Abstract
Background/Objectives: Motivational Enhancement Treatment in Spanish (METS) is a brief intervention aimed at resolving patient ambivalence towards behavior change that has demonstrated efficacy in substance use disorder treatment to reduce use and increase treatment engagement in different populations. In order to have evidence for its implementation in Mexico, a multi-site, randomized, two-arm, controlled clinical trial was conducted at three outpatient addiction treatment centers in the country to compare the effect of METS with Counseling as Usual (CAU). Method: One hundred and twenty patients were randomized to receive three sessions of METS (n = 54) or CAU (n = 66) during the first four weeks of treatment and were assessed during the following 12 weeks. Primary outcome measures were self-reported days of substance use and of treatment services utilization, which were tested using Generalized Estimating Equations. Results: Results associated both conditions with significant changes in substance use over, whereas there were no differences between conditions in substance use or in service utilization. Conclusions: Findings do not support the hypothesis that METS is more effective than CAU, but suggest that brief interventions at treatment initiation may improve patient outcomes.
Keywords: Motivational interviewing, Substance use disorder, Evidence-based practice, México, Experimental trial
Resumen
Antecedentes/Objetivos: La Intervención de Incremento Motivacional (METS) es una intervención breve para resolver la ambivalencia del paciente con respecto a su comportamiento y ha demostrado eficacia en distintas poblaciones para reducir el consumo de sustancias e incrementar la asistencia al tratamiento en adicciones. Con el objetivo de generar evidencia para su implementación en México, se desarrolló un ensayo clínico controlado, multi-sede, aleatorizado, de dos brazos en tres centros de tratamiento ambulatorio para adicciones, para comparar el efecto de METS con el del tratamiento usual (CAU). Método: Ciento veinte pacientes fueron aleatorizados a tres sesiones de METS (n = 54) o CAU (n = 66) durante las primeras cuatro semanas de tratamiento y evaluados durante las siguientes doce. Se midieron resultados mediante autoinforme de días con consumo de sustancias y días de utilización de servicios, los cuales fueron analizados mediante ecuaciones de estimación generalizadas. Resultados: Los resultados asociaron ambas condiciones a cambios significativos en uso de sustancias a lo largo del tiempo, pero no demostraron diferencias entre condiciones en el uso de sustancias o en la utilización de servicios. Conclusiones: Los hallazgos contradicen la hipótesis de superioridad de METS sobre CAU, pero sugieren que las intervenciones breves al inicio del tratamiento pudieran mejorar la respuesta del paciente.
Palabras clave: Entrevista motivacional, Trastorno por consumo de sustancias, Prácticas basadas en evidencia, México, Experimento
Early patient engagement in substance abuse treatment has been widely reported as a strong predictor of positive treatment outcomes (Simpson & Joe, 2004). In Mexico, achieving such engagement and retention has proven to be a challenge. Data from the National Addiction Survey reports that only 1.0% of alcohol and 9.4% of drug users seek specialized treatment of which only 17.5% and 35% finish treatment (Secretaría de Salud, 2012a, Secretaría de Salud, 2012b). In recent years, efforts have been made to make specialized treatment more available to the population (Marín-Navarrete et al., 2014) and while they have enhanced the capacity to reach patients in need of treatment (e.g. treatment utilization in alcohol users increased 13% between 2008 and 2011); reported dropout rates suggest there is still a need for interventions that improve patients’ engagement in treatment (Secretaría de Salud, 2012a).
Motivational Interviewing (MI) is a client-centered therapeutic approach aimed at improving treatment engagement and outcomes (Lundahl & Burke, 2009). MI focuses on the enhancement of the patient's intrinsic motivation to change their substance use by exploring and resolving ambivalence towards behavior change (Miller and Rollnick, 2002, Miller and Rose, 2009). Various meta-analyses and reviews support the effectiveness of MI-based interventions for treating alcohol and drug use (Lundahl and Burke, 2009, Rubak et al., 2005, Smedslund et al., 2011, Vasilaki et al., 2006); highlighting its low cost and ease of implementation in primary and secondary healthcare settings by non-specialized professionals as some of its strongest attributes (Rubak et al., 2005). Manual-based adaptations of MI have been developed for clinical trials to test its effect as a brief intervention delivered in the early phases of treatment in different populations (Ball et al., 2007, Carroll et al., 2006, Project MATCH Research Group, 1997). A three-session Spanish-language adaptation (Motivational Enhancement Treatment in Spanish; METS) was tested with Hispanics in the U.S. and reported a significant effect in alcohol-users (Carroll et al., 2009).
