Abstract
Background
The aim of this study was to investigate differences in the imaging features of mass-like tuberculosis and lung cancer on conventional MR sequences to improve the diagnostic ability for pulmonary masses.
Methods
Thirty patients with suspicious pulmonary lesions were enrolled and diagnosed with tuberculosis by pathology or comprehensive clinical diagnoses. Twenty-six cases of lung cancer were retrospectively analyzed. Transverse fat-suppressed T2-weighted (T2W) imaging and T1-weighted (T1W) imaging were obtained at 1.5 Tesla. The imaging characteristics of lesions on the T2W and T1W images were compared between the two groups. The imaging features of enlarged mediastinal lymph nodes on T2W images were studied and compared.
Results
On T2W images, there was a higher percentage of lesions containing hypointensity in the tuberculosis group (GTB) than in the lung cancer group (GLC) (P=0.004).The incidence of lesions demonstrating heterogeneous intensity was significantly greater in the GTB than in the GLC (70.0% vs. 7.7%, P=0.001). Approximately 92.3% of the lung cancer cases showed hyperintensity, a proportion substantially greater than that in the GTB (6.7%). On T1W images, more cases showed hyperintensity in the GTB than in the GLC (43.3% vs. 7.7%, P=0.003). The signal intensity ratios (SIRs) of the lesion to rhomboid muscle on T2W and T1W images were significantly different between the two groups. The mean intrasubject standard deviation (SD) of lesions in the GTB was markedly greater than that in the GLC on both T2W and T1W images. Benign mediastinal lymph nodes in the GTB showed a variety of signals on T2W images, whereas 80% of metastatic mediastinal lymph nodes displayed slight homogeneous hyperintensity, and this difference between the two groups was statistically significant.
Conclusions
Conventional MR sequences can reveal the essential differences between mass-like tuberculosis and lung cancer and may be helpful for discriminating pulmonary masses.
Keywords: Tuberculosis, lung neoplasm, magnetic resonance imaging, differential diagnosis
Introduction
Pulmonary tuberculosis is a common pulmonary infectious disease. There was an estimated 10.4 million newly reported TB cases worldwide in 2015 (1). Although the morbidity of tuberculosis has decreased in most developed countries, it continues to affect high-risk urban groups, such as people who originate from areas with a high tuberculosis burden in Asia and Africa, the poor, and those with immunological deficiency. Tuberculosis can lead to high rates of disability and mortality, which confers a heavy burden on families and society.
Tuberculosis exhibits miscellaneous imaging features in different stages of the disease. The radiological signs of tuberculosis are frequently misleading. Tuberculosis may be initially misinterpreted as lung cancer in approximately 25% of cases (2), leading to delayed treatment, invasive biopsy and even unnecessary resection. CT, a major imaging modality for chest disease, can provide important diagnostic and differential information, especially when used with contrast-enhanced examination. However, the low resolution of soft tissue is a major limitation of CT for the differential diagnosis of pulmonary masses, even with contrast medium. Furthermore, the radiation exposure is non-negligible, particularly for young patients and pregnant women. Therefore, an alternative noninvasive modality would be helpful for discriminating pulmonary masses or nodules.
MRI might be a good choice for this purpose due to its higher contrast resolution, absence of radiation, and multiple-parameter imaging. With the rapid development of imaging and post-processing, MRI has found relevant applications in chest disease and for the differential diagnosis of benign and malignant pulmonary lesions (3-5). A previous study indicated comparable abilities of MRI and CT to identify the location and distribution of lung tuberculosis lesions, and the higher resolution of MRI enabled the improved identification of parenchymal inhomogeneity, caseation, and pleural or nodal involvement (6). Although many advanced imaging techniques and post-processing algorithms, such as contrast-enhanced MR imaging and diffusion-weighted imaging (7-9), are available for the diagnosis and differential diagnosis of pulmonary lesions, conventional MR sequences are fundamental, convenient and practical for clinical work with a short imaging time. Therefore, the aim of this study was to investigate the imaging features of mass-like tuberculosis on T2-weighted (T2W) and T1-weighted (T1W) images compared with lung cancer to improve the diagnostic ability for pulmonary masses.
Methods
Patient enrollment and characteristics
This was a prospective study of MR imaging of tuberculosis patients, and the analysis involved a retrospective component that compared these tuberculosis patients with lung cancer patients who were scanned with MR. The institutional review board of our hospital approved this study. Informed consent was obtained from all enrolled tuberculosis patients. The entry criteria for the tuberculosis group (GTB) included the following: (I) undetermined mass or nodule detected by chest CT; (II) no contraindications to MRI scanning; and (III) pathology-proven pulmonary tuberculosis or comprehensive diagnosis of pulmonary tuberculosis proven by radiologic follow-up after anti-tuberculosis treatment. Cases without a clear diagnosis of the lesions were excluded. Thirty patients with 30 lesions were enrolled in the GTB from January 2012 to December 2014.
CT and MRI images of a group of consecutive lung cancer cases (GLC) were retrospectively reviewed with the purpose of studying mediastinal lymph nodes, and informed consent was waived. The enrollment criteria for the GLC included the following: (I) pathologically proven diagnosis; (II) lesion manifesting as a solid mass or nodule; and (III) initial diagnosis without treatment. Twenty-six patients with 26 lesions met all of these criteria and were enrolled in the GLC.
