Abstract
Reactive oxygen species (ROS) impair neovascularization and perfusion recovery following limb ischemia in patients with peripheral arterial disease (PAD). Hydrogen molecules (H2) comprise an antioxidant gas that has been reported to neutralize cytotoxic ROS. The present study investigated whether H2 may serve as a novel therapeutic strategy for PAD. H2-saturated water or dehydrogenized water was supplied to mice with experimental PAD. Laser Doppler perfusion imaging demonstrated that H2-saturated water improved perfusion recovery, decreased the rate of necrosis, increased the capillary density in the gastrocnemius muscle and increased the artery density in the abductor muscle in the ischemic limbs, at 14 and 21 days post-hindlimb ischemia. Ischemic muscle tissue was harvested 7 days after experimental PAD for biochemical testing and H2 was observed to reduce the levels of malondialdehyde and increase the levels of cyclic guanine monophosphate (cGMP). In cultured endothelial cells, H2-saturated culture medium resulted in reduced ROS levels, increased tube formation and increased cGMP levels. In macrophages, H2 decreased cellular ROS levels and promoted M2 polarization. H2-saturated water increases angiogenesis and arteriogenesis and subsequently improves perfusion recovery in a mouse PAD model via reduction of ROS levels.
Keywords: hydrogen molecule, reactive oxygen species, peripheral arterial disease, angiogenesis, arteriogenesis
Introduction
Peripheral arterial disease (PAD) is caused by atherosclerotic occlusion of the arteries to the lower extremities. PAD affects >200 million people worldwide and puts them at risk of lower extremity amputation and mortality (1–3). As total occlusions along the path of the major inflow arteries to the lower extremities are common in patients with PAD, the blood flow that is able to be delivered to the distal tissue becomes dependent on the extent of neovascularization in the ischemic leg (4–6). In the ischemic muscle, reactive oxygen species (ROS) impair ischemia-stimulated angiogenesis and perfusion recovery (7–10). Gene delivery of ROS scavengers was demonstrated to improve perfusion recovery and reduce tissue loss in an experimental PAD model (11,12).
Molecular hydrogen (H2), a physiological regulatory gas molecule, is able to neutralize numerous types of cytotoxic ROS and therefore acts as an antioxidant within the body (13). Clinical trials have demonstrated that H2 therapy improves the outcome of a variety of diseases, including cerebral ischemia (14,15) and diabetes (16). However, to the best of our knowledge, the effects of H2 on PAD have not been studied. The present study investigated the hypothesis that H2 therapy may improve angiogenesis and perfusion recovery by neutralizing ROS in experimental PAD.
Materials and methods
Hindlimb ischemia (HLI) model and H2 treatment
A total of 36 male Balb/c mice (25–30 g) were used in the present study; the mice were housed in a specific pathogen-free laboratory environment with free access to food and water under a 12-h light/dark cycle, an ambient temperature of 21±2°C and a constant humility of 50±10%. Following induction of anesthesia (30–40 mg/kg pentobarbital via an intraperitoneal injection), unilateral femoral artery ligation and excision were performed on the left side of the mice, as described previously (17,18). Immediately following surgery, hydrogen-saturated water (1.6 ppm or 0.8 mM) or dehydrogenized water was supplied to the mice (n=18 per group), and the water was changed daily to ensure adequate H2 levels in the drinking water. Hydrogen-saturated water was prepared daily using an AquelaBlue electrolysis instrument (MiZ Co., Ltd, Kanagawa, Japan), as described previously (19,20). All procedures involving animal use conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Bethesda, MD, USA), and the protocol was approved by the Institutional Animal Care Committee of Wuhan University (Wuhan, China).
Perfusion recovery
Perfusion flow in the ischemic and contralateral non-ischemic limbs was measured as described previously, with the use of a laser Doppler perfusion imaging system (Perimed AB, Järfälla, Sweden) (17,18). Perfusion was expressed as the ratio of the left (ischemic) to the right (non-ischemic) hind limb and was performed on days 0, 7, 14 and 21 following surgery. In mice that developed autoamputation (2 in the hydrogen-saturated water group and 6 in the control group), the perfusion ratio obtained from the limb prior to autoamputation was used.