In Mexico, behavioral interventions for substance use have been tested in controlled settings; but to date there have been no randomized controlled clinical trials (RCT) testing manual-based behavioral interventions in ‘real world’ settings (Rojas, Real, García, & Medina-Mora, 2011). Considering the evidence supporting MI-based interventions in other populations, and the fact that a trial testing METS showed good results with Hispanics in the U.S. (in which 49.4% of the sample were of Mexican origin) (Carroll et al., 2009), we conducted a study to test the effect of METS compared with Counseling as Usual (CAU) in three outpatient addiction care centers in Mexico. We hypothesized that METS would be more effective than CAU in reducing number of days of substance use and increasing engagement in treatment (i.e., utilization of treatment services offered within and outside the treatment centers and retention to counseling services). In light of findings from the METS trial (Carroll et al., 2009), we also hypothesized that METS would be more effective than CAU in patients reporting alcohol as their primary substance of use.
Method
This RCT was the first trial implemented in the Mexican Clinical Trials Network (Horigian et al., 2016, Horigian et al., 2015, Marín-Navarrete et al., 2014). Considering the need to improve mental health research in low and middle-Income countries (Collins et al., 2011), the network was the result of a technology transfer process between the Mexican National Institute of Psychiatry Ramón de la Fuente Muñiz (INPRFM) in Mexico and the University of Miami in the United States to develop research infrastructure and capacity to conduct rigorous RCTs in community-based addiction and mental health treatment centers in Mexico (Horigian et al., 2015). This study was conducted and monitored in compliance with Good Clinical Practice (GCP) guidelines, which inform the Mexican regulations for clinical research with human subjects (Organización Panamericana de la Salud [OPS], 2005). Before trial implementation, all protocol procedures, informed consent forms, and case report forms were approved by the ethics committees/institutional review boards (IRB) of the participating institutions. All study participants and counselors provided written informed consent before participation. This clinical trial can be found at the International Standard Randomized Clinical Trial Number (ISRCTN) registry (Protocol No. ISRCTN91657311).
Study design
This was a multi-site parallel group superiority trial with participants randomly assigned to either METS or CAU in an allocation ratio of 1:1. This trial was an adaptation of a previous protocol conducted by the National Institute of Drug Abuse Clinical Trials Network (NIDA-CTN) (Carroll et al., 2009), which tested METS in Hispanic substance users in the U.S.; therefore, it shares its basic design and primary outcome measures. Additional adaptations were required to ensure adequate cultural fit with the Mexican population. For instance, most case report forms went through a back-translation process and were reviewed using cognitive laboratories methodology (Nolin and Chandler, 1996, Ramada-Rodilla et al., 2013).
Study assessment schedule was as follows: eligibility and baseline assessments were performed at treatment intake, after which, participants were randomized to receive 3 sessions of METS or 3 sessions of CAU over a 28-day period (active phase). Participants were assessed three times during the active phase and again at the end of this period (day 28). Follow-up assessments were conducted at 8 and 16 weeks after randomization.
Study sample
Participants were adults (ages between 18 and 65 years) who requested outpatient treatment for substance use at the study sites, with any substance use in the 28 days before initiating treatment, and were willing to participate in all study procedures (e.g., accept randomization, availability during 16 weeks of participation). Participants that required immediate specialized care other than Substance Use Disorder outpatient treatment (e.g., crisis intervention for suicidality, detoxification), faced imminent incarceration, or had a significant other enrolled in the study were excluded from participation. Women on their eight-month of pregnancy or beyond were also excluded.
Counselors inclusion criteria were: being a member of the participating site clinical staff, volunteering to participate in the study, having never received prior training in MI, agreeing to have their sessions audio-taped and to be randomized to deliver either METS or CAU. Fourteen counselors from three outpatient addiction care centers were randomized using a computer-based unblocked procedure performed by the research team at the INPRFM, where all participating counselors were individually informed of their assignment. Eight counselors were assigned to CAU and six to METS. 12 of the 14 counselors were women. Counselor mean age was 37.6 years (SD = 11.7). Years of experience in addiction treatment ranged from less than one year to more than 20. All counselors had at least the Mexican equivalent to a bachelor-degree level of education in Psychology. When inquired about their usual practice before study participation, the majority endorsed an approach based on cognitive-behavioral therapy or an “eclectic” counseling approach with no dominant theoretical orientation. There were no statistically significant differences in counselor mean age, years of experience, level of education and baseline counseling approach between METS and CAU.
Study interventions
METS consists of three individual manualized counseling sessions. During the sessions, in addition to strategies like open-questions, affirmations, reflections and summary statements, counselor and patient engage in structured exercises, with the aid of printed handouts, aimed at providing personal feedback on substance use, explore ambivalence and discrepancies, and (if the patient readiness is appropriate) jointly develop a change plan to be followed in the course of treatment (Ball et al., 2007). The METS manual and patient handouts from the NIDA-CTN study (Farentinos & Obert, 2000) were translated to Spanish and adapted for Mexican population by a clinical supervisor from the INPRFM aided by two bilingual METS experts who collaborated in the U.S. trial.
