Abstract
Intervertebral disc degeneration (IDD) is a multifactorial disease with few efficacious clinical drugs, which has been demonstrated to be associated with nucleus pulposus (NP) cells apoptosis and degeneration of the extracellular matrix (ECM). Interleukin (IL)-1β, a common proinflammatory cytokine, is considered to be one of key regulators in IDD development. Andrographolide (AG), extracted from Andrographis paniculata, has been suggested to possess marked anti-inflammatory properties. However, the effects of AG on IDD has not been well explored. The present study aimed to investigate the effects and the mechanisms of AG on IDD in human NP cells. NP cells were treated with IL-1β in the absence or presence of AG to investigate the effects on cell viability, cellular apoptosis, production of ECM and matrix metalloproteinase (MMP)-3, MMP-9 and MMP-13, and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and ADAMTS-5. It was identified that IL-1β-induced NP cellular apoptosis was significantly inhibited by AG treatment. Furthermore, AG mitigated the IL-1β-induced degeneration of the ECM, which was paralleled by a decrease in MMPs and ADAMTS levels. In addition, AG exhibited marked inhibitory properties against the activation of Toll-like receptors (TLRs), Myeloid differentiation factor 88 (MyD88) and the nuclear translocation of Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Taken together, these results demonstrated that AG treatment mitigated IL-1β-induced NP cells degeneration through the TLR4/MyD88/NF-κB signaling pathway, and suggested that AG may be a potential agent for IDD prevention and therapy.
Keywords: intervertebral disc degeneration, nucleus pulposus cells, inflammation, apoptosis, andrographolide, toll-like receptor 4/MyD88/nuclear factor kappa-light-chain-enhancer of activated B cells
Introduction
Intervertebral disc degeneration (IDD) is considered to be closely associated with low back pain-associated diseases, including lumbar disc herniation, lumbar spinal stenosis, and degenerative lumbar spondylolisthesis (1,2). Low back pain is one of the common symptoms of IDD, which is a leading cause of disability with a high economic burden worldwide (3). Stimulation of the inflammatory response, mechanical stress and biochemical effectors have been implicated as active components in a number of events and processes associated with IDD (4,5). However, the specific pathological mechanisms and effective treatment methods remain unknown (6).
Intervertebral discs are composed of the nucleus pulposus (NP), annulus fibrosus and cartilaginous endplates. NP cells have been proposed to serve a crucial role in the physiological function of intervertebral discs via maintaining homeostasis among various components of the extracellular matrix (ECM) (7). When the homeostasis of the catabolic and anabolic activities within the ECM, which include collagen I, collagen II and proteoglycans, are disrupted, IDD may occur (8,9). The process of ECM breakdown is caused and promoted by matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) (10,11). Among these molecules, MMP-3, MMP-9, MMP-13, and ADAMTS-4 and ADAMTS-5 are specific typical representatives of degradative matrix components, which have been identified to be highly expressed in IDD tissues and are involved in normal cell turnover and pathological degradation of the major structural components of intervertebral discs (12,13). The activities of MMPs may be controlled by tissue inhibitors of metalloproteinases (TIMPs), which also participate in the maintenance of ECM homeostasis. Among TIMPs, TIMP-1 is closely associated with MMP-3 and MMP-13, and downregulates their activities (14).
Maintaining the stability of viable NP cell numbers is also considered to serve a pivotal role in maintaining the normal function of the NP. Apoptosis, or type I programmed cell death, is an important part of normal cell growth cycle. However, excessive NP cell apoptosis, induced by various pathogenic factors, often causes a decrease in the number of viable cells in the NP, which is associated with IDD (15). Interleukin (IL)-1β, a key proinflammatory cytokine, is an important mediator that causes uncontrolled NP cells apoptosis and degeneration of the ECM (16). As a result, suppression of IL-1β-mediated apoptosis and degeneration of the ECM may be a potential strategy to alleviate IDD development.
The nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway serves as a crucial mediator of inflammatory responses and induces the expression of various pro-inflammatory genes, including those encoding cytokines and chemokines (17). In addition, the NF-κB pathway becomes active when responding to a number of different types of stimuli, including oxidative, mechanical, genotoxic and chemical factors, which finally results in the nuclear accumulation of NF-κB transcription factors, consequently affecting the expression of target genes (18). Previous studies have suggested that the NF-κB pathway may markedly increase the production of specific MMPs and ADAMTS to compromise the normal ECM by degrading it in animal NP cells (19). Similar to the NF-κB pathway, the toll-like receptor (TLR)-4/myeloid differentiation primary response protein MyD88 (MyD88)/NF-κB signaling pathway was identified to be critical in molecular modulation, and has also been demonstrated to be involved in the inflammatory response (20–24).
Andrographolide (AG), a type of diterpenoid extracted from Andrographis paniculata, has been used for patients suffering from infectious diseases of different etiologies (25). It has been well documented that AG possesses potent anti-inflammatory capabilities in conditions associated with inflammation, including certain bacterial or viral infections, tumors and other chronic diseases (24,26–28). It has been reported that AG is a potential therapeutic agent for IL-1β-induced cartilage degeneration and the production of inflammatory factors involved in the pathogenesis of osteoarthritis (29). Additionally, the study of tumor and immune-associated diseases also revealed that the therapeutic effects of AG are closely associated with the TLR4/MyD88/NF-κB signaling pathway (20,24). In addition, it has been well established that abnormal apoptosis may be modulated by AG treatment in certain pathological states (30,31). All of these data indicate that AG may be a therapeutic agent for treating and preventing inflammatory diseases; however, the effects in IDD have not been well explored.
Therefore, on the basis of these data, we hypothesized that AG may have protective properties against the degeneration of NP cells via inhibiting the TLR4/MyD88/NF-κB signaling pathway. In the present study, human NP cells were used to mimic the model of IDD in vitro, and the anti-inflammatory effect and underlying mechanism of AG on the IL-1β-induced degeneration of NP cells was investigated.
