is a high-resolution two-dimensional detector adapted for use with a C-arm. It rotates 200° around a stationary target (the operating room table) and acquires a 3-dimensional data set. The patient is under general anesthesia with volume control ventilation and respiration is paused during image acquisition. Using the 3-dimensional cross section images the target can be identified and manually contoured on a workstation and then be superimposed on live fluoroscopy and provide real time imaging.
Electromagnetic navigational bronchoscopy
In all instances, we used the SuperDimension navigation system 6.0 (Medtronic; Inc.), the 180° or 90° angle Edge extended working channel (EWC) catheter (Medtronic; Inc.), and the locatable guide (LG). Using the standard approach, we attempted to access all target lesions and confirmed each navigation with r-EBUS (Figure 1) and a single spin of CBCT (Figure 2). If apposition of the catheter to the lesion was not in the appropriate 3-dimensional plane after subsequent manipulation, the lesion was then accessed by the TBAT (Figure 3). All samples were taken with at least two separate biopsy tools and included either forceps, fine needle aspiration, brush or GenCutTM core biopsy system (Medrtonic; Inc.). Regardless of the tool, at least 7 samples from each biopsy were collected. Decision on biopsy tool depended on a variety of different factors including the apposition of the lesion to vessels and pleural surface.
Figure 1.
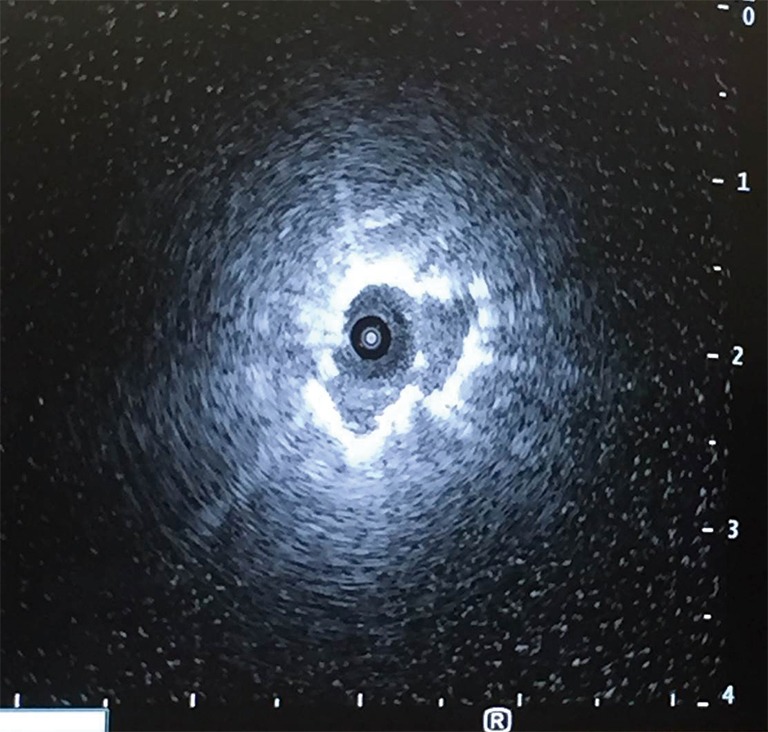
Radial ultrasound image of peripheral pulmonary nodule after successful electromagnetic navigational bronchoscopy.
Figure 2.
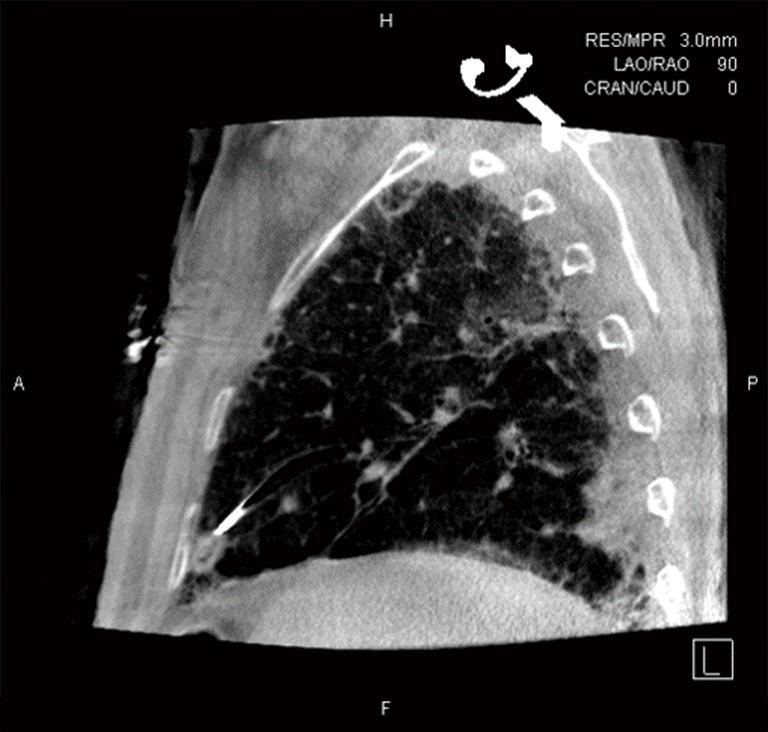
CBCT image following deployment of the TBAT. CBCT, cone beam computed tomography; TBAT, Transbronchial Access Tool.