Immunofluorescence
For the assessment of capillary density, ischemic gastrocnemius muscle sections from mice treated with hydrogen-saturated water or dehydrogenized water at 21 days post-HLI were used for immunofluorescent staining, as described previously (17,21). Anti-platelet endothelial cell adhesion molecule (CD31) antibody (rat anti-mouse CD31; 550274; 1:100; BD Pharmingen; BD Biosciences, San Jose, CA, USA) was applied to acetone-fixed (−20°C for 30 min) cryosections (7 µm) of ischemic gastrocnemius muscle specimens at 4°C overnight in blocking solution (1% goat serum in saline solution; Wuhan Boster Biological Technology Co., Ltd., Wuhan, China). Following rinsing with PBS, Alexa Fluor 488 phalloidin (for muscle fiber staining; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the secondary reagent goat anti-rat Alexa Fluor 555 (1:100; A-21434; Thermo Fisher Scientific, Inc.) were applied for 1 h at room temperature. Secondary antibody only, without primary antibody, was used as a negative control to assess non-specific binding. Stained sections were examined at ×200 magnification using an Olympus IX71 high-magnification microscope (Olympus Corporation, Tokyo, Japan). Capillary densities were analyzed by counting five random high-power fields (magnification, ×200), and were expressed as the number of CD31+ cells per muscle fiber area, as described previously (17,22).
For the assessment of arteries, α-smooth muscle actin (1:50; Wuhan Boster Biological Technology Co., Ltd.) was applied to the abductor muscle in the ischemic side 21 days after HLI. A total of five microscopic fields were randomly selected and counted in three tissue sections from each animal. Artery density was expressed as the number of arteries counted in ×200 high-power magnification fields.
Biochemical assay
Malondialdehyde (MDA) in the mouse ischemic muscle homogenates was measured using thiobarbituric acid, as previously described (23). The absolute amount of MDA was read from a standard curve prepared from serial dilutions of the primary standard. Activation of nitric oxide (NO)-sensitive guanylyl cyclase (GC) leads to enhanced production of the intracellular messenger cyclic guanosine monophosphate cyclic (cGMP) (24). Therefore, tissue cGMP levels in the ischemic muscle were measured in order to assess tissue NO bioactivity using a cGMP Parameter Assay Kit (KGE3; R&D Systems, Minneapolis, MN).
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated and used for qPCR, as previously described (21,25). qPCR was performed using primers/probes for arginase-1 (arg1), tumor necrosis factor (tnf) and 18S rRNA from Applied Biosystems, Thermo Fisher Scientific, Inc. (Hs00163660_m1, Hs00174128_m1 and #Hs03003631_g1, respectively). Quantitative normalization of cDNA in each sample was performed using the expression of 18S rRNA as an internal control. The generated Cq value of each gene was normalized to the respective Cq value of 18S rRNA (ΔCq). Each gene was further normalized to the average ΔCq value of its control group (ΔΔCq). The final fold expression changes were calculated using the equation 2−ΔΔCq (26).
Cell culture and in-vitro angiogenesis assay
Human umbilical vein endothelial cells (HUVECs) were purchased from Cyagen Biosciences (Santa Clara, CA, USA), and grown in standard endothelial cell growth medium (Cell Applications, Inc., San Diego, CA, USA) with 10% fetal bovine serum (FBS; Wuhan Boster Biological Technology Co., Ltd.). To generate bone marrow-derived macrophages (BMDMs), femurs from four 2-month-old male Balb/c mice (18–22 g, purchased from the animal center of Wuhan University, China), which were housed in the same conditions as those mentioned above, were flushed with sterile Dulbecco's modified Eagle's medium (DMEM; Wuhan Boster Biological Technology Co., Ltd.). Following lysis of the red blood cells, total bone marrow cells were plated in a Petri dish in DMEM medium with 10% FBS and 100 ng/ml macrophage colony-stimulating factor (R&D systems, Inc., Minneapolis, MN, USA) and were allowed to differentiate for 7 days.
The in-vitro angiogenesis assay was performed as previously described (27). Briefly, HUVECs were plated at a density of 1×104 cells/well in 96-well dishes that were coated with growth factor-reduced Matrigel (BD Biosciences). The cells were exposed to hypoxia serum starvation conditions (HSS; mimic in vivo ischemia) for 12 h with H2-saturated endothelial starvation medium or dehydrogenized endothelial starvation medium to assess tube formation. Hydrogen-saturated medium was prepared daily using an AquelaBlue electrolysis instrument (MiZ Co., Ltd., Kanagawa, Japan). Each condition was applied to eight wells. The degree of tube formation was determined by measuring the length of the tubes and the number of loops in each well under ×40 magnification using Image J version 6.0 (National Institutes of Health).