Materials and methods
Reagents and antibodies
AG was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific, Inc., (Waltham, MA, USA). Anti-collagen (cat. no. MA1-37493; 1:200), anti-aggrecan (cat. no. MA3-16888; 1:200) and anti-TRL-4 (cat. no. 48-2300; 1:50) were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Anti-MyD88 (cat. no. 4283; 1:500), anti-transcription factor p65 (p65; cat. no. 8242; 1:100), anti-phosphorylated (p)-p65 (cat. no. 3033; 1:500), anti-B-cell lymphoma 2 (Bcl-2; cat. no. 3498; 1:1,000), anti-Bcl-2-associated X protein (Bax; cat. no. 5023; 1:1,000), anti-cleaved caspase 3 (cat. no. 9661; 1:1,000), and goat anti-rabbit (cat. no. 7074; 1:1,000) and anti-mouse (cat. no. 7076; 1:1,000) IgG-horseradish peroxidase secondary antibodies were purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA). β-actin antibodies (cat. no. sc-81178; 1:200) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). An enhanced chemiluminescence (ECL) kit was purchased from Bio-Rad Laboratories, Inc., (Hercules, CA, USA). All of the other reagents were purchased from Sigma-Aldrich; Merck KGaA unless specified otherwise.
NP cell isolation and culture
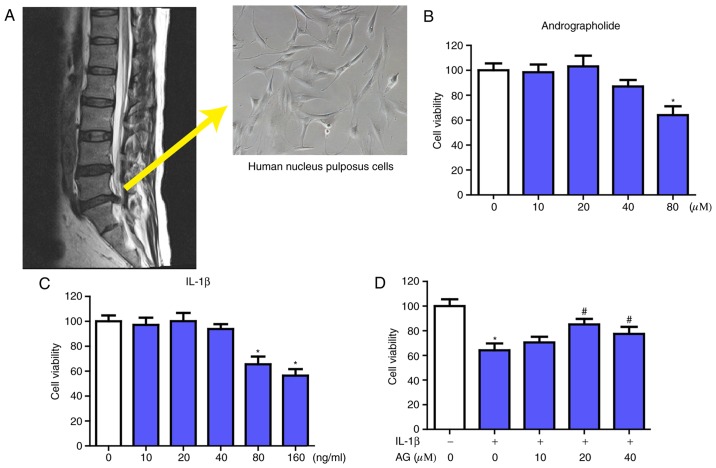
All the tissues from human discs were collected as surgical waste from patients undergoing spinal surgical procedures (Fig. 1A). Young patients diagnosed with lumbar disc herniation were considered ideal candidates. In addition, mild or absent degeneration of the lumbar intervertebral discs in MRI were required. The present study was approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University (Wenzhou, China), and informed consent for the collection of tissues was acquired from patients or relatives. The Pfirrmann grading system was used to evaluate the degenerative conditions of the discs (32). Subsequent to collection of the NP tissues, the NP cells were isolated using 0.25% trypsin and 0.2% type II collagenase for ~3 h at 37°C and used within the first 3 passages. Then, the NP cells were cultured in DMEM supplemented with 10% FBS and antibiotics (1% penicillin/streptomycin) at 37°C in a humidified atmosphere of 5% CO2. During passage, no significant changes in the morphology of cells was identified between the primary cells (passage 0) and later passage cells (passage 2).
Figure 1.
Acquisition and morphology of NP cells and the effect of AG and IL-1β on NP cells viability. (A) The NP cells were collected from patients undergoing spinal surgical procedures, and the morphology of NP cells (magnification, ×400) was investigated. The NP cells obtained were then cultured with increasing concentrations of (B) andrographolide (0, 10, 20, 40 and 80 µM) and (C) IL-1β (0, 10, 20, 40, 80 and 160 ng/ml) for 24 h, followed by the Cell Counting kit-8 analysis for cell viability. (D) NP cells were pretreated for 2 h with various concentrations of andrographolide (0, 10, 20 and 40 µM) and then stimulated with IL-1β (160 ng/ml) for 24 h, followed by Cell Counting kit-8 analysis of cell viability. The values are presented as the mean ± standard deviation of 3 independent experiments. *P<0.05 vs. control group; #P<0.05 vs. IL-1β only group (0 µM AG). NP, nucleus pulposus; IL-1β, interleukin 1β; AG, andrographolide.
Cell viability assays
The viability of the NP cells was assessed by the Cell Counting kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan). For this assay, the cells were seeded at a density of 5,000 cells/well in 96-well plates with 6 replicate wells. DMEM was used to dilute the CCK-8 reagent 10-fold (10:100 µl) prior to addition to each well. Following incubation at 37°C for 3 h, the absorbance of each well was then measured at 450 nm by a microplate reader. The optical density at 450 nm is proportional to the degree of cell viability.
RNA isolation and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted by a TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to manufacturer's protocol. RNA was reverse transcribed into cDNA using a PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China), and RT-qPCR was conducted using SYBR Green Supermix (QPK-212; Toyobo Life Science, Osaka, Japan) and a Light Cycler480 system (Roche Diagnostics GmbH, Mannheim, Germany). The reverse transcription reaction was conducted under the following conditions: 37°C for 15 min and 85°C for 5 sec, then held at 4°C. To amplify genomic DNA, qPCR was performed under the following conditions: 95°C for 5 min, followed by 40 cycles of 95°C for 10 sec, 60°C for 10 sec, and 72°C for 10 sec. The cycle threshold (Cq) values were collected and normalized to the level of the housekeeping gene GAPDH. The relative expression levels were analyzed using the 2−ΔΔCq method (33). The RT-qPCR was performed with the following specific primers: MMP-3 forward, 5′-CAAGGAGGCAGGCAAGACAGC-3′ and reverse, 5′-GCCACGCACAGCAACAGTAGG-3′; MMP-9 forward, 5′-CTTTGAGTCCGGTGGACGAT-3′ and reverse, 5′-TCGCCAGTACTTCCCATCCT-3′; MMP-13 forward, 5′-TGCTTCCTGATGACGATGTAC-3′ and reverse, 5′-TCCTCGGAGACTGGTAATGG-3′; TMIP-1 forward, 5′-TGGCTTCTGGCATCCTGTTGTTG-3′ and reverse, 5′-CGCTGGTATAAGGTGGTCTGGTTG-3′; ADAMTS-4 forward, 5′-GGATTACAGGTGTGAGCCACCA-3′ and reverse, 5′-GGATGCAACCACATCTGTCTGA-3′; ADAMTS-5 forward, 5′-GCAGTATGACAAGTGCGGAGT-3′ and reverse, 5′-CAGGGCTAAATAGGCAGTGAA-3′.
