Abstract
Tendon repair follows a slow course of early inflammatory, proliferative and remodeling phases, which commonly results in the failure and loss of normal biomechanical properties. Previous studies have demonstrated that tendon-derived stem cells (TDSCs) are vital healing cells and that mRNA expression of anti-inflammatory cytokine interleukin (IL)-10 is significantly upregulated at the late inflammatory phase. To explore how IL-10 may impact tendon healing, the present study investigated the in vitro effects of IL-10 on TDSCs isolated from rat Achilles tendons. Cellular activities of TDSCs and the expression levels of tendon cell markers were measured treatment with IL-10 and subsequent performance of wound healing assays, reverse transcription-quantitative polymerase chain reaction and western blot analyses. The results demonstrated that IL-10 treatment markedly increased the proliferative capacity of TDSCs. In addition, IL-10 significantly enhanced cell migration when compared with the control cells. Furthermore, IL-10 treatment significantly activated the JAK/Stat3 signaling pathway and inhibited the protein expression of tendon cell markers, including scleraxis and tenomodulin. Notably, IL-10 treatment also reduced the gene expression levels of type 1 collagen, type 3 collagen, lumican and fibromodulin in TDSCs. These findings indicated that IL-10 enhanced cell proliferation and migration, and inhibited tenogenic differentiation in TDSCs in vitro. Reducing the negative effects whilst enhancing the positive effects of IL-10 may be a potential therapeutic target in tendon repair.
Keywords: interleukin-10, tendon, tendon-derived stem cells, injury
Introduction
The tendon is a vital component of the musculoskeletal system. Its strong mechanical properties enable it to transmit muscle-contraction force to the skeleton in order to maintain posture or produce motion (1). This property is a consequence of its highly organized structure, consisting of hypocellular connective tissue arranged in a specific spatial organization of type I collagen (Col1) fibers (2). Tendon injuries are common, and account for nearly one third of all musculoskeletal conditions (3). In addition, the repair process of a tendon injury is slow, and commonly results in scar tissue and incomplete recovery (4). Therefore, a full understanding of the pathogenesis and healing mechanism of tendon repair is urgently required. Degeneration is considered the intrinsic pathological mechanism of chronic tendon injury or tendinopathies (5). Previous studies have highlighted the importance of inflammatory cell infiltration and inflammatory cytokine gene expression in animal and human tendon disease (6,7), indicating that inflammation may have an important role in the tendon healing process (8).
It has been demonstrated that interleukin (IL)-10 gene expression is significantly upregulated 2 weeks following the occurrence of Achilles tendon rupture in humans, between the inflammatory and the proliferative phases during the healing process (9,10). Notably, in vivo overexpression of IL-10 has been demonstrated to significantly increase the maximum stress in a tendon-healing model (11). In other tissues and cells, IL-10 has been demonstrated to: i) provide pro-survival cues to melanocytes by exerting anti-apoptotic effects (12); ii) inhibit bone marrow fibroblast progenitor cells from homing and transdifferentiating into myofibroblasts, thereby modulating cardiac fibrosis (13); and iii) reduce type I collagen in cultured human skin fibroblasts (14). However, the exact impact of upregulated IL-10 gene expression on injured tendons has not been fully elucidated.
Recently, tendon-derived stem cells (TDSCs) have been identified in various species including humans, rabbits, rats and mice (15–17). The characteristic properties of stem cells, including proliferation, cloning and multipotency, allow them to differentiate into tendon-like tissues in vitro and/or in vivo (15). A previous study indicated that TDSCs form tendon-like tissues in nude mouse or nude rat models (17), which suggests that TDSCs may contribute to tendon repair. To understand how inflammatory cytokines impact the regenerative and degenerative potentials of TDSCs, the present study focused on IL-10, a cytokine that is upregulated in injured tendons, and examined the effects of IL-10 on the function of TDSCs.
Materials and methods
Animals
All aspects of the research were approved by the Institutional Animal Care and Use Committee of Nanfang Hospital, Southern Medical University (Guangzhou, China). Female Sprague-Dawley rats (n=2; 6-weeks-old; 170–200 g) were purchased from the Laboratory Animal Center of Southern Medical University (Guangzhou, China).
Isolation of TDSCs
TDSCs were isolated from the Achilles tendons of Sprague-Dawley rats as previously reported (18,19). Briefly, rats were anesthetized via an intramuscular injection of pentobarbital (30 mg/kg) and were subsequently sacrificed. Following this, the Achilles tendons were dissected and incubated in 600 U/ml (3 mg/ml) type I collagenase (cat. no. C0130; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and PBS for 2 h at 37°C with gentle shaking. The dissociated cells were plated at a density of 140 cells/cm2 in 100 mm dishes and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) for 8–10 days at 5% CO2 and 37°C. TDSCs at passage 3 or 4 were used in the subsequent experiments. The stem cell characteristics of TDSCs, including the proliferation, clonogenicity and multi-lineage differentiation potential, were confirmed prior to use in subsequent experiments using standard assays, including colony-forming unit fibroblast assays, Oil red O or Alizarin red staining and Alcian blue staining, as described previously (19).
Cell proliferation assay
To perform the cell proliferation assay, TDSCs were plated at a density of 103 cells/well in a 96-well plate cultured in DMEM containing 10% FBS and allowed to adhere overnight at 5% CO2 and 37°C. Following this, DMEM containing 10% FBS with 0.1, 1, 10 and 100 ng/ml rat IL-10 (cat. no. 400-19; PeproTech, Inc., Rocky Hill, NJ, USA) was added to TDSCs, which were then cultured for 1, 3 or 5 days at 37°C. Untreated cells cultured for 1, 3 or 5 days at 37°C were treated as the control. TDSC proliferation was subsequently determined using a Cell Counting Kit-8 assay (CCK-8; cat. no. KL640; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to previously published protocol (20,21).