Counseling as Usual (CAU) consisted of three individual non-manualized counseling sessions, delivered in the therapeutic style that counselors who were not trained in METS would usually offer at the participating sites. CAU therapists at all sites had periodic meetings with a site director to discuss progress of all site patients and did not receive supervision on the delivery of their individual counseling.
Treatment process assessment
All sessions from both study interventions were audio-recorded for independent adherence and competence rating. Two certified independent raters assessed randomly selected audio-recorded sessions using the Supervisory Rater Form (STR) from the original METS study (Santa Ana et al., 2009). The STR is a 39-item adaptation of the Yale Adherence and Competence Scale (YACS) (Carrol et al., 2000) that measures adherence and competence in METS-specific consistent fundamental strategies (e.g., open-ended questions, reflective statements) METS-specific advanced strategies (e.g. pros, cons and ambivalence, change planning), METS inconsistent strategies (e.g. therapeutic authority, unsolicited advice, etc.) and general substance abuse counseling strategies (e.g., assessing substance use, program orientation) using a 7-point Likert scale (Martino, Ball, Nich, Frankforter, & Carroll, 2008; 2009; Santa Ana et al., 2009).
METS training and supervision
METS supervisors were proposed by each site director based on their leadership and years of professional experience in addiction treatment and were supervised by METS experts from the U.S. All METS counselors and supervisors participated in a 2-day centralized training conducted by the bilingual METS experts. Site supervisors attended a third training day on supervision techniques. Following training, counselors completed two certification cases that were audio-recorded and rated by each site supervisor and by the METS experts with the same STR form used by independent raters. Adherence and competence ratings from the original METS study were used for counselor certification (Martino et al., 2008, Martino et al., 2009). After certification, site supervisors rated one randomly selected session each week and had weekly supervision sessions with the counselors where they provided feedback along with the METS experts. If a counselor drifted below initial certification ratings, s/he was suspended from seeing study participants and repeated the certification process.
Study procedures
Participants were recruited from three outpatient addiction care centers, two in Mexico City and one in the city of Puebla, between April and November 2012. Each of the selected sites represented one of the three government funded leading treatment institutions in the country: National Institute of Psychiatry Ramon de la Fuente Muñiz, National Center for Prevention and Control of Addictions, and Youth Integration Centers. Recruitment target was of 120 randomized participants, with a goal of 40 participants per site. A trained research assistant (RA) recruited participants at each site, screened them for eligibility, and conducted randomization and study assessment procedures on-site. Randomization was performed with a computer-based “urn randomization” procedure (Wei & Lachin, 1988) balanced by gender and primary substance of use, which was programmed into an online clinical trial management software. After randomization, the RA arranged a first visit with a site counselor corresponding to the assigned treatment condition. Site RAs contacted all participants by phone or e-mail before a scheduled visit to confirm visit appointments. Participants received gift cards for completing study assessments.
The safety-monitoring plan involved a continuous assessment of Adverse Events (AE), queried through a semi-structured interview at each study visit by the site RA. Good Clinical Practice (GCP) definitions and classifications of AEs for seriousness, severity, relatedness and resolution status were used (Organización Panamericana de Salud, 2005). Only events that were serious or study-related were reported to the IRBs within 10 days of occurrence. All other adverse events were reported quarterly.
Outcome measures
Primary outcomes for this study were: days of substance use and days of treatment services utilization. The following measures were used.
Substance Use Calendar (SUC). Based on the Timeline Follow-Back interview (Fals-Stewart et al., 2000, Sobell and Sobell, 1992), this measure uses a calendar method to record daily substance use (alcohol, cocaine, marijuana, opioids, tranquilizers, amphetamine-like stimulants, heroin, hallucinogens, and tobacco). The SUC was used to assess substance use at baseline, over the course of treatment and follow-up. Urine toxicology screens were used to confirm reported substance use at each study visit (Donovan et al., 2012).
Treatment Utilization Form (TUF). An interview-based assessment was used to register self-reported utilization of treatment services from the active phase of treatment through the last follow-up assessment. Utilization of treatment services was assessed in days of attendance to study treatment sessions and to other treatment services offered within and outside the sites. Treatment services considered for this measure were: counseling services (group or individual counseling sessions), mutual-aid groups (Alcoholics Anonymous, 12-step based programs), childcare, medical, psychiatric, social work, legal, and family services.
Statistical analysis
Because participants, RAs and counselors were aware of the allocated group, investigators and data analysts were blinded during data analysis. Statistical power analysis was calculated using PASS 12 (Hintze, 2013). 120 participants were sufficient to have 80% power to show a rate ratio of 1.50 between the two conditions. Count distributed outcomes were tested using Generalized Estimating Equations (GEE). Data was preliminarily examined to assess for over dispersion. Examination of fit statistics and estimated scale parameters showed that a negative binomial specification fitted better than a Poisson and an autoregressive correlation structure fitted better than other alternatives. Treatment effect was assessed using an “intention-to-treat” approach. This analysis procedure allows the inclusion of all participants regardless of the presence of any missing data at particular assