Abstract
(−)Epigallocatechin-3-gallate (EGCG) is a type of polyphenol monomer and is the predominant component of catechin compounds extractable from green tea. Previous studies have demonstrated that EGCG exhibits numerous bioactivities both in vitro and in vivo, including antitumor, antioxidant and anti-inflammatory activities, as well as lowering blood lipid levels and protecting against radiation. The present study aimed to investigate whether administration of EGCG may attenuate anesthesia-induced memory deficit in young mice and to reveal the associated underlying mechanisms. The present study revealed that EGCG administration significantly attenuated memory deficit, oxidative stress and cell apoptosis exhibited by anesthesia-induced mice, as determined by Morris water maze testing and ELISA analysis. Furthermore, the results of ELISA and western blot analysis demonstrated that EGCG administration restored acetylcholinesterase activity and modulated the expression levels of neuronal nitric oxide synthase (nNOS), β-amyloid and amyloid precursor protein in anesthesia-induced mice. The present study also employed L-arginine as an nNOS substrate and 7-nitroindazole as an nNOS inhibitor, which were demonstrated to inhibit or potentiate the effects of EGCG, respectively, on anesthesia-induced memory deficit in mice. Therefore, the present study demonstrated that the administration of EGCG attenuated anesthesia-induced memory deficit in young mice, potentially via the modulation of nitric oxide expression and oxidative stress.
Keywords: (−)epigallocatechin-3-gallate, anesthesia, memory deficit, nitric oxide, oxidative stress
Introduction
Postoperative cognitive dysfunction (POCD) refers to alterations in cognitive abilities experienced by patients following anesthesia, affecting functions such as orientation, memory, attention and insight (1). Furthermore, changes in personality, social skills and other cognitive functions may have a negative impact upon patient recovery, such as extension of hospital stays and regression of quality of life (2). POCD is a common central nervous system complication affecting elderly people following surgery and presents as a mild cognitive dysfunction (2).
Learning and memory are closely associated with the central nervous system, particularly with the function of the hippocampus. The hippocampus is a major component of the limbic system and is an important structure for learning, memory and the regulation of behavior (3). Neuronal nitric oxide synthase (nNOS) in the hippocampus is a key enzyme involved in the synthesis of the neurotransmitter nitric oxide (NO), which participates in mechanisms of learning and memory (4). Furthermore, the long-term potentiation of synaptic transmission efficiency in the hippocampus is recognized as the synaptic plasticity model of memory, which underpins the neuronal mechanisms responsible for behavior, learning and memory (5).
Oxidative stress refers to the process in which the body has been exposed to harmful stimuli and an imbalance between antioxidative and oxidative systems in the body develops, thus predisposing the body towards oxidation (3). Oxidative stress results in the infiltration of neutrophils, increased secretion of proteases and the production of a large number of oxide intermediates, including reactive nitrogen species (RNS) and reactive oxygen species (ROS) (6). Furthermore, excessive levels of oxidation intermediates have been reported to cause tissue damage, which is considered to be an important factor leading to aging and the development of disease (7). RNS include NO, nitrogen dioxide and peroxide nitride, while ROS include the ultra-oxygen anion, oxygen free radicals and hydrogen peroxide (8).
Highly enriched in green tea, polyphenols account for ~22–30% of the dry weight of tea leaves and are primarily composed of four components: Catechin, flavonoids, phenolic acids and flower pigment composition. (−)Epigallocatechin-3-gallate (EGCG) is an antioxidant that protects the adenosine triphosphate enzyme in erythrocytes from oxidative damage (9). Previous studies have indicated that when used as a food additive, EGCG may attenuate the effects of POCD. Green tea leaves contain numerous natural active compounds (9,10). For example, as the predominant catechin in green tea in terms of content, EGCG exhibits important physiological and pharmacological effects (11). As a result, further research is required in order to investigate the therapeutic potential of EGCG (11). Furthermore, numerous plant polyphenols with diverse bioactivities require further investigation in order to determine their therapeutic potential (11). Considering this, the present method of investigation into the effects of EGCG administration may represent a valuable reference for future research aiming to investigate the therapeutic potentials of other polyphenols. The present study investigated whether EGCG administration may attenuate anesthesia-induced memory deficit in young mice, as well the potential underlying mechanisms.
Materials and methods
Experimental animals
All experiments were performed with the approval of the Ethics Committee of Yinzhou People's Hospital (Ningbo, China). Following the purchasing at postnatal day 14, C57BL/6J mice (male; weight, 4–7 g, n=110) were purchased from Animal laboratory of Zhejiang University, housed in polypropylene cages at 22–24°C and 55–60% humidity with free access to food and water on a 12 h light/dark cycle. A total of 110 mice were used as follows: 30 in the first set of experiments followed by 40 (n=10 each for control, model, EGCG treatment and EGCG + L-arginine treatment) and 40 (n=10 each for control, model, EGCG treatment and EGCG + 7-nitroindazole treatment) in the second set of experiments.