Chest CT scanning and image reconstruction
Chest CT was performed with two 64-row CT scanners (GE Discovery CT750 HD; GE Lightspeed VCT; GE Healthcare, Milwaukee, Wisconsin, USA). The range of the scans included the upper supraclavicular area and the lower adrenal area on both sides. The scanning parameters were 120 kVp and auto milliampere settings (tube current, 400-50 mA ; noise index, 13; pitch, 0.984:1; 40 mm/rotation; 5-mm slice thickness; 0.8 s/rotation). Contrast material (1.5 mL/kg, 350 mg I/mL; Bayer Schering, Berlin, Germany) was administered intravenously at a rate of 2.5 mL/s for twenty cases in the GTB and thirteen cases in the GLC. The contrast-enhanced CT was scanned at 30 seconds and 90 seconds after the injection of contrast agents. Axial CT images with a slice thickness of 1.25 mm were obtained, and 5.0-mm axial, coronal and sagittal section images were reconstructed.
MR scans
All patients were scanned on a 1.5 T MR scanner (GE OPTIMA MR360) with an eight-channel phased-array body coil. All MR imaging examinations were performed with the patients in the supine position. Transverse T2W and T1W images were acquired for all enrolled cases. Fat-suppressed T2W fast spin-echo images with respiratory triggering were scanned with the following settings: repetition time/echo time, 6,000–8,000 ms (1–2 R-R interval of the respiratory cycle)/86 ms; section thickness, 5 mm; slice gap,1 mm; field of view, 32–38 cm; echo train length, 18; matrix, 288×256; number of excitations, 4; scan time = 2–4 min. 3D T1W imaging (LAVA-FLEX) (10) was scanned with the breath held to obtain in-phase and out-of-phase images simultaneously with the following settings: repetition time/echo time, 6.1 ms/4.2 ms for in-phase; repetition time /echo time, 6.1 ms/2.1 ms for out-of-phase; section thickness, 5 mm; slice gap, 1 mm; field of view, 32–38 cm, matrix, 260×192; flip angle, 10°; band width, 62.5 kHz; phase FOV, 0.80; number of excitations, 0.7; scan time =19 seconds.
Image analysis
Two experienced chest radiologists reviewed the MR and CT images and reached agreement by consultation. All images were reviewed on a PACS. The location of the lesion was recorded, and the largest diameter was measured on CT. The region of interest (>20 mm2) was placed on a solid area, avoiding liquefactive necrotic areas. The peak enhancement CT value and plain scan CT value of the region of interest were measured, and the net enhancement value (peak enhancement CT value − plain scan CT value) was computed for the contrast-enhanced CT scans. Lesions with a net enhancement value ≥15 HU were considered significantly enhanced (11). Non-calcified lymph nodes with a short axis ≥8 mm were measured, and the stations were recorded according to the lymph node map for AJCC cancer staging 7th version (12). Lymph nodes with hyperattenuation in the hilum and mediastinum on unenhanced CT were classified as benign and were excluded. Abnormalities in other lung fields were also recorded.
The signal characteristics of the lesions on T2W images in the GTB and the GLC were visually analyzed. Compared with the rhomboid muscle, the signal intensity of the lesions was classified as follows: (I) isointensity/hypointensity; (II) slight hyperintensity; and (III) heterogeneous intensity. Fused nodules and lesions with cavities were recorded.
The signal intensity of the lesions on out-of-phase T1W images was classified into four types compared to the rhomboid muscle: (I) hypointensity; (II) isointensity; (III) hyperintensity; and (IV) heterogeneous intensity. The signal intensity of the lesions on T2W and out-of-phase T1W images was measured by placing a round or oval region of interest over the slice in which the lesion had maximal extension. The signal intensity ratio (SIR) of the lesion to rhomboid muscle on both T2W and T1W images was computed as follows: SIR = SL/SM·100%, where SL is the signal intensity of the lesion and SM is the signal intensity of rhomboid muscle. The intrasubject standard deviation (SD) was recorded for each lesion.
The mediastinal enlarged lymph nodes (MLNs) on T2W images were recognized according to the location shown on CT. The signal intensity was compared to that of the rhomboid muscle and classified as follows: (I) hypointensity/isointensity; (II) slight hyperintensity; (III) bullseye sign (with hyperintensity in the periphery and isointensity/slight hyperintensity in the center of the lymph nodes); (IV) rim-like (even thickening of the cortex with low intensity in the hilum of the lymph node); and (V) heterogeneous intensity.
Diagnostic criteria
Pathological diagnosis of tuberculosis was made by resection of the lesion (n=5), CT-guided core-needle biopsy (n=5), and mediastinoscopy biopsy or mediastinal lymphadenectomy (n=2). Clinical diagnosis of tuberculosis was made based on comprehensive laboratory tests in the tuberculosis hospital and a radiologic follow-up study over more than one year that revealed significant regression of the mass or nodules after anti-tuberculosis chemotherapy in eighteen cases.
Pathologic diagnosis of lung cancer was made by CT-guided core-needle biopsy, bronchoscopy biopsy or transbronchial needle aspiration. Lung cancer comprised adenocarcinoma (n=18), squamous cell carcinoma (n=5), adeno-squamous carcinoma (n=1), carcinoid (n=1) and small cell lung cancer (n=1).
Thirty-five stations of MLNs were observed in twelve cases in the GTB. Fifteen benign MLNs were pathologically proven in 5 cases. The other 20 benign MLNs in seven cases were proven by radiologic follow-up showing decreased size or no significant change after at least one year of follow-up.
Twenty cases of metastatic MLNs in ten cases of the GLC were all pathologically proven by mediastinoscopy biopsy or mediastinal lymphadenectomy (n=7), transbronchial needle aspiration biopsy (n=2), or CT-guided core-needle biopsy (n=1).