Cell cycle analysis
Based on the results of aforementioned cell proliferation assays, TDSCs that were either untreated or treated with IL-10 (10 ng/ml) cultured in DMEM containing 10% FBS for 3 days were washed once in PBS and fixed with 500 µl cold 70% ethanol in PBS for 2 h at 4°C. TDSCs were centrifuged at 800 × g at 4°C for 5 min and washed again in PBS, then resuspended in 100 µl RNase A (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) and incubated at 37°C for 30 min. Following this, TDSCs were incubated with 400 µl propidium iodide (PI; Nanjing KeyGen Biotech Co., Ltd.) at 4°C for 30 min, and analyzed with a flow cytometer (FACScan; BD Biosciences, San Jose, CA, USA) and FlowJo 7.6.1 software (FlowJo LLC, Ashland, OR, USA).
Wound healing assay
Cell migration was determined with a wound-healing assay, as previously described (22). TDSCs were grown to 100% confluence in 6-well plates and treated with IL-10 (10 ng/ml) or DMEM containing 2% FBS at 37°C. A sterile P200 pipette tip was used to create a scratch across the cell monolayer. Cultures were subsequently washed once with 1 ml growth medium to remove the damaged and detached cells. Following medium replacement, TDSCs were cultured for 24 h at 37°C. Cell cultures were examined with a phase-contrast microscope (Olympus Corporation, Tokyo, Japan), and images of the entire strip, including three from the same scratch fields, were acquired at 0, 12 and 24 h after cells were scratched. The recovered gap area of the entire strip was measured at different time points using ImageJ 1.8.0 software (National Institutes of Health, Bethesda, MD, USA). The migration rate was based on the calculated recovered gap area and was expressed as a percentage.
Signal transducer and activator of transcription 3 (Stat3) inhibitor application
To demonstrate that IL-10 exerts its functions through the janus kinase (JAK)/Stat3 signaling pathway, TDSCs were plated at a density of 4×105/well on a 60 mm dish, cultured in DMEM containing 10% FBS and allowed to adhere overnight at 5% CO2 and 37°C. TDSCs were subsequently cultured in DMEM containing 10% FBS, supplemented with or without 10 ng/ml IL-10, and with or without 5 µM Stat3 inhibitor (WP1066; cat. no. S2796; Selleck Chemicals, Shanghai, China) for 3 days at 37°C. Following the above treatments, cells were collected and used in further experiments.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
To investigate the effect of IL-10 on spontaneous tenogenic differentiation, spontaneously differentiated tenocytes were used as the control group, in which TDSCs were treated with basic culture medium without IL-10 for 3 days. Following the treatment of cells with 0, 0.1, 1, 10 or 100 ng/ml IL-10 for 3 days, total RNA was isolated from TDSCs with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. Following this, total RNA was reverse-transcribed into cDNA using the PrimeScript™ RT Master Mix kit (cat. no. RR036A; Takara Biotechnology Co., Ltd., Dalian, China). Reactions were incubated in a LightCycler® 480 system (Roche Diagnostics, Basel, Switzerland) for 15 min at 37°C, followed by 5 sec at 85°C and then cooling to 4°C. The resulting cDNA was subjected to qPCR in a LightCycler® 480 system with SYBR green reagent (cat. no. AK8307; Takara Biotechnology Co., Ltd.). The thermocycling conditions used were as follows: Pre-denaturation at 95°C for 30 sec; followed by 40 cycles of denaturation at 95°C for 5 sec, and annealing and extension at 60°C for 30 sec. The mean Cq value was calculated from triplicate reactions. The relative expression levels of scleraxis (Scx), Col1, tenomodulin (Tnmd), collagen type 3 (Col3), mohawk (Mkx), early growth response gene 1 (Egr1), fibromodulin (Fmod), lumican (Lum), decorin (Dcn) and biglycan (Bgn) were calculated based on a standard curve and normalized to GAPDH (23). The primer sequences used are listed in Table I.
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward primers | Reverse primers | NCBI Accession no.a |
|---|---|---|---|
| GAPDH | 5′-AAGCTCATTTCCTGGTATGACA-3′ | 5′-TCTTACTCCTTGGAGGCCATGT-3′ | NM_017008.4 |
| Scx | 5′-AGAACACCCAGCCCAAACA-3′ | 5′-CGGTCTTTGCTCAACTTTCT-3′ | NM_001130508 |
| Col1 | 5′-GTGCTAAGGGTGAAGCTGGT-3′ | 5′-CATCAGCACCAGGGTTTCCAG-3′ | NM_053304 |
| Tnmd | 5′-GTCACATTCTAAATGCAGAAG-3′ | 5′-CTCCCCCAAAACAGGACAAT-3′ | NM_022290 |
| Col3 | 5′-CTGGAGATAAGGGTGAAGGT-3′ | 5′-GAGGGCCTCCTTCACCTTTCT-3′ | NM_032085 |
| Mkx | 5′-CTATCGCACAGGTAAGCCCA-3′ | 5′-CCCACGTATCAGTTTCTCCCA-3′ | XM_017600733 |
| Egr1 | 5′-AACAACCCTACGAGCACCTG-3′ | 5′-ACCAGCGCCTTCTCGTTATT-3′ | NM_012551 |
| Fmod | 5′-CCCGTGATTGTCCCCAAGAA-3′ | 5′-CAGGTACTTGAGGTTGCGGT-3′ | NM_080698 |
| Lum | 5′-GCTTCACCGGGCTTCAATAC-3′ | 5′-AAATGAGTTTCCAGGCACGC-3′ | NM_031050 |
| Dcn | 5′-CCTAAAGGAGCTGCCCGAAA-3′ | 5′-GCCGCCCAGTTCTATGACAA-3′ | NM_024129 |
| Bgn | 5′-CTGCATTGAGATGGGTGGGA-3′ | 5′-GGTAGTTGAGCTTCAGGCCA-3′ | NM_017087 |
NCBI Accession nos. available at https://www.ncbi.nlm.nih.gov/. Scx, scleraxis; Col1, collagen type 1; Tnmd, tenomodulin; Col3, collagen type 3; Mkx, mohawk; Egr1, early growth response gene 1; Fmod, fibromodulin; Lum, lumican; Dcn, decorin; Bgn, biglycan.