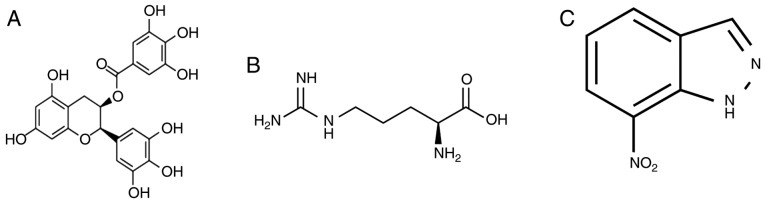
A total of 30 mice were randomly divided into three groups (n=10 per group): Control group, anesthesia model group and EGCG treatment group. In the anesthesia model and EGCG treatment groups, mice were administered 2% sevoflurane delivered via humidified 30% O2 carrier gas for 5 h. Following this, mice belonging to the EGCG treatment group were intragastrically administered EGCG (2 mg/kg/day, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for a total of 4 weeks. The chemical structure of EGCG is presented in Fig. 1A.
Figure 1.
Chemical structure of EGCG and a neuronal nitric oxide synthase inhibitor and substrate. Chemical structures are presented for (A) EGCG, (B) 7-nitroindazole and (C) L-arginine. EGCG, (−)epigallocatechin-3-gallate.
Following this, a total of 40 mice were randomly divided into four groups (n=10 per group): Control group, anesthesia model group, EGCG treatment group (2 mg/kg/day of EGCG) and EGCG + L-arginine (Sigma-Aldrich; Merck KGaA) group (50 mg/kg once a week administered intragastrically; L-arginine for a total of 4 weeks).
Following this, a total of 40 mice were randomly divided into four groups (n=10 per group): Control group, anesthesia model group, EGCG treatment group (2 mg/kg/day of EGCG) and EGCG + L-arginine (Sigma-Aldrich; Merck KGaA) or 7-nitroindazole (Sigma-Aldrich; Merck KGaA) group (50 mg/kg once a week administered intragastrically; 7-nitroindazole administered for a total of 4 weeks). The chemical structures of 7-nitroindazole and L-arginine are presented in Fig. 1B and C, respectively.
Morris water maze
A round pool (90×50 cm) was filled with warm water (25–27°C) and painted black in order to appear opaque. An escape platform (10 cm diameter) was fixed 0.5 cm below the water line in one of four quadrants of the round pool. Mice (n=3/group) were placed in a fixed position of one quadrant facing the wall of the pool. Mice were allowed 120 sec to swim and discover the hidden platform in order to escape. Mice that discovered the hidden platform were permitted to remain on the platform for a further 30 sec. The duration of the escape latencies (finding the hidden platform) was recorded for each mouse and the experiment was repeated three times for each mouse. The mean time was considered to represent the result for any given mouse for that day. Following this, mice were wiped dry before being returned to their cages. On the fifth day of experimentation, the platform was removed and mice were permitted to swim in the pool for a 1 min time period. The number of times mice crossed the original platform site and the time spent in the target quadrant were recorded.
ELISA
Brain tissue was homogenized using a radioimmunoprecipitation assay (Beyotime Institute of Biotechnology, Haimen, China) and protein concentrations of the homogenates were determined using a bicinchoninic assay (Beyotime Institute of Biotechnology). A total of 5 µg protein samples were analyzed for the detection of oxidative stress via determination of malondialdehyde (MDA; cat. no. A003-1), superoxide dismutase (SOD; cat. no. A001-1-1) and glutathione (GSH; cat. no. A006-2) expression levels, while caspase-3/9 activities (cat. nos. G015/G018) were analyzed to determine apoptosis and acetylcholinesterase (AChE; cat. no. A024) activity was also measured, using ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Western blot analysis
