Abstract
Background
A decrease in serum albumin is commonly observed after lung cancer surgery, however, whether it is associated with postoperative outcomes is unknown. The objective of this study was to evaluate whether the reduction of serum albumin (ΔALB) on postoperative day one could serve as a predictor of postoperative pulmonary complications (PPCs) after thoracoscopic anatomical resection in lung cancer patients.
Methods
Patients characteristics were compared between groups of whether they experienced PPCs or not. The cutoff value of ΔALB was examined by receiver operating characteristic curve to find out the threshold value of ΔALB in predicting PPCs. Logistic regression analysis was conducted to identify potential risk factors for PPCs.
Results
Totally 533 patients were included into analysis, and among them, 52 experienced PPCs. The ΔALB was significant in the PPCs group than in the non-PPCs group (P<0.001), and ΔALB was observed an independent risk factor for PPCs (OR =2.268, 95% CI: 1.153–4.460). The cutoff value of ΔALB in predicting PPCs was 14.97%. Patients with ΔALB ≥14.97% were more likely to have PPCs (P<0.001).
Conclusions
A reduction of serum albumin with a cut-off value of 14.97% can be served as a predictor to identify patients at high risk of developing PPCs following thoracosopic anatomical lung cancer surgery.
Keywords: Lung cancer, postoperative pulmonary complications (PPCs), serum albumin, risk factor
Introduction
Since the introduction of video-assisted thoracoscopic surgery (VATS), it has rapidly developed to become a major method in treating operable non-small cell lung cancer (NSCLC). Though the surgical techniques and the perioperative management levels developed rapidly, postoperative complications are still important factors that trouble both patients and physicians. Postoperative complications may prolong the length of hospital stay, increase the in-hospital cost and influence the recovery of patients’ function status (1). Postoperative pulmonary complications (PPCs) are the most common complications after lung cancer surgery.
As a nutritional status indicator and inflammatory factor, the role of perioperative albumin levels and postoperative outcomes has been explored (2). Several previous studies have demonstrated that preoperative serum albumin was significantly associated with postoperative complications in malignancies including colorectal, gastric, and gynecologic cancer and non-cancer patients (3-6). Likewise, the relationship between postoperative hypoalbuminemia and poor postoperative outcomes has been discussed (7). Increasing evidence also indicated that the decrease of albumin after surgery was associated with surgery outcomes (8-11).
However, whether perioperative albumin is related with postoperative short-term morbidity after lung cancer resection is still unknown. The objective of this study was to define how the postoperative decrease of serum albumin (ΔALB) was related with PPCs for lung cancer patients who underwent VATS anatomical resection.
Methods
Patients
This is a retrospective cohort study from a single center. Consecutive patients underwent VATS lobectomy or segmentectomy for NSCLC at the Department of Thoracic Surgery, West China Hospital, between May 1, 2015 and December 30, 2016 were retrospectively examined. Finally, a total of 533 patients were enrolled in the study.
Patients’ inclusion criteria: (I) a defined diagnosis of primary NSCLC confirmed by pathology; (II) underwent VATS lobectomy or segmentectomy. Exclusion criteria: (I) transfusion of albumin during the perioperative period; (II) underwent neoadjuvant therapy; (III) a history of surgery within 3 months preoperatively; (IV) patients with signs of infection and those combined with disease which may affect the serum albumin; (V) data incomplete or could not be obtained.
Data collection
Data including: age, gender, body mass index (BMI), smoking history, American Society of Anesthesiologists (ASA) grade, preoperative comorbidities, surgical procedures (surgical approach, type of resection), pathology and American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging (the eighth edition) were collected.
Usually, blood tests were performed within 7 days preoperatively and on the morning of postoperative day 1 (POD1) in our institution, so serum albumin levels were obtained and ΔALB were calculated according to previous studies (8-12).
Definition of outcomes
The primary outcome of our study was the incidence of PPCs that occurred within 30 days postoperatively. PPCs were defined including: pneumonia, atelectasis, acute respiratory distress syndrome (ARDS), pulmonary embolism, re-intubation. The criteria used to define PPCs were according to the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definitions (13). Hypoalbuminemia was defined as a serum albumin level less than 35 grams per liter (g/L).