Immunofluorescence staining
Following the aforementioned treatments, Col1 immunofluorescence staining was performed on the cell monolayer in a two-step procedure. Specifically, Cells were cultured until a 40–60% confluence was reached prior to immunofluorescence staining. Following this, cells were fixed in 4% paraformaldehyde at room temperature for 10 min, lysed using 0.5% Triton X-100 and then blocked with 5% bovine serum albumin (cat. no. 36101ES25; Shanghai Yusheng Biotechnology Co., Ltd., Shanghai, China) at room temperature for 30 min. Cells were then incubated overnight at 4°C with primary rabbit anti-Col1 antibodies (1:100; cat. no. 14695-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) and subsequently incubated with tetramethylrhodamine-tagged goat anti-rabbit IgG secondary antibodies (1:100; cat. no. HA1016; Hangzhou HuaAn Biotechnology Co., Ltd., Hangzhou, China) for 2 h at room temperature. Following this, cells were incubated with 4′,6-diamidino-2-phenylindole at room temperature for 5 min prior to being photographed using a fluorescence microscope (Olympus BX51; magnification, ×100; Olympus Corporation, Tokyo, Japan).
Western blot analysis
TDSCs were collected and radioimmunoprecipitation assay lysis buffer was used to extract total cell protein. Protein concentration in the samples was measured using a bicinchoninic acid protein assay kit (Nanjing KeyGen Biotech Co., Ltd.). Protein samples (25 µg) were separated via 10% SDS-PAGE, electrotransferred onto polyvinylidene fluoride membranes and then blocked using 5% bovine serum albumin (cat. no. 36101ES25; Shanghai Yusheng Biotechnology Co., Ltd.) at room temperature 1 h. Following this, membranes were incubated with primary antibodies [rabbit polyclonal antibodies against Scx (1:500; cat. no. ab58655; Abcam, Cambridge, MA, USA), Tnmd (1:500; cat. no. ab203676; Abcam), Col1 (1:1,000; cat. no. 14695-1-AP; ProteinTech Group, Inc., Chicago, IL, USA), Col3 (1:1,000; cat. no. 13548-1-AP; ProteinTech Group, Inc.), Stat3 (1:1,000; 10253-2-AP; ProteinTech Group, Inc.), phosphorylated (p)-Stat3 (1:1,000; Tyr705; cat. no. AF3295; Affinity Biosciences, Cincinnati, OH, USA), protein kinase B (Akt; 1:500; cat. no. 10176-2-AP; ProteinTech Group, Inc.), p-Akt (1:500; cat. no. 66444-1-Ig; ProteinTech Group, Inc.) and GAPDH [1:5,000; cat. no. 10494-1-AP; ProteinTech Group, Inc.)] overnight at 4°C. Membranes were subsequently incubated with horseradish peroxidase-conjugated Affinipure Goat Anti-Rabbit IgG (H+L) secondary antibodies (1:5,000; cat. no. SA00001-2; ProteinTech Group, Inc.) for 2 h at room temperature. Proteins were visualized with the electrochemiluminescence kit (cat. no. P0018; Beyotime Institute of Biotechnology, Shanghai, China) and images were captured using the Tanon imaging system (Tanon-5200; Tanon Science and Technology Co., Ltd., Shanghai, China).
Statistical analysis
Results were analyzed using the SPSS version 20 (IBM Corp., Armonk, NY, USA) and expressed as the mean ± standard error of the mean. Data represents at least three replicates for each experimental condition. A Student's t-test, or one-way analysis of variance followed by Dunnett's post-hoc test was used to identify statistical differences. P<0.05 was considered to indicate a statistically significant difference.