Brain tissue was homogenized using a radioimmunoprecipitation assay. Total protein was determined using a bicinchoninic assay. A total of 50 µg protein was subjected to 8–12% SDS-PAGE analysis and transferred to nitrocellulose membranes. Membranes were blocked using 5% skimmed milk powder in TBS with 0.1% Tween-20 for 1 h at room temperature and then incubated at 4°C overnight with the following primary antibodies: nNOS (cat. no. c-17825, 1:1,000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), β-amyloid (Aβ; cat. no. 8243, 1:2,000, Cell Signaling Technology, Inc.), amyloid precursor protein (APP; cat. no. sc-374527, 1:5,000, Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. sc-51631, 1:5,000, Santa Cruz Biotechnology, Inc.). Following this, membranes were incubated at 37°C for 1 h with corresponding horseradish peroxidase-conjugated secondary antibodies (cat. no. sc-2004, 1:2,000; Santa Cruz Biotechnology, Inc.). Membranes were visualized using the ECL Plus western blotting detection system (Beyotime Institute of Biotechnology), developed on a C-DiGit Blot Scanner (LI-COR Biosciences, Lincoln, NE, USA) and quantified using Image Lab 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error (n=3) and were analyzed by one-way analysis of variance followed by Tukey's post-hoc tests using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
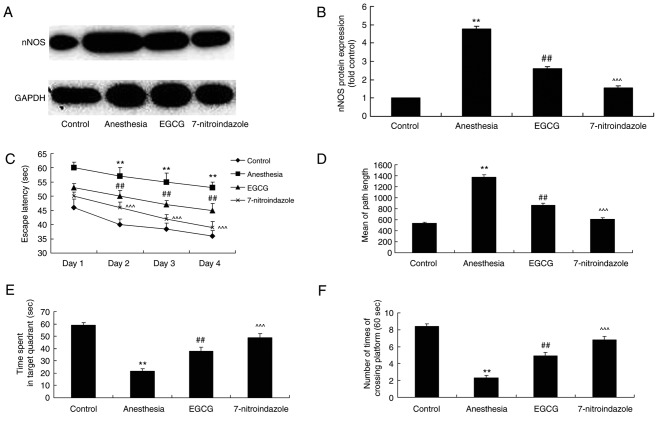
EGCG administration attenuates memory deficit in anesthesia-induced mice
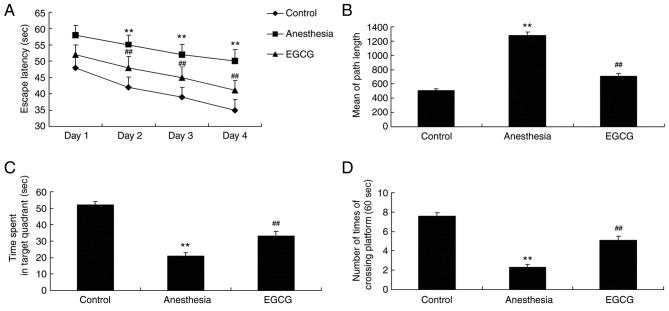
After EGCG treatment, mice from each group were subjected to Morris water maze analysis in order to investigate the effects of EGCG administration. As revealed in Fig. 2A and B, the duration of escape latency and the mean path length (on day 5) exhibited by the anesthesia model group were significantly increased compared with the control group. In addition, the duration of time spent in the target quadrant and the number of times crossing the platform exhibited by the anesthesia model group were significantly decreased compared with the control group (Fig. 2C and D). However, treatment with EGCG was demonstrated to significantly decrease the escape latency duration and the mean path length, and significantly increase the time spent in the target quadrant and the number of times crossing the platform, compared with the anesthesia model group (Fig. 2).
Figure 2.
Effects of EGCG on memory deficit in anesthesia-induced mice. The effects of EGCG on the duration of escape latency. Results are presented as (A) time of escape latency, (B) mean path length, (C) duration of time spent in target quadrant and (D) number of times crossing platform. **P<0.01 vs. control group; ##P<0.01 vs. anesthesia model group. EGCG, (−)epigallocatechin-3-gallate.
EGCG administration attenuates oxidative stress in anesthesia-induced mice
In order to investigate the effect of EGCG administration on oxidative stress levels in mice, samples were subjected to ELISA analysis in order to determine the expression levels of MDA, SOD and GSH. The results demonstrated that there was a significant increase in the expression of MDA, and a significant suppression of SOD and GSH expression, in the anesthesia model group compared with the control group (Fig. 3). However, administration of EGCG was revealed to significantly suppress the expression of MDA, and increase the expression of SOD and GSH, compared with the anesthesia model group (Fig. 3).
Figure 3.