Diagnostic criteria of MRI
Two diagnostic criteria for differentiating the GTB from the GLC were used. According to the first criterion, a lesion showing isointensity/hypointensity or heterogeneous intensity on T2W images was judged as TB, whereas that presenting as slight hyperintensity was judged as lung cancer. In addition, a lesion showing hyperintensity on T1W images was judged as TB; otherwise, the lesion was judged as lung cancer. If the signal features of the lesion met the TB diagnostic criterion on either T1W or T2W, the lesion was judged as TB. As the second criterion, patients were divided by using the cut-off point of T2W SIR or T1W SIR.
Statistical analyses
Statistical tests were conducted using statistical software (SPSS, version 24.0.; SPSS/IBM, Chicago, IL, USA). The chi-square test was used to detect differences in the location distribution, enhancement rate of contrast-enhanced CT, and signal characteristics on T2W and T1W images between the GTB and the GLC. The Mann-Whitney U test was applied to compare the SIR of lesions to the rhomboid muscle between the two groups. Box plots were used to compare SIR for both T2W and T1W images between the two groups. Receiver operating characteristic curve analysis was applied to evaluate the diagnostic performance of SIR, and a cut-off value was selected by using the maximum Youden index. The independent samples t-test was used to detect differences in the mean size of MLNs and mean intrasubject SD between the two groups under the assumption of a normal distribution. Statistical significance was considered at P<0.05.
Results
Case characteristics in the GTB and the GLC
The detailed characteristics of the two groups are presented in Table 1. The cohort was younger in the GTB than in the GLC (49.7±14.3 vs. 59.5±9.6 yrs, Mann-Whitney U, P=0.012). The enhancement ratio on contrast-enhanced CT was lower in the GTB than in the GLC (25.0% vs. 84.6%, P=0.001). The mean size and distribution of the lesion locations did not differ significantly between the two groups. Patchy or small nodules were found in 20 cases of the GTB, including eight around the lesions and twelve distributed in other pulmonary lobes.
Table 1. Characteristics of the GTB and GLC.
| Clinical features | GTB | GLC | P value |
|---|---|---|---|
| Male: female | 23:7 | 12:14 | 0.019 |
| Mean age (years) | 49.7 | 59.5 | 0.012 |
| Location | 0.116 | ||
| RUL | 17/30 | 8/26 | |
| RML | 3/30 | 2/26 | |
| RLL | 5/30 | 3/26 | |
| LUL | 4/30 | 9/26 | |
| LLL | 1/30 | 4/26 | |
| Size (mm, mean ± SD) | 45.15±19.30 | 44.92±18.17 | 0.965 |
| CT | – | ||
| CE-CT | 20 | 13 | |
| Plain CT | 10 | 13 | |
| Net enhancement (HU) | 0.001 | ||
| <15 | 15 (75.0%) | 2 (15.4%) | |
| ≥15 | 5 (25.0%) | 11 (84.6%) |
GTB, tuberculosis group; GLC, lung cancer group; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
Mass characteristics and SIR on T2W and T1W images
Table 2 shows the characteristics and SIR of TB and lung cancer on T2W and T1W images. A typical TB mass that exhibited homogeneous hypointensity on T2W images is shown in Figure 1. The TB shown in Figure 2 exhibited heterogeneous intensity with patchy and scattered spot hypointensity. When these two types of hypointensity on the T2W images were combined, there was a higher percentage (12/30 vs. 2/26) of lesions containing hypointense areas in the GTB than in the GLC (P=0.004).
Table 2. MRI features of pulmonary lesions on T2W images and T1W images.
| Signal features | GTB | GLC | |||||
|---|---|---|---|---|---|---|---|
| n (%) | Average* | Intrasubject SD* | n (%) | Average* | Intrasubject SD* | ||
| T2W | |||||||
| Isointensity/hypointensity | 7 (23.3) | 168.71±42.44 | 53.44±26.54 | 0 | – | – | |
| Slightly hyperintensity | 2 (6.7) | 321.81±93.69 | 42.40±26.59 | 24 (92.3) | 329.82±108.32 | 28.68±25.89 | |
| Heterogeneous intensity | 21 (70.0) | 292.30±113.94 | 79.36±47.27 | 2 (7.7) | 323.56±160.59 | 53.50±48.79 | |
| Total | 30 | 268.76±112.56 | 70.85±43.50 | 26 | 329.34±108.76 | 30.59±27.52 | |
| Fused nodules | 1 (3.3) | – | – | 0 | – | – | |
| Cavity | 6 (20.0) | – | – | 1 (3.8) | – | – | |
| T1W | |||||||
| Hypointensity | 1 (3.3) | 79.88 | 10.30 | 1 (3.8) | 43.18 | 1.20 | |
| Isointensity | 9 (30.0) | 88.83±14.16 | 59.18±29.22 | 20 (76.9) | 93.70±16.05 | 12.96±16.15 | |
| Hyperintensity | 13 (43.3) | 124.06±29.75 | 52.92±29.93 | 2 (7.7) | 112.63±1.48 | 39.05±38.82 | |
| Heterogeneous intensity | 7 (23.3) | 113.01±21.52 | 72.16±98.53 | 3 (11.5) | 111.50±0.83 | 54.40±41.00 | |
| Total | 30 | 110.44±27.33 | 57.86±52.47 | 26 | 94.04±18.79 | 19.25±24.88 | |
*, mean ± SD. GTB, tuberculosis group; GLC, lung cancer group;
Figure 1.
Tuberculosis was pathologically proven by surgical removal of the lesion from the right middle lobe in a 65-year-old male patient. (A) The lobulated mass (arrow) showed hypointensity on T2W imaging; (B) on T1W imaging, the mass showed hyperintensity. T2W, T2-weighted.
Figure 2.