Figure 3.
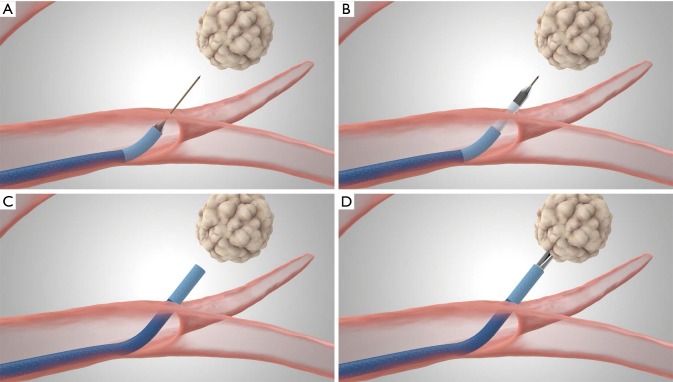
Deployment of the TBAT (Medtronic; Inc.). (A) The guidewire needle punctures the airway wall and traverses the lung parenchyma into the target lesion; (B) the dilation catheter is advanced over the guidewire into the target lesion; (C) EWC is guided over the dilation catheter and TBAT is removed; (D) biopsy tools are advanced through the EWC. TBAT, Transbronchial Access Tool; EWC, extended working channel. All rights reserved. Used with the permission of Medtronic.
TBAT
TBAT can be utilized through a flexible bronchoscope with an EWC. The TBAT comes with a straight wire and requires a 2mm working channel. It is inserted through the EWC of the ENB catheter. It is designed to puncture the airway wall and traverse tissue to the selected target thus facilitating access of additional tools for patients with peripheral lung nodules or lung masses. The guidewire traverses the lung parenchyma into the target lesion (Figure 3A). A dilation catheter is advanced over the guidewire into the target lesion (Figure 3B). The EWC is then guided over the dilation catheter and the TBAT is removed (Figure 3C), biopsy tools can then be introduced (Figure 3D).
Statistics
Statistical analysis was performed using SPSS 17.0 for windows. Continuous variables were summarized by mean, standard deviation and range, if the outcomes were normally distributed. Where the continuous variables were not normally distributed, median and quartiles were expressed. Basic line characteristics of this project were analyzed by Student’s t-tests for continuous variables and the chi-square test for dichotomous variables. Diagnostic yield was analyzed by chi-square test or Fisher’s exact test. P value of less than 0.05 indicated statistical significance.
Results
Basic characteristics of PNs
The basic line characteristics of patients with PNs in this study were performed in Table 1. There were 22 patients enrolled in this study (8 males and 14 females) with mean age of 69 (range, 55–83) years. Of the 22 patients, 17 patients (81%) had smoking history and 13 patients (65%) had chronic obstructive pulmonary disease (COPD), 7 patients (32%) had cancer history before this study. Four of the seven patients had lung cancer (2 adenocarcinoma of lung, 1 squamous cell of lung and 1 unknown), and 3 of 7 patients had other types of cancer including breast cancer, pancreatic cancer, brain cancer, colon cancer and melanoma.
