Abstract
Background
The purpose of this study was to systematically assess the subjective and objective outcomes of macrolide therapy for chronic rhinosinusitis (CRS).
Methods
PubMed, Embase and Cochrane databases were searched for clinical trials detailing the effects of macrolide therapy in patients with CRS and published up to December 2017. Sino-Nasal Outcome Test (SNOT), endoscopic scores and computed tomography scans (CT) scores were assessed by mean difference (MD) or standardized mean difference (SMD) with 95% confidence interval. Subgroup analyses were performed to evaluate the source of heterogeneity according to study design and geographic locations. I2 metric was used to assess the heterogeneity.
Results
Seven randomised clinical trials (RCTs) and four cohort trials meeting pre-determined selection criteria were enrolled in this meta-analysis. Assessment of the findings for SNOT after 12 weeks’ macrolide treatment demonstrated a significant improvement in subgroup of trials in Asian patients (SMD =−0.51; 95% CI: −0.96, −0.02; P=0.04), but not in non-Asians (SMD =−0.01; 95% CI: −0.65, 0.63; P=0.98). At 12 or 24 weeks’ visit no significant difference in SNOT was noted compared with control group, either in RCTs or cohort trials subgroups. However, findings for endoscopic scores were found to be significantly improved compared to placebo in the subgroup of non-RCT studies after 8 weeks (SMD =−0.77; 95% CI: −1.07, −0.46; P<0.00001) and 12 weeks (SMD=−1.40; 95% CI: −1.97, −0.82; P<0.00001) of macrolide therapy. Similarly, findings for CT scores showed significant improvements in CT scores compared to baseline after 12 weeks’ treatment (MD=−5.81; 95% CI: −8.10, −3.52; P<0.00001) in cohort trials.
Conclusions
Macrolide therapy can significantly improve endoscopic and CT scores in CRS patients, compared to baseline. Further well-designed studies are needed to confirm the efficacy and safety of macrolides in CRS treatment.
Keywords: Macrolide antibiotics, chronic rhinosinusitis (CRS), Asian, non-Asian, meta-analysis
Introduction
Chronic rhinosinusitis (CRS) is a condition characterised by chronic inflammation of the paranasal sinuses, which shows a high prevalence worldwide and significantly affects patients’ quality of life (1). The treatment of CRS according to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2012 consists of intranasal or oral corticosteroids, nasal saline irrigation, antibiotics and surgery. The efficacy of antibiotics, however, remains controversial in the treatment of CRS (1).
Of the available antibiotics, the macrolides have been shown to have good bioavailability and tissue penetration, following oral administration (2,3). Since the first demonstration by Kikuchi and colleagues (4) of the effectiveness of long-term treatment with low-dose erythromycin in a cohort of 26 patients with CRS, this macrolide has been recommended in the treatment of CRS in Japan (5), as well as in the treatment of CRS without nasal polyps (CRSsNP) by the EPOS (1). Although the macrolides were widely applied for treating bacterial pathogens in CRS, increasing evidence has demonstrated that the macrolides possessed both anti-inflammatory and immunomodulatory effect (6-10), and lead to the concept of macrolides being immune-modulatory rather than anti-bacterial. However, several studies have shown some macrolides to have no significant benefits compared to placebo in the treatment of CRS (11-13), and therefore a matter of much debate presently. Pynnonen and colleagues (14) have conducted a meta-analysis of studies investigating the outcomes of long-term macrolide therapy for CRS and shown limited evidences to support long-term macrolide therapy. In particular, while studies in patients from Asian countries such as China and Japan have demonstrated macrolide and nasal steroids to provide a similar clinical effect for CRSsNP and both improving symptoms of CRS (13,15,16); some studies in patients from western countries have demonstrated different outcomes (11,17). Indeed, it is as been demonstrated that there was a difference of macrolide efficacy between Asian and western patients due to CRS endotypes (18), possibly as a result of differences in the inflammatory mechanisms in the ethnicity of the patients.
Since the publication of the meta-analysis by Pynnonen and colleagues (14), several studies investigating the curative effects of long-term macrolide therapy for CRS have been published. Thus, we have performed another meta-analysis of all available studies to date to re-evaluate the efficacy of macrolide therapy for CRS with additional objective measurements (endoscopic examination and CT examination) as well as distinguish the possible different curative effect between Asian and Caucasian.
Methods
The study followed recommendations of the Cochrane (http://www.cochrane.org) and the PRISMA 2009 guidelines (http://www.prisma-statement.org).