A 46-year-old male patient with a comprehensive clinical diagnosis of tuberculosis. (A) CT showed a mass in the right upper lobe; (B) the mass exhibited heterogeneous intensity with patchy hypointensity (arrow) and multiple scattered low intensity and focal hyperintensity on T2W imaging; (C) the mass showed hyperintensity on T1W imaging. T2W, T2-weighted; T1W, T1-weighted.
The incidence of lesions exhibiting a heterogeneous signal on T2W images was significantly greater in the GTB than in the GLC (21/30 vs. 2/26, P<0.001). Six cases with a lamellate structure (hypointensity or isointensity in the center and single or double ring peripherally located) were observed only in the GTB and pathologically corresponded to granulomatous inflammation with necrosis (Figure 3). By contrast, 92.3% (24/26) of the GLC showed homogeneous slight hyperintensity on T2W images.
Figure 3.
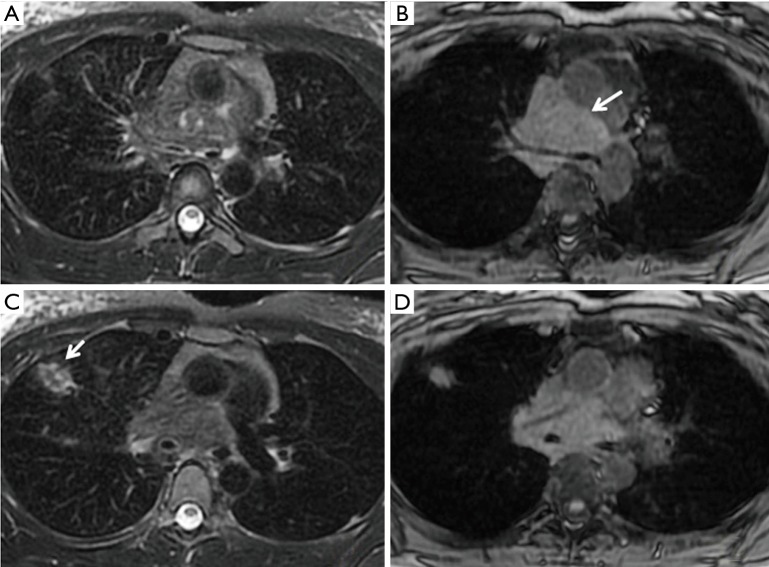
Tuberculosis was pathologically proven by resection of the lesion in the left lower lobe in a 43-year-old male patient. The nodule (arrow) showed a lamellate structure on T2W imaging (A) and heterogeneous intensity on T1W imaging (B). (C) Pathology (H&E 40×) showed tuberculous granulomatous inflammation with large organizing necrosis; immunohistochemical results showed acid fast stain positivity. T2W, T2-weighted; T1W, T1-weighted.
Thirteen cases showed hyperintensity on T1W images in the GTB (Figure 4), in contrast to two cases in the GLC (P=0.003).
Figure 4.
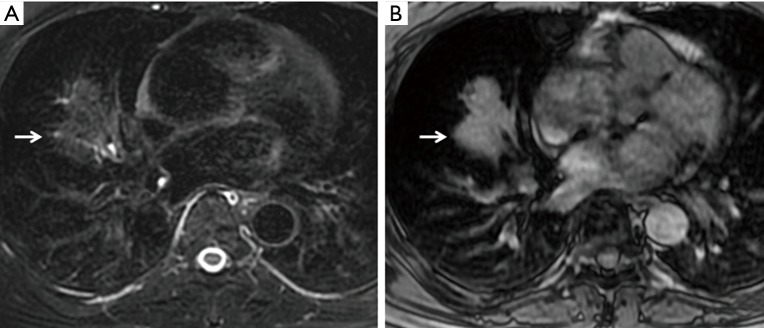
CT detected a large mass in the mediastinum in a 20-year-old female who was twice negative upon transbronchial needle aspiration biopsy. The mediastinal mass (long arrow) showed isointensity on T2W imaging (A,C) and hyperintensity on T1W imaging (B,D). A small nodule (short arrow) in the right upper lobe showed heterogeneous intensity on T2W imaging. After six months of anti-tuberculosis chemotherapy, radiologic follow-up showed a marked decrease of the mediastinal mass (not shown). T2W, T2-weighted; T1W, T1-weighted.
The signal intensity of the rhomboid muscle was comparable between the GTB and GLC (T2W: 143.10±214.34 vs. 90.73±70.71, P=0.162; T1W: 602.67±536.73 vs. 434.77±452.33, P=0.203), and no difference was detected in the intrasubject SD of muscle signal intensity between the GTB and GLC (T2W: 16.45±15.60 vs. 14.11±12.03, P=0.419; T1W: 29.6±31.12 vs. 24.41±25.29, P=0.507). The SIR of lesions to the rhomboid muscle was significantly different on both T2W and T1W images between the GTB and the GLC (Mann-Whitney U, P=0.049 and P=0.042, respectively) (Figure 5). The mean intrasubject SD of the GTB was dramatically greater than that of the GLC on both T2W and T1W images (P<0.001 and P=0.001).
Figure 5.