Statistical analysis
According to whether they experienced PPCs, patients were divided into two groups (the PPCs group and non-PPCs group). Clinicopathologic characteristics were compared between the two groups. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal threshold ΔALB value to predict PPCs. The optimal cut-off value of ΔALB was estimated using the Youden index (sensitivity + specificity−1) which is a common summary measure of the ROC curve. Patients were categorized and compared based on the cut-off value identified by the ROC curve analysis. Binomial logistic regression analysis was conducted to identify potential preoperative risk factors for PPCs and to examine the associations between ΔALB with PPCs. Firstly, univariate analysis was conducted, and then variables with P value <0.1 were included into the adjusted multivariable analysis. The results of regression analysis were reported as odds ratio (ORs) and 95% confidence interval (95% CI).
Continuous normally distributed data were expressed as mean ± standard deviation (SD) and non-normal data as medians with interquartile range (IQR) (25th and 75th percentiles). Student t-test was used to compare normally distributed continuous variables, and non-normal data were tested with Mann-Whitney U text. Categorical variables are expressed as absolute frequencies and proportions (%) and between group difference were compared using the χ2 or Fisher’s exact tests. All significance tests were two tailed with P<0.05 considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics Version 23.
Results
Study population and baseline characteristics
A total of 533 female and male patients who underwent VATS anatomical resection for lung cancer were identified for eligibility this study. The baseline characteristics are summarized in Table 1. The mean age was 61.63±8.64 years. Nearly half of the patients (50.7%) had a history of smoking. The preoperative mean albumin level was 42.39±4.12 g/L. Only 16 patients (3%) with a preoperative albumin level less than 35 g/L were defined with hypoalbuminemia. While on POD1, the mean ALB level was decreased to 37.63±4.93 g/L, and more patients (131, 24.6%) were at hypoalbuminemia status. Four hundred patients (75%) underwent lobectomies and 133 patients (25%) underwent segmentectomies.
Table 1. Characteristics of two group patients divided by PPCs.
| Characteristic | PPCs | P | |
|---|---|---|---|
| Yes (n=52) | No (n=481) | ||
| Age (years), mean ± SD | 59.67±7.98 | 61.84±8.69 | 0.086 |
| Gender (female), n (%) | 19 (36.5) | 206 (42.8) | 0.460 |
| BMI (kg/m2), mean ± SD | 23.62±3.29 | 23.29±2.96 | 0.456 |
| Current or former smokers, n (%) | 30 (57.7) | 240 (49.9) | 0.309 |
| Preoperative ALB (g/L) | 41.80±4.90 | 42.46±4.03 | 0.273 |
| Preoperative hypoalbuminemia, n (%) | 3 (5.8) | 13 (2.7) | 0.198 |
| Postoperative ALB (g/L), mean ± SD | 34.94±4.61 | 37.92±4.88 | <0.001 |
| Postoperative hypoalbuminemia, n (%) | 23 (44.2) | 108 (22.5) | 0.001 |
| ÄALB, median (95% CI) | 0.16 (0.09–0.21) | 0.12 (0.06–0.16) | <0.001 |
| Blood loss (mL), median (95% CI) | 100 (32.5–127.5) | 80 (40–150) | 0.302 |
| ASA score, n (%) | <0.001 | ||
| 2 | 45 (86.5) | 384 (79.8) | |
| 3 | 7 (13.5) | 90 (18.7) | |
| 4 | 0 | 7 (1.5) | |
| Perioperative morbidity, n (%) | |||
| COPD | 13 (25.0) | 111 (23.1) | 0.732 |
| Hypertension | 16 (30.8) | 120 (24.9) | 0.402 |
| Diabetes | 3 (5.6) | 51 (10.6) | 0.341 |
| CHD | 2 (3.8) | 26 (5.4) | 1.000 |
| Previous malignancy | 5 (9.6) | 38 (7.9) | 0.596 |
| Pulmonary function (L), mean ± SD | |||
| FEV1 | 2.13±0.62 | 2.47±2.22 | 0.276 |
| TNM stage, n (%) | 0.595 | ||
| I | 39 (75) | 340 (70.7) | |
| II | 7 (13.5) | 74 (15.4) | |
| III | 4 (7.7) | 58 (12.1) | |
| IV | 2 (3.8) | 9 (1.9) | |
| Extent of resection, n (%) | 0.866 | ||
| Lobectomy | 40 (76.9) | 360 (74.8) | |
| Segmentectomy | 12 (23.1) | 121 (25.2) | |
| Pathology, n (%) | 0.815 | ||
| Adenocarcinoma | 36 (69.2) | 342 (71.1) | |
| SCC | 10 (19.2) | 97 (20.2) | |
| Others | 6 (11.5) | 42 (8.7) | |
PPCs, postoperative pulmonary complications; BMI, body mass index; ALB, albumin; ÄALB, postoperative decrease in serum albumin; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease; FEV1, forced expiratory volume in 1 second; SCC, squamous cell carcinoma.