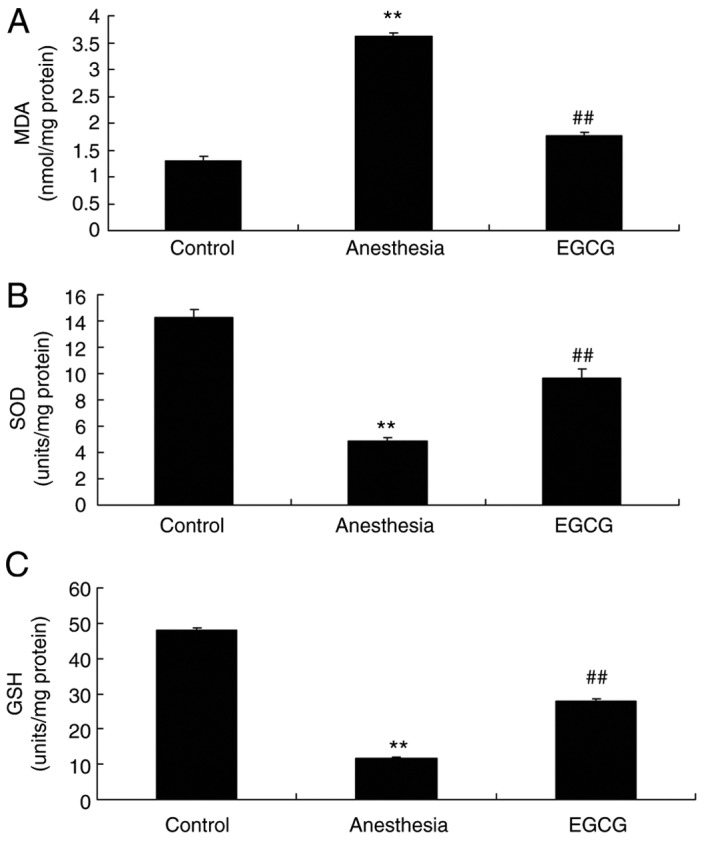
Effects of EGCG on oxidative stress in anesthesia-induced mice. Effects of EGCG on (A) MDA, (B) SOD and (C) GSH levels. **P<0.01 vs. control group; ##P<0.01 vs. anesthesia model group. EGCG, (−)epigallocatechin-3-gallate; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
EGCG administration attenuates apoptosis in anesthesia-induced mice
The effects of EGCG on apoptosis in anesthesia-induced mice were investigated by ELISA. It was demonstrated that caspase-3 and caspase-9 activities were significantly enhanced in the anesthesia model group compared with the control group (Fig. 4). However, treatment with EGCG significantly suppressed both caspase-3 and caspase-9 activity compared with the anesthesia model group (Fig. 4).
Figure 4.
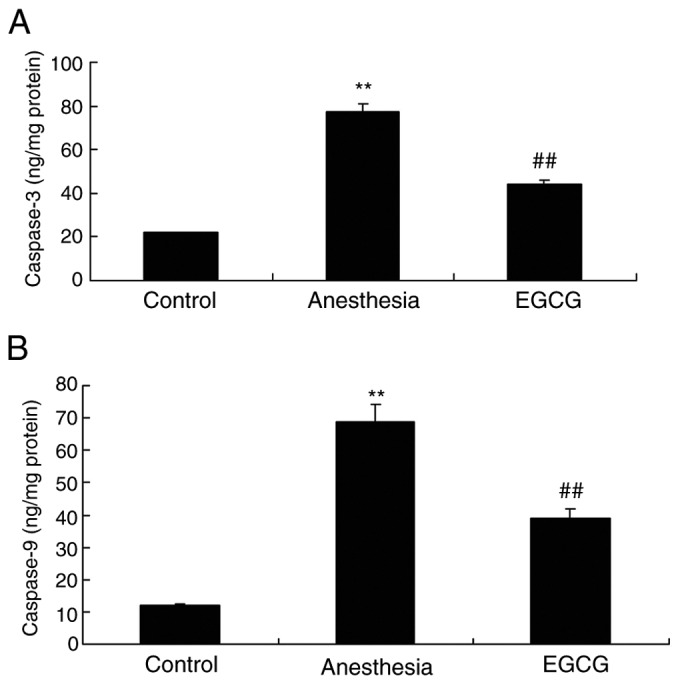
Effects of EGCG on apoptosis in anesthesia-induced mice. Effects of EGCG administration on (A) casapse-3 and (B) caspase-9 activity. **P<0.01 vs. control group; ##P<0.01 vs. anesthesia-induced model. EGCG, (−)epigallocatechin-3-gallate.
EGCG administration suppresses nNOS, Aβ and APP expression and AChE activity in anesthesia-induced mice
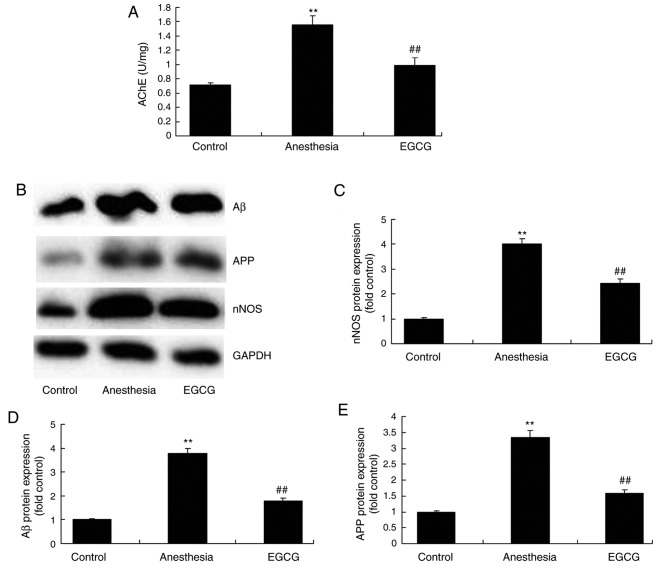
In order to investigate the biological alterations underlying cognitive impairment induced by anesthesia, nNOS, Aβ and APP expression, as well as AChE activity, were analyzed by western blotting and ELISA, respectively. Notably, in the anesthesia model group, nNOS, Aβ and APP protein expression levels, as well as AChE activity, were enhanced compared with the control group (Fig. 5). However, EGCG administration significantly suppressed nNOS, Aβ and APP protein expression levels, as well as AChE activity, compared with the anesthesia model group (Fig. 5).