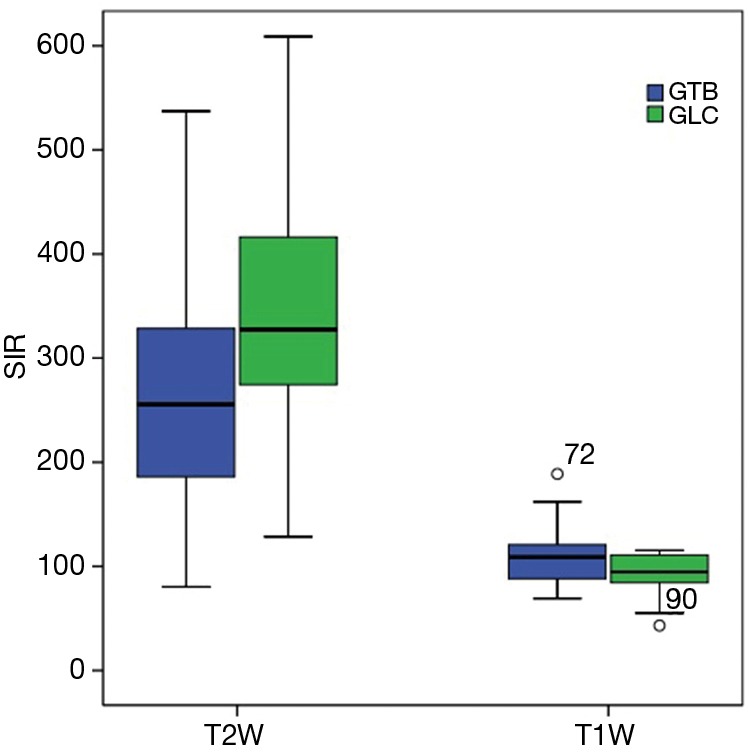
SIR of the lesion to rhomboid muscle on T1W and T2W images compared between the GTB and GLC. SIR, signal intensity ratio; GTB, tuberculosis group; GLC, lung cancer group; T2W, T2-weighted; T1W, T1-weighted.
Characteristics of MLNs on T2W images
The mean size of benign MLNs in the GTB was 13.55±5.50 mm (mean ± SD) and did not differ from that of metastatic lymph nodes in the GLC, 14.71±3.30 mm (P=0.419). The signal characteristics of MLNs on T2W images in the GTB and the GLC are presented in Table 3. In the GTB, the benign enlarged lymph nodes exhibited a variety of intensities, with 42.8% showing hypointensity and 28.6% showing a bullseye sign (corresponding to granuloma with necrosis on pathology), as exemplarily shown in Figure 6. However, 80.0% of metastatic lymph nodes in the GLC displayed homogeneous slight hyperintensity, a much higher proportion than in the GTB (14.3%).
Table 3. Characteristics of mediastinal enlarged lymph nodes on T2W images in the GTB and GLC.
| Signal features on T2W | Benign MLN in the GTB (%) | Metastatic MLN in the GLC (%) |
|---|---|---|
| Hypointensity/isointensity | 15 (42.8) | 0 |
| Slight hyperintensity | 5 (14.3) | 16 (80.0) |
| Bullseye sign | 8 (28.6) | 0 |
| Rim-like | 4 (5.7) | 0 |
| Heterogeneous intensity | 3 (8.6) | 4 (20.0) |
T2W, T2-weighted; GTB, tuberculosis group; GLC, lung cancer group; MLN, mediastinal lymph nodes.
Figure 6.
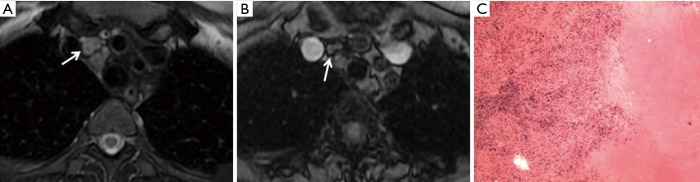
A 61-year-old female patient with a history of fever. Consolidation in the right middle lobe and multiple enlarged lymph nodes were detected by CT. (A) The multiple clustering of enlarged lymph nodes showed a bullseye sign (arrow) on T2W imaging; (B) T1W image of the same slice; (C) pathology (H&E 20×) of lymph nodes by mediastinoscopy biopsy: granuloma with necrosis and tuberculosis was suspected. After ten months of anti-tuberculosis chemotherapy, her symptoms disappeared, and radiologic follow-up showed a marked decrease in the mediastinal lymph nodes. T2W, T2-weighted; T1W, T1-weighted.
Diagnostic performance of MRI
The detailed diagnostic performance of the signal characteristics of lesions and the SIR of the lesion to rhomboid muscle on both T2W and T1W images is shown in Table 4. The signal features of lesions on T2W images demonstrated a superior diagnostic accuracy of 92.9%. Hyperintensity of lesions presented on T1W images showed a high true negative rate of 92.3% and a positive predictive value of 86.7%. The combination of T2W and T1W imaging achieved the highest overall accuracy of 94.6%, with both a high true positive rate of 96.7% and a true negative rate of 92.3%.The areas under the receiver operating characteristic curve of T2W SIR and T1W SIR were 0.660 (95% CI, 0.516 to 0.805) and 0.659 (95% CI, 0.516 to 0.802), respectively, and the selected cut-off values were 276 and 116, respectively. The overall diagnostic accuracy of T2W SIR and T1W SIR was only 66.1%.