Western blot analysis
The western blot analysis protocol was performed as described previously (34). Briefly, NP cells were homogenized in ice-cold lysis buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 10 mM Na2P2O7, 10 mM NaF, 1 mg/ml aprotinin, 10 mg/ml leupeptin, 1 mM sodium vanadate and 1 mM PMSF. The cells homogenates were incubated for 15 min at 4°C and centrifuged at 11,792 × g for 15 min at 4°C. The protein concentration of each sample was determined using the Bicinchoninic Acid method. The total protein (60 µg) was loaded onto SDS-PAGE (8–10%) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc.). Then, the 5% non-fat milk dissolved in TBS was used to block the PVDF membranes for 2 h at room temperature and the membranes were incubated overnight at 4°C with the aforementioned primary antibodies. Following washing with TBS 3 times for 5 min, the PVDF membranes were incubated at room temperature with horseradish peroxidase-conjugated secondary antibodies to detect the primary antibodies. Signals were visualized using the ChemiDocTM XRS + Imaging System (Bio-Rad Laboratories, Inc.,). Densitometric quantification of the membranes was performed using Image J version 1.4 (National Institutes of Health, Bethesda, MD, USA). Experiments were repeated 3 times.
Immunofluorescence
The NP cells grown on 14×14 mm microscopic glass were washed with ice-cold PBS and fixed with ice-cold 4% paraformaldehyde for 30 min at 4°C. Following washing with ice-cold PBS, the cells were blocked in 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) at room temperature for 1 h. Then, the NP cells were incubated with primary antibodies (against collagen-II, 1:200; aggrecan, 1:200; and p65, 1:100) at 4°C overnight. Following washing with PBS, the cells were incubated with Alexa Fluor 488-conjugated anti-IgG or Texas red-conjugated anti-IgG secondary antibodies for 1 h at 37°C to detect the primary antibodies. The nuclei of cells were stained at room temperature with DAPI (10 µg/ml) for 5 min and finally washed in PBS and sealed with a coverslip. All images were captured on a Nikon ECLIPSE Ti-U fluorescence microscope (Nikon Corporation, Tokyo, Japan; magnification, ×400).
Statistical analysis
The experiments were repeated at least 3 times. The results were analyzed using SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA) and presented as the mean ± standard error of the mean from 3 independent experiments. Statistical significance was examined using one-way analysis of variance and Dunnett's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of AG on the viability of NP cells with or without IL-1β treatment
The source and morphology of NP cells are presented in the Fig. 1A. Firstly, the potential cytotoxicity of AG in NP cells was evaluated using a CCK-8 assay. Increasing concentrations of AG (0, 10, 20, 40 and 80 µM) and IL-1β (0, 10, 20, 40, 80 and 160 ng/ml) were used to culture the NP cells for 24 h, followed by the CCK-8 assay. As indicated in Fig. 1B, AG did not exhibit significant cytotoxic effects on human NP cells at the concentrations of 0–40 µM. When treated with IL-1β only, it demonstrated marked cytotoxic effects in NP cells at the concentrations of 80 and 160 ng/ml, while there was no significant change at concentrations of 0–40 ng/ml (Fig. 1C). The viability of the NP cells following treatment with IL-1β combined with various concentrations of AG (0–40 µM) was also measured by the CCK-8 assay. The results indicated that AG mitigated the cytotoxic effects of IL-1β treatment in a dose-dependent manner, and the concentration of AG that exhibited the most marked effect was 20 µM (Fig. 1D). Therefore, these results indicated that AG had no cytotoxic effects, but did exhibit protective effects on NP cells at concentrations of 20 µM in the assay prior to IL-1β treatment. Therefore, 20 µM AG was used in the subsequent experiments.
AG attenuates IL-1β induced apoptosis of NP cells
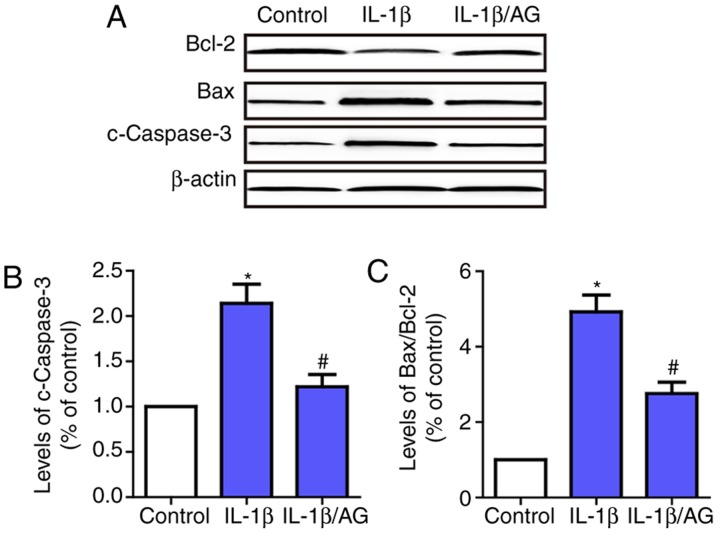
Subsequently, the effect of AG on IL-1β-induced apoptosis of NP cells was examined by investigating the changes in apoptosis-associated enzymes, including caspase-3, Bax and Bcl-2. As indicated in Fig. 2, the results suggested that the level of caspase-3 and the Bax/Bcl2 ratio were increased significantly following IL-1β stimulation compared with the control group. Conversely, AG markedly inhibited IL-1β-induced expression of caspase-3 and Bax. These results demonstrated that AG attenuated IL-1β-induced apoptosis of NP cells.