On POD1, the mean value of albumin level in the PPCs groups was significantly lower than in the non-PPCs group (34.94±4.61 vs. 37.92±4.88, P<0.001), and more patients had hypoalbuminemia status in the PPCs group than in the non-PPCs group [23 (44.2%) vs. 108 (22.5%), P=0.001]. Patients who experienced PPCs were at higher rate of ALB decrease after surgery [0.16 (0.09–0.21) vs. 0.12 (0.06–0.16), P<0.001]. Patients in the PPCs group tended to have higher rate of comorbidities of chronic obstructive pulmonary disease (COPD) and coronary heart disease (CHD), while there was no difference regarding hypertension, diabetes and history of malignant neoplasm. There was no difference regarding baseline characteristics including age, gender, smoking history, preoperative albumin level, the rate of preoperative hypoalbuminemia and oncological characteristics including TNM stage, extent of resection, and pathology in the PPCs group compared to the non-PPCs group (Table 1).
ROC curve analysis of ΔALB in predicting PPCs
Based on the ROC curve analysis, the ΔALB of 14.97% was found the cut-off value in predicting PPCs. The area under the value (AUC) was 0.655 (95% CI: 0.578–0.732, P<0.001), with the specificity of 69.6% and sensitivity of 57.7% (Figure 1).
Figure 1.
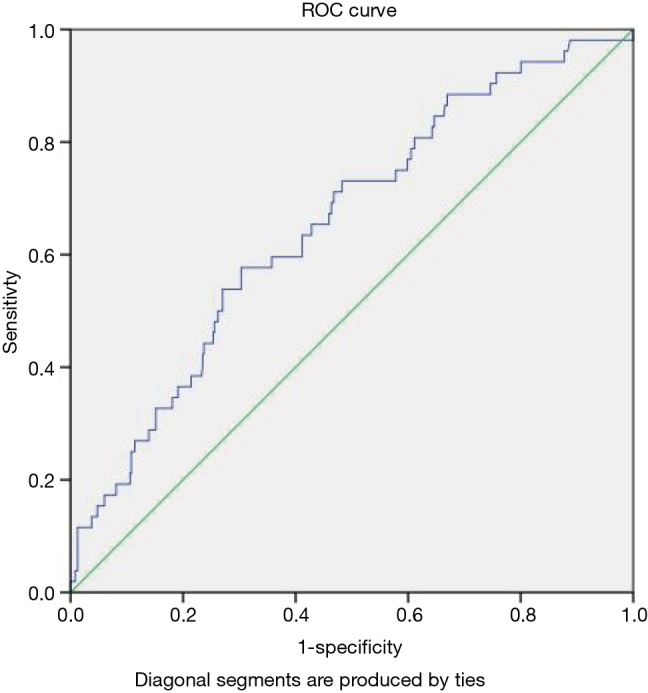
Receiving operator curve of ΔALB in predicting PPCs. PPC, postoperative pulmonary complication.
Association between threshold ΔALB and perioperative parameters
The demographic differences between patients with ΔALB ≥14.97% and patients with ΔALB <14.97% were summarized in Table 2. Compared with patients with ΔALB ≥14.97%, patients with ΔALB <14.97% were older, had lower BMI.