For the assessment of macrophage polarization, bone marrow-derived macrophages (BMDMs) were treated with H2-saturated or dehydrogenized medium under HSS for 24 h. Arg1 and tnf mRNA expression levels were measured as the markers for M2/M1 polarization by qPCR, respectively.
Intracellular ROS assay
Intracellular ROS production was detected using the nonfluorescent cell permeating compound, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), as previously described (28). In the presence of ROS, DCFH-DA is hydrolyzed to the fluorescent product DCF. HUVECs were incubated with 10 µM DCFH-DA for 45 min at 37°C, and the fluorescence intensity of DCFH was read at 525 nm emission when excited at 488 nm in a 96-well plate reader. Results are expressed as a percentage of the control (cells cultured under normoxic conditions) fluorescence intensity.
Statistical analysis
All results are presented as the mean ± standard error and statistical analysis was performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). All the in vitro studies were repeated 3 times. An unpaired t-test was used for comparison between two groups, and comparisons in experiments with ≥3 groups were performed using one-way analysis of variance and the Tukey post-hoc test. Differences in necrosis rate were analyzed by χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Hydrogen-saturated water improves perfusion recovery, angiogenesis and arteriogenesis in experimental PAD
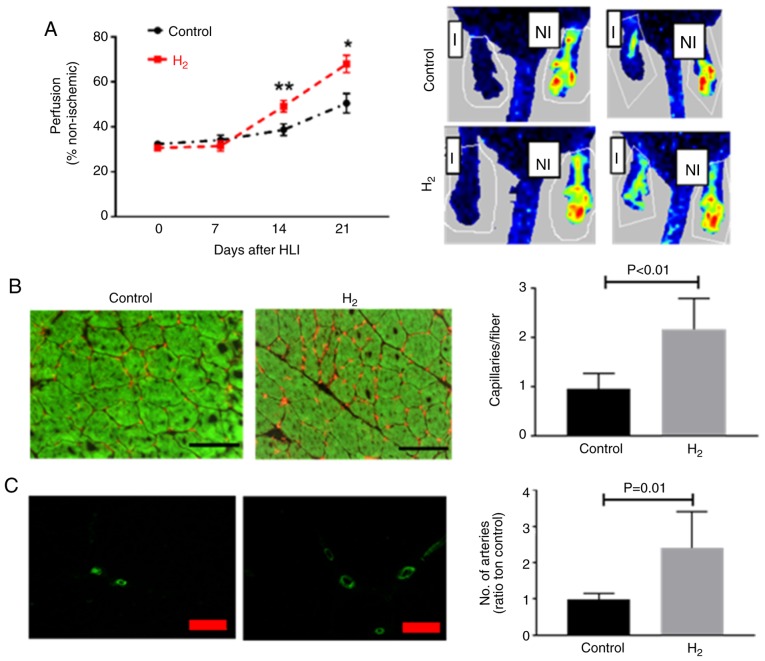
Balb/c mice receiving hydrogen-saturated water exhibited improved perfusion recovery at 14 (49.2±2.5% vs. 38.7±2.6%; P<0.01) and 21 (67.9±3.8% vs. 50.5±4.3%; P=0.012) days post HLI (Fig. 1A), and less tissue necrosis (4 out of 18 vs. 10 out of 18, P=0.02) compared with those receiving dehydrogenized water, 2 mice in hydrogen-saturated water group and 6 in control group developed auto-amputation in the first 7 days after HLI. The capillary density was determined in the ischemic gastrocnemius muscle, and mice receiving hydrogen-saturated water had a higher capillary density compared with those receiving dehydrogenized water (2.2±0.2 vs. 0.96±0.1 capillaries/fiber; n=10 per group; P<0.01) 21 days post-HLI (Fig. 1B). As Fig. 1C demonstrates, 21 days post-HLI, mice received hydrogen-saturated water had a higher artery density (2.4±0.3 vs. 1.0±0.06 capillaries/fiber; n=10 per group; P=0.01) in the abductor muscle from the ischemic limb.
Figure 1.