Table 1. Characteristics of peripheral pulmonary lesions of patients with available demographic data.
| Demographic data | Value |
|---|---|
| Age (mean ± SD, range) | 69±8.8 [55–83] |
| BMI (mean ± SD, range) | 26.38±6.38 (13.68–38.95) |
| Sweeps of CBCT (mean ± SD, range) | 2.63±1.06 [1–4] |
| Tunnel length (mean ± SD, range) | 1.5±0.41 [1–2] |
| Procedure time, min (mean ± SD, range) | 79.95±21.15 [50–124] |
| Fluoroscopy time, min (mean ± SD, range) | 10.39±5.14 (1–21.7) |
| Size of PN, cm (mean ± SD, range) | 2.1±0.98 (0.7–5.2) |
| Sex (%) | |
| Male | 8 [36] |
| Female | 14 [64] |
| Smoke | |
| No | 4 [19] |
| Yes | 17 [81] |
| COPD | |
| No | 7 [35] |
| Yes | 13 [65] |
| Cardiac disease | |
| No | 9 [41] |
| Yes | 13 [59] |
| Hypertension | |
| No | 8 [36] |
| Yes | 14 [64] |
| Diabetes | |
| No | 16 [76] |
| Yes | 5 [24] |
| History of tumor | |
| No | 15 [68] |
| Yes | 7 [32] |
| Visible at fluoroscopy | |
| No | 10 [45] |
| Yes | 12 [55] |
| CBCT | |
| No | 0 |
| Yes | 22 [100] |
| TBAT | |
| No | 15 [68] |
| Yes | 7 [32] |
SD, standard deviation; BMI, body mass index; PN, peripheral nodule; CBCT, cone beam computed tomography; COPD, chronic obstructive pulmonary disease; TBAT, transbronchial access tool.
There were 12 cases (55%) whose PNs were visible on fluoroscopy. The mean duration of procedure was 79.95 (range, 50–124) minutes. And the length of tunnel from the airway wall point of entry to the nodule ranged from 1 to 2 cm, with a mean length of 1.5 cm. The mean size of the PN was 2.1 (range, 0.7–5.2) centimeters in long axis diameter.
Feasibility and safety
The safety and procedural aspects, and overall diagnostic yield of the TBAT for PN were summarized in Table 2 and Table 3. The number of PN located in left upper lung (LUL) was 11, accounted for 50% of total patients with PN. Two of the 11 patients had a history of lung cancer (all were classified as NSCLC). Of the PNs in the LUL, 4 (46.4%) were visible on fluoroscopy. The mean size of PN in LUL was 1.98 (range, 1.2–2.8) cm.
Table 2. Safety and procedural aspects of CrossCountryTM Transbronchial Access Tool.
| Site | Total number (%) | Size, cm (mean ± SD, range) | Visibility on fluoroscopy (%) | Adverse events | Tunnel length of 7 lesions accessed by TBAT, cm (mean ± SD, range) |
|---|---|---|---|---|---|
| RUL | 4 (18.2) | 2.38±1.37, 0.7–3.5 | 3 (75.0) | 0 | 1.33±0.29, 1–1.5 |
| LUL | 11 (50.0) | 1.98±0.43, 1.2–2.8 | 4 (46.4) | 0 | 1.55±0.44, 1–2 |
| RLL | 2 (9.1) | 3.25±2.76, 1.3–5.2 | 1 (50.0) | 0 | 1.25±0.35, 1–1.5 |
| LLL | 3 (13.6) | 1.4±0.26, 1.1–1.6 | 2 (66.7) | 0 | 1.75±0.35, 1.5–2.0 |
| RML | 2 (9.1) | 2.05±.5, 1.7–2.4 | 2 (100.0) | 0 | 1.5±0.71, 1–2 |
RUL, right upper lobe; LUL, left upper lobe; RLL, right lower lobe; LLL, left lower lobe; RML, right middle lobe; TBAT, Transbronchial Access Tool.
Table 3. Diagnosis of all cases.
| Diagnosis | Number | Rate |
|---|---|---|
| Benign | 3 | 17.6 (3/17) |
| Non caseating granulomas | 2 | 66.7 (2/3) |
| Mycobacterium avium complex | 1 | 33.3 (1/3) |
| Malignant | 14 | 82.3 (14/17) |
| Adenocarcinoma | 6 | 42.8 (6/14) |
| Squamous | 6 | 42.8 (6/14) |
| Carcinoid tumor of the lung | 1 | 7.1 (1/14) |
| MALT lymphoma | 1 | 7.1 (1/14) |
| Non-diagnostic | 5 | 22.7 (5/22) |
| Total diagnostic | 17 | 77.2 (17/22) |
MALT, mucosa-associated lymphoid tissue.
Sufficient samples for a histological diagnosis were obtained in 21 patients, in the remaining 1 patient the procedure was aborted due to lack of catheter apposition and presence of significant vessel interposed between the catheter and lesion. Histological diagnoses were successfully achieved in 17 of 22 patients and the total diagnostic yield was 77.2%. In contrast to yield of benign diagnosis (17.6%), the yield of malignant diagnosis was 82.3%, including 6 adenocarcinomas (42.8%), 6 squamous (42.8%) and 1 carcinoid tumor of lung cancer (7.1%).