Table 2. Characteristics classified by ÄALB cutoff value.
| Variables | ÄALB <14.97% (n=357) | ÄALB ≥14.97% (n=176) | P |
|---|---|---|---|
| Age (years), mean ± SD | 62.53±8.47 | 59.80±8.72 | 0.001 |
| Gender (female), n (%) | 161 (45.1) | 64 (36.4) | 0.062 |
| BMI (kg/m2), mean ± SD | 22.95±2.86 | 24.09±3.12 | <0.001 |
| Smoking history, n (%) | 180 (50.4) | 90 (51.1) | 0.927 |
| ASA score, n (%) | 0.393 | ||
| 2 | 290 (81.2) | 139 (79.0) | |
| 3 | 61 (17.1) | 36 (20.5) | |
| 4 | 6 (1.7) | 1 (0.6) | |
| Perioperative morbidity, n (%) | |||
| COPD | 83 (23.2) | 41 (23.3) | 1.000 |
| Hypertension | 94 (26.3) | 42 (23.9) | 0.598 |
| Diabetes | 35 (9.8) | 19 (10.8) | 0.761 |
| CHD | 21 (5.9) | 7 (4) | 0.414 |
| Previous malignancy | 33 (9.2) | 10 (5.7) | 0.178 |
| Pulmonary function (L), mean ± SD | |||
| FEV1 | 2.52±2.54 | 2.26±0.63 | 0.178 |
| TNM stage, n (%) | 0.011 | ||
| I | 247 (69.2) | 132 (75.0) | |
| II | 66 (18.5) | 15 (8.5) | |
| III | 39 (10.9) | 23 (13.1) | |
| IV | 5 (1.4) | 6 (3.4) | |
| Extent of resection, n (%) | 0.09 | ||
| Lobectomy | 276 (77.3) | 124 (70.5) | |
| Segmentectomy | 81 (22.7) | 52 (29.5) | |
| Pathology, n (%) | 0.184 | ||
| Adenocarcinoma | 244 (68.3) | 134 (76.1) | |
| SCC | 78 (21.8) | 29 (16.5) | |
| Others | 35 (9.8) | 13 (7.4) |
BMI, body mass index; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease; FEV1, forced expiratory volume in 1 second; SCC, squamous cell carcinoma.
Association between threshold ΔALB and the development of postoperative complications
The incidence of common complications including pulmonary complications and surgery related complications is summarized in Table 3. In our cohort, the most frequent pulmonary complication was postoperative pneumonia (6.2%), followed by atelectasis (3.4%), pulmonary embolism (0.9%), ARDS (0.4%) and reintubation (0.4%).
Table 3. Main postoperative complications classified by ΔALB cutoff value.
| Classification | Total | ÄALB <14.97% (n=357) | ÄALB ≥14.97% (n=176) | P |
|---|---|---|---|---|
| Total complications, n (%) | 100 (18.8) | 50 (14.0) | 50 (28.4) | <0.001 |
| PPCs, n (%) | 52 (9.8) | 22 (6.2) | 30 (17.0) | <0.001 |
| Pneumonia | 33 (6.2) | 10 (2.8) | 23 (13.1) | <0.001 |
| Atelectasis | 18 (3.4) | 9 (2.5) | 9 (5.1) | 0.131 |
| Pulmonary embolism | 5 (0.9) | 1 (0.3) | 4 (2.3) | 0.043 |
| ARDS | 2 (0.4) | 2 (0.6) | 0 (0.0) | 1 |
| Reintubation | 2 (0.4) | 2 (0.6) | 0 (0.0) | 1 |
| SRCs, n (%) | 56 (10.5) | 33 (9.2) | 23 (13.1) | 0.179 |
| BPF | 1 (0.2) | 0 (0.0) | 1 (0.6) | 0.330 |
| Pulmonary air leak | 42 (7.9) | 24 (6.7) | 18 (10.2) | 0.173 |
| Subcutaneous emphysema | 13 (2.4) | 9 (2.5) | 4 (2.3) | 1 |
| Chylothorax | 5 (0.9) | 3 (0.8) | 2 (1.1) | 0.667 |
| Pleural effusion | 4 (0.8) | 3 (0.8) | 1 (0.6) | 1 |
PPCs, postoperative pulmonary complications; ARDS, acute respiratory distress syndrome; SRCs, surgical related complications; BPF, bronchopleural fistula.
The overall incidence of PPCs of patients with ΔALB ≥14.97% was significantly higher than that of patients with ΔALB <14.97% [30 (17.0%) vs. 22 (6.2%), P<0.001]. Compared with patients with ΔALB <14.97%, patients with ΔALB ≥14.97% had higher rate of postoperative pneumonia [23 (13.1%) vs. 10 (2.8%), P<0.001] and pulmonary embolism [4 (2.3%) vs. 1 (0.3%), P=0.043], while no significant difference was found in the other PPCs between these two groups.