Results
IL-10 stimulates cell proliferation of TDSCs
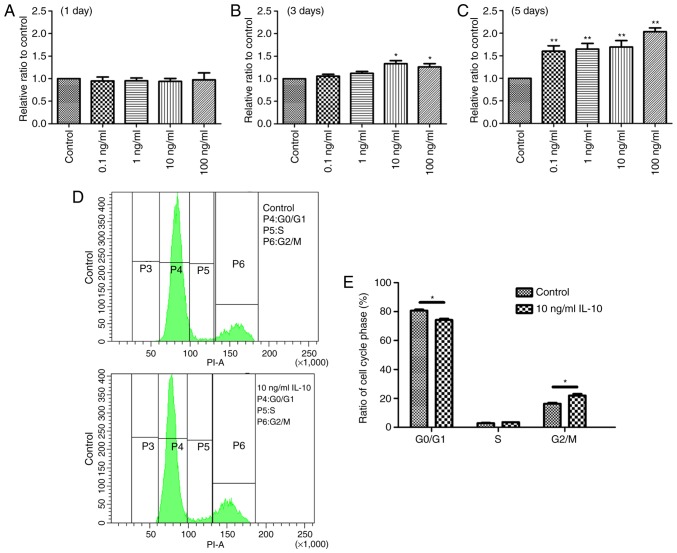
The effect of IL-10 on cell proliferation was initially examined. TDSCs were treated with 0, 0.1, 1, 10 or 100 ng/ml IL-10 for 1, 3 or 5 days, and cell proliferation was determined with a CCK-8 assay. The results revealed a statistically significant increase in cell proliferation in the IL-10-treated cells compared with the controls. At day 1, there was no significant difference between any groups (Fig. 1A). However, the optical density values of TDSCs treated with 10 and 100 ng/ml IL-10 were significantly higher after 3 days compared with the control (P<0.05; Fig. 1B). At day 5, all experimental groups exhibited a significant increase in cell proliferation compared with the control group (Fig. 1C). Based on these results, it was concluded that a 3 day incubation with 10 ng/ml IL-10 would be the condition used to induce the desired effects in the subsequent experiments.
Figure 1.
IL-10 promotes cell proliferation and leads to G2 phase activation in TDSCs. TDSCs were treated with the indicated concentrations of IL-10 for (A) 1, (B) 3 and (C) 5 days. Cell proliferation was subsequently assessed with a Cell Counting Kit-8 assay. (D) TDSCs were treated with 10 ng/ml IL-10 for 3 days and subjected to cell cycle analysis by flow cytometry. (E) The percentage of TDSCs in the G1 phase decreased, whereas the percentage in the G2/M phase increased. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. control cells. IL-10, interleukin-10; TDSCs, tendon-derived stem cells; P, population.
IL-10 leads to cell cycle progression in TDSCs
IL-10 altered the cell cycle of TDSCs. Following incubation of TDSCs with 10 ng/ml IL-10 for 3 days, cell cycle progression was analyzed by flow cytometry. The results demonstrated that the G1 phase was activated and an increased number of cells were in the G2/M phase in IL-10-treated cells, compared with the control (Fig. 1D). In the control and 10 ng/ml IL-10-treated TDSCs, the percentages of cells in G1 phase was 80.7±0.78 and 74.2±0.95%, respectively (Fig. 1E), indicating that IL-10 accelerated G1 phase cell cycle division, compared with the controls (P<0.05). By contrast, the percentage of control and 10 ng/ml IL-10-treated TDSCs in the G2/M phase was 16.3±0.71 and 21.9±1.12%, respectively (P<0.05; Fig. 1E). However, the percentage of TDSCs in the S phase with and without IL-10 treatment was almost identical.
IL-10 promotes TDSC migration
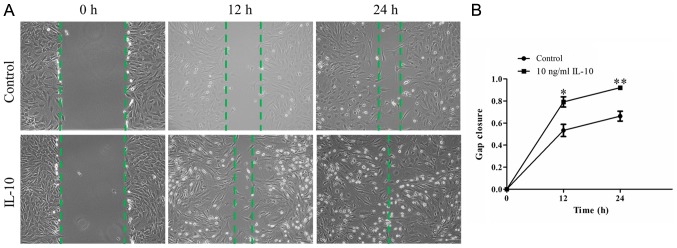
To examine whether IL-10 promotes the repair capacity of TDSCs, an in vitro wound-healing assay was performed. A confluent monolayer of TDSCs was wounded and treated with or without 10 ng/ml IL-10 for 24 h. Microscopy indicated that wound closure in IL-10-treated TDSCs appeared to be significantly greater at 12 and 24 h compared with the control cells (Fig. 2A). Quantitative analyses indicated the cells in the IL-10-treated group had a quicker migration velocity, compared with that in the control group at the two different time points. The mean relative gap closure in cells treated with 0 and 10 ng/ml IL-10 was 53.35±5.54 and 79.18±4.65% after 12 h, and 66.31±4.50 and 92.10±1.04% after 24 h, respectively (Fig. 2B; P<0.01).
Figure 2.
IL-10 promotes TDSC migration (A) Representative time-lapse migration images of control and IL-10-treated cells from the wound healing assay. Images were acquired 0, 12 and 24 h after scratching. Original magnification, ×100. (B) The migration rate was measured by quantifying the total area of the entire strip lacking cells. The relative migration rate of recovered area at 12 and 24 h was calculated from three independent experiments. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. control cells. IL-10, interleukin-10; TDSCs, tendon-derived stem cells.
