Abstract
Background
Recently, studies have demonstrated that microRNA-497 (miR-497) plays an important role in modulating tumor cell sensitivity to chemotherapeutic drugs; however, its role in cellular resistance to the effects of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in treatment of non-small cell lung cancer (NSCLC) is not fully understood. In this study, we explored the potential of miR-497 in targeting the insulin-like growth factor-1 receptor (IGF-1R) signaling pathways to overcome gefitinib resistance.
Methods
A gefitinib resistant human lung adenocarcinoma A549 cell line (A549/GR) was established by the method of gefitinib mutagenesis culture. Next, the A549/GR cells were transfected with miR-497 mimics to establish an miR-497 overexpression model, designated A549/GR-miR497-mimic. MTT assay was used to assess cell resistance to gefitinib, and western blot assay was employed to evaluate alterations of IGF-1R and the AKT1 signaling pathway.
Results
We found that A549/GR-miR497-mimic cells (IC50 =33.76±0.97 µmol/L) were more sensitive to gefitinib than the control group (P<0.01). In addition, the expression levels of IGF-1R and phosphorylated AKT1 (p-AKT1) in A549/GR-miR497-mimic cells were reduced.
Conclusions
We demonstrated that miR-497 may have the effect of reversing gefitinib resistance and increasing the sensitivity of NSCLC cells to EGFR-TKIs by inhibiting the expression of IGF-1R and reducing activation of the downstream AKT signaling pathway. Thus, miR-497 plays a vital role in the acquired resistance to EGFR-TKIs, and it may represent a potential therapeutic strategy to treat NSCLC exhibiting resistance to EGFR-TKIs.
Keywords: Gefitinib, insulin-like growth factor-1 receptor (IGF-1R), microRNAs (miRNAs), non-small cell lung carcinoma
Introduction
Lung cancer remains the leading cause of cancer death worldwide. In 2012, an estimated 1.8 million new lung cancer cases were diagnosed, accounting for about 13% of all new cancers (1,2). In China, as the most frequently diagnosed cancer, lung cancer account for about 23.86% of all new cancers (3). Due to the lack of early screening of lung cancer, most patients have been diagnosed late and missed the best opportunity for surgical treatment. Currently, in addition to traditional therapy, molecular targeted drugs achieve good clinical efficacy in the treatment of these late diagnosed lung cancer patients. The most common molecular targeted drugs are epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib, afatinib, erlotinib and AZD9291 (4).
The EGFR-TKIs can inhibit the phosphorylation of EGFR and block its activation, thus interfering with the activation of Ras-Raf-MEK-ERK, PI3K-AKT and JAK-STAT. By mediating these 3 main signal transduction pathways, EGFR can ultimately inhibit the proliferation, differentiation, invasion, and metastasis of tumor cells. However, advanced lung cancer patients undergoing continuous EGFR-TKI treatment show a progression-free survival of only 9–13 months, and eventually develop disease progression and acquired resistance to EGFR-TKIs (5). Subsequently, based on the great potential therapeutic value of EGFR-TKIs in advanced lung cancer, it is important to study and overcome the EGFR-TKI resistance. Recent studies have shown that the mechanisms underlying acquired resistance to EGFR-TKIs include secondary EGFR mutations, abnormal activation of non-EGFR-dependent signal transduction pathway and downstream signaling molecules (such as PI3K and JAK2) mediated by EGFR, phenotypic transformation (such as epithelial-mesenchymal transformation and phenotypic transformation of small cell lung cancer) (6).
The insulin-like growth factor-1 receptor (IGF-1R), similar to the insulin receptor, is a transmembrane tyrosine kinase receptor. When combined with insulin-like growth factors (IGFs), IGF-1R can be activated by autophosphorylation, inducing its downstream Ras-Raf-MEK-ERK and PI3K-AKT signaling pathways. Ultimately, IGF-1R can regulate cell proliferation, differentiation, growth and development, metabolism, and anti-apoptotic processes (7). When IGF-1R is abnormally expressed, the downstream PI3K-AKT signaling pathway is abnormally activated as the non-EGFR-dependent signal transduction pathway, and then lead to acquired resistance to EGFR-TKI in non-small cell lung cancer (NSCLC) (8). Guix has shown that gefitinib can significantly reduce the phosphorylation of EGFR, ErbB-3 and Erk in gefitinib-resistant tumor cells in which IGF-1R is abnormally activated, but cannot decrease the phosphorylation of AKT. However, after inhibiting the activity of IGF-1R, the PI3K-AKT signaling pathway can be inhibited, and the growth of the tumor cells can be decreased (9). It is suggested that the abnormal activation of IGF-1R may further activate the PI3K-AKT signaling pathway and induce resistance to EGFR-TKIs. Moreover, recent studies have found that microRNA (miRNA) plays an important regulatory role in this process. For example, miRNA-21 can promote acquired resistance to EGFR-TKIs in NSCLC cells by abnormally activating PI3K-AKT signals (10); whereas, miRNA-200b can reduce the activity of the PI3K-AKT signaling pathway by inhibiting the expression of IGF-1R, and ultimately enhance the sensitivity of tumor cells to gefitinib (11). Our previous study demonstrated that the expression of some miRNAs, which was detected by a miRNA microarray assay, was increased after IGF-1R was silenced by small interfering RNA (siRNA) in A549/GR cells that had obtained resistance to gefitinib. One of the most significantly increased miRNAs was miR-497, which was correlated with the acquisition of resistance to gefitinib in NSCLC (12), but its mechanism of action is not clear.
To investigate the relationship between miR-497 and acquired gefitinib resistance in NSCLC and the associated mechanism, we developed an A549/GR cell line of NSCLC with acquired gefitinib resistance. The cells were induced and cultured in vitro, and overexpressed with miR-497. After that, the changes in drug resistance and the expression levels of IGF-1R and p-AKT1 proteins were detected in order to further understand the possible mechanism of interaction between miR-497 and acquired EGFR-TKI resistance in NSCLC.
Methods
Cell lines and reagents
The human lung adenocarcinoma A549 cell line was purchased from the Sun Yat-sen University Experimental Animal Center Cell Bank and cultured in RPMI-1640 medium (Gibco, USA) with 10% newborn bovine serum (Ausgenex, Australia) and 100 U/mL penicillin/streptomycin (Sigma-Aldrich, USA). Gefitinib (R&D Systems, USA) was dissolved by dimethyl sulfoxide (DMSO) and stored at −20 °C. RiboFECTTMCP transfection reagents, miR-497-mimic, and miR-497-NC were purchased from Guangzhou Ruibo Biotechnology Co. (China). IGF-1R and GAPDH antibody were purchased from Proteintech (USA), and p-AKT1 antibody was purchased from Abcam (UK).
Establishment of gefitinib-resistant A549/GR cell line
The A549 cell line acquired gefitinib resistance following 12 months of exposure to gradually increasing concentrations of gefitinib and grew stably in medium with 40 µM gefitinib. All cells were cultured at 37 °C in a humidified atmosphere of 95% air/5% CO2.
Drug resistance of A549/GR cells
A549 and A549/GR cells in the logarithmic growth phase were each individually inoculated into a 96-well cell culture plate. Different concentrations of gefitinib (2.5, 5.0, 10.0, 20.0, 30.0, 40.0, 60.0, 80.0 and 120.0 µM; as Table 1) were added to the RPMI-1640 culture medium. For the negative control and blank groups, the same amount of culture medium and PBS, respectively, were added instead of gefitinib. The cells were placed in an incubator for conventional culture. After 72 h, the viability of cells was detected by MTT assay, and then the absorbance [optical density (OD)] of each hole at the wavelength of 570 nm was measured by a microplate reader. Three parallel wells were measured in each group, and the experiment was repeated 3 times. The cell survival rate of each hole was calculated according to the following formula: cell survival rate (%) = (drug group OD − blank group OD)/(negative control group OD − blank group OD) ×100%. A linear regression method between the logarithmic value of drug concentration and the corresponding cell survival rate was used to calculate the half inhibitory concentration (IC50) of the drug. The resistance index (RI) was calculated to determine cell resistance (RI = IC50 value of drug-resistant cells/IC50 value of parent cells).
Table 1. Preparation of gefitinib culture medium with different concentrations.
| Component | Concentration of gefitinib (ìM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 5.0 | 10.0 | 20.0 | 30.0 | 40.0 | 60.0 | 80.0 | 120.0 | |
| RPMI-1640 culture medium (mL) | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| DMSO (ìL) | 6.00 | 5.75 | 5.50 | 5.00 | 4.00 | 3.00 | 2.00 | 0 | 0 | 0 |
| 40 mmol/L gefitinib storage solution (ìL) | 0 | 0.25 | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 6.00 | 8.00 | 12.00 |
DMSO, dimethyl sulfoxide.