Figure 2.
Effects of AG on IL-1β-induced apoptosis of NP cells. Cells were stimulated by IL-1β with or without AG treatment. (A) Western blot analysis was used to detect the levels of c-caspase-3 and Bax/Bcl-2. (B) Quantification of c-caspase levels. (C) Quantification of the Bax/Bcl-2 ratio. β-actin was used as an internal control. The values are presented as the mean ± standard deviation of 3 independent experiments. *P<0.05 vs. control group; #P<0.05 vs. IL-1β group. AG, andrographolide; NP, nucleus pulposus; IL-1β, interleukin 1β; c-caspase-3, cleaved caspase-3; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
AG suppresses IL-1β induced MMPs and ADAMTS expression in NP cells
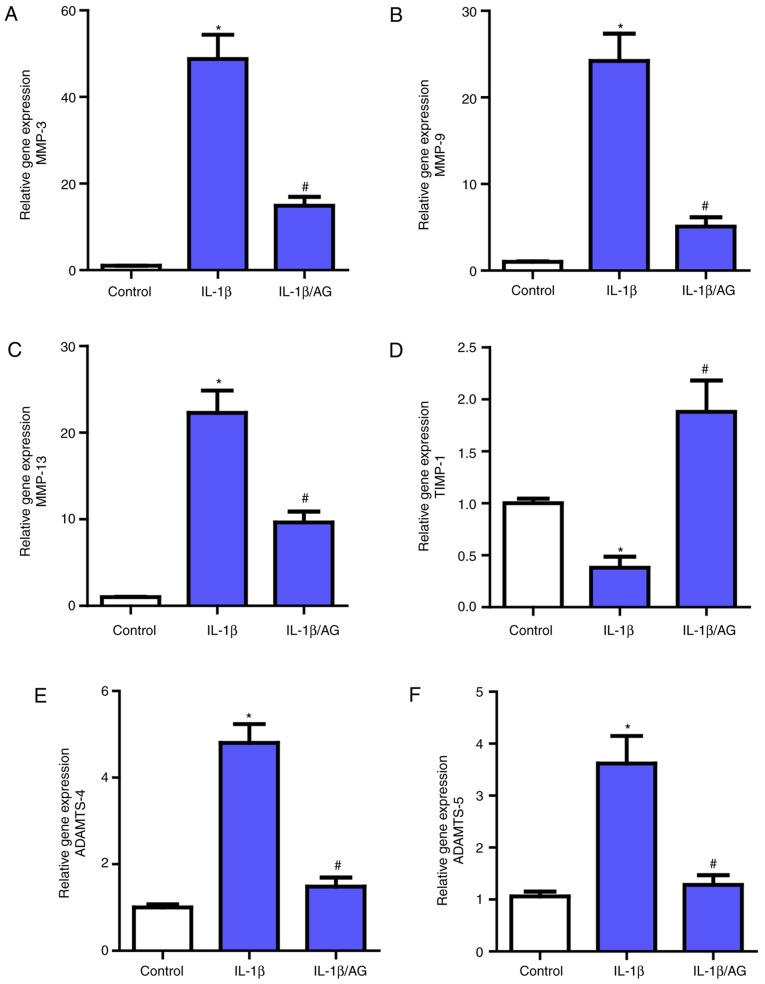
Then, the effect of AG on MMPs (MMP3, MMP-9, MMP-13) and ADAMTS (ADAMTS-4 and ADAMTS-5) expression in NP cells was measured and analyzed by RT-qPCR. As demonstrated in Fig. 3, a marked upregulation of the mRNA expression of these MMPs and ADAMTS following IL-1β stimulation compared with the control group was observed, which was consistent with the previously mentioned association between these catabolic enzymes and NP cell ECM. However, following treatment with AG, there was a marked inhibition of the effect of IL-1β on these enzymes, as demonstrated by RT-qPCR. Additionally, as the endogenous inhibitors of MMPs, the opposite result was observed for TIMP-1 levels compared with MMPs. These results indicated that AG may suppress the expression levels of the catabolic enzymes.
Figure 3.
Effects of AG on MMPs and ADAMTS expression in NP cells. Cells were stimulated by IL-1β with or without AG treatment. The mRNA expression levels of (A) MMP-3, (B) MMP-9, (C) MMP-13, (D) TIMP-1, (E) ADAMTS-4 and (F) ADAMTS-5 were assayed by reverse transcription quantitative polymerase chain reaction. The values are presented as the mean ± standard deviation of 3 independent experiments. *P<0.05 vs. control group; #P<0.05 vs. IL-1β group. AG, andrographolide; MMP, matrix metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; IL-1β, interleukin 1β; TIMP-1, tissue inhibitors of metalloproteinase 1.