Predictive factors for PPCs
The results of univariate and multivariate analysis are summarized in Table 4. Potential risk factors were included into analysis. From the univariate analysis, postoperative hypoalbuminemia (OR =0.365, 95% CI: 0.203–0.657, P=0.001), ΔALB ≥14.97% (OR =3.129, 95% CI: 1.746–5.608, P<0.001) and FEV1 (OR =0.556, 95% CI: 0.349–0.855, P=0.013) were found to be significantly associated with PPCs. Then a multivariate analysis model was conducted to identify the independent predictive factors for PPCs. ΔALB ≥14.97% (OR =2.268, 95% CI: 1.153–4.460, P=0.018) and FEV1 (OR =0.596, 95% CI: 0.369–0.963, P=0.034) were maintained in the model after adjustment (Table 4).
Table 4. Predictive factors for PPCs: univariate and Multivariable analysis.
| Variables | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Age | 0.972 (0.940–1.004) | 0.087 | 0.981 (0.947–1.015) | 0.261 | |
| Gender (female) | 0.769 (0.425–1.390) | 0.384 | – | – | |
| BMI | 1.037 (0.943–1.140) | 0.456 | – | – | |
| Smoking history | 1.369 (0.768–2.4442) | 0.287 | – | – | |
| Preoperative hypoalbuminemia | 0.454 (0.125–1.647) | 0.230 | – | – | |
| Postoperative hypoalbuminemia | 0.365 (0.203–0.657) | 0.001 | 0.627 (0.317–1.241) | 0.180 | |
| Blood loss | 0.999 (0.998–1.001) | 0.409 | – | – | |
| ÄALB ≥14.97% | 3.129 (1.746–5.608) | <0.001 | 2.268 (1.153–4.460) | 0.018 | |
| ASA score | 0.601 (0.273–1.322) | 0.206 | – | – | |
| Hypertension | 1.337 (0.716–2.496) | 0.362 | – | – | |
| Diabetes | 0.516 (0.155–1.716) | 0.281 | – | – | |
| Previous malignancy | 1.240 (0.466–3.303) | 0.667 | – | – | |
| COPD | 1.111 (0.573–2.155) | 0.755 | – | – | |
| CHD | 0.700 (0.161–3.037) | 0.634 | – | – | |
| FEV1 | 0.556 (0.349–0.855) | 0.013 | 0.596 (0.369–0.963) | 0.034 | |
| TNM stage | 0.922 (0.628–1.353) | 0.677 | – | – | |
| Extent of resection | 1.120 (0.569–2.205) | 0.742 | – | – | |
| Pathology | 1.114 (0.727–1.707) | 0.620 | – | – | |
OR, odds ratio; CI, confidence interval; BMI, body mass index; ÄALB, postoperative decrease in serum albumin; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease; FEV1, forced expiratory volume in one second.
Discussion
The serum albumin level indicating the nutrition status is regarded as an acute phase protein. Both pre- and postoperative albumin levels have been proven to be significantly related with perioperative morbidity (3-7,14). However, if we focus on only pre- or postoperative albumin levels, we might not perceive the dynamic change of serum albumin during the perioperative period and its relationship with postoperative outcomes. To our knowledge, no studies have investigated the meaning of serum albumin change postoperatively in predicting postoperative morbidity in lung cancer patients who underwent anatomical resection.
Our results revealed that ΔALB was an independent risk factor in predicting PPCs after anatomical resection in lung cancer patients. And a ΔALB of 14.97% was found to be the threshold value to predict PPCs. The rates of PPCs including postoperative pneumonia and pulmonary embolism, were significant higher in the high ΔALB group than in the low ΔALB group. No such relation was discovered regarding surgery related complications.