Search strategy
PubMed, Embase and Cochrane databases were systematically searched by two independent reviewers for appropriate studies published up to December 2017. The search terms were “Macrolide” or “Clarithromycin” or “Erythromycin” or “Roxithromycin” or “Azithromycin” and “Chronic rhinosinusitis”. All published studies were included in the meta-analysis if they met the following criteria: (I) the criteria for diagnosis of CRS employed in all studies were clear and as recommended outline by the Rhinosinusitis Task Force (19) or European position paper on rhinosinusitis and nasal polyps (1); (II) the patients were aged over 14 years old; (III) all patients had received oral macrolide therapy; (IV) outcomes such as Sino-Nasal Outcome Test-20 (SNOT-20), SNOT-22, endoscopic scores, and computed tomography (CT) scan scores were reported; (V) all patients had signed informed consent before participation in the study; and (VI) published in English or Chinese language. Articles published as reviews, and abstracts or reports presented at scientific/medical meetings were excluded.
Data extraction and quality assessment
Two independent researchers extracted information from the selected studies; including details of patient characteristics, experimental and control interventions employed, and main outcome measurements; according to a pre-established information table. Any uncertainty and inconformity of the extracted information was discussed between the two researchers until a general consensus was reached on the information in question. The quality of the published studies was assessed by two researchers using the risk of bias tool from Cochrane to assess the RCT clinic trials, and the Newcastle-Ottawa Scale (NOS) to assess the cohort trials.
Statistical analysis
The meta-analysis was performed using the Cochrane Review Manager, version 5.3. The differences in SNOT, endoscopic scores, and CT scores between the CRS and control groups were expressed as mean ± standard difference (SD) and 95% confidence interval (95% CI). Test of homogeneity was used to measure heterogeneity in multiple similar studies, according to the formula I2= (Q-df)/Q ×100%; where Q follows a χ2 distribution and df indicates the degrees of freedom (20). I2>50% indicates occurrence of significant heterogeneity. Fixed effect model was used in studies without heterogeneity (I2≤50%), because otherwise the fixed effect model would turn to random effect model. Values of P≤0.05 for differences in outcomes were considered to be statistically significant.
Results
Study selection and characteristics
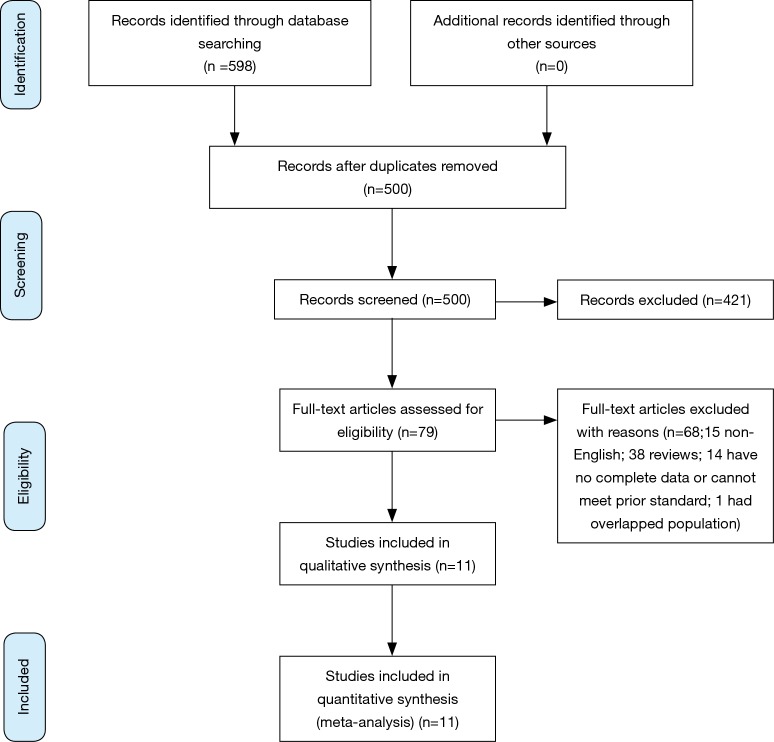
Figure 1 shows the process of selecting studies included in the meta-analysis. Searching PubMed, Embase and Cochrane databases revealed a total of 598 studies, of which 98 studies were duplicates and excluded. Thus, a total of 500 potential studies were screened and further evaluated for specific relevance to the present meta-analysis. Based on examination of the title and abstract, 421 articles were excluded because these did not meet pre-established inclusion criteria. The remaining 79 articles were obtained as full articles and following detailed examination. 68 of these were excluded (15 non-English articles; 38 reviews; 14 could not extract data or meet prior inclusion criteria; 1 had overlapped population). Finally, eleven articles were included in the meta-analysis (11-13,16,17,21-26).