Western blot analysis
Total protein was extracted from the A549 and A549/GR cells using radio immunoprecipitation assay (RIPA) buffer and quantified with a BCA protein assay kit (Beijing Dingguo Changsheng Biotechnology Co. Ltd, China). The proteins were separated by electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were then blocked for 2 h at room temperature with 5% skim milk and subsequently incubated overnight at 4 °C in a shaking bed with the following antibodies: polyclonal rabbit anti-IGF-1R (1:5,000), polyclonal rabbit anti-phosphorylated (p-)Akt (1:3,000), and polyclonal rabbit anti-GAPDH (1:10,000). Following 3 washes with Tris-buffered saline containing Tween-20, the membranes were incubated with the secondary antibody, anti-rabbit IgG (1:5,000), for 1 h at room temperature. The blots were visualized using enhanced chemiluminescence (ECL) and analyzed by the Tanon 5200 chemiluminescence imaging system. The gray-scale value of each protein band was measured with the ImageJ. GAPDH was probed as the reference of internal control. All the data were normalized to the signal of GAPDH.
Cell transfection
Based on the A549/GR cell line, the A549/GR-miR497-mimic group was transfected with miR-497 mimics (sequence: GAGCAGCACACUGUGGUUUGU) by RiboFECTTMCP transfection reagent. miR-497-NC, that contained a sequence that lacked homology with other miRNA, was transfected under identical conditions into A549/GR cells as the negative control and designated the A549/GR-miR497-NC group. The A549/GR group represented the A549/GR cell line without transfection. The 3 groups were prepared in a 96-well plate, respectively, and cultured for 24 h. The transfection efficiency was observed under an inverted fluorescence electron microscope.
Detection of drug resistance by MTT assay
The old cell culture media were discarded and replaced with culture media containing various concentrations of gefitinib. After 72-h culture, the survival rate of the cells was determined by MTT assay, and the IC50 of each cell line was calculated.
Expression of IGF-1R and p-AKT1 by western blot
A549/GR cells in the logarithmic growth stage were divided into 3 groups and inoculated at 2×105/well into 6-well cell culture plates. After 24-h culture, the transfection of miR-497-mimic and miR-497-NC was performed with RiboFECTTMCP transfection reagent. Then, the A549/GR-miR497-mimic, A549/GR-miR497-NC, and A549/GR groups were placed into a cell incubator and cultured for 48 h. The expression of IGF-1R and p-AKT1 in each experimental group was detected by western blot assay after total protein extraction with RIPA buffer.
Statistical analysis
The statistical analysis of the experimental results was carried out with SPSS 13.0 statistical software. Quantitative data are presented as the mean ± standard deviation (SD). The results were compared and analyzed with the t-test between the two groups. The differences among groups were compared with one-way ANOVA. A value of P<0.05 was considered to indicate a statistically significant difference. All experiments were independently repeated at least 3 times.
Results
Establishment of gefitinib-resistant A549/GR cell line
The MTT assay results showed that the survival rate of A549 and A549/GR cells decreased gradually with increasing gefitinib concentration, but the survival rate of A549/GR cells was higher than that of A549 cells (Figure 1). The IC50 of A549 and A549/GR cells was significantly different at 8.90±0.48 and 51.96±2.39 µmol/L, respectively (P<0.01). The drug RI of A549/GR was 5.84, indicating that the A549/GR cell line was successfully established.
Figure 1.
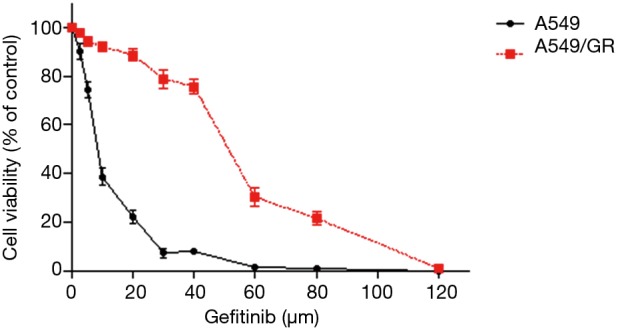
Cell viability curves of A549 and A549/GR cells.
Expression of IGF-1R and p-AKT1 proteins in A549/GR and A549 cells
The expression level of IGF-1R protein in the A549/GR cell line was higher than that in the A549 cell line (P<0.05), and the expression of p-AKT1 also significantly increased (P<0.05) (Figure 2).
Figure 2.
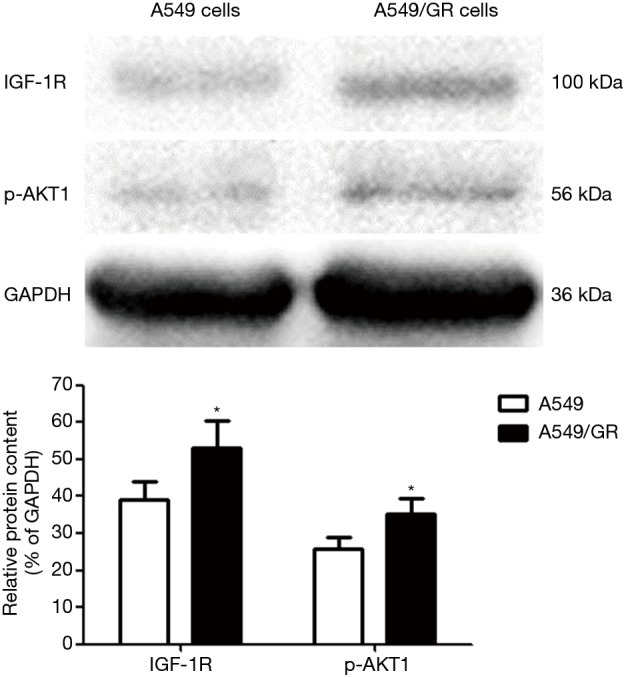
Expression level of IGF-1R and p-AKT1 protein in A549 and A549/GR cells (*, P<0.05 vs. A549 cells).