Our results agree with several previous studies that investigated the relationship between the ΔALB and perioperative outcomes. Liu et al. (9) investigated the relationship between ΔALB and short-term complications after gastric cancer resection. They found that a ΔALB value of 14.0% was the cut-off value in predicting postoperative complications after gastrectomy and patients whose serum ALB levels were reduced by >14.0% should be identified by physicians for complications. Ge et al. (10) retrospectively analyzed 626 patients who underwent colorectal surgery, and the authors discovered that a ΔALB value of ≥15% after 2 days postoperatively was an independent risk factor for overall complications. Though their studies were not focused on lung cancer patients, their results were quite similar to ours regarding the ΔALB cut-off value in predicting postoperative complications. Another study revealed that the serum albumin dropped rapidly and a definite decrease of serum albumin was significantly associated with postoperative complications and prolonged hospital stay (8,11).
The decrease in serum albumin can be attributed to the blood loss intraoperatively and postoperatively including the hemodilution during fluid transfusion and capillary permeability into the interstitial space (15). However, in our study, we did not find a relationship between blood loss and PPCs, and in the regression analysis blood loss was not an independent risk factor for PPCs. This may be explained by the fact that most of the albumin decrease after surgery was on account for the redistribution (11). For pulmonary resection patients, the lung injure from the operation and single lung ventilation during the procedure could increase the inflammatory response (16,17).
Unlike traditional inflammatory factors of C-reactive protein (CRP) and IL-6, IL-8 and IL-10, serum albumin is a negative acute phase protein, where its level drops in response to inflammation and surgery trauma. Studies demonstrated that the response of serum albumin might occur earlier than CRP to surgical stress, and this may be attributed to its quicker kinetics than CRP (10,18-20). The relationship between CRP and albumin during the perioperative period had been studied (20), and the authors found that the antecedent CRP levels could predict hypoalbuminemia to some extent in the future, which reflected the close relationship between CRP and albumin. Increased inflammatory cytokines during the perioperative period promote the degradation of the serum albumin and decrease its synthesis (20,21). This mechanism combined with the capillary permeability and fluid transfusion result in the decrease of serum albumin postoperatively.
On the other hand, the serum albumin level is the most frequently used parameter to reflect one’s nutritional status (20,22). The nutritional status plays an important role in patient recovery from surgery and may account for the incidence of perioperative morbidity after surgery (23,24). However, in our study, the preoperative serum albumin was not an independent risk factor for PPCs, which was not consistent with other studies. This could be explained by the low rate of preoperative malnutrition of 3%. Despite this inconsistency, we found that the decrease of serum albumin on POD1 is an independent risk factor for PPCs, which was consistent with two other studies in gastric and colorectal resection patients (9,10).
There are some limitations in our study. This is a retrospective, observational cohort study from one single institution that only included patients who underwent anatomical resection, which may limit its applicability. The primary endpoint of this study was the occurrence of PPCs, and the other perioperative morbidities and mortality was not explored. We failed to assess the predictive value of ΔALB combined with other factors that may have a close relationship with the drop of serum albumin including the amount and type of transfusion since these records were not available. Finally, the present findings need to be further validated by large prospective cohort studies.
Conclusions
The current study revealed that a decrease of serum albumin on POD1 predicted PPCs after VATS anatomical lung cancer resection. An ΔALB of 14.97% was confirmed as the cut-off value in predicting PPCs, and patients with albumin levels decrease ≥14.97% were at higher risk of developing PPCs. Surgeons are advised to monitor the change of serum albumin after lung cancer anatomical resection to detect potential pulmonary complications.
Acknowledgements
None.