Figure 1.
Flowchart of study selection.
The specific details of the studies selected for inclusion in the meta-analysis are summarized in Table 1. Seven studies were randomized controlled trials (RCTs) and four were cohort trials. Among the RCTs, four studies were performed in patients from Asian countries and three studies in patients from non-Asian countries. Thus, the Cochrane risk of bias tool was used to assess the findings from the RCTs (Figure 2) and NOS was used to assess the findings from the cohort trials. When the NOS score for any trial was ≥4, the quality of the study was considered acceptable. Overall, all eleven studies selected were found to be suitable for inclusion in the meta-analysis with a moderate risk of bias.
Table 1. Characteristic of studies.
| Author, year | Study design | Patient characteristics | Sample size | Treatment | Comparator | Median follow-up | Treatment duration (wk) | NOS |
|---|---|---|---|---|---|---|---|---|
| Amali 2015 (13) | RCT | CRS | 63 | Azithromycin 250 mg QD + nasal saline solution irrigation TID + fluticasone nasal spray 2puffs/BID | Nasal saline solution irrigation TID + fluticasone nasal spray 2puffs/BID | Baseline and 12 weeks | 12 | – |
| Deng 2018 (16) | RCT | CRS | 74 | budesonide 64 ìg/spray BID + Clarithromycin 250 mg QD | budesonide 64 ìg/spray BID | Baseline, 4, 8, 12 weeks | 12 | – |
| Jiang 2012 (21) | RCT | CRSsNPs | 53 | Erythromycin (250 mg) + a Chinese herbal medicine placebo capsule BID | Chinese herbal medicine + Erythromycin placebo capsule BID | Baseline, 8 weeks | 8 | – |
| Videler 2011 (11) | RCT | CRSsNPs | 60 | Azithromycin 500 mg QD 3 days, 500 mg QW 11 weeks + nasal saline irrigation BID | Placebo + nasal saline irrigation BID | 6, 12, 14, 24 weeks | 12 | – |
| Wallwork 2006 (17) | RCT | CRSsNPs | 64 | Roxithromycin 150 mg QD | Placebo | 6, 12, 24 weeks | 12 | – |
| Korkmaz 2014 (22) | RCT | CRSwNP | 44 | Clarithromycin 500 mg BID 2 weeks and 250 mg QD 6 weeks + metasone furoate nasal spray 200 ug once daily for 8 weeks | Metasone furoate nasal spray 200 ìg QD | Baseline, 8 weeks | 8 | – |
| Haxel 2015 (12) | RCT | CRS | 58 | Erythromycin 250 mg QD + nasal saline irrigation BID + fluticasone furoate QD | Placebo + nasal saline irrigation BID + fluticasone furoate QD | Baseline, 12, 24 weeks | 12 | – |
| Bewick 2017 (23) | Cohort | CRSsNPs | 54 | Clarithromycin 250 mg + nasal douching + intranasal mometasone (two squirts, each nostril) BID | – | 12, 24 weeks | 12 | 8 |
| Peric 2010 (24) | Cohort | CRSwNPs (atopic and nonatopic) | 40 | Clarithromycin 500 mg QD | – | Baseline, 8weeks | 8 | 8 |
| Li 2014 (25) | Cohort | CRS | 54 | Flixonase two squirts QD + Clarithromycin 250 mg QD + myrtol standardized enteric capsules 300 mg BID + nasal saline irrigation BID | – | Baseline, 12 weeks | 12 | 8 |
| Luo 2014 (26) | Cohort | CRS (CRSsNP and CRSwNP) | 50 | Clarithromycin 250 mg QD | – | Baseline, 8, 12 weeks | 12 | 8 |
NOS, Newcastle-Ottawa Scale; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps.
Figure 2.