Figure 5.
Effects of EGCG administration on the activity of AChE, and nNOS, Aβ and APP protein expression, in anesthesia-induced mice. (A) ELISA was performed to determine the effect of EGCG administration on AChE activity in anesthesia-induced mice. (B) Representative protein bands for nNOS, Aβ and APP protein expression by western blotting analysis. Densitometric analysis of western blotting results was performed to quantify and statistically analyze the protein expression of (C) nNOS, (D) Aβ and (E) APP. **P<0.01 vs. control group; ##P<0.01 vs. anesthesia model group. EGCG, (−)epigallocatechin-3-gallate; AChE, acetylcholinesterase; nNOS, nitric oxide synthase; Aβ, β-amyloid; APP, amyloid precursor protein.
L-arginine administration reverses the protective effects of EGCG on memory deficit in anesthesia-induced mice
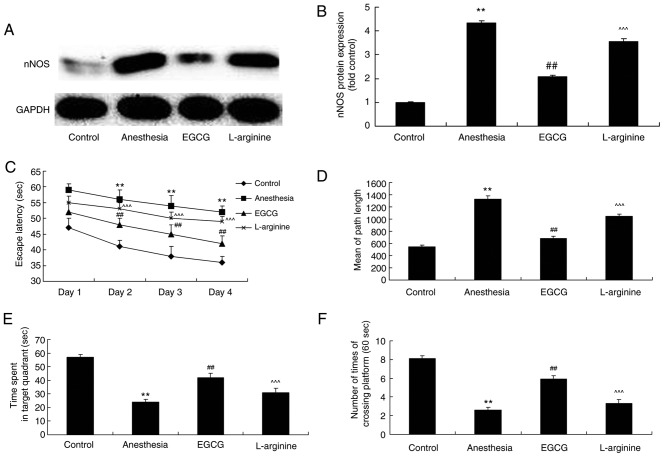
The role of nNOS expression in ECGC-induced attenuation of memory deficit was investigated via administration of L-arginine (an nNOS substrate) alongside EGCG. L-arginine was revealed to significantly enhance nNOS protein expression in anesthesia-induced mice following EGCG treatment, compared with the EGCG-only treatment group (Fig. 6A and B). Furthermore, following treatment with L-arginine, the effects of EGCG treatment on the results of the Morris water maze experiment for the investigation of memory deficit in anesthesia-induced mice were significantly attenuated (Fig. 6C-F).
Figure 6.
nNOS substrate influence on the effects of EGCG administration on memory deficit. (A) Effect of L-arginine, an nNOS substrate, on nNOS protein expression in EGCG-treated anesthetized mice was revealed by western blotting analysis. (B) Densitometric analysis was performed to quantify and statistically analyze nNOS protein expression. A Morris water maze was employed to determine the effects of L-arginine on (C) time of escape latency, (D) mean path length, (E) time spent in target quadrant and (F) number of times crossing platform in EGCG-treated anesthetized mice. **P<0.01 vs. control group; ##P<0.01 vs. anesthesia-induced model; ^^^P<0.01 vs. EGCG-treated anesthetized mice. nNOS, nitric oxide synthase; EGCG, (−)epigallocatechin-3-gallate.
Inhibition of nNOS with 7-nitroindazole enhances the effects of EGCG administration with regards to memory deficit
The effect of the inhibition of nNOS on memory deficit exhibited by anesthesia-induced mice treated with EGCG was also investigated. 7-nitroindazole was employed as an nNOS inhibitor, and the results of western blotting demonstrated that 7-nitroindazole significantly inhibited nNOS protein expression in anesthesia-induced mice treated with EGCG (Fig. 7A and B). However, the inhibition of nNOS via administration of 7-nitroindiazole significantly enhanced the effects of EGCG treatment with regards to memory deficit in anesthesia-induced mice, as measured by the Morris water maze tests (Fig. 7C-F).