miR-497 overexpression in A549/GR cells
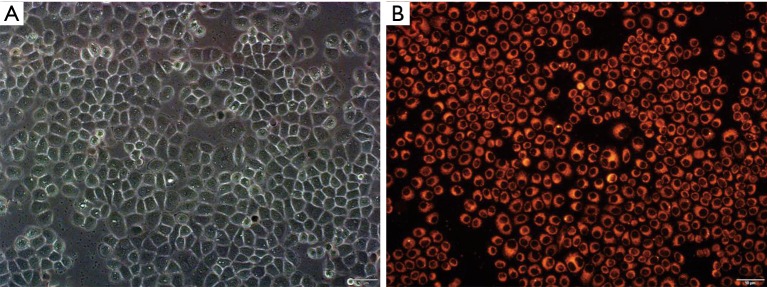
The miR-497-mimic labeled with Cy3 dye emits red fluorescence at the excitation wavelength of 555 nm. Uniform red fluorescence was observed in the cytoplasm of the cells under a fluorescence microscope (as shown in Figure 3), indicated that the miR-497-mimic was successfully transfected into the A549/GR cells.
Figure 3.
Microscopic observation of A549/GR-miR497-mimic cells. (A) Morphology of A549/GR-miR497-mimic cells under white light (×200); (B) morphology of cells in the same field of view under 555-nm excitation (×200).
Overexpression of miR-497 enhances the sensitivity of A549/GR cells to gefitinib
The results of MTT assay showed that the cell survival rate of the A549/GR-miR497-mimic, A549/GR-miR497-NC, and A549/GR groups decreased gradually with the increase in gefitinib concentration, but the cell survival rate of the A549/GR-miR497-mimic group decreased faster than that of the negative control and blank control group (Figure 4). Then, we calculated the IC50 of each group that A549/GR-miR497-mimic cells were 33.76±0.97 µmol/L, A549/GR-miR497-NC cells were 51.84±2.50 µmol/L and A549/GR cells were 52.55±0.53 µmol/L. The statistical analysis of IC50 among the 3 groups revealed a significant difference between the cells of the A549/GR-miR497-mimic group and the negative control group (P<0.01), and between the cells of the A549/GR-miR497-mimic group and the blank control group (P<0.01), but no significant difference between cells of the negative control group and blank control group was observed (P>0.05).
Figure 4.
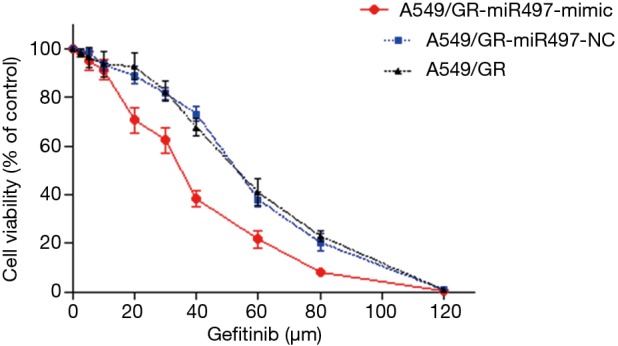
Cell viability curves of A549/GR-miR497-mimic, A549/GR-miR497-NC, and A549/GR cells.
miR-497 inhibits IGF-1R/AKT signaling
The expression level of IGR-1R and p-AKT1 protein in the A549/GR-miR497-mimic cell line was lower than that in the A549/GR-miR497-NC and A549/GR cell lines (Figure 5). Specifically, the expression of IGF-1R protein was 0.75 times lower than that in the negative control group (P=0.018), while the expression of p-AKT1 was 0.69 times lower than that in the negative control group (P=0.010). In contrast, there was no significant difference in the expression of IGF-1R and p-AKT1 protein between the A549/GR-miR497-NC and A549/GR group cells (P>0.05). Therefore, miR-497 may inhibit the IGF-1R/AKT signaling pathway by targeting IGF-1R.
Figure 5.