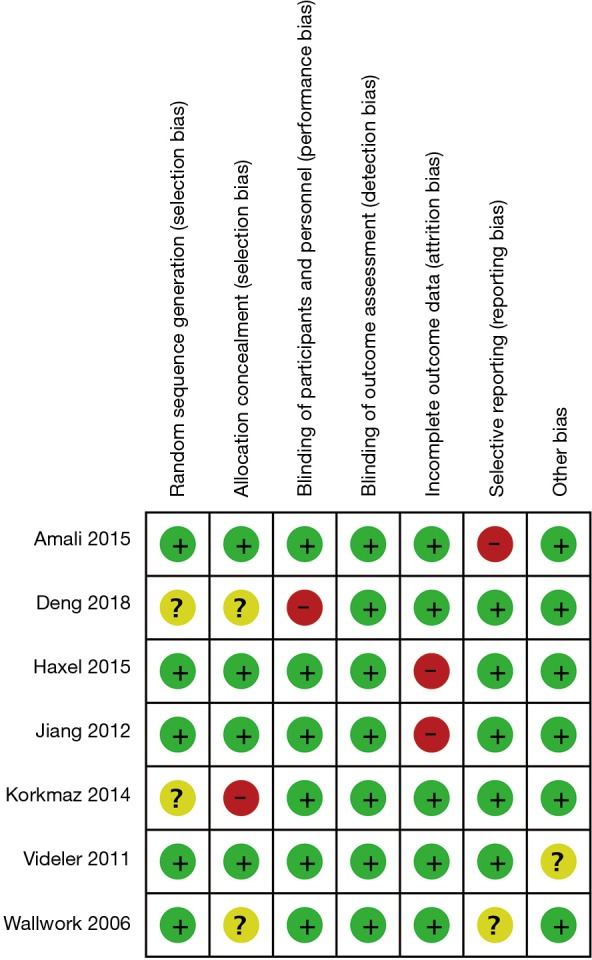
Risk of bias summary for RCT studies. RCT, randomised clinical trial.
SNOT evaluation
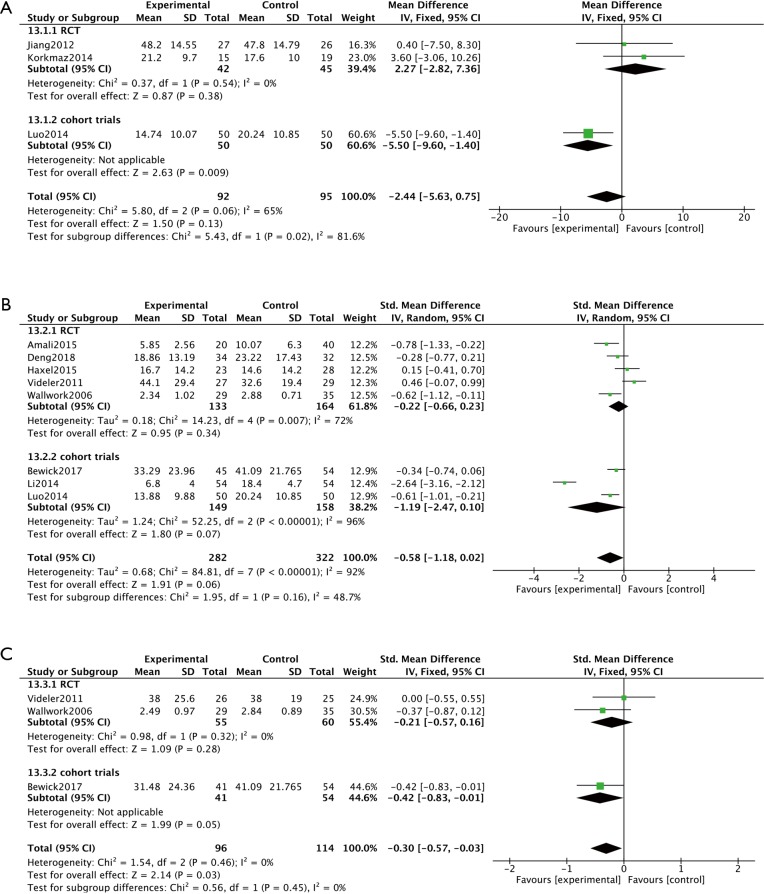
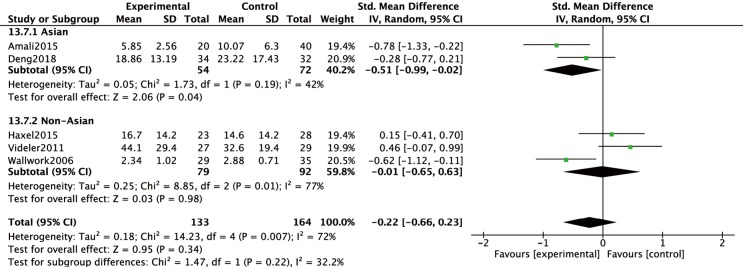
Subjective symptoms of CRS were assessed at 8-, 12- or 24-week visits by SNOT-20 or SNOT-22; whereby patients were required to answer 20 or 22 questions, respectively, according to their nasal symptoms. Assessment of findings for the subgroup of cohort trials, indicated a significant difference in SNOT scores between the macrolide-treated and control groups (MD =−5.50; 95% CI: −9.60, −1.40; P=0.009) at the 8-week visit (Figure 3A). In contrast, the findings for SNOT in the macrolide-treated group were not significantly different from those for the control group in the RCTs subgroup (MD =2.27; 95% CI: −2.28, 7.36; P=0.38) was found (Figure 3A). Similarly, no significant differences were found in SNOT scores between macrolide-treated and control groups in RCTs or cohort trials subgroups, at 12- and 24-week visits (Figure 3B,C). Furthermore, five RCTs were subdivided according to studies conducted in Asian or non-Asian patients (Figure 4). Analysis of SNOT in these subgroups demonstrated that the SNOT scores at the 12-week visit was significantly different between the macrolide-treated and control groups in the Asian subgroup (SMD =−0.51; 95% CI: −0.99, −0.02; P=0.04), but not significantly different in the non-Asian subgroup (Figure 4).