Figure 7.
nNOS inhibitor influence on the effects of EGCG administration on memory deficit. (A) Effect of 7-nitroindazole, an nNOS inhibitor, on nNOS protein expression in EGCG-treated anesthetized mice was revealed by western blotting analysis. (B) Densitometric analysis was performed to quantify and statistically analyze nNOS protein expression. A Morris water maze was employed to determine the effects of 7-nitroindazole on (C) time of escape latency (D) mean path length, (E) time spent in target quadrant and (F) number of times crossing platform in EGCG-treated anesthetized mice. **P<0.01 vs. control group; ##P<0.01 vs. anesthesia-induced model; ^^^P<0.01 vs. EGCG-treated anesthetized mice. nNOS, nitric oxide synthase; EGCG, (−)epigallocatechin-3-gallate.
Discussion
The elderly population represents a high-risk group for the development of POCD following clinical anesthesia (12). As a result, ketamine increases the incidence of POCD in elderly people, thus limiting the clinical application of ketamine for anesthetic purposes with regards to elderly patients (12). Sevoflurane is a frequently used intravenous anesthetic in clinical practice. Previous studies have also demonstrated that a combinatory administration of propofol and ketamine reduces the required dosage of ketamine as well as the associated adverse side effects, such as mental and cardiovascular excitation (13,14). In the present study, it was demonstrated that EGCG administration attenuated memory deficit in anesthesia-induced mice. He et al (15) revealed that EGCG administration attenuated acrylamide-induced apoptosis and astrogliosis in the cerebral cortex of rats (12). These results indicate that EGCG may represent a novel therapeutic agent for the treatment of anesthesia-induced memory deficit; however, determining the exact molecular mechanism underlying this process requires further investigation.
Sevoflurane has previously been demonstrated to attenuate cell apoptosis meditated by tumor necrosis factor through the suppression of caspase-3 activity and inhibition of apoptosis via regulation of apoptosis-associated protein expression, such as affecting the Bcl-2/Bcl-2-associated X protein ratio (16). The caspase family refers to a group of regulator genes closely associated with apoptosis, and caspase-3 represents an important apoptotic protein responsible for the induction and execution of cell apoptosis in mammals (17). Hippocampal neurons are the most responsive to total cerebral ischemia and thus are the most susceptible to apoptotic induction (18). The present study revealed that treatment with EGCG significantly suppressed caspase-3 and caspase-9 activity in anesthesia-induced mice.
Previous studies have demonstrated that isoflurane administration affects the formation and degradation process of APP, which may lead to alterations in the secondary structure of Aβ (19,20). Following this, Aβ aggregates form oligomers and eventually plaque formation surrounding brain neurons (19). Furthermore, Aβ sediments were reported to trigger immune inflammatory responses and activate neurotoxic pathways, resulting in the degeneration and induction of apoptosis in neurons (20). In addition, Aβ polypeptides inhibit the release of endogenous acetylcholine (19). Based on the aforementioned factors, it may be hypothesized that the use of isoflurane in anesthesia may damage hippocampal cholinergic neurons, inhibit the release of acetylcholine in the hippocampus and induce the development of POCD (21). The present study demonstrated that EGCG treatment significantly inhibited the activity of AChE and the protein expression of Aβ and APP in mice exposed to anesthesia. In the present study, the regulation of AChE activity by EGCG, as well as the modulation of Aβ and APP expression, was a potentially important mechanism for the attenuation of anesthesia-induced memory deficit in mice. Furthermore, we will investigate the underlying regulatory mechanisms of EGCG administration in anesthesia-induced mice with regards to AChE activity, as well as Aβ and APP expression, in a future study.
Oxidative stress is a predominant factor involved in the aging process. In 1956, a study demonstrated that free radicals were implicated in the aging process of the body (22). Numerous studies have since demonstrated that free radical damage represents part of the aging process, and oxidative stress causes damage to cells and may induce numerous diseases, including brain damage, arteriosclerosis, cognitive dysfunction and dementia (3). As oxidative stress has been reported to accelerate neurodegeneration in Alzheimer's disease, it may be hypothesized that oxidative stress represents part of the neural pathological process associated with POCD (23). Therefore, oxidative stress may represent a predominant source of the damage experienced by neurons and ROS may be considered an important causal factor for the development of POCD and numerous other neuropathies (23). In addition, the present study revealed that treatment with EGCG significantly suppressed MDA levels and significantly enhanced SOD and GSH levels in anesthesia-induced mice. Furthermore, He et al (24) demonstrated that EGCG administration inhibited acrylamide-induced oxidative stress and apoptosis in PC12 cells via the reduction of oxidative stress.