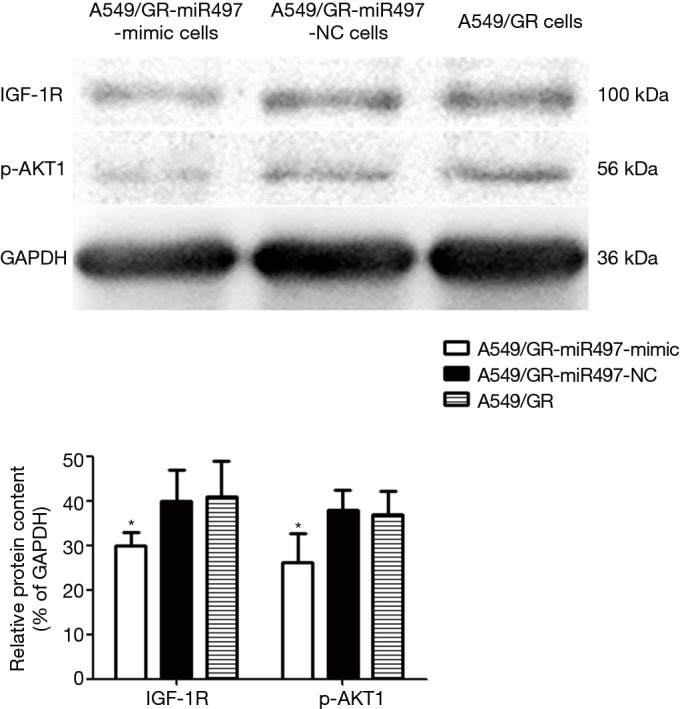
Expression of IGF-1R and p-AKT1 protein in A549/GR-miR497-mimic, A549/GR-miR497-NC, and A549/GR cells (*, P<0.05 vs. A549/GR-miR497-NC or A549/GR cells).
Discussion
miRNAs are single-stranded noncoding RNAs of 19–25 nucleotides in length and encoded by endogenous genes. The main mechanism of miRNAs involves interference with the translation of the target gene and regulation of the expression of the target gene by complete or incomplete complementation of the mRNA. miRNAs are not only involved in cellular functions, including differentiation, proliferation, and apoptosis, but also play important roles in tumor initiation and progression. Research has shown that miR-17-92 clusters (including miR-17, 18a, 19a, 19b, 20a, and 92a), the miR-183 family (including miR-183 and 182), miR-21, and miR-494 can promote the occurrence and development of NSCLC (13-17). On the contrary, the development of NSCLC was inhibited by miR-7, miR-34b, miR-449a, miR-223, and others (18-21). It is evident that miRNA plays a bidirectional role in the development of NSCLC. Recent researches also demonstrate that miRNA is closely related to the occurrence of acquired EGFR-TKI resistance in patients with NSCLC. Zhang et al. found that miR-223 can increase the resistance of NSCLC cells to erlotinib by regulating the FBXW7 signaling pathway (22). On the contrary, Li et al. showed that NSCLC patients with high expression of miR-200c were more sensitive to treatment with gefitinib or erlotinib (23). Further studies have revealed that miR-200c can increase the sensitivity of drug-resistant NSCLC cells to gefitinib by inhibiting activation of the PI3K/AKT signaling pathway (24). In addition, the results of Izumchenko showed that miR-200 can negatively regulate the expression of mitogen-inducible gene 6 (MIG6) protein to reverse the resistance to erlotinib in NSCLC cells (25). Therefore, miRNA can also regulate the EGFR-TKI resistance of NSCLC in both directions.
miR-497 is a tumor-suppressive miRNA that has been reported in various types of cancer such as NSCLC, breast cancer, osteosarcoma, cervical cancer, bladder cancer, and ovarian cancer (26-31). Recent studies have found that miR-497 is also involved in the regulation of sensitivity to chemotherapeutic drugs. In colorectal cancer, miR-497 can regulate the expression of proto-oncogene KSR1 and then enhance the sensitivity of cancer cells to 5-fluorouracil (32). Moreover, the high expression of miR-497 in ovarian cancer can reduce the resistance of tumor cells to cisplatin (33). In addition, studies have shown that miR-497 can modulate the sensitivity of NSCLC to chemotherapeutic drugs. Zhu et al. found a significant decrease in the expression of miR-497 in a human lung adenocarcinoma A549 cell line with multidrug resistance (MDR) to chemotherapeutics. Then they found that the high expression of miR-497 restored the sensitivity to vincristine and cisplatin in the multidrug-resistant A549 cells and promoted apoptosis compared with those in cells with low expression of miR-497 (34). However, the role of miR-497 in resistance to EGFR-TKIs in NSCLC during targeted molecular therapy remained unclear. In the present study, we found that miR-497 could also regulate the resistance of NSCLC to gefitinib, which is an EGFR-TKI. Figure 4 reveals a significant difference in the response to gefitinib between the A549/GR-miR497-mimic and A549/GR-miR497-NC cells, indicating that miR-497, which was a promoter of tumor cell apoptosis, could enhance the sensitivity of resistant cells to gefitinib. Therefore, miR-497 may reverse EGFR-TKI resistance in NSCLC. Yet, the mechanisms that miR-497 regulate the resistance of NSCLC to gefitinib were still need to be elucidated.