The diagnostic yield of PN was summarized in Table 4. The diagnostic yield of lesions ≤2 and >2 cm in diameter were 75% and 76.9%, respectively (P=0.99). And in lesion >3 cm, the diagnostic yield was 100%, compared with 72.2% of diagnostic yield in lesion ≤3 cm (P=0.572). No difference in nodule size was observed. There was no significant difference in diagnostic yield which was seen depending on the localization of PN (P=0.201). Only the lesion in LUL achieved a 50% diagnostic yield, and the yields of PNs located in right upper lobe (RUL), right lower lobe (RLL), left lower lobe (LLL) and right middle lobe (RML) were all 100%. There was no adverse event during or after procedure.
Table 4. Diagnostic yield of peripheral nodules by size, location and navigation methods.
| Variables | Number of lesions (diagnostic/total) | Diagnostic yield (%) | P value | Duration of procedure (min) | P value |
|---|---|---|---|---|---|
| ≤2 cm | 6/8 | 75.0 | 1.000 | 80±23.47 | 0.990 |
| >2 cm | 10/13 | 76.9 | 79.88±17.84 | ||
| ≤3 cm | 13/18 | 72.2 | 0.549 | 81±22.03 | 0.572 |
| >3 cm | 3/3 | 100.0 | 73.33±15.89 | ||
| RUL | 4/4 | 100.0 | 0.201 | 72.25±14.77 | 0.118 |
| LUL | 5/10 | 50.0 | 73.09±17.76 | ||
| RLL | 2/2 | 100.0 | 71±15.56 | ||
| LLL | 3/3 | 100.0 | 107±24.43 | ||
| RML | 2/2 | 100.0 | 101.5±10.61 | ||
| CBCT + RUS | 8/13 | 61.5 | 0.447 | 71.38±5.4 | 0.067 |
| CBCT | 3/5 | 100.0 | 85.5±29.58 | ||
| CBCT + RUS | 5/5 | 100.0 | 97.8±17.71 |
RUL, right upper lobe; LUL, left upper lobe; RLL, right lower lobe; LLL, left lower lobe; RML, right middle lobe; CBCT, cone beam computed tomography; RUS, radial ultrasound.
In five cases (22.8%), the pathological results were non-diagnostic, all non-diagnostic cases were located in LUL.
Chest X-ray examination was performed 2 hours after the procedure, and did not reveal any complications, such as bleeding, pneumothorax and infection. And during the follow-up conducted at 3 and 6 months after the procedure, no adverse event was detected.
Discussion
The advent and introduction of tools such as CBCT and TBAT suggest the potential to improve bronchoscopy diagnostic yield in discrete lesions. Combined with TBAT, there is the suggestion of further improvement especially relative to the ‘off-airway’ type of aberrancies. Herein, we present a series utilizing ENB and CBCT guided biopsies of peripheral pulmonary nodules and in certain cases done in conjunction with the use of TBAT. To our knowledge this is only the second and the largest series of its kind. In this series, there were no procedural complications. Overall diagnostic yield was 77.2% and TBAT was used in 32% (7) of the cases. In all seven of the cases where the TBAT was deployed, a diagnosis was made. TBAT was not deployed in the remainder of the cases for two reasons. First, given the data from the various systems, r-EBUS, CBCT, ENB, etc. it appeared as if the catheter was positioned appropriately relative to lesion. Alternatively, we would have desired to deploy the TBAT device in one case but we were unable to as a consequence of the inability to approximate an appropriate trajectory between the catheter and the lesion in a three-dimensional volume. In addition, there appeared to be vasculature interposed between the position of the catheter and the target such that even if we were able to better approximate the trajectory that would have also potentially prevented deployment. In the remaining non-TBAT cases we navigated with combination of modalities and, as discussed, appeared to be able to position the catheter either adjacent to or within the target area without having to go off-airway. Interestingly, all non-diagnostic samples were obtained from the LUL and correlated with higher BMI. We hypothesize that several factors may play a role: First air flow distribution within the left upper lobe bronchus in a recumbent mechanically ventilated obese patient may predispose that area to greater atelectasis and more airway collapse especially with posterior lesions. This would, therefore, potentially obviate the benefits of any of these tools. Second, three of the four LUL biopsies were conducted relatively early in the process and may reflect on the understanding of where to best deploy the various tools when compared with the more traditional approach. For example, similar to Bowling and colleagues, we found that in all TBAT cases the EWC would straighten as soon as the catheter deployed. After some effort, we accounted for this by manipulating the EWC to a position that was at a position to compensate for relative tool stiffness once the biopsy tool was introduced the catheter would straighten into the lesion.