Table 4. Diagnostic performance of MRI.
| MRI | Group | TPR (%) | TNR (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|---|
| GTB | GLC | ||||||
| T2W* | |||||||
| GTB | 28 | 2 | 93.3 | 92.3 | 93.3 | 92.3 | 92.9 |
| GLC | 2 | 24 | |||||
| T1W** | |||||||
| GTB | 13 | 2 | 43.4 | 92.3 | 86.7 | 58.5 | 66.1 |
| GLC | 17 | 24 | |||||
| T2W + T1W& | |||||||
| GTB | 29 | 2 | 96.7 | 92.3 | 93.5 | 4.0 | 94.6 |
| GLC | 1 | 24 | |||||
| T2W-SIR# | |||||||
| GTB | 18 | 7 | 60.0 | 67.9 | 72.0 | 61.3 | 66.1 |
| GLC | 12 | 19 | |||||
| T1W-SIR## | |||||||
| GTB | 11 | 0 | 36.7 | 100 | 100 | 57.8 | 66.1 |
| GLC | 19 | 26 | |||||
*, isointensity/hypointensity or heterogeneous intensity was judged as GTB; slight hyperintensity was judged as GLC; **, hyperintensity was judged as GTB; hypointensity, isointensity or heterogeneous intensity was judged as GLC; &, if the signal features of the lesion met the GTB diagnostic criterion on either T1W or T2W, the lesion was judged as GTB; otherwise, the lesion was judged as GLC; #, T2W-SIR <276 was judged as GTB; T2W-SIR ≥276 was judged as GLC; ##, T1W-SIR ≥116 was judged as GTB; T1W-SIR <116 was judged as GLC. TPR, true positive rate; TNR, true negative rate; PPV, positive predictive value; NPV, negative predictive value; GTB, tuberculosis group; GLC, lung cancer group; T2W, T2-weighted; T1W, T1-weighted; SIR, signal intensity ratio.
Discussion
The imaging features of pulmonary tuberculosis are often diverse in different stages of the disease, ranging from exudation, consolidation, and the proliferation of nodules to fibrosis and calcification. Tuberculosis can occasionally mimic a tumor with lobulated and/or atypical spiculated appearance in morphology, and such mimics are the main entities of benign lesions that must be discriminated from lung cancer. Contrast-enhanced CT plays an important role in differentiating benign from malignant lesions. In general, a net enhancement value of less than 15 HU indicates a high possibility of a benign lesion (11). However, the net enhancement value of a mass might overlap between benign and malignant lesions, as observed in the present study. A noninvasive imaging modality is needed to improve the diagnosis of pulmonary mass-like lesions.
The results of the present study showed that mass-like tuberculosis may exhibit hypointensity and heterogeneous intensity on T2W images; some lesions showed hyperintensity on T1W images, which was fundamentally different from the features of lung cancer. MLNs in tuberculosis may present as a variety of signal attenuations, which differs from metastatic lung cancer. This fuller understanding of the imaging features of mass-like tuberculosis will aid the differentiation of tuberculosis from lung cancer.
Our study demonstrated that more lesions showed hypointensity on T2W images in the GTB than in the GLC. A previous study also reported this feature in a group of tuberculoma cases (13). The cause of this hypointensity in tuberculosis remains unclear. Some researchers have attributed the hypointensity to paramagnetic substances produced during phagocytosis, such as macrophage-laden oxygen-free radicals that cause shortening of the T2 value (14). Brown et al. (15) hypothesized that calcification, air cysts, fibrous tissue, collagen tissue, and paramagnetic material in low-intensity pulmonary lesions might all contribute to T2 shortening or magnetic susceptibility effects on T2W images. In this study, we observed that mass-like tuberculosis not only had a higher ratio of hypointensity on T2W images but also had a higher SIR of lesion to muscle than lung cancer on T1W images. Furthermore, the pathology of one resected TB mass revealed epithelioid cells and hyperplasia of fibrous tissue surrounding a caseous necrosis area. These results support the hypothesis that the effects of paramagnetic substances and fibrous tissue both contribute to the hypointensity of TB masses on T2W images.
Furthermore, our results also showed that more lesions exhibited heterogeneous intensity on T2W images in the GTB than in the GLC. The characteristic features of heterogeneous intensity include multiple hypointensity and/or multiple, small focal hyperintensity. The distribution of hyperintensity in mass-like tuberculosis was eccentric or scattered, reflecting liquefactive necrosis, and differed significantly from the central necrosis of lung cancer. Six lesions showed a lamellate structure embodying the histopathological features of tuberculoma: central intensity corresponding to caseous necrosis or scarring surrounded by fibrous tissue, which, in one resected case in this study, surrounded the epithelioid granulomas. The high resolution for soft tissue is an absolute advantage of MR imaging over CT for revealing the pathological features of tuberculosis. Moreover, a previous study showed that it was practical to monitor therapeutic efficacy by analyzing the signals of lesions on T2W images to distinguish exudation from fibrosis (16).
The clarification of MLNs is vital to the diagnosis and staging of lung cancer but remains challenging because tuberculosis, inflammation, and sarcoidosis can also cause enlargement of lymph nodes. CT, a primary modality for thoracic imaging, shows limited value in identifying MLNs in the presence of other confounding factors (17). MRI is even more valuable for the discrimination of enlarged lymph nodes than PET-CT (18,19).
On MRI, the imaging findings of tuberculous enlarged lymph nodes may depend on the presence and degree of granuloma formation, caseous/liquefactive necrosis, fibrosis and calcifications and may reflect different stages of the tuberculous process (20,21). In our study, approximately half of CT-imaged non-calcified enlarged lymph nodes (15/35) in the GTB showed low intensity on T2W images, corresponding pathologically to fibrocalcified nodes. One third (10/35) of lymph nodes in 5 patients showed a bullseye sign and pathologically corresponded to central caseous or liquefactive necrosis within the nodes. These findings are consistent with those of a previous study (21). Rim-like lymph nodes showed even thickening with a typical fatty hilum, which is considered a feature of benign nodes (22). Although the bullseye sign and rim-like lymph nodes have similar imaging features on T2W images, the central signal has a different pathological basis and therefore should be classified into different types. This study showed that benign MLNs can exhibit hypointensity, a bullseye sign and rim-like characteristics on T2W images. Based on these features, 77% (30/35) of lymph nodes in the GTB could be accurately diagnosed.