H2-saturated water improves perfusion recovery following HLI. (A) Laser Doppler imaging indicated a significant increase in perfusion recovery in Balb/c mice treated with H2-saturated water on days 14 and 21 following HLI (n=10 per group). (B) At day 21 following HLI, the ischemic gastrocnemius muscle from mice treated with H2-saturated water had a significantly higher capillary density (CD31, red) when compared with those treated with dehydrogenized water (n=8 mice/group). Scale bar, 60 µM. (C) Artery density (α-smooth muscle actin, green) in the abductor muscle from mice treated with H2-saturated water was higher compared with the control mice (n=8 per group). Scale bar, 100 µM. Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. respective control. H2, hydrogen molecule-saturated water; control, mice with dehydrogenized water. HLI, hindlimb ischemia; I, ischemic; NI, non-ischemic.
Hydrogen-saturated water decreases MDA levels, increases cGMP levels and promotes M2-like macrophage polarization in ischemic muscle
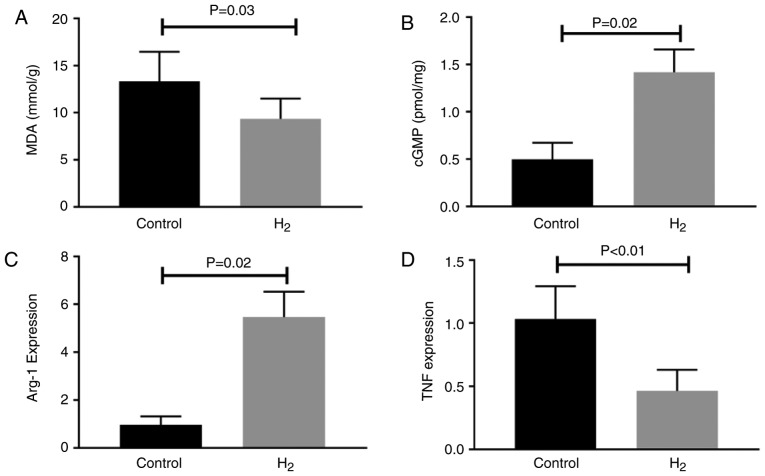
Day 7 following HLI was selected to study the molecular alterations since, although hydrogen-saturated water improved the long-term (14 and 21 days post-HLI) outcome in experimental PAD, it did not alter perfusion recovery at this time point. Lipid peroxidation, established by measuring MDA, is widely used to assess ROS bioactivity in tissues (29). In the present study, hydrogen-saturated water significantly decreased MDA levels in the ischemic muscle 7 days subsequent to HLI (Fig. 2A).
Figure 2.
H2-saturated water decreases oxidative stress, increases macrophage M2 polarization in ischemic muscle. H2-saturated water intake (A) decreases MDA levels, (B) increases cGMP levels and increases M2-like macrophage polarization levels, as indicated by (C) increased expression levels of an M2-like macrophage marker (arg-1) and (D) decreased expression levels of an M1-like macrophage marker (tnf) in the ischemic muscle 7 days after experimental peripheral arterial disease. Data are presented as the mean ± standard error of the mean. H2, hydrogen molecule-saturated water; control, mice with dehydrogenized water. arg-1, arginase-1; tnf, tumor necrosis factor; MDA, malondialdehyde; cGMP, cyclic guanine monophosphate.
NO exerts its effects via stimulation of NO-sensitive GC in blood vessels, which leads to enhanced production of the intracellular messenger cGMP (30). Levels of cGMP, the product of NO-activated GC, were assessed in the present study. cGMP levels were 2.8-fold higher (P<0.01) in ischemic hind-limbs from the hydrogen-saturated water group compared with the control group, thus providing evidence that H2 increases NO bioactivity in ischemic muscles (Fig. 2B).
Macrophages have been reported to modulate arteriogenesis and angiogenesis, which are important vascular remodeling processes following PAD. In the present study, arg1 and tnf, as the respective markers for M2-like and M1-like macrophages, were measured. In the ischemic muscle from experimental PAD, H2-saturated water significantly increased the mRNA expression levels of arg-1 (Fig. 2C) and decreased the mRNA expression levels of tnf (Fig. 2D).