IL-10 suppresses spontaneous tenogenic differentiation
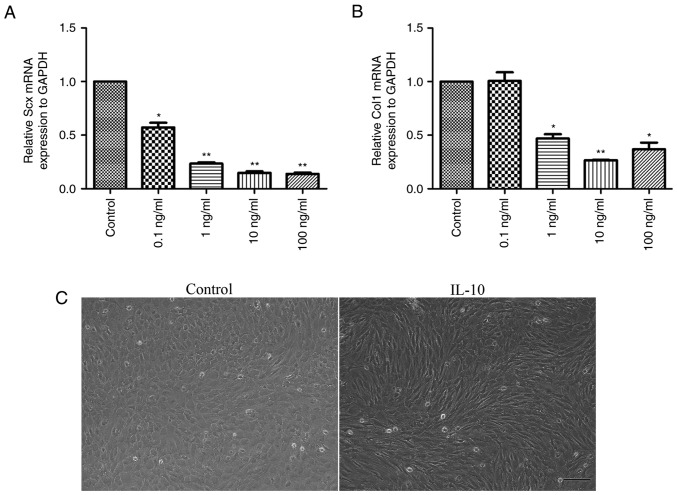
Following the treatment of cells with 0, 0.1, 1, 10 or 100 ng/ml IL-10 for 3 days, the gene expression levels of tendon cell markers, Scx and Col1, were analyzed. The results demonstrated that a significant dose-dependent reduction in the mRNA expression levels of Scx (Fig. 3A) and Col1 (Fig. 3B) occurred. Notably, cells treated with 10 or 100 ng/ml IL-10 were suppressed to a larger extent when compared with the control cells (P<0.01). Furthermore, it was revealed that cells in the IL-10-treated group exhibited long spindle-like shapes and orderly arrangement, while those in control group were ovoid in shape (Fig. 3C).
Figure 3.
IL-10 inhibits spontaneous tenogenic differentiation. Tendon-derived stem cells were treated with the indicated concentrations of IL-10 for 3 days and subjected to quantitative polymerase chain reaction to detect the gene expression of (A) Scx and (B) Col1. (C) Representative images of the morphological alterations in control and IL-10-treated TDSCs (magnification, ×100). Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. control cells. IL-10, interleukin-10; Col1, collagen type 1; Scx, scleraxis.
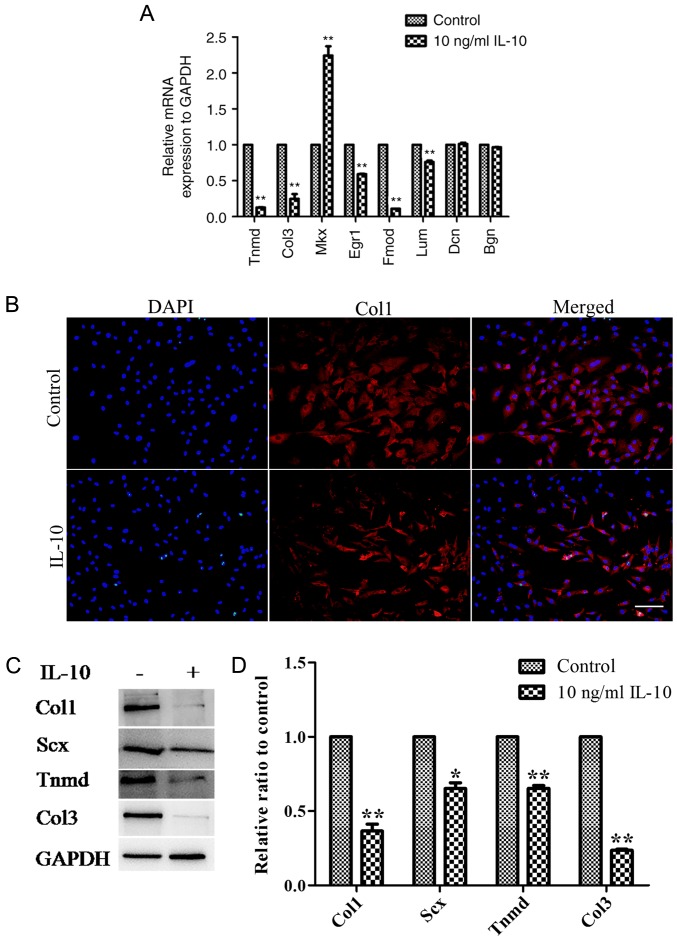
RT-qPCR analysis revealed that IL-10 significantly downregulated the gene expression levels of Tnmd, Col3 and Egr1, Fmod and Lum; however MKx expression was significantly upregulated compared with the control (Fig. 4A). Additionally, no distinctions were observed in the gene expression of Dcn and Bgn between the control and IL-10-treated cells (Fig. 4A). Immunofluorescence analysis demonstrated that the cytoplasm of the control group contained more Col1 than that of the IL-10-treated group (Fig. 4B). At the protein level, western blot analysis consistently revealed significant reductions in the expression levels of Col1 (P<0.01), Scx (P<0.05), Tnmd (P<0.01) and Col3 (P<0.01) in TDSCs incubated with 10 ng/ml IL-10, compared with the control (Fig. 4C and D).
Figure 4.
IL-10 alters the mRNA and protein expression levels tendon-associated molecules in TDSCs. (A) The mRNA expression Tnmd, Col3, Mkx, Egr1, Fmod and Lum was altered by IL-10 treatment. (B) Fluorescent staining of Col1 expression following treatment with IL-10 for 3 days. (C) Rat TDSCs were treated with the indicated concentrations of IL-10 for 3 days and subjected to western blot analysis for Scx, Col1, Tnmd and Col3 protein expression. (D) Densitometric analysis of the western blotting results. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. control cells. IL-10, interleukin-10; TDSCs, tendon-derived stem cells; Tnmd, tenomodulin; Col3, collagen type 3; Mkx, mohawk; Egr1, early growth response gene 1; Fmod, fibromodulin; Lum, lumican; Dcn, decorin; Bgn, biglycan.