Figure 3.
No significant differences were found in SNOT between macrolide-treated and control groups at 8, 12, and 24 weeks. (A) In cohort trials, there was a significant difference in SNOT scores between the macrolide-treated and control groups (P=0.009) at the 8-week visit. In contrast, there was no significantly different in the RCTs subgroup (P=0.38). (B) No significant differences were found in SNOT scores between macrolide-treated and control groups in RCTs (P=0.34) or cohort trials subgroups (P=0.07) at 12-week visit. (C) No significant differences existed in SNOT scores between macrolide-treated and control groups in RCTs (P=0.28) or cohort trials (P=0.05) at 24-week visit. SNOT, Sino-Nasal Outcome Test; RCTs, randomised clinical trials.
Figure 4.
The SNOT scores collected at the 12-week visit was significantly different between the macrolide-treated and control groups in the Asian subgroup (P=0.04), but not in the non-Asian subgroup (P=0.98). SNOT, Sino-Nasal Outcome Test.
Objective measurements
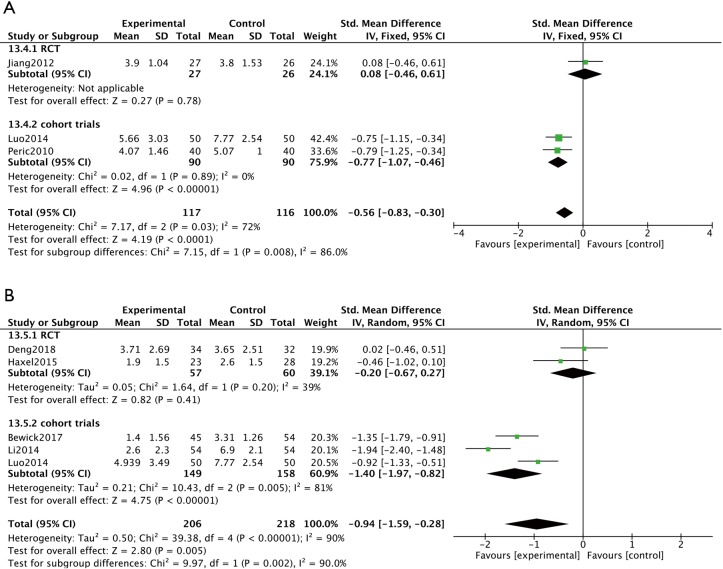
Endoscopic examination was employed to evaluate the effect of macrolide treatment in four non-RCTs, using the Lund-Kennedy scoring system or having similar scoring system which evaluated swelling, mucosal color, nasal secretions and appearance of polyps (12,21,24). Analysis of these findings demonstrated significantly reduced endoscopic scores in the macrolide treated groups compared to control groups at both the 8-week visit (SMD =−0.77; 95% CI: −1.07, −0.46; P<0.00001) (Figure 5A) and the 12-week visit (SMD =−1.40; 95% CI: −1.97, −0.82; P<0.00001) (Figure 5B).
Figure 5.
Macrolide therapy can significantly reduce endoscopic scores compared to control groups at both the 8-week visit and the 12-week visit in cohort trials. (A) In cohort trials, compared to control groups at the 8-week visit, the endoscopic scores were significantly reduced in the macrolide treated groups (P<0.00001). (B) At the 12-week visit, the endoscopic scores were significantly different between macrolide treated groups and baseline in cohort trials (P<0.00001).