As a relatively recently identified neurotransmitter, NO administration is effective in POCD to a certain extent as it aids learning and memory processing via the regulation of synaptic transmission as well as the induction and maintenance of long-term potentiation (25). Despite this, a dual role for NO in POCD has been reported. A low concentration of NO was reported to boost nerve growth and protect against apoptosis, while a high concentration of NO exerted neurotoxic effects via the induction of mitochondrial dysfunction and the activation of apoptotic pathways (26). However, other studies have indicated that different types of NOS have different effects on POCD (27). The present study demonstrated that EGCG treatment significantly suppressed nNOS expression in anesthesia-induced mice. In addition, the present study also demonstrated that treatment with L-arginine, an nNOS substrate, and 7-nitroindazole, an nNOS inhibitor, inhibited and potentiated the effects of EGCG, respectively, on anesthesia-induced memory deficit in mice. Furthermore, Wei et al (28) revealed that EGCG attenuated NADPH-diaphorase/nNOS expression in the motor neurons of rats. In addition, the present study demonstrated that EGCG administration modulated nNOS levels in brain tissues and attenuated memory deficit in anesthesia-induced mice. However, the underlying therapeutic mechanisms of EGCG administration on nNOS levels have not yet been determined and require further investigation.
In conclusion, the results of the present study demonstrated that EGCG administration attenuated memory deficit, and suppressed apoptosis and oxidative stress, in anesthesia-induced mice. Furthermore, it was revealed that the mechanism underlying this process may involve the regulation of nNOS expression. Thus, the protective effects of EGCG, functioning as a memory deficit suppressor during anesthesia, may represent a novel therapeutic agent for the treatment of POCD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SY designed the study; LD, XG and JH performed the experiments; SY analyzed the data; LD, XG and JH wrote the manuscript.
Ethics approval and consent to participate
All experiments were performed with the approval of the Ethics Committee of Yinzhou People's Hospital (Ningbo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: The influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. doi: 10.1186/s12871-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu YZ, Yao R, Zhang Z, Xu H, Wang LW. Parecoxib prevents early postoperative cognitive dysfunction in elderly patients undergoing total knee arthroplasty: A double-blind, randomized clinical consort study. Medicine (Baltimore) 2016;95:e4082. doi: 10.1097/MD.0000000000004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MR, Abo-Youssef AM, Messiha BA, Khattab MM. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:637–656. doi: 10.1007/s00210-016-1234-6. [DOI] [PubMed] [Google Scholar]
- 4.Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 2013;65:1655–1662. doi: 10.1016/S1734-1140(13)71527-5. [DOI] [PubMed] [Google Scholar]
- 5.Shen F, Li YJ, Shou XJ, Cui CL. Role of the NO/sGC/PKG signaling pathway of hippocampal CA1 in morphine-induced reward memory. Neurobiol Learn Mem. 2012;98:130–138. doi: 10.1016/j.nlm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Sohanaki H, Baluchnejadmojarad T, Nikbakht F, Roghani M. Pelargonidin improves memory deficit in amyloid β25–35 rat model of Alzheimer's disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed Pharmacother. 2016;83:85–91. doi: 10.1016/j.biopha.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Nieradko-Iwanicka B, Borzęcki A. Subacute poisoning of mice with deltamethrin produces memory impairment, reduced locomotor activity, liver damage and changes in blood morphology in the mechanism of oxidative stress. Pharmacol Rep. 2015;67:535–541. doi: 10.1016/j.pharep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.De Moura AC, Brito VB, Porawski M, Saffi J, Giovenardi M. Low maternal care is associated with increased oxidative stress in the brain of lactating rats. Brain Res. 2017;1655:17–22. doi: 10.1016/j.brainres.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Koeberle D, Betticher DC, von Moos R, Dietrich D, Brauchli P, Baertschi D, Matter K, Winterhalder R, Borner M, Anchisi S, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: A randomized phase III non-inferiority trial (SAKK 41/06) Ann Oncol. 2015;26:709–714. doi: 10.1093/annonc/mdv011. [DOI] [PubMed] [Google Scholar]