This series highlights the unique challenges faced by the endoscopist during a biopsy of a discrete lung aberrancies and peripheral pulmonary nodules. The three main challenges to a successful biopsy are intraprocedural localization (i.e., successful navigation), appropriate position of the catheter to the target as well as appropriate biopsy tool selection.
Intraprocedural localization, has been addressed with several advanced guiding techniques. Advent of these modalities has raised the diagnostic yield anywhere from 61% to 80%. CBCT offers a unique real-time, albeit static 3-dimensional localization. Its advantages include allowing onsite decision regarding type of access and biopsy tool needed as well as outlining the lesion and its apposition to other intrathoracic structures. Additionally, some nodules are eccentrically positioned relative to the catheter and others may or may not have an airway leading to them and thus utilization tools such as CBCT, r-EBUS, ENB, etc. would again allow for different choices depending on the relative position of the catheter and the target with respect to the potential tools’ choices available as well as for potential deployment of a TBAT. This can also allow for successful navigation to be less dependent on a “bronchus sign”.
Further, once successful localization and positioning is controlled for, it would seem reasonable to suggest that the remaining variable in a non-diagnostic sample is appropriate tool selection. This is also likely dependent on catheter’s apposition to the lesion and how it changes after each biopsy sample is obtained. Thus, the decision regarding appropriate tool selection requires continuous real time re-assessment with combination of static and dynamic diagnostic modalities such as r-EBUS, CBCT and ENB.
In our series, the combination of dynamic and static mapping and the use of TBAT allows safe and effective positioning of the biopsy tool regardless of the presence of an airway and with the potential concomitant benefit of improving yield and negative predictive value. The combination of these modalities may be an attractive option for patients who are poor surgical candidate and have peripheral lung lesions that are difficult to access with trans-thoracic needle aspiration (difficult angle of approach, depth of location, underlying emphysema). Additionally, in some circumstances, it can provide an option of one procedure that allows for not only diagnosis, but also for mediastinal staging with EBUS and placement of fiducial markers into difficult to reach lesions.
Further study to confirm these approaches and demonstrate true diagnostic yield will be necessary as these tools become increasingly part of bronchoscopy.
Acknowledgments
None.
Ethical Statement: Permission to access this information was approved by the MedStar Health Research Institute Institutional Review Board (MHRIRB 2016-189).
Footnotes
Conflict of Interest: William Krimsky: (I) Part-time employee, Medtronic; (II) Gala Therapeutics, consultant with options; (III) Innovital Systems, consultant with options; (IV) Peytant Solutions, consultant with options; (V) CSA medical. The other authors have no conflicts of interest to declare.
References
- 1.Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017;17:59. 10.1186/s12890-017-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. 10.1378/chest.11-1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loo FL, Halligan AM, Port JL, et al. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol 2014;122:191-9. 10.1002/cncy.21373 [DOI] [PubMed] [Google Scholar]
- 4.Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187-92. 10.1183/09031936.00165306 [DOI] [PubMed] [Google Scholar]
- 5.Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging 2015;15:21. 10.1186/s12880-015-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anciano C, Brown C, Bowling M. Going Off Road: The First Case Reports of the Use of the Transbronchial Access Tool With Electromagnetic Navigational Bronchoscopy. J Bronchology Interv Pulmonol 2017;24:253-6. 10.1097/LBR.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 7.Harzheim D, Sterman D, Shah PL, et al. Bronchoscopic Transparenchymal Nodule Access: Feasibility and Safety in an Endoscopic Unit. Respiration 2016;91:302-6. 10.1159/000445032 [DOI] [PubMed] [Google Scholar]
- 8.Bowling MR, Brown C, Anciano CJ. Feasibility and Safety of the Transbronchial Access Tool for Peripheral Pulmonary Nodule and Mass. Ann Thorac Surg 2017;104:443-9. 10.1016/j.athoracsur.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 9.Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone Beam Computertomography (CBCT) in Interventional Chest Medicine - High Feasibility for Endobronchial Realtime Navigation. J Cancer 2014;5:231-41. 10.7150/jca.8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohenforst-Schmidt W, Banckwitz R, Zarogoulidis P, et al. Radiation Exposure of Patients by Cone Beam CT during Endobronchial Navigation - A Phantom Study. J Cancer 2014;5:192-202. 10.7150/jca.8395 [DOI] [PMC free article] [PubMed] [Google Scholar]