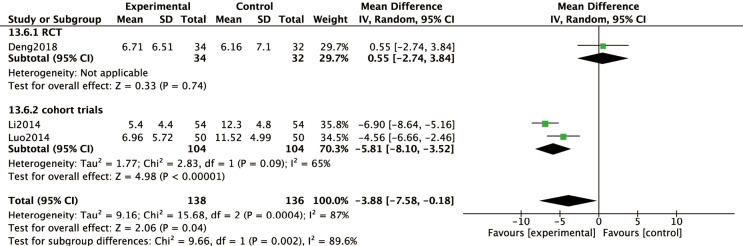
Assessment of Lund Mackay scores on CT scans were also employed to measure the effect of macrolide treatment in two cohort trials (Figure 6). These studies indicated that macrolide treatment significantly decreased the CT scores, compared to baseline (MD =−5.81; 95% CI: −8.10, −3.52; P<0.00001) (Figure 6) after 12 weeks’ treatment.
Figure 6.
Significant improvements existed in CT scores compared to baseline after 12 weeks’ treatment (P<0.00001) in cohort trials.
Discussion
The efficacy of macrolides and the mechanisms underlying their activity in the treatment of CRS have been widely investigated; with EPOS 2012 guidelines recommending the use of long-term low-dose macrolide therapy for CRSsNP (1). While some studies have shown macrolides to improve both subjective and objective outcomes (13,15,17,23,26,27), other studies have shown no significant benefits after macrolide therapy, as compared to placebo treatment (11,12). Although Wallwork and colleagues (17) recommended the use of macrolide therapy in CRSsNP patients with low level IgE, this is not strongly recommended for CRS patients with polyps (CRSwNP) patients (1,28). However, a recent study by Peric and colleagues (24) has reported that long-term low-dose clarithromycin was effective in the treatment of nasal polyps. Evidence based on existing studies, however, has been a matter of some debate due to the diverse results obtained from different studies.
The recommendation for use of macrolides in CRS has been reduced to grade-C, due to the lack of efficacy observed in Videler’s study (11). The meta-analysis by Pynnonen et al. (14) showed there was limited evidence to support the use of long-term macrolide to treat CRS, similar to a recent Cochrane review (29). However, Zeng and colleagues (15) indicated clarithromycin shows similar clinical effect as mometasone furoate (we excluded this article because of unavailable raw data). The present meta-analysis was thus conducted based on the initial meta-analysis by Pynnonen and colleagues (14), and included additional studies published subsequently. Furthermore, we compared the differences of macrolide effect in CRS patients from different areas (Asian and non-Asian countries) and different study designs (RCTs and cohort studies).
We included four cohort studies in this meta-analysis. According to cohorts the outcome of this meta-analysis showed macrolide therapy significantly improved endoscopic and CT scores in CRS patients. Different from most RCTs performed among Caucasian population, two of four cohort trails were designed by Chinese researchers. The heterogeneity of patients from different regions and ethnics might be responsible for the disparity between the findings of the RCTs and cohort studies. Additionally, RCTs compare the difference between two randomized groups, while cohort studies compare the difference before and after treatment in the same cohort group. The design of trials would lead to the different outcomes as well. Lastly, the significant difference of sample size between RCTs and cohorts might lead to the disparity when analyzed scores of endoscopic examination and CT.
Although in some RCT studies (16,21,22) there were no significant differences between experimental and control groups, we found the score of SNOT, endoscopic examination and CT were improved compared to its own baseline in experimental arm (P<0.05). These findings were similar to the outcomes in cohort studies. The minimal clinically important difference (MCID) was also considered. Previous article indicated that anchor-based approaches cannot be used for SNOT-22 (30), so we used distribution-based methods (31) for MCID of SNOT-20 or SNOT-22. We found the improvement of symptoms in Korkmaz et al. (22), Luo et al. (26), Amali et al. (13), Deng et al. (16), Wallwork et al. (17) and Li et al. (25) have clinical significance. In this regard, we demonstrated that SNOT scores were significantly improved after 12 weeks’ macrolide treatment compared with control therapy in Asian patients, but not in non-Asian patients. Moreover, objective measures, such as endoscopic scores and CT scores, showed statistical difference after 8 and 12 weeks’ macrolide therapy in cohort trials. Indeed, the findings for the differences between Asian and non-Asian CRS patients are in accordance with previous studies which have demonstrated clear differences in CRS endotypes between eastern and western patients. In particular, while the inflammatory pattern in white patients with CRS is predominantly eosinophilic, Chinese patients have been reported to demonstrate a predominantly TH1-predominant cytokine profile in CRSsNP and half of CRSwNP demonstrating a non-eosinophilic inflammatory pattern (32). Furthermore, Zhang and colleagues (18) have reported that nasal polyps of Asian patients are TH1/TH17 dominated and biased toward neutrophil inflammation, whereas nasal polyps of white patients are TH2-biased and with predominantly eosinophilic inflammation.