- 10.Malcomson FC, Willis ND, McCallum I, Xie L, Lagerwaard B, Kelly S, Bradburn DM, Belshaw NJ, Johnson IT, Mathers JC. Non-digestible carbohydrates supplementation increases miR-32 expression in the healthy human colorectal epithelium: A randomized controlled trial. Mol Carcinog. 2017;56:2104–2111. doi: 10.1002/mc.22666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sha D, Lee AM, Shi Q, Alberts SR, Sargent DJ, Sinicrope FA, Diasio RB. Association study of the let-7 miRNA-complementary site variant in the 3′ untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 Clinical Trial) Clin Cancer Res. 2014;20:3319–3327. doi: 10.1158/1078-0432.CCR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silbert BS, Evered LA, Scott DA. Incidence of postoperative cognitive dysfunction after general or spinal anaesthesia for extracorporeal shock wave lithotripsy. Br J Anaesth. 2014;113:784–791. doi: 10.1093/bja/aeu163. [DOI] [PubMed] [Google Scholar]
- 13.Saleh AJ, Tang GX, Hadi SM, Yan L, Chen MH, Duan KM, Tong J, Ouyang W. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: A randomized controlled trial. Med Sci Monit. 2015;21:798–805. doi: 10.12659/MSM.893359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N, Ou C, Liu Y, Zuo Y, Bai Y. Effect of inhalational anaesthetic on postoperative cognitive dysfunction following radical rectal resection in elderly patients with mild cognitive impairment. J Int Med Res. 2014;42:1252–1261. doi: 10.1177/0300060514549781. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Tan D, Bai B, Wu Z, Ji S. Epigallocatechin-3-gallate attenuates acrylamide-induced apoptosis and astrogliosis in rat cerebral cortex. Toxicol Mech Methods. 2017;27:298–306. doi: 10.1080/15376516.2017.1279251. [DOI] [PubMed] [Google Scholar]
- 16.Tagawa T, Sakuraba S, Kimura K, Mizoguchi A. Sevoflurane in combination with propofol, not thiopental, induces a more robust neuroapoptosis than sevoflurane alone in the neonatal mouse brain. J Anesth. 2014;28:815–820. doi: 10.1007/s00540-014-1822-x. [DOI] [PubMed] [Google Scholar]
- 17.Jia Z, Geng L, Xie G, Chu Q, Zhang W. Sevoflurane impairs acquisition learning and memory function in transgenic mice model of Alzheimer's disease by induction of hippocampal neuron apoptosis. Int J Clin Exp Med. 2015;8:15490–15497. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang DX, Zhang LM, Zhao XC, Sun W. Neuroprotective effects of erythropoietin against sevoflurane-induced neuronal apoptosis in primary rat cortical neurons involving the EPOR-Erk1/2-Nrf2/Bach1 signal pathway. Biomed Pharmacother. 2017;87:332–341. doi: 10.1016/j.biopha.2016.12.115. [DOI] [PubMed] [Google Scholar]
- 19.Harach T, Marungruang N, Duthilleul N, Cheatham V, McCoy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T, Bolmont T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep46856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somers C, Goossens J, Engelborghs S, Bjerke M. Selecting Aβ isoforms for an Alzheimer's disease cerebrospinal fluid biomarker panel. Biomark Med. 2017;11:169–178. doi: 10.2217/bmm-2016-0276. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Lv S, Huang Q, Wei J, Zhang S, Huang R, Lu Z, Lin X. Pratensein attenuates Aβ-induced cognitive deficits in rats: Enhancement of synaptic plasticity and cholinergic function. Fitoterapia. 2015;101:208–217. doi: 10.1016/j.fitote.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Mehta V, Parashar A, Udayabanu M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol Behav. 2017;171:69–78. doi: 10.1016/j.physbeh.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Soodi M, Saeidnia S, Sharifzadeh M, Hajimehdipoor H, Dashti A, Sepand MR, Moradi S. Satureja bachtiarica ameliorate beta-amyloid induced memory impairment, oxidative stress and cholinergic deficit in animal model of Alzheimer's disease. Metab Brain Dis. 2016;31:395–404. doi: 10.1007/s11011-015-9773-y. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Tan D, Mi Y, Bai B, Jiang D, Zhou X, Ji S. Effect of epigallocatechin-3-gallate on acrylamide-induced oxidative stress and apoptosis in PC12 cells. Hum Exp Toxicol. 2017;36:1087–1099. doi: 10.1177/0960327116681648. [DOI] [PubMed] [Google Scholar]
- 25.Zhou XY, Zhang F, Ying CJ, Chen J, Chen L, Dong J, Shi Y, Tang M, Hu XT, Pan ZH, et al. Inhibition of iNOS alleviates cognitive deficits and depression in diabetic mice through downregulating the NO/sGC/cGMP/PKG signal pathway. Behav Brain Res. 2017;322:70–82. doi: 10.1016/j.bbr.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Orzelska J, Talarek S, Listos J, Fidecka S. Effects of NOS inhibitors on the benzodiazepines-induced memory impairment of mice in the modified elevated plus-maze task. Behav Brain Res. 2013;244:100–106. doi: 10.1016/j.bbr.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Yu SY, Zhang M, Luo J, Zhang L, Shao Y, Li G. Curcumin ameliorates memory deficits via neuronal nitric oxide synthase in aged mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:47–53. doi: 10.1016/j.pnpbp.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Wei IH, Tu HC, Huang CC, Tsai MH, Tseng CY, Shieh JY. (−)-Epigallocatechin gallate attenuates NADPH-d/nNOS expression in motor neurons of rats following peripheral nerve injury. BMC Neurosci. 2011;12:52. doi: 10.1186/1471-2202-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.