To illuminate the underlying mechanisms involved in the miR-497-induced apoptosis of A549/GR cells, we detected the expression of related proteins by western blot and found that the expression of IGF-1R and p-AKT1 in A549/GR-miR497-mimic cells was lower than that in the negative control cells. Related research has indicated that IGF-1R is a target of miR-497; specifically, miR-497 can modulate the expression of IGF-1R and reduce the activity of its downstream signaling pathway to inhibit the growth of liver cancer and osteosarcoma cells (35-37). To further corroborate this, Luo et al. confirmed that miR-497 can directly inhibit the translation of mRNA into IGF-1R protein by incomplete binding with the 3'UTR sequence of IGF-1R mRNA, resulting in the decrease in IGF-1R protein expression and further inhibition of the activation of its downstream PI3K-AKT signal transduction pathway (38). Combined with the results of our study, it was concluded that the overexpression of miR-497 can inhibit the expression of IGF-1R protein and further block the activation of downstream AKT1 signaling in the NSCLC A549/GR cells. In addition, it was found that miR-497 can restore the sensitivity of tumor cells to chemotherapeutic drugs by inhibiting the expression of IGF-1R protein. In the study of colorectal cancer, overexpression of miR-497 could inhibit the expression of IGF-1R and its downstream PI3K-AKT signaling pathway, which not only plays a role in inhibiting the proliferation of tumor cells but also promotes the apoptosis of tumor cells induced by cisplatin and 5-fluorouracil (39). In the study of pancreatic cancer, it was also determined that the overexpression of miR-497 restores the sensitivity of pancreatic cancer cells to gemcitabine by regulating the expression of IGF-1R protein and activation of the AKT pathway (40). The current study suggested that miR-497 can affect not only the response of tumor cells to chemotherapeutic drugs but also the resistance of tumor cells to EGFR-TKI drugs by regulating the expression of IGF-1R protein and its downstream AKT signaling pathway.
At present, numerous studies have confirmed that miRNA can reverse the resistance to EGFR-TKI drugs in NSCLC by inhibiting the IGF-1R signaling pathway. For example, miR-200b can reduce the activity of the PI3K-AKT and MAPK signaling pathways by inhibiting the expression of IGF-1R, ultimately promoting gefitinib-induced proliferation inhibition and apoptosis (11). miR-30a-5p can also restore the sensitivity of NSCLC cells to EGFR-TKIs by inhibiting the expression of IGF-1R and its downstream PI3K-AKT signaling pathway (41). Zhao et al. enhanced the expression of miR-223 by lentiviral transfection of NSCLC cells and found that miR-223 can reverse the resistance of cells to erlotinib by inhibiting the expression of IGF-1R and phosphorylation of AKT, thus restoring the sensitivity of NSCLC cells to erlotinib (42). In the present study, we observed that the expression of IGF-1R and p-AKT1 protein was lower in gefitinib-resistant NSCLC cells with high expression of miR-497, and the sensitivity of resistant cells to gefitinib was increased. It is suggested that miR-497, as a miRNA, can reverse the drug resistance of NSCLC cells by inhibiting the expression of IGF-1R protein and blocking the activation of its downstream AKT1 signaling pathway, thus enhance the sensitivity of drug-resistant cells to gefitinib.
Nevertheless, there are some limitations in the present study. We have not analyzed the expression of RAS, RAF, MEK and ERK proteins. Thus, it was not known whether miR-497 can interfere with the activation of RAS-RAF-MEK-ERK signaling pathway by inhibiting the expression of IGF-1R or not. In addition, our research, which only explored with A549 cell line, has not repeated the results with other lung cancer cell lines. This should influence the extrapolation of miR-497 effects found in this study to all lung cancer cell lines. However, miR-497 did decrease the expression of IGF-1R and AKT1 proteins, and thus enhance the sensitivity of A549/GR cells to gefitinib. Therefore, further studies are warranted to validate our findings.