The differences noted in Asian and white CRS patients are of particular significance, because macrolides have been reported to potentially contribute to treatment of CRS by inhibiting proinflammatory cytokines such as IL-8, IL-1 and IL-6 (33), as well as decreasing neutrophil infiltration by reducing neutrophil chemoattractant and inducing the apoptosis of neutrophil (34). Moreover, these findings provide a possible explanation for the greater efficacy of macrolide therapy for CRS in Asian patients than in non-Asian white patients. The differences in efficacy of the macrolide therapy may also be a consequence of differences in the subtypes of nasal polyps predominating, of which five types have been reported; including plasma cell-dominant, lymphocyte-dominant, mixed inflammation, neutrophil-dominant subtype and eosinophil-dominant subtype (35). In this regard, as macrolides can inhibit IL-8, a strong neutrophil chemoattractant, the macrolide therapy may be particularly effective in the treatment of neutrophil-dominant and mixed inflammation endotype.
Conclusions
This meta-analysis indicated that macrolide therapy significantly improved endoscopic and CT scores in CRS patients, compared to baseline. However, these findings are limited, because presently relatively few high quality RCTs assessing the efficacy of macrolides in CRS patients are available. Secondly, there is no standard macrolide dose and treatment course in clinical practice, which contributes to appreciable heterogeneity of studies that could be selected for inclusion in the meat-analysis. What’s more, the different scenarios of patients in different trials (i.e., post-operation treatment versus non-operated or not recently operated cases) and distinction of scales cause high heterogeneity. Thus, further well-designed multicentre studies investigating the efficacy and safety of macrolides in the treatment of different phenotypes of CRS; with particular emphasis on the dose and duration of treatment; are clearly needed.
Acknowledgements
We thank Dr. Hui Chen for her assistance in statistics.
Funding: This work was supported by grants from the national natural science foundation of China (81630023, 81420108009, 81400444 and 81470678), the national key R&D program of China (2016YFC20160905200), the Program for Changjiang Scholars and Innovative Research Team (IRT13082), Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150203), Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150202), and Beijing Advanced Innovation Center for Food Nutrition and Human Health [Beijing Technology and Business University (BTBU) 20181045].
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012;23:3 p preceding table of contents, 1-298. [PubMed]
- 2.Aminov R. History of antimicrobial drug discovery: Major classes and health impact. Biochem Pharmacol 2017;133:4-19. 10.1016/j.bcp.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Bearden DT, Rodvold KA. Penetration of macrolides into pulmonary sites of infection. Infections in Medicine 1999;16:480-484A. [Google Scholar]
- 4.Kikuchi S, Suzaki H, Aoki A, et al. Clinical effect of long-term low-dose erythromycin therapy for chronic sinusitis. Practotol 1991;84:41-7. [Google Scholar]
- 5.Society JR. Macrolide therapy. In: The handbook of management of chronic rhinosinusitis. Tokyo: Kanehara Press 2007:49–51 [in Japanese]. [Google Scholar]
- 6.Cervin A, Wallwork B. Macrolide therapy of chronic rhinosinusitis. Rhinology 2007;45:259-67. [PubMed] [Google Scholar]
- 7.Nonaka M, Pawankar R, Saji F, et al. Effect of roxithromycin on IL-8 synthesis and proliferation of nasal polyp fibroblasts. Acta Otolaryngol Suppl 1998;539:71-5. [DOI] [PubMed] [Google Scholar]
- 8.Akamatsu H, Yamawaki M, Horio T. Effects of roxithromycin on adhesion molecules expressed on endothelial cells of the dermal microvasculature. Journal of International Medical Research 2001;29:523-7. 10.1177/147323000102900609 [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka N, Eguchi K, Kawakami A, et al. Inhibitory effect of clarithromycin on costimulatory molecule expression and cytokine production by synovial fibroblast-like cells. Clinical & Experimental Immunology 1996;104:501-8. 10.1046/j.1365-2249.1996.46752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu T, Suzaki H. Past, present and future of macrolide therapy for chronic rhinosinusitis in Japan. Auris Nasus Larynx 2016;43:131-6. 10.1016/j.anl.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 11.Videler WJ, Badia L, Harvey RJ, et al. Lack of efficacy of long-term, low-dose azithromycin in chronic rhinosinusitis: a randomized controlled trial. Allergy 2011;66:1457-68. 10.1111/j.1398-9995.2011.02693.x [DOI] [PubMed] [Google Scholar]