Hydrogen-saturated water decreases ROS levels and increases the levels of cGMP and angiogenesis in endothelial cells and promotes M2-like-macrophage polarization in vitro
In the cultured endothelial cells under HSS conditions, hydrogen-saturated medium resulted in a significant increase in tube formation as indicated by increased tube length and number of loops (Fig. 3A). Compared with normoxic conditions, ROS levels were increased, as indicated by DCFH-DA assay, under HSS; however, hydrogen-saturated medium significantly decreased ROS levels in HUVECs (Fig. 3B). cGMP levels were detected in the cultured endothelial cell lysates, and hydrogen-saturated medium was found to increase cGMP levels (Fig. 3C).
Figure 3.
H2-saturated medium increases tube formation and cellular cGMP levels and decreases cellular ROS levels in cultured HUVECs. (A) HUVECs were plated on Matrigel with reduced growth factors and incubated for 12 h in HSS conditions with H2-saturated medium or medium without H2. H2 treatment resulted in enhanced tube formation, which was quantified as total length of the cords per visual field, and total loops per visual field as represented by the bar graph. Scale bar, 100 µm. (B) H2-saturated medium resulted in reduced ROS levels as indicated by 2′,7′-dichlorodihydrofluorescein diacetate staining in cultured HUVECs 1–6 h under HSS. *P<0.05, **P<0.01 vs. respective control. (C) H2-saturated medium resulted in increased cellular cGMP levels 24 h under HSS. Data are representative of 2–3 separate batches of HUVECs (n=8–12 per group). Data are presented as the mean ± standard error of the mean. H2, hydrogen molecule-saturated medium; control, medium without H2. ROS, reactive oxygen species; cGMP, cyclic guanine monophosphate; HUVEC, human umbilical vein endothelial cell; HSS, hypoxia serum starvation.
Consistent with the findings in the endothelial cells, HSS increased ROS levels in the cultured BMDMs. H2-saturated water significantly reduced ROS levels in the BMDMs 1–6 h after exposure to HSS (Fig. 4A), which led to increased expression levels of the M2-like macrophage marker (arg1) and decreased expression levels of the M1-like macrophage marker (tnf) (Fig. 4B and C). These findings are consistent with our in vivo findings in the mouse ischemic muscle.
Figure 4.

H2 decreases ROS, increases macrophage M2 polarization in vitro. H2-saturated medium (A) decreases cellular ROS levels, (B) increases the expression of an M2-like macrophage marker (arg-1) and (C) decreases the expression of an M1-like macrophage marker (tnf) in cultured bone marrow-derived macrophages. Data are representative of 2–3 separate batches of macrophages (n=8–12 per group). Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01 vs. respective control. H2, hydrogen molecule-saturated medium; control, medium without H2. ROS, reactive oxygen species; arg-1, arginase-1; tnf, tumor necrosis factor.
Discussion
To best of our knowledge, this is the first study to demonstrate that H2 improves perfusion recovery angiogenesis and arteriogenesis in experimental PAD. Furthermore, it was identified that H2-saturated water is sufficient to neutralize ROS in ischemic muscle tissue, cultured endothelial cells and macrophages under simulated ischemia, and subsequently increases NO bioactivity and promotes M2-like macrophage polarization.
The most important finding of the present study is that molecular hydrogen improves perfusion recovery, angiogenesis and arteriogenesis following PAD. Currently, there is no known pharmaceutical therapy that is able to improve blood perfusion in the ischemic limbs of patients with PAD (2,6). Hydrogen gas inhalation or hydrogen-saturated water has been widely used in a variety of clinical conditions, including strokes, and its efficacy and safety in patients has been demonstrated in previous studies (31,32). In line with these studies, the present study demonstrated that oral administration of H2 was effective for the treatment of PAD, which is a convenient method for clinical use. Thus, the findings of the present study may initiate the clinical study of H2 in patients with PAD.
Another important finding of the present study is that H2-saturated water increased NO bioavailability in ischemic muscle tissue, as indicated by higher cGMP levels in ischemic muscles that received treatment with molecular hydrogen. Neovascularization is a physiological repair process that primarily depends on NO, a bioactive gas that induces multiple pathways in order to promote angiogenesis and tissue repair (30,33). However, NO is not stable and may be converted to peroxynitrite in the presence of ROS (34). Unlike NO, peroxynitrite does not promote angiogenesis. H2 is known to neutralize the most cytotoxic ROS, including ·OH and ONOO- (13,35). In the present study, H2 decreased MDA levels in the ischemic muscle and ROS levels in endothelial cells and macrophages. These results indicated that H2 decreases the levels of ROS in experimental PAD, and subsequently leads to increased bioavailability of NO.