In conclusion, abnormal activation of IGF-1R and its downstream AKT signaling pathway can promote acquisition of gefitinib resistance by NSCLC cells to bypass EGFR blocking. However, increased miR-497 level may inhibit activation of the AKT1 pathway caused by IGF-1R, representing a potential biomarker of gefitinib-resistant NSCLC. Thus, the overexpression of miR-497 in EGFR-TKI-resistant NSCLC may prove to be beneficial. These results suggested a promising therapeutic strategy against acquired resistance to EGFR-TKIs in NSCLC. Nonetheless, additional potential links and interaction mechanisms between miR-497 and acquired EGFR-TKI resistance should be further explored.
Acknowledgements
We would like to acknowledge the Guangzhou First People’s Hospital.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol. Biomarkers Prev 2016;25:16-27. 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett 2017;401:63-71. 10.1016/j.canlet.2017.04.024 [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol 2016 12;9:34. [DOI] [PMC free article] [PubMed]
- 5.Lee DH. Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): The road to a success, paved with failures. Pharmacol Ther 2017;174:1-21. 10.1016/j.pharmthera.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Morgillo F, Della Corte CM, Fasano M, et al. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open 2016;1:e000060. 10.1136/esmoopen-2016-000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer G, Price J, Bourgeois S, et al. Insulin-like growth factor 1 receptor mediated tyrosine 845 phosphorylation of epidermal growth factor receptor in the presence of monoclonal antibody cetuximab. BMC Cancer 2016;16:773. 10.1186/s12885-016-2796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JM, Jang J, Choi Y, et al. Reduced expression of EI24 confers resistance to gefitinib through IGF-1R signaling in PC9 NSCLC cells. Lung Cancer 2015;90:175-81. 10.1016/j.lungcan.2015.08.019 [DOI] [PubMed] [Google Scholar]
- 9.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008;118:2609-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, Zhu F, Liu J, et al. Alteration in Mir-21/PTEN expression modulates gefitinib resistance in non-small cell lung cancer. PLoS One 2014;9:e103305. 10.1371/journal.pone.0103305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Yao L, Tan B, et al. Detection of microRNA-200b may predict the inhibitory effect of gefitinib on non-small cell lung cancer and its potential mechanism. Oncol Lett 2016;12:5349-55. 10.3892/ol.2016.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma W, Kang Y, Ning L, et al. Identification of microRNAs involved in gefitinib resistance of non-small-cell lung cancer through the insulin-like growth factor receptor 1 signaling pathway. Exp Ther Med 2017;14:2853-62. 10.3892/etm.2017.4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guinot A, Oeztuerk-Winder F, Ventura JJ. miR-17-92/p38 Dysregulation Enhances Wnt Signaling and Selects Lgr6+ Cancer Stem-like Cells during Lung Adenocarcinoma Progression. Cancer Res 2016;76:4012-22. 10.1158/0008-5472.CAN-15-3302 [DOI] [PubMed] [Google Scholar]
- 14.Li L, Song W, Yan X, et al. Friend leukemia virus integration 1 promotes tumorigenesis of small cell lung cancer cells by activating the miR-17-92 pathway. Oncotarget 2017;8:41975-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Quan H, Wang S, et al. MiR-183 promotes growth of non-small cell lung cancer cells through FoxO1 inhibition. Tumour Biol 2015;36:8121-6. 10.1007/s13277-015-3550-8 [DOI] [PubMed] [Google Scholar]
- 16.Markou A, Zavridou M, Lianidou ES. miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer (Auckl) 2016;7:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faversani A, Amatori S, Augello C, et al. miR-494-3p is a novel tumor driver of lung carcinogenesis. Oncotarget 2017;8:7231-47. 10.18632/oncotarget.13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Q, Mao ZD, Shi YJ, et al. MicroRNA-7 inhibits cell proliferation, migration and invasion in human non-small cell lung cancer cells by targeting FAK through ERK/MAPK signaling pathway. Oncotarget 2016;7:77468-81. 10.18632/oncotarget.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LG, Ni Y, Su BH, et al. MicroRNA-34b functions as a tumor suppressor and acts as a nodal point in the feedback loop with Met. Int J Oncol 2013;42:957-62. 10.3892/ijo.2013.1767 [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Huang B, Li Z, et al. MicroRNA-449a is downregulated in non-small cell lung cancer and inhibits migration and invasion by targeting c-Met. PLoS One 2013;8:e64759. 10.1371/journal.pone.0064759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dou L, Han K, Xiao M, et al. MiR-223-5p suppresses tumor growth and metastasis in non-small cell lung cancer by targeting E2F8. Oncol Res 2018. [Epub ahead of print]. 