AG mitigates IL-1β-induced NP cells ECM degradation
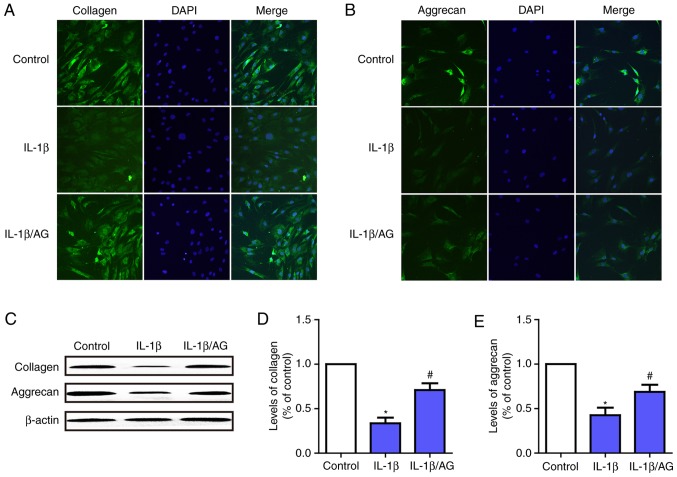
To investigate the function of AG on IL-1β-induced ECM (aggrecan and collagen-II) degradation in NP cells, immunofluorescence staining and western blot analysis were used. As presented in Fig. 4A and B, immunofluorescence staining revealed that IL-1β stimulation resulted in a significant downregulation of aggrecan and collagen-II at the mRNA and protein levels. Conversely, this downregulation was markedly attenuated by AG. Fig. 4C-E demonstrate the results of the western blot analysis, which were consistent with the results of the immunofluorescence assay. These results suggested that AG was likely to serve a role in protecting intervertebral discs by attenuating ECM degeneration in NP cells.
Figure 4.
Effect of AG on IL-1β-induced extracellular matrix degradation in human NP cells. NP cells were stimulated by IL-1β with or without AG treatment. Immunofluorescence images of (A) collagen-II and (B) aggrecan expression following treatment (magnification, ×400). (C) The protein expression levels of aggrecan and collagen were determined western blot analysis. (D) Quantification of collagen levels. (E) Quantification of aggrecan levels. The values are presented as the mean ± standard deviation of 3 independent experiments. *P<0.05 vs. control group; #P<0.05 vs. IL-1β group. AG, andrographolide; IL-1β, interleukin 1β; NP, nucleus pulposus.
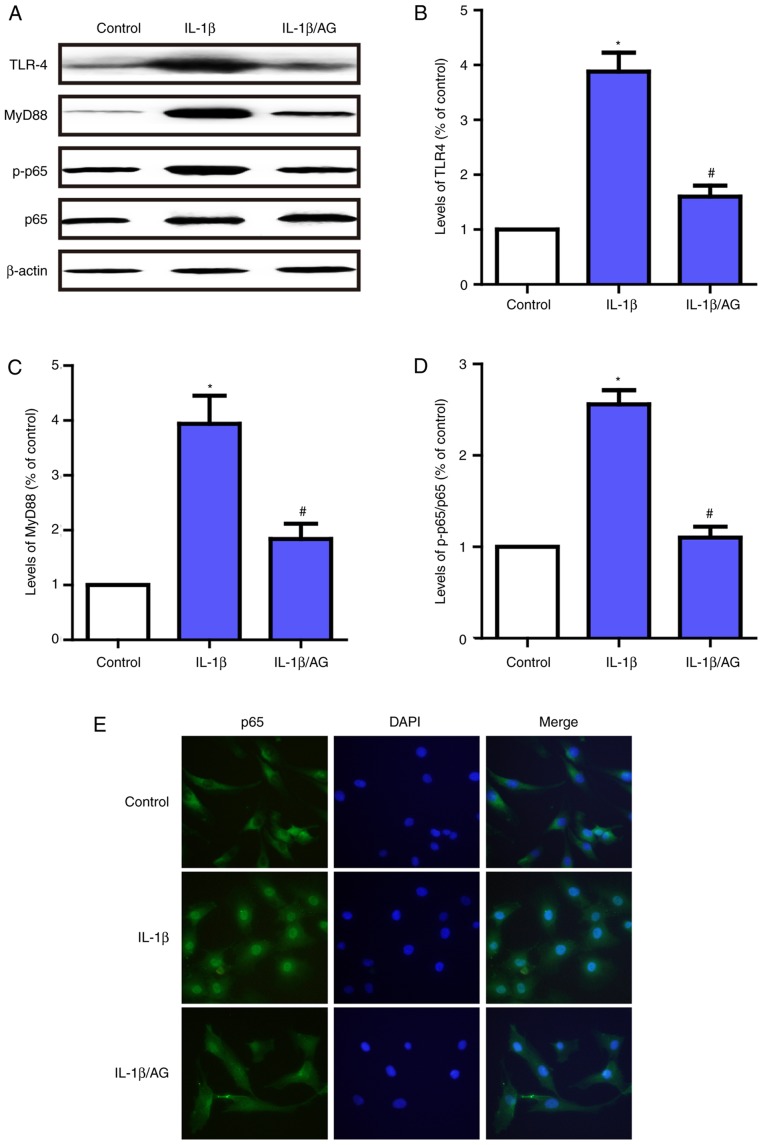
AG protects against NP cell degradation via the TLR4/MyD88/NF-κB signaling pathway
In order to additionally examine the protective mechanism of AG in NP cell degeneration, immunofluorescent staining and western blot analysis were used to detect the effect of AG on IL-1β-induced TLR4/MyD88/NF-κB activation. As demonstrated in Fig. 5A-D, the levels of TLR-4 and MyD88, and the phosphorylation of p65 were significantly upregulated following stimulation with IL-1β. By contrast, AG inhibited the effect on IL-1β-induced activation of the TLR4/MyD88/NF-κB signaling pathway. In addition, the immunofluorescence results suggested that p65 was primarily distributed in the cytoplasm of unstimulated NP cells and exhibited marked localization in the nucleus upon IL-1β stimulation; however, AG significantly inhibited this translocation induced by IL-1β (Fig. 5E). These results suggest that AG has the potential to suppress the expression of TLR-4 and MyD88, and inhibit NF-κB activation in NP cells. The therapeutic effects of AG may be exerted via the TLR4/MyD88/NF-κB signaling pathway.
Figure 5.