IL-10 inhibits spontaneous tenogenic differentiation of TDSCs by activating the JAK/Stat3 signaling pathway
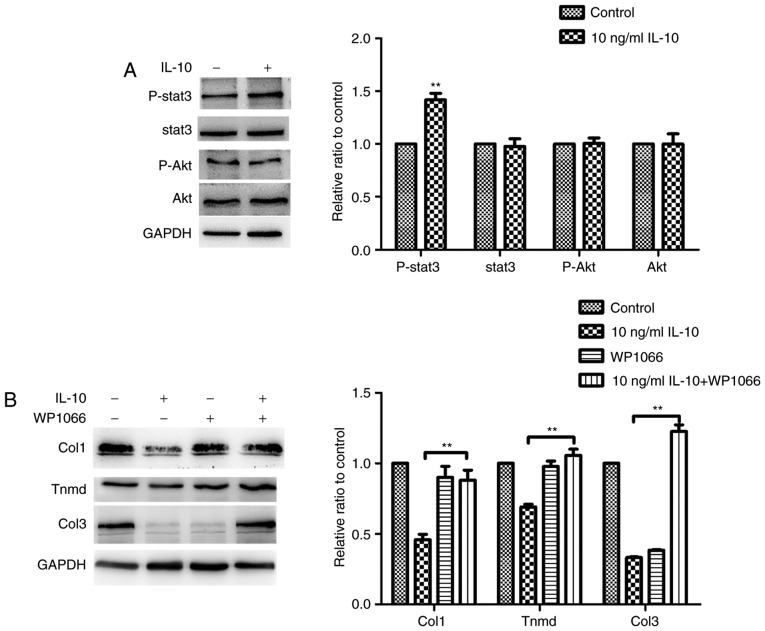
In order to investigate the molecular pathways responsible for the effects of IL-10, the response of TDSCs to IL-10 (10 ng/ml) was examined. It was demonstrated that Stat3 was phosphorylated to a significantly higher degree following IL-10 treatment, compared with the control group, although the PI3K/Akt signaling pathway was not activated (Fig. 5A). These results demonstrated that TDSCs responded directly to IL-10 stimulation. The effect of IL-10 on the gene expression of tendon cell markers, Col1, Col3 and Tnmd, was significantly suppressed by treatment of TDSCs with the Stat3 inhibitor, WP1066, which confirmed that IL-10 inhibited spontaneous tenogenic differentiation of TDSCs by activating the JAK/Stat3 pathway (Fig. 5B).
Figure 5.
IL-10 activates Stat3 but has no effect on Akt expression in TDSCs. (A) Rat TDSCs were treated with the indicated concentrations of IL-10 and were subjected to western blot analysis to detect p-Stat3, Stat3, p-Akt and Akt protein expression. (B) TDSCs were treated with the indicated concentrations of IL-10 with or without the Stat3 inhibitor WP1066 and subjected to western blot analysis for Col1, Tnmd and Col3 protein expression. Data are expressed as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. 10 ng/ml IL-10. IL-10, interleukin-10; TDSCs, tendon-derived stem cells; p-, phosphorylated; Stat3, Signal transducer and activator of transcription 3; Akt, protein kinase B; Tnmd, tenomodulin; Col3, collagen type 3.
Discussion
The tendon is a unique connective tissue with few cells, blood vessels or nerves. Its healing follows a typical wound healing course, which is divided into three overlapping phases: The early inflammatory, proliferative and remodeling phases (24). A commonly injured tendon never regains complete recovery and may result in heterotopic ossification (25,26). The inferior scar tissue formation greatly increases the risk of reinjury (27). Recruitment of a sufficient number of TDSCs is the premise of effective healing, which occurs at the late inflammatory phase and proliferative phase (24). Furthermore, correct tenogenic differentiation of TDSCs is key to exerting beneficial effects, including collagen synthesis, regulation of the tendon microenvironment and regaining normal tendon tissue (17,28). It has been reported that IL-10 is upregulated at the post-inflammatory phase in early human tendon repair (9). IL-10 inhibits the release of pro-inflammatory mediators (29), including IL-1β, which was demonstrated to irreversibly inhibit tenogenic differentiation of injured tendon-derived progenitor cells in our previous study (30). Moroguchi et al (31) demonstrated that IL-10 suppresses the proliferation and expression of type I collagen in human skin fibroblasts. In addition, IL-10 exerts an anti-apoptotic effect, as well as homing and differentiation effects in cells (12,13,32). However, the exact impact of increased IL-10 on injured tendons has not been fully elucidated.
The results of the present study revealed that the proliferative capacity of TDSCs was significantly increased when the cells were treated with IL-10. This indicated that the upregulation of IL-10 at the later inflammatory phase may promote tendon healing by stimulating the proliferation of TDSCs. Notably, it was demonstrated that IL-10 induced cell cycle activation and transition into the G2/M phase from the G1 phase. Additionally, IL-10 significantly promoted cell migration when compared with the control cells, which reflected the repair capacity of TDSCs. In the regenerative phase, healing cells migrate into the injury/repair site, actively proliferate and deposit abundant extracellular matrix components in the tissue (33). Therefore, the proliferation and migration of TDSCs serves a pivotal role in tendon healing. The findings suggest that IL-10, which promotes the proliferation and migration of TDSCs, has a positive impact on tendon healing.