10.3727/096504018X15219188894056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Chen F, He Y, et al. Sensitivity of non-small cell lung cancer to erlotinib is regulated by the Notch/miR-223/FBXW7 pathway. Biosci Rep 2017;37:R20160478. 10.1042/BSR20160478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Li X, Ren S, et al. miR-200c overexpression is associated with better efficacy of EGFR-TKIs in non-small cell lung cancer patients with EGFR wild-type. Oncotarget 2014;5:7902-16. 10.18632/oncotarget.2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou G, Zhang F, Guo Y, et al. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1. Biomed Pharmacother 2017;85:113-9. 10.1016/j.biopha.2016.11.100 [DOI] [PubMed] [Google Scholar]
- 25.Izumchenko E, Chang X, Michailidi C, et al. The TGF -miR200-MIG6 Pathway Orchestrates the EMT-Associated Kinase Switch That Induces Resistance to EGFR Inhibitors. Cancer Res 2014;74:3995-4005. 10.1158/0008-5472.CAN-14-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z, Li A, Yu Z, et al. MicroRNA-497-5p Suppresses Tumor Cell Growth of Osteosarcoma by Targeting ADP Ribosylation Factor-Like Protein 2. Cancer Biother Radiopharm 2017;32:371-8. 10.1089/cbr.2017.2268 [DOI] [PubMed] [Google Scholar]
- 27.Tao L, Zhang CY, Guo L, et al. MicroRNA-497 Accelerates Apoptosis While Inhibiting Proliferation, Migration, and Invasion through negative regulation of the MAPK/ERK signaling pathway via RAF-1. J Cell Physiol 2018;233:6578-88. 10.1002/jcp.26272 [DOI] [PubMed] [Google Scholar]
- 28.Wei Z, Hu X, Liu J, et al. MicroRNA-497 upregulation inhibits cell invasion and metastasis in T24 and BIU-87 bladder cancer cells. Mol Med Rep 2017;16:2055-60. 10.3892/mmr.2017.6805 [DOI] [PubMed] [Google Scholar]
- 29.Lin Z, Zhao J, Wang X, et al. Overexpression of microRNA-497 suppresses cell proliferation and induces apoptosis through targeting paired box 2 in human ovarian cancer. Oncol Rep 2016;36:2101-7. 10.3892/or.2016.5012 [DOI] [PubMed] [Google Scholar]
- 30.Gu A, Lu J, Wang W, et al. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. Int J Biochem Cell Biol 2016;70:118-25. 10.1016/j.biocel.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Cai X, Huang C, et al. miR-497 suppresses angiogenesis in breast carcinoma by targeting HIF-1alpha. Oncol Rep 2016;35:1696-702. 10.3892/or.2015.4529 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Jiang CF, Li DM, et al. MicroRNA-497 inhibits tumor growth and increases chemosensitivity to 5-fluorouracil treatment by targeting KSR1. Oncotarget 2016;7:2660-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S, Fu GB, Tao Z, et al. MiR-497 decreases cisplatin resistance in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget 2015;6:26457-71. 10.18632/oncotarget.4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu W, Zhu D, Lu S, et al. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol 2012;29:384-91. 10.1007/s12032-010-9797-4 [DOI] [PubMed] [Google Scholar]
- 35.Ding WZ, Ni QF, Lu YT, et al. MicroRNA-497 regulates cell proliferation in hepatocellular carcinoma. Oncol Lett 2016;11:1081-8. 10.3892/ol.2015.3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Wang H, Singh A, et al. Expression and function of microRNA-497 in human osteosarcoma. Mol Med Rep 2016;14:439-45. 10.3892/mmr.2016.5256 [DOI] [PubMed] [Google Scholar]
- 37.Cheng H, Xue J, Yang S, et al. Co-targeting of IGF1R/mTOR pathway by miR-497 and miR-99a impairs hepatocellular carcinoma development. Oncotarget 2017;8:47984-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo M, Shen D, Zhou X, et al. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery 2013;153:836-47. 10.1016/j.surg.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 39.Guo ST, Jiang CC, Wang GP, et al. MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene 2013;32:1910-20. 10.1038/onc.2012.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu JW, Wang TX, You L, et al. Insulin-like growth factor 1 receptor (IGF-1R) as a target of MiR-497 and plasma IGF-1R levels associated with TNM stage of pancreatic cancer. PLoS One 2014;9:e92847. 10.1371/journal.pone.0092847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng F, Wang F, Wang L, et al. MiR-30a-5p Overexpression May Overcome EGFR-Inhibitor Resistance through Regulating PI3K/AKT Signaling Pathway in Non-small Cell Lung Cancer Cell Lines. Front Genet 2016;7:197. 10.3389/fgene.2016.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao F Y, Han J, Chen X W, et al. miR-223 enhances the sensitivity of non-small cell lung cancer cells to erlotinib by targeting the insulin-like growth factor-1 receptor. Int J Mol Med 2016;38:183-91. 10.3892/ijmm.2016.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]