Effect of AG on IL-1β-induced activation of the TLR4/MyD88/NF-κB signaling pathway in human NP cells. Cells were stimulated by IL-1β with or without AG treatment. (A) The levels of TLR-4, MyD88, p65 and p-p65 were determined by western blot analysis. (B) Quantification of TLF4 levels. (C) Quantification of MyD88 levels. (D) Quantification of p65/p-p65 ratio. (E) p65 expression was also measured by immunofluorescence (magnification, ×400). The values are presented as the mean ± standard deviation of 3 independent experiments. *P<0.05 vs. control group; #P<0.05 vs. IL-1β group. AG, andrographolide; IL-1β, interleukin 1β; TLR-4, toll-like receptor 4; MyD88, myeloid differentiation primary response protein MyD88; p65, Transcription factor p65; p, phosphorylated.
Discussion
At present, the primary clinical therapy for low back pain-associated disease is symptomatic, or surgical treatment to relieve symptoms, and there is no efficacious medication to mitigate the degenerative progress of the discs. A number of studies have verified the presence of lumbar IDD as an important factor for the promotion of the onset and development of low back pain-associated disease (1,6). It has been established that the degeneration of ECM and the apoptosis of NP cells serve an important role in the basic pathological mechanisms of IDD. Due to the vital role of IDD in low back pain, therapeutic approaches that may postpone or even reverse the IDD process are urgently required. AG, an important component of A. paniculata, has been confirmed to exhibit potential anti-inflammatory and protective effects in infections, tumors and other diseases (24,26). In addition, it has been also suggested that AG possesses therapeutic properties in IL-1β-induced osteoarthritis (29), and has the ability to attenuate abnormal apoptosis under pathological conditions (30,31). In the present study, the primary results indicated that AG treatment effectively mitigated the IL-1β-induced inflammatory and apoptosis effects on the degeneration of human NP cells. The primary mechanism of function is the suppression of the expression of certain MMPs and ADAMTS, and attenuation of the degradation of aggrecan and collagen-II. Additionally, the inflammatory cytokine-mediated changes in apoptosis-associated proteins were modulated by AG. The present study also indicated that the molecular mechanisms of AG treatment may be partly associated with the TLR4/MyD88/NF-κB signaling pathway.
It has been established that IDD is primarily associated with the inflammatory response (35), and inflammatory cytokines have been suggested to have a vital role in the pathological processes of osteoarthritis and IDD. It has been well documented that the expression of inflammatory mediators in degraded tissue is increased compared with normal tissues (36,37). IL-1β is a key inflammatory cytokine, which may significantly induce the occurrence of IDD (38). Concomitantly, NP cell degradation may also be mitigated by the inhibition of IL-1β (39). Therefore, in the present study, the pathophysiology of IDD was mimicked by stimulation with IL-1β.
Apoptosis, also termed type I programmed cell death, is involved in a number of physiological processes. However, uncontrolled apoptosis may be detrimental and serve a causative factor in certain diseases, including IDD (15). Abnormal mechanical stress, oxidative stress, cytokines and nitric oxide may all induce apoptosis of intervertebral disc cells, in particular NP cells (40,41). Adequate, viable NP cells form the physiological basis of intervertebral discs; conversely, the excessive apoptosis of NP cells will lead to the development of IDD (16). The data from the present study suggested that IL-1β accelerated the apoptosis of NP cells, but this effect was significantly inhibited by AG, which indicated that AG may mitigate IL-1β-induced NP cell degeneration by ameliorating the increased levels of apoptosis.
The normal physiological function of intervertebral discs is also considered to depend on the homeostasis between ECM catabolic and anabolic activities, and disturbance of this balance will result in the occurrence of degeneration (8). The ECM of NP cells is primarily composed of aggrecan and collagen-II, and it performs essential functions in intervertebral discs, including absorption of nutrition, maintenance of disc height and withstanding mechanical load (8,42). In the present study, following treatment with IL-1β, the levels of aggrecan and collagen-II decreased significantly; this decrease was suppressed by AG. From a molecular perspective, the MMPs and ADAMTS families are two major groups of catabolic enzymes involved in ECM regulation. MMPs have been demonstrated as vital proteases in the progression of the irreversible breakdown of collagen-II and proteoglycans, with a high expression in degenerative disc tissues. It was also suggested that ADAMTS-4 and ADAMTS-5 are 2 primary proteolytic enzymes responsible for the cleavage of aggrecan. Furthermore, certain studies provided evidence that inhibiting the expression and activity of MMPs and ADAMTS may prevent the progression of IDD (43). For example, Zhongyi et al (19) identified that IL-1β-dependent gene upregulation of MMP-3, MMP-9, MMP-13, ADAMTS-4 and ADAMTS-5 was significantly decreased by NF-κB inhibition. Therefore, the inhibition of MMPs and ADAMTS may be considered potential therapeutic targets for treating IDD. In addition, TIMPs, the endogenous inhibitors of MMPs, also participate in the maintenance of ECM homeostasis. Among TIMPs, TIMP-1 is closely associated with MMP-3 and MMP-13, and downregulates their activities (14). Therefore, TIMP-1 is also considered to have an inhibitory effect on IDD. In the present study, it was identified that IL-1β upregulated the expression of MMP-3, MMP-9, MMP-13, ADAMTS-4, and ADAMTS-5; concomitantly, TIMP-1 exhibited a marked decrease. However, AG treatment significantly suppressed these IL-1β-induced changes in the ECM and metabolic enzymes in NP cells. These changes suggested that AG inhibited IL-1β-induced NP cells degeneration via decreasing the level of ECM degeneration and suppressing the expression these catabolic enzymes.