In experimental PAD, monocyte/macrophage recruitment into tissue supplied by the occluded vessel may induce potent effects on neovascularization and tissue repair. The ability of macrophages to modulate these processes is dependent on their polarization state. M2-like macrophages serve critical roles in inflammation resolution by secreting growth factors that induce arteriogenesis and angiogenesis (36). In the present study, H2 increased the levels of M2-like macrophages, which may be explained by their effects on ROS neutralization as ROS elevation has been reported to induce macrophage M1 polarization through activation of hypoxia-inducible factor 1 (37).
In conclusion, hydrogen-saturated water improves perfusion recovery and increases angiogenesis and arteriogenesis by neutralizing ROS, at least partially, in endothelial cells and macrophages under ischemia. The present study may initiate further experimental and clinical studies that examine pharmaceutical approaches towards the treatment of PAD.
Acknowledgements
The authors would like to thank Dr Mingjie Yuan (Department of Cardiology, Renmin Hospital of Wuhan University, Hubei, China) for his comments on the writing of this manuscript.
Funding
The present study was supported by a Grant from the Planned Science and Technology Project of Hubei Province, China (grant no. 2006A301A04).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JF conceived the project and designed experiments. JF, JZ, CC, HL, LW and YZ performed the experiments. JZ, CC and JF wrote and edited the manuscript and all authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures involving animal use conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Bethesda, MD, USA), and the protocol was approved by the Institutional Animal Care Committee of Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Guerchet M, Aboyans V, Mbelesso P, Mouanga AM, Salazar J, Bandzouzi B, Tabo A, Clément JP, Preux PM, Lacroix P. Epidemiology of peripheral artery disease in elder general population of two cities of Central Africa: Bangui and Brazzaville. Eur J Vasc Endovasc Surg. 2012;44:164–169. doi: 10.1016/j.ejvs.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: Epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 4.Annex BH, Beller GA. Towards the development of novel therapeutics for peripheral artery disease. Trans Am Clin Climatol Assoc. 2016;127:224–234. [PMC free article] [PubMed] [Google Scholar]
- 5.Ko SH, Bandyk DF. Therapeutic angiogenesis for critical limb ischemia. Semin Vasc Surg. 2014;27:23–31. doi: 10.1053/j.semvascsurg.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–396. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 7.Gardner AW, Montgomery PS, Zhao YD, Silva-Palacios F, Ungvari Z, Csiszar A, Sonntag WE. Association between daily walking and antioxidant capacity in patients with symptomatic peripheral artery disease. J Vasc Surg. 2017;65:1762–1768. doi: 10.1016/j.jvs.2016.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loffredo L, Marcoccia A, Pignatelli P, Andreozzi P, Borgia MC, Cangemi R, Chiarotti F, Violi F. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur Heart J. 2007;28:608–612. doi: 10.1093/eurheartj/ehl533. [DOI] [PubMed] [Google Scholar]
- 9.Loffredo L, Pignatelli P, Cangemi R, Andreozzi P, Panico MA, Meloni V, Violi F. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: Effect of an antioxidant treatment. J Vasc Surg. 2006;44:525–530. doi: 10.1016/j.jvs.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol. 2012;590:6237–6246. doi: 10.1113/jphysiol.2012.241281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 12.Saqib A, Prasad KM, Katwal AB, Sanders JM, Lye RJ, French BA, Annex BH. Adeno-associated virus serotype 9-mediated overexpression of extracellular superoxide dismutase improves recovery from surgical hind-limb ischemia in BALB/c mice. J Vasc Surg. 2011;54:810–818. doi: 10.1016/j.jvs.2011.03.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 14.Shui M, Liu X, Zhu Y, Wang Y. Exogenous hydrogen sulfide attenuates cerebral ischemia-reperfusion injury by inhibiting autophagy in mice. Can J Physiol Pharmacol. 2016;94:1187–1192. doi: 10.1139/cjpp-2016-0100. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang L, Zhao W, Liu T. The protective effects of hydrogen on HO-1 expression in the brainafter focal cerebral ischemia reperfusion in rats. Turk J Med Sci. 2016;46:1534–1539. doi: 10.3906/sag-1502-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Liu J, Jin K, Xu H, Wang C, Zhang Z, Kong M, Zhang Z, Wang Q, Wang F. Subcutaneous injection of hydrogen gas is a novel effective treatment for type 2 diabetes. J Diabetes Investig. 