Ethical Statement: The study was approved by the Ethics Committee of West China Hospital, Sichuan University, Chengdu, China (No. 2017-403) and written informed consent was waived due to the retrospective nature.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Simonsen DF, Søgaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. 10.1016/j.rmed.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 2.Aldebeyan S, Nooh A, Aoude A, et al. Hypoalbuminemia-a marker of malnutrition and predictor of postoperative complications and mortality after hip fractures. Injury 2017;48:436-40. 10.1016/j.injury.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 3.Hardt J, Pilz L, Magdeburg J, et al. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int J Colorectal Dis 2017;32:1439-46. 10.1007/s00384-017-2884-7 [DOI] [PubMed] [Google Scholar]
- 4.Uppal S, Al-Niaimi A, Rice LW, et al. Preoperative hypoalbuminemia is an independent predictor of poor perioperative outcomes in women undergoing open surgery for gynecologic malignancies. Gynecol Oncol 2013;131:416-22. 10.1016/j.ygyno.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 5.Peacock MR, Farber A, Eslami MH, et al. Hypoalbuminemia Predicts Perioperative Morbidity and Mortality after Infrainguinal Lower Extremity Bypass for Critical Limb Ischemia. Ann Vasc Surg 2017;41:169-75.e4. 10.1016/j.avsg.2016.08.043 [DOI] [PubMed] [Google Scholar]
- 6.Bohl DD, Shen MR, Hannon CP, et al. Serum Albumin Predicts Survival and Postoperative Course Following Surgery for Geriatric Hip Fracture. J Bone Joint Surg Am 2017;99:2110-8. 10.2106/JBJS.16.01620 [DOI] [PubMed] [Google Scholar]
- 7.Lee JI, Kwon M, Roh JL, et al. Postoperative hypoalbuminemia as a risk factor for surgical site infection after oral cancer surgery. Oral Dis 2015;21:178-84. 10.1111/odi.12232 [DOI] [PubMed] [Google Scholar]
- 8.Labgaa I, Joliat GR, Kefleyesus A, et al. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open 2017;7:e013966. 10.1136/bmjopen-2016-013966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu ZJ, Ge XL, Ai SC, et al. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroenterol 2017;23:4978-85. 10.3748/wjg.v23.i27.4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge X, Dai X, Ding C, et al. Early Postoperative Decrease of Serum Albumin Predicts Surgical Outcome in Patients Undergoing Colorectal Resection. Dis Colon Rectum 2017;60:326-34. [DOI] [PubMed] [Google Scholar]
- 11.Hübner M, Mantziari S, Demartines N, et al. Postoperative Albumin Drop Is a Marker for Surgical Stress and a Predictor for Clinical Outcome: A Pilot Study. Gastroenterol Res Pract 2016;2016:8743187. 10.1155/2016/8743187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spolverato G, Kim Y, Ejaz A, et al. Effect of Relative Decrease in Blood Hemoglobin Concentrations on Postoperative Morbidity in Patients Who Undergo Major Gastrointestinal Surgery. JAMA Surg 2015;150:949-56. 10.1001/jamasurg.2015.1704 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. 10.1016/j.athoracsur.2014.05.104 [DOI] [PubMed] [Google Scholar]
- 14.Saji H, Ueno T, Nakamura H, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: JACS1303. Eur J Cardiothorac Surg 2018;53:835-41 10.1093/ejcts/ezx415 [DOI] [PubMed] [Google Scholar]
- 15.Ryan AM, Hearty A, Prichard RS, et al. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg 2007;11:1355-60. 10.1007/s11605-007-0223-y [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Shu SH, Wang D, et al. Goal-directed fluid restriction using stroke volume variation and cardiac index during one-lung ventilation: a randomized controlled trial. J Thorac Dis 2017;9:2992-3004. 10.21037/jtd.2017.08.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Conno E, Steurer MP, Wittlinger M, et al. Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology 2009;110:1316-26. 10.1097/ALN.0b013e3181a10731 [DOI] [PubMed] [Google Scholar]
- 18.Marik PE, Flemmer M. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg 2012;73:801-8. 10.1097/TA.0b013e318265cf87 [DOI] [PubMed] [Google Scholar]
- 19.Easton R, Balogh ZJ. Peri-operative changes in serum immune markers after trauma: a systematic review. Injury 2014;45:934-41. 10.1016/j.injury.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 20.Sonoda A, Ohnishi S, Nakao S, et al. Factors affecting serum albumin in the perioperative period of colorectal surgery: a retrospective study. BMC Res Notes 2015;8:638. 10.1186/s13104-015-1632-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth 2000;85:599-610. 10.1093/bja/85.4.599 [DOI] [PubMed] [Google Scholar]
- 22.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol 1997;50:693-703. 10.1016/S0895-4356(97)00015-2 [DOI] [PubMed] [Google Scholar]
- 23.Okada S, Shimada J, Kato D, et al. Clinical Significance of Prognostic Nutritional Index After Surgical Treatment in Lung Cancer. Ann Thorac Surg 2017;104:296-302. 10.1016/j.athoracsur.2017.01.085 [DOI] [PubMed] [Google Scholar]
- 24.Shoji F, Morodomi Y, Akamine T, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 2016;98:15-21. 10.1016/j.lungcan.2016.05.010 [DOI] [PubMed] [Google Scholar]