The NF-κB signaling pathway is known for its crucial regulation in a series of catabolic processes active in response to inflammation, stress, and cellular damage (17,19). For example, following stimulation with IL-1β, the inactive NF-κB combined with the inhibitory protein NF-κB inhibitor α may be activated and released, subsequently translocated from the cytoplasm into the nucleus, and finally activate the transcription of its target genes, including MMPs (44). It has been demonstrated that the activation of the NF-κB signaling pathway contributes to ECM degradation by increasing the activity of matrix-degrading enzymes in the NP cells (19). Therefore, the targeted inhibition of NF-κB may be a critical therapeutic target for IDD. Additionally, The p65 binding site has also been identified to be in the promoter regions of several MMP genes (45). Therefore, in the present study, it was determined whether the anti-inflammatory effects of AG against ECM degradation functioned through NF-κB signaling pathways by investigating the changes in p65 and nuclear translocation. Notably, the IL-1β-induced phosphorylation of p65 and nuclear translocation were significantly inhibited by AG. These results were consistent with Peng et al (46), who identified that AG markedly decreased the p65 phosphorylation level following ovalbumin stimulation. The TLR4/MyD88 signaling pathway is also a pivotal pathway involved in inflammation response (20,21), which is considered to function in conjunction with NF-κB signaling pathway (22–24). The TLRs are a family of receptor proteins used by the innate immune system in mammals; activation of TLRs is involved in the production of a number of proinflammatory cytokines. MyD88 is a signal adaptor molecule with roles in signaling via the TLRs, including TLR4 (47). The activation of the TLR4/MyD88 pathway is considered as an activating factor for the NF-κB signaling pathway (23,24). The results of the present study demonstrated that the IL-1β-mediated upregulation of TLR4 and MyD88 was inhibited by AG treatment, which was consistent with the changes of p65 observed. Taken together, these data suggest that the inhibition of the IL-1β-induced inflammatory response by AG may be partly associated with TLR4/MyD88/NF-κB signaling pathway. It should be also noted that additional studies, which reconfirm this mechanism by using gene knockout mice, are expected to clarify this issue.
In conclusion, the data from the present study revealed that AG may alleviate IL-1β-induced human NP cells apoptosis. Furthermore, AG may also attenuate IL-1β-induced degeneration of the ECM, and the expression of MMPs and ADAMTS via inhibiting the TLR4/MyD88/NF-κB signaling pathway. Therefore, AG may be a potential agent for IDD prevention and treatment. However, the exact mechanism of AG-based regulation of inflammation in NP cells remains unclear, and additional studies are required.
Acknowledgements
The authors would like to thank the Laboratory of Orthopedics and Scientific Research Center of Second Affiliated Hospital of Wenzhou Medical University (Zhejiang, China).
Glossary
Abbreviations
- IDD
intervertebral disc degeneration
- NP
nucleus pulposus
- ECM
extracellular matrix
- IL-1β
interleukin-1β
- MMP
matrix metalloproteinase
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- TLRs
toll-like receptors
- MyD88
myeloid differentiation primary response protein MyD88
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- TIMPs
tissue inhibitors of metalloproteinases
- AG
andrographolide
Funding
The present study was supported by Zhejiang Province Medical Science and Technology Project (grant no. 2017171281) and the Wenzhou Bureau of Science and Technology Project (grant no. Y20160136).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LZ and SS conceived and designed the experiments. LZ, QC and JY performed the experiments and analyzed the data. HW prepared and assessed the figures. SS provided guidance for experiments. LZ was primarily responsible for the preparation of the manuscript. SS contributed to revision of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University (Zhejiang, China) and the patients provided written informed consent.
Patient consent for publication
The patients provided written informed consent.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Livshits G, Popham M, Malkin I, Sambrook PN, Macgregor AJ, Spector T, Williams FM. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 4.Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: Review of animal model data. J Bone Joint Surg Am. 2006;88(Suppl 2):76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 5.Vo N, Niedernhofer LJ, Nasto LA, Jacobs L, Robbins PD, Kang J, Evans CH. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31:831–837. doi: 10.1002/jor.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Froud R, Patterson S, Eldridge S, Seale C, Pincus T, Rajendran D, Fossum C, Underwood M. A systematic review and meta-synthesis of the impact of low back pain on people's lives. BMC Musculoskel Dis. 2014;15:50. doi: 10.1186/1471-2474-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH, Smit TH. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Hangai M, Kaneoka K, Kuno S, Hinotsu S, Sakane M, Mamizuka N, Sakai S, Ochiai N. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732–740. doi: 10.1016/j.spinee.2007.07.392. [DOI] [PubMed] [Google Scholar]
- 10.Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 11.Bachmeier BE, Nerlich A, Mittermaier N, Weiler C, Lumenta C, Wuertz K, Boos N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18:1573–1586. doi: 10.1007/s00586-009-1031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: Their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 13.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Wu Y, Tan Y, Fei X, Deng Y, Cao H, Chen B, Wang H, Magdalou J, Chen L. Cytotoxic effects of the quinolone levofloxacin on rabbit meniscus cells. J Appl Toxicol. 2014;34:870–877. doi: 10.1002/jat.2903. [DOI] [PubMed] [Google Scholar]
- 15.Zhou GQ, Yang F, Leung VVL, Cheung KMC. Molecular and cellular biology of the intervertebral disc and the use of animal models. Curr Orthopaed. 2008;22:267–273. doi: 10.1016/j.cuor.2008.05.008. [DOI] [Google Scholar]