In the GTB, 23% of MLNs showed slight hyperintensity and heterogeneous hyperintensity overlapping with that of metastatic MLNs in the GLC. For these MLNs, it was difficult to distinguish benign from malignant nodes based on T2W images alone. Other parameters, such as the apparent diffusion coefficient, should be investigated (23,24) to provide additional information beyond conventional T1W and T2W images.
In this study, mass-like tuberculosis occasionally showed hyperintensity on T1W images. The contrast between the lesion and muscle was more significant on out-of-phase images than on in-phase images because of fat suppression. The mean SIR of lesion to rhomboid muscle on T1W images was significantly higher in the GTB than in the GLC. We therefore recommend greater attention to the features of lesions on T1W images. The intrasubject SD in the GTB was markedly greater than that in the GLC on both T2W and T1W images, confirming the inhomogeneity of TB lesions. Masses showing hypointensity or heterogeneous intensity on T2W images and hyperintensity on T1W images indicate a possible diagnosis of tuberculosis. Comprehensive analysis of the signal features of the lesion on T2W and T1W images is essential for an accurate diagnosis of a pulmonary mass. Based on the above criteria, twenty-nine cases in the GTB (29/30) in this study could be accurately diagnosed (true positive rate 96.7% and true negative rate 92.3%).The application of conventional MRI may play an important role in the management of patients who are suspicious for malignancy but have negative biopsy results. Indicative diagnosis of tuberculosis by MRI might avoid unnecessary repeated biopsy and guide tuberculosis-related laboratory tests or anti-tuberculosis treatment.
In this study, we found that many cases (20/30) in the GTB were accompanied by multiple patchy or small nodules around the lesion (satellite lesions) or in different lobes, whereas these findings were rare in the GLC. Moreover, T2W could display the heterogeneous intensity feature of TB nodules, which is uncommon for metastasis of lung cancer without treatment. Multiple coexisting lesions might provide some clues to the differential diagnosis of a pulmonary mass.
There are shortcomings of this study. First, the cohort size was small, with a total of 30 tuberculosis patients. Second, SIR on T1W and T2W images was measured and calculated on the largest slice, which might incompletely reflect the characteristics of the lesion. In the future, it might be possible to use 3D segmentation for a more accurate quantitative measure. Third, the signal intensity of lesions was visually compared to that of rhomboid muscle in this study. The comparison was more robust on fat-suppressed T2W images than without fat suppression, which decreased the variation of signal intensity of rhomboid muscle among different subjects (e.g., fat/slim patients, women/men, etc.). In the future, fat-suppressed T1W imaging should be studied to determine if it could better demonstrate the signal comparison between a lesion and rhomboid muscle than the presently used T1W sequence. Finally, previous studies have shown that diffusion-weighted imaging and the apparent diffusion coefficient are useful for the differential diagnosis of pulmonary masses or nodules (25,26). Furthermore, DCE-MRI and contemporary quantitative MR imaging techniques are helpful for the differentiation of TB vs. cancer (7,27,28). The combination of conventional and advanced MR imaging sequences will improve the differential diagnosis of TB and lung cancer.
In conclusion, conventional MR sequences can reveal the characteristics of mass-like tuberculosis and may be valuable for discriminating benign and metastatic MLNs. A sufficient understanding of the MR features of mass-like tuberculosis may improve the diagnostic ability for pulmonary masses and facilitate patient management.
Acknowledgements
The authors would like to thank Zhen Liang, Zhong-Jiu Li of Peking University Cancer Hospital and Institute for providing support for this study, and Drs. Kejia Cai and Muge Karaman of University of Illinois at Chicago for helpful discussions.
Funding: This work was supported by Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education, Peking University Cancer Hospital and Institute, China. Program No. 1122-01-1431.
Ethical Statement: The institutional review board of Peking University Cancer Hospital approved this study (# 2012011009). Informed consent was obtained from all prospectively enrolled tuberculosis patients. Informed consent was waivered for retrospectively collected lung cancer patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tuberculosis, Global Tuberculosis Report 2016. Available online: http://www.who.int/tb/publications/factsheet_global.pdf. Accessed 10 January 2017.