Scx and Tnmd represent tenogenic differentiation markers (2). Under self-differentiated culture conditions, the present study revealed that IL-10 reduced the expression levels of Scx and Tnmd, as well as Egr-1, a transcriptional factor that functions in tendon development (34), indicating that IL-10 may have suppressed TDSC tenogenic differentiation. In addition, IL-10 reduced the expression levels of the main tendon-associated collagens, Col1 and Col3, which was consistent with the results obtained by Abbah et al (35) in human tenocytes. Similarly, gene expression levels of Fmod and Lum, two leucine-rich repeat proteins influencing collagen fibrillogenesis (36), were also inhibited in TDSCs by IL-10. Notably, as Fmod is a critical component of the TDSC niche, reduction of Fmod affects TSDC differentiation (17). It was also revealed that overexpression of Fmod enhanced tendon healing in vivo and in vitro (37). Furthermore, Fmod-deficient mice developed abnormal tendon and ectopic ossification (38). It has been demonstrated Lum has a combined effect together with Fmod at different developmental stages (36). Taken together, these effects of IL-10 may have altered the microenvironment of the TDSCs niche to determine cell fate, consequentially impairing the recovery of biomechanical properties of regenerating tendons. Furthermore, IL-10 is predominantly regulated by JAK and Stat3 transcription factors, and considering that Stat3 is situated downstream of JAK. Stat3 is phosphorylated by JAK (32,39,40). The results of the present study determined that IL-10 inhibited the gene expression of tenogenic differentiation markers in TDSCs by activating the JAK/Stat3 pathway, which was similar to results obtained in previous report in ventral mesencephalic neurons (41).
Upregulated expression of Mkx, a homeobox gene involved in tendon development, was detected in the present study, which was similar to our previous results regarding IL-1β (30). Ito et al (42) reported that Mkx mutant mouse tendons were hypoplastic throughout the body and collagen fibril diameters were smaller, indicating that Mkx has a critical role in tendon development by regulating type I collagen production (42). Although the role of Mkx in tendon repair has not been fully elucidated, upregulation of Mkx by IL-10 may be involved in collagen fibril formation during tendon healing. However, the specific significance of Mkx upregulation by IL-10 and other cytokines requires further investigation.
The present study demonstrated that IL-10 exerted dual effects on TDSCs in vitro. On the one hand, IL-10 enhanced the proliferation and migration of TDSCs. Conversely, IL-10 also inhibited tenogenic differentiation of TDSCs. However, in a murine patellar tendon model, Ricchetti et al (11) revealed that overexpression of IL-10 markedly increased its maximum stress, indicating that complex mechanisms regulate the in vivo effect of IL-10. Although it remains unclear whether the same pathway regulates of the promotion of the proliferation of TDSCs and the inhibition of tenogenic differentiation, it was concluded that timely control of the negative effect of IL-10 and interventional promotion of its positive effect may benefit the stimulation of tendon regeneration. Therefore, effective regulation of IL-10 expression may represent a therapeutic strategy for the clinical treatment of tendon diseases.
In summary, the present study revealed that IL-10 exerted dual effects on TDSCs in vitro via strongly enhancing cell proliferation and migration, as well as inhibiting TDSC tenogenic differentiation through activation of the JAK/Stat3 pathway.
Acknowledgements
The authors thank Professor Liang Ping for his revision of this manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81601900) and the Science and Technology Project of Guangdong Province (grant no. 2016A020214010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Authors' contributions
The experiments were designed by GD and KZ. Experiments were performed by GD, KL, SC, PC and HZ. Data were analyzed by GD, KZ and BY. The paper was written by GD, KZ and BY. All authors read and approved the final manuscript, were involved in revising the manuscript critically for important intellectual content, study conception and design, as well as data interpretation and analysis.
Ethical approval and consent to participate
All aspects of the research were approved by the Institutional Animal Care and Use Committee of Nanfang Hospital, Southern Medical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nourissat G, Berenbaum F, Duprez D. Tendon injury: From biology to tendon repair. Nat Rev Rheumatol. 2015;11:223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 2.Gaut L, Duprez D. Tendon development and diseases. Wiley Interdiscip Rev Dev Biol. 2016;5:5–23. doi: 10.1002/wdev.201. [DOI] [PubMed] [Google Scholar]
- 3.Kaux JF, Forthomme B, Goff CL, Crielaard JM, Croisier JL. Current opinions on tendinopathy. J Sports Sci Med. 2011;10:238–253. [PMC free article] [PubMed] [Google Scholar]
- 4.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–578. [PubMed] [Google Scholar]
- 5.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battery L, Maffulli N. Inflammation in overuse tendon injuries. Sports Med Arthrosc Rev. 2011;19:213–217. doi: 10.1097/JSA.0b013e31820e6a92. [DOI] [PubMed] [Google Scholar]
- 7.Millar NL, Dean BJ, Dakin SG. Inflammation and the continuum model: Time to acknowledge the molecular era of tendinopathy. Br J Sports Med. 2016;50:1486. doi: 10.1136/bjsports-2016-096419. [DOI] [PubMed] [Google Scholar]
- 8.Abate M, Silbernagel KG, Siljeholm C, Di Iorio A, De Amicis D, Salini V, Werner S, Paganelli R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann PW, Domeij-Arverud E, Leclerc P, Amoudrouz P, Nader GA. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc. 2013;21:1801–1806. doi: 10.1007/s00167-012-2197-x. [DOI] [PubMed] [Google Scholar]