- 16.Yang SD, Yang DL, Sun YP, Wang BL, Ma L, Feng SQ, Ding WY. 17β-estradiol protects against apoptosis induced by interleukin-1β in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis. 2015;20:348–357. doi: 10.1007/s10495-015-1086-4. [DOI] [PubMed] [Google Scholar]
- 17.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: The roles of NF-κB and MAP kinases. Eur Cells Mater. 2012;23:103–119. doi: 10.22203/ecm.v023a08. discussion 119–119. [DOI] [PubMed] [Google Scholar]
- 19.Zhongyi S, Sai Z, Chao L, Jiwei T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine (Phila Pa 1976) 2015;40:224–232. doi: 10.1097/BRS.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 20.Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, Joyce SK, Panda B, Paidas MJ, Abrahams VM. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62:96–111. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyo F, Futani H, Matsui K, Terada M, Adachi K, Nagata K, Sano H, Tateishi H, Tsutsui H, Nakanishi K. Endogenous interleukin-6, but not tumor necrosis factor alpha, contributes to the development of toll-like receptor 4/myeloid differentiation factor 88-mediated acute arthritis in mice. Arthritis Rheum. 2005;52:2530–2540. doi: 10.1002/art.21213. [DOI] [PubMed] [Google Scholar]
- 22.Biragyn A, Coscia M, Nagashima K, Sanford M, Young HA, Olkhanud P. Murine beta-defensin 2 promotes TLR-4/MyD88-mediated and NF-kappaB-dependent atypical death of APCs via activation of TNFR2. J Leukoc Biol. 2008;83:998–1008. doi: 10.1189/jlb.1007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayan V, Khandelwal M, Manglani K, Gupta S, Surolia A. Methionine down-regulates TLR4/MyD88/NF-κB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br J Pharmacol. 2014;171:107–121. doi: 10.1111/bph.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang QQ, Ding Y, Lei Y, Qi CL, He XD, Lan T, Li JC, Gong P, Yang X, Geng JG, Wang LJ. Andrographolide suppress tumor growth by inhibiting TLR4/NF-κB signaling activation in insulinoma. Int J Biol Sci. 2014;10:404–414. doi: 10.7150/ijbs.7723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Sheeja K, Kuttan G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharmacol Immunotoxicol. 2007;29:81–93. doi: 10.1080/08923970701282726. [DOI] [PubMed] [Google Scholar]
- 26.Wintachai P, Kaur P, Lee RC, Ramphan S, Kuadkitkan A, Wikan N, Ubol S, Roytrakul S, Chu JJ, Smith DR. Activity of andrographolide against chikungunya virus infection. Sci Rep. 2015;5:14179. doi: 10.1038/srep14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiou WF, Chen CF, Lin JJ. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br J Pharmacol. 2000;129:1553–1560. doi: 10.1038/sj.bjp.0703191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu WJ, Lin KH, Hsu MJ, Chou DS, Hsiao G, Sheu JR. Suppression of NF-κB signaling by andrographolide with a novel mechanism in human platelets: Regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochem Pharmacol. 2012;84:914–924. doi: 10.1016/j.bcp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Ding QH, Ji XW, Cheng Y, Yu YQ, Qi YY, Wang XH. Inhibition of matrix metalloproteinases and inducible nitric oxide synthase by andrographolide in human osteoarthritic chondrocytes. Mod Rheumatol. 2013;23:1124–1132. doi: 10.3109/s10165-012-0807-6. [DOI] [PubMed] [Google Scholar]
- 30.Burgos RA, Seguel K, Perez M, Meneses A, Ortega M, Guarda MI, Loaiza A, Hancke JL. Andrographolide inhibits IFN-gamma and IL-2 cytokine production and protects against cell apoptosis. Planta Med. 2005;71:429–434. doi: 10.1055/s-2005-864138. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Zhu D, Wang Y, Ju Y. Andrographolide attenuates LPS-induced cardiac malfunctions through inhibition of IκB phosphorylation and apoptosis in mice. Cell Physiol Biochem. 2015;37:1619–1628. doi: 10.1159/000438528. [DOI] [PubMed] [Google Scholar]
- 32.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Choi Y, Ahn M, Jung K, Shin T. Olfactory dysfunction in autoimmune central nervous system neuroinflammation. Mol Neurobiol. 2018;55:8499–8508. doi: 10.1007/s12035-018-1001-4. [DOI] [PubMed] [Google Scholar]
- 35.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 37.Smith LJ, Chiaro JA, Nerurkar NL, Cortes DH, Horava SD, Hebela NM, Mauck RL, Dodge GR, Elliott DM. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur Cells Mater. 2011;22:291–301. doi: 10.22203/eCM.v022a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JM, Song JY, Baek M, Jung HY, Kang H, Han IB, Kwon YD, Shin DE. Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res. 2011;29:265–269. doi: 10.1002/jor.21210. [DOI] [PubMed] [Google Scholar]
- 40.Zhang CC, Cui GP, Hu JG, Xiao YZ, Zhou XS, Shao C, Lin Q, Zhou JS. Effects of adenoviral vector expressing hIGF-1 on apoptosis in nucleus pulposus cells in vitro. Int J Mol Med. 2014;33:401–405. doi: 10.3892/ijmm.2013.1586. [DOI] [PubMed] [Google Scholar]
- 41.Yang D, Wang D, Shimer A, Shen FH, Li X, Yang X. Glutathione protects human nucleus pulposus cells from cell apoptosis and inhibition of matrix synthesis. Connect Tissue Res. 2014;55:132–139. doi: 10.3109/03008207.2013.876421. [DOI] [PubMed] [Google Scholar]
- 42.Feng H, Danfelter M, Strömqvist B, Heinegård D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 43.Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–341. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang JP, Hori M, Sanda T, Okamoto T. Identification of a novel inhibitor of nuclear factor-kappaB, RelA-associated inhibitor. J Biol Chem. 1999;274:15662–15670. doi: 10.1074/jbc.274.22.15662. [DOI] [PubMed] [Google Scholar]
- 45.Frank S, Peters MA, Wehmeyer C, Strietholt S, Koers-Wunrau C, Bertrand J, Heitzmann M, Hillmann A, Sherwood J, Seyfert C, et al. Regulation of matrixmetalloproteinase-3 and matrixmetalloproteinase-13 by SUMO-2/3 through the transcription factor NF-κB. Ann Rheum Dis. 2013;72:1874–1881. doi: 10.1136/annrheumdis-2012-202080. [DOI] [PubMed] [Google Scholar]
- 46.Peng S, Gao J, Liu W, Jiang C, Yang X, Sun Y, Guo W, Xu Q. Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget. 2016;7:80262–80274. doi: 10.18632/oncotarget.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.