- 2.Rolston KV, Rodriguez S, Dholakia N, et al. Pulmonary infections mimicking cancer: a retrospective three-year review. Support Care Cancer 1997;5:90-3. 10.1007/BF01262563 [DOI] [PubMed] [Google Scholar]
- 3.Biederer J, Beer M, Hirsch W, et al. MRI of the lung (2/3). Why …when …how? Insights Imaging 2012;3:355-71. 10.1007/s13244-011-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer JF, Vollmar J, Schick F, et al. Solitary pulmonary nodules: dynamic contrast-enhanced MR imaging--perfusion differences in malignant and benign lesions. Radiology 2004;232:544-53. 10.1148/radiol.2322030515 [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto K. Usefulness of contrast enhanced magnetic resonance imaging for evaluating solitary pulmonary nodules. Cancer Imaging 2008;8:36-44. 10.1102/1470-7330.2008.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzi EB, Schinina' V, Cristofaro M, et al. Detection of Pulmonary tuberculosis: comparing MR imaging with HRCT. BMC Infect Dis 2011;11:243. 10.1186/1471-2334-11-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kono R, Fujimoto K, Terasaki H, et al. Dynamic MRI of solitary pulmonary nodules: comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol 2007;188:26-36. 10.2214/AJR.05.1446 [DOI] [PubMed] [Google Scholar]
- 8.Sakai F, Sone S, Maruyama A, et al. Thin-rim enhancement in Gd-DTPA-enhanced magnetic resonance imaging of tuberculoma: a new finding of potential differential diagnosis importance. J Thorac Imaging 1992;7:64-9. 10.1097/00005382-199206000-00007 [DOI] [PubMed] [Google Scholar]
- 9.Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology 2016;278:13-32. 10.1148/radiol.2015150244 [DOI] [PubMed] [Google Scholar]
- 10.Li XH, Zhu J, Zhang XM, et al. Abdominal MRI at 3.0 T: LAVA-Flex compared with conventional fat suppression T1-weighted images. J Magn Reson Imaging 2014;40:58-66. 10.1002/jmri.24329 [DOI] [PubMed] [Google Scholar]
- 11.Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73-80. 10.1148/radiology.214.1.r00ja1473 [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, et al. eds. AJCC cancer staging manual. 7th ed. New York, NY: Springer, 2009. [Google Scholar]
- 13.Chung MH, Lee HG, Kwon SS, et al. MR imaging of solitary pulmonary lesion: emphasis on tuberculomas and comparison with tumors. J Magn Reson Imaging 2000;11:629-37. [DOI] [PubMed] [Google Scholar]
- 14.Sze G, Zimmerman RD. The magnetic resonance imaging of infections and inflammatory diseases. Radiol Clin North Am 1988;26:839-59. [PubMed] [Google Scholar]
- 15.Brown JJ, Gilbert T, Gamsu G, et al. MR Imaging of low signal intensity pulmonary lesions using flow-sensitive techniques. J Comput Assist Tomogr 1988;12:560-4. 10.1097/00004728-198807000-00003 [DOI] [PubMed] [Google Scholar]
- 16.Berthezène Y, Vexler V, Kuwatsuru R, et al. Differentiation of alveolitis and pulmonary fibrosis with a macromolecular MR imaging contrast agent. Radiology 1992;185:97-103. 10.1148/radiology.185.1.1523341 [DOI] [PubMed] [Google Scholar]
- 17.Arita T, Matsumoto T, Kuramitsu T, et al. Is it possible to differentiate malignant mediastinal nodes from benign nodes by size? Reevaluation by CT, transesophageal echocardiography, and nodal specimen. Chest 1996;110:1004-8. 10.1378/chest.110.4.1004 [DOI] [PubMed] [Google Scholar]
- 18.Ohno Y, Hatabu H, Takenaka D, et al. Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative and qualitative assessment with STIR turbo spin-echo MR imaging. Radiology 2004;231:872-9. 10.1148/radiol.2313030103 [DOI] [PubMed] [Google Scholar]
- 19.Usuda K, Sagawa M, Motono N, et al. Advantages of diffusion-weighted imaging over positron emission tomography-computed tomography in assessment of hilar and mediastinal lymph node in lung cancer. Ann Surg Oncol 2013;20:1676-83. 10.1245/s10434-012-2799-z [DOI] [PubMed] [Google Scholar]
- 20.De Backer AI, Mortelé KJ, Van Den Heuvel E, et al. Tuberculous adenitis: comparison of CT and MRI findings with histopathological features. Eur Radiol 2007;17:1111-7. 10.1007/s00330-006-0412-1 [DOI] [PubMed] [Google Scholar]
- 21.Moon WK, Im JG, Yu IK, et al. Mediastinal tuberculous lymphadenitis: MR imaging appearance with clinicopathologic correlation. AJR Am J Roentgenol 1996;166:21-5. 10.2214/ajr.166.1.8571880 [DOI] [PubMed] [Google Scholar]
- 22.Yeh DW, Lee KS, Han J, et al. Mediastinal nodes in patients with non-small cell lung cancer: MRI findings with PET/CT and pathologic correlation. AJR Am J Roentgenol 2009;193:813-21. 10.2214/AJR.08.2083 [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa I, Boiselle PM, Kuwabara K, et al. Mediastinal lymph nodes in patients with non-small cell lung cancer: preliminary experience with diffusion-weighted MR imaging. J Thorac Imaging 2008;23:157-61. 10.1097/RTI.0b013e318166d2f5 [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Tian J, Liu Y, et al. Accuracy of diffusion-weighted (DW) MRI with background signal suppression (MR-DWIBS) in diagnosis of mediastinal lymph node metastasis of nonsmall-cell lung cancer (NSCLC). J Magn Reson Imaging 2014;40:200-5. 10.1002/jmri.24343 [DOI] [PubMed] [Google Scholar]
- 25.Shen G, Jia Z, Deng H. Apparent diffusion coefficient values of diffusion-weighted imaging for distinguishing focal pulmonary lesions and characterizing the subtype of lung cancer: a meta-analysis. Eur Radiol 2016;26:556-66. 10.1007/s00330-015-3840-y [DOI] [PubMed] [Google Scholar]
- 26.Deng Y, Li X, Lei Y, et al. Use of diffusion-weighted magnetic resonance imaging to distinguish between lung cancer and focal inflammatory lesions: a comparison of intravoxel incoherent motion derived parameters and apparent diffusion coefficient. Acta Radiol 2016;57:1310-7. 10.1177/0284185115586091 [DOI] [PubMed] [Google Scholar]
- 27.Gai ND, Malayeri AA, Bluemke DA. Three-dimensional T1 and T2* mapping of human lung parenchyma using interleaved saturation recovery with dual echo ultrashort echo time imaging (ITSR-DUTE). J Magn Reson Imaging 2017;45:1097-104. 10.1002/jmri.25487 [DOI] [PubMed] [Google Scholar]
- 28.Bauman G, Santini F, Pusterla O, et al. Pulmonary relaxometry with inversion recovery ultra-fast steady-state free precession at 1.5T. Magn Reson Med 2017;77:74-82. 10.1002/mrm.26490 [DOI] [PubMed] [Google Scholar]