2018;9:83–90. doi: 10.1111/jdi.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazarika S, Farber CR, Dokun AO, Pitsillides AN, Wang T, Lye RJ, Annex BH. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127:1818–1828. doi: 10.1161/CIRCULATIONAHA.112.000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical Hindlimb ischemia. Circulation. 2008;117:1207–1215. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto Y, Kato T, Ito M, Azuma Y, Fukasawa Y, Ohno K, Kojima S. Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and antioxidant effects. J Thorac Cardiovasc Surg. 2015;150:645–654. doi: 10.1016/j.jtcvs.2015.05.052. e3. [DOI] [PubMed] [Google Scholar]
- 20.Nakai Y, Sato B, Ushiama S, Okada S, Abe K, Arai S. Hepatic oxidoreduction-related genes are upregulated by administration of hydrogen-saturated drinking water. Biosci Biotechnol Biochem. 2011;75:774–776. doi: 10.1271/bbb.100819. [DOI] [PubMed] [Google Scholar]
- 21.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after Hindlimb ischemia in type 2 diabetes Mellitus: Differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 22.Meisner JK, Song J, Annex BH, Price RJ. Myoglobin overexpression inhibits reperfusion in the ischemic mouse hindlimb through impaired angiogenesis but not arteriogenesis. Am J Pathol. 2013;183:1710–1718. doi: 10.1016/j.ajpath.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papastergiadis A, Mubiru E, Van Langenhove H, De Meulenaer B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J Agric Food Chem. 2012;60:9589–9594. doi: 10.1021/jf302451c. [DOI] [PubMed] [Google Scholar]
- 24.Denninger JW, Marletta MA. Guanylate cyclase and the. NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/S0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 25.Hazarika S, Angelo M, Li Y, Aldrich AJ, Odronic SI, Yan Z, Stamler JS, Annex BH. Myocyte specific overexpression of myoglobin impairs angiogenesis after hind-limb ischemia. Arterioscl Throm Vas Biol. 2008;28:2144–2150. doi: 10.1161/ATVBAHA.108.170951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Cunningham A, Dokun AO, Hazarika S, Houston K, Chen L, Lye RJ, Spolski R, Leonard WJ, Annex BH. Loss of interleukin-21 receptor activation in hypoxic endothelial cells impairs perfusion recovery after hindlimb ischemia. Arterioscl Throm Vas Biol. 2015;35:1218–1225. doi: 10.1161/ATVBAHA.115.305476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Yotnda P. Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp. 2011 doi: 10.3791/3357. pii: 3357. doi: 10.3791/3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirinccioglu AG, Gokalp D, Pirinccioglu M, Kizil G, Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin Biochem. 2010;43:1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Murohara T, Asahara T. Nitric oxide and angiogenesis in cardiovascular disease. Antioxid Redox Signal. 2002;4:825–831. doi: 10.1089/152308602760598981. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama M, Itami N, Suzuki H, Hamada H, Osaka N, Yamamoto R, Tsunoda K, Nakano H, Watanabe K, Zhu WJ, et al. Possible clinical effects of molecular hydrogen (H2) delivery during hemodialysis in chronic dialysis patients: Interim analysis in a 12 month observation. PLoS One. 2017;12:e0184535. doi: 10.1371/journal.pone.0184535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimaki K, Asada T, Ohsawa I, Nakajima E, Ikejima C, Yokota T, Kamimura N, Ohta S. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr Alzheimer Res. 2018;15:482–492. doi: 10.2174/1567205014666171106145017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009;2:ra41. doi: 10.1126/scisignal.2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohta S. Hydrogen gas and hydrogen water act as a therapeutic and preventive antioxidant with a novel concept. Nihon Ronen Igakkai Zasshi. 2008;45:355–362. (In Japanese) [PubMed] [Google Scholar]
- 36.Takeda Y, Costa S, Delamarre E, Roncal C, de Oliveira Leite R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyère F, Wenes M, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Covarrubias A, Byles V, Horng T. ROS sets the stage for macrophage differentiation. Cell Res. 2013;23:984–985. doi: 10.1038/cr.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.