- 10.Tarafder S, Chen E, Jun Y, Kao K, Sim KH, Back J, Lee FY, Lee CH. Tendon stem/progenitor cells regulate inflammation in tendon healing via JNK and STAT3 signaling. FASEB J. 2017;31:3991–3998. doi: 10.1096/fj.201700071R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricchetti ET, Reddy SC, Ansorge HL, Zgonis MH, Van Kleunen JP, Liechty KW, Soslowsky LJ, Beredjiklian PK. Effect of interleukin-10 overexpression on the properties of healing tendon in a murine patellar tendon model. J Hand Surg Am. 2008;33:1843–1852. doi: 10.1016/j.jhsa.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Ling J, Song J, Wang Y, Feng B, Ping F. Interleukin 10 protects primary melanocyte by activation of Stat-3 and PI3K/Akt/NF-κB signaling pathways. Cytokine. 2016;83:275–281. doi: 10.1016/j.cyto.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Verma SK, Garikipati VNS, Krishnamurthy P, Schumacher SM, Grisanti LA, Cimini M, Cheng Z, Khan M, Yue Y, Benedict C, et al. Interleukin-10 inhibits bone marrow fibroblast progenitor cell-mediated cardiac fibrosis in pressure-overloaded myocardium. Circulation. 2017;136:940–953. doi: 10.1161/CIRCULATIONAHA.117.027889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitamo S, Remitz A, Tamai K, Uitto J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94:2489–2492. doi: 10.1172/JCI117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskel Dis. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–1558. doi: 10.1089/ten.tea.2009.0529. [DOI] [PubMed] [Google Scholar]
- 17.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Zhang S, Li Q, Yang J, Dong W, Wang S, Cheng Y, Al-Qwbani M, Wang Q, Yu B. Effects of celecoxib on proliferation and tenocytic differentiation of tendon-derived stem cells. Biochem Biophys Res Commun. 2014;450:762–766. doi: 10.1016/j.bbrc.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 19.Asai S, Otsuru S, Candela ME, Cantley L, Uchibe K, Hofmann TJ, Zhang K, Wapner KL, Soslowsky LJ, Horwitz EM, Enomoto-Iwamoto M. Tendon progenitor cells in injured tendons have strong chondrogenic potential: The CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 2014;32:3266–3277. doi: 10.1002/stem.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YH, Liu W, Zhang L, Liu XY, Wang Y, Xue B, Liu B, Duan R, Zhang B, Ji Y. Effects of microRNA-24 targeting C-myc on apoptosis, proliferation, and cytokine expressions in chondrocytes of rats with osteoarthritis via MAPK signaling pathway. J Cell Biochem. 2017 Nov 16; doi: 10.1002/jcb.26514. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Ji D, Wang P, Liang D, Jin L, Shi H, Liu X, Meng Q, Yu R, Gao S. The atypical protein kinase RIOK3 contributes to glioma cell proliferation/survival, migration/invasion and the AKT/mTOR signaling pathway. Cancer Lett. 2018;415:151–163. doi: 10.1016/j.canlet.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33:832–839. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliva F, Via AG, Maffulli N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012;10:95. doi: 10.1186/1741-7015-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien EJ, Frank CB, Shrive NG, Hallgrímsson B, Hart DA. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012;93:319–331. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–329. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Chan KM, Zhang JF, Li G. Tendon-derived stem cells undergo spontaneous tenogenic differentiation. Exp Cell Res. 2016;341:1–7. doi: 10.1016/j.yexcr.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 30.Zhang K, Asai S, Yu B, Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 2015;463:667–672. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moroguchi A, Ishimura K, Okano K, Wakabayashi H, Maeba T, Maeta H. Interleukin-10 suppresses proliferation and remodeling of extracellular matrix of cultured human skin fibroblasts. Eur Surg Res. 2004;36:39–44. doi: 10.1159/000075073. [DOI] [PubMed] [Google Scholar]
- 32.Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Tsai WC, Yu TY, Lin LP, Cheng ML, Chen CL, Pang JH. Prevention of simvastatin-induced inhibition of tendon cell proliferation and cell cycle progression by geranylgeranyl pyrophosphate. Toxicol Sci. 2016;149:326–334. doi: 10.1093/toxsci/kfv239. [DOI] [PubMed] [Google Scholar]
- 34.Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin M, Ruggiu M, Olivera-Martinez I, Robert N, Lu Y, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564–3576. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbah SA, Thomas D, Browne S, O'Brien T, Pandit A, Zeugolis DI. Co-transfection of decorin and interleukin-10 modulates pro-fibrotic extracellular matrix gene expression in human tenocyte culture. Sci Rep. 2016;6:20922. doi: 10.1038/srep20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delalande A, Gosselin M, Suwalski A, Guilmain W, Leduc C, Berchel M, Jaffrès P, Baril P, Midoux P, Pichon C. Enhanced achilles tendon healing by fibromodulin gene transfer. Nanomedicine. 2015;11:1735–1744. doi: 10.1016/j.nano.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed ST, Ivashkiv LB. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J Immunol. 2000;165:5227–5237. doi: 10.4049/jimmunol.165.9.5227. [DOI] [PubMed] [Google Scholar]
- 40.Tanuma N, Shima H, Nakamura K, Kikuchi K. Protein tyrosine phosphatase epsilonC selectively inhibits interleukin-6- and interleukin-10-induced JAK-STAT signaling. Blood. 2001;98:3030–3034. doi: 10.1182/blood.V98.10.3030. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Liu Z, Peng YP, Qiu YH. Interleukin-10 inhibits neuroinflammation-mediated apoptosis of ventral mesencephalic neurons via JAK-STAT3 pathway. Int Immunopharmacol. 2017;50:353–360. doi: 10.1016/j.intimp.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, Nishida K, Akimoto T, Takahashi M, Miyaki S, Asahara H. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Nat Acad Sci. 2010;107:10538–10542. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.