- 12.Haxel BR, Clemens M, Karaiskaki N, et al. Controlled trial for long-term low-dose erythromycin after sinus surgery for chronic rhinosinusitis. Laryngoscope 2015;125:1048-55. 10.1002/lary.25052 [DOI] [PubMed] [Google Scholar]
- 13.Amali A, Saedi B, Rahavi-Ezabadi S, et al. Long-term postoperative azithromycin in patients with chronic rhinosinusitis: A randomized clinical trial. Am J Rhinol Allergy 2015;29:421-4. 10.2500/ajra.2015.29.4244 [DOI] [PubMed] [Google Scholar]
- 14.Pynnonen MA, Venkatraman G, Davis GE. Macrolide therapy for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg 2013;148:366-73. 10.1177/0194599812470427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng M, Long XB, Cui YH, et al. Comparison of efficacy of mometasone furoate versus clarithromycin in the treatment of chronic rhinosinusitis without nasal polyps in Chinese adults. Am J Rhinol Allergy 2011;25:e203-7. 10.2500/ajra.2011.25.3728 [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Chen F, Lai Y, et al. Lack of additional effects of long-term, low-dose clarithromycin combined treatment compared with topical steroids alone for chronic rhinosinusitis in China: a randomized, controlled trial. Int Forum Allergy Rhinol 2018;8:8-14. 10.1002/alr.22041 [DOI] [PubMed] [Google Scholar]
- 17.Wallwork B, Coman W, Mackay-Sim A, et al. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006;116:189-93. 10.1097/01.mlg.0000191560.53555.08 [DOI] [PubMed] [Google Scholar]
- 18.Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 2008;122:961-8. 10.1016/j.jaci.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117:S1-7. 10.1016/S0194-5998(97)70001-9 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang RS, Wu SH, Tsai CC, et al. Efficacy of Chinese herbal medicine compared with a macrolide in the treatment of chronic rhinosinusitis without nasal polyps. Am J Rhinol Allergy 2012;26:293-7. 10.2500/ajra.2012.26.3778 [DOI] [PubMed] [Google Scholar]
- 22.Korkmaz H, Ocal B, Tatar EC, et al. Biofilms in chronic rhinosinusitis with polyps: is eradication possible? Eur Arch Otorhinolaryngol 2014;271:2695-702. 10.1007/s00405-013-2756-x [DOI] [PubMed] [Google Scholar]
- 23.Bewick J, Ahmed S, Carrie S, et al. The value of a feasibility study into long-term macrolide therapy in chronic rhinosinusitis. Clin Otolaryngol 2017;42:131-8. 10.1111/coa.12685 [DOI] [PubMed] [Google Scholar]
- 24.Peric A, Vojvodic D, Baletic N, et al. Influence of allergy on the immunomodulatory and clinical effects of long-term low-dose macrolide treatment of nasal polyposis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010;154:327-33. 10.5507/bp.2010.049 [DOI] [PubMed] [Google Scholar]
- 25.Li S, Chen J, Yu Y, et al. Short-term efficacy of standardized medication offer chronic rhinosinusitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2014;28:75-9. [PubMed] [Google Scholar]
- 26.Luo Q, Deng J, Xu R, et al. Clinical effect of clarithromycin therapy in patients with chronic rhinosinusitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2014;49:103-8. [PubMed] [Google Scholar]
- 27.Oliveira IS, Crosara PF, Cassali GD, et al. Evaluation of the improvement of quality of life with Azithromycin in the treatment of eosinophilic nasal polyposis. Braz J Otorhinolaryngol 2016;82:198-202. 10.1016/j.bjorl.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl 2007;20:1-136. [PubMed] [Google Scholar]
- 29.Head K, Chong LY, Piromchai P, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4:CD011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhury NI, Mace JC, Bodner TE, et al. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol 2017;7:1149-55. 10.1002/alr.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582-92. 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- 32.Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 2009;124:478-84, 484.e1-2. [DOI] [PubMed]
- 33.Cervin A, Kalm O, Sandkull P, et al. One-year low-dose erythromycin treatment of persistent chronic sinusitis after sinus surgery: clinical outcome and effects on mucociliary parameters and nasal nitric oxide. Otolaryngol Head Neck Surg 2002;126:481-9. 10.1067/mhn.2002.124849 [DOI] [PubMed] [Google Scholar]
- 34.Inamura K, Ohta N, Fukase S, et al. The effects of erythromycin on human peripheral neutrophil apoptosis. Rhinology 2000;38:124-9. [PubMed] [Google Scholar]
- 35.Lou H, Meng Y, Piao Y, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology 2016;54:150-9. 10.4193/Rhin15.271 [DOI] [PubMed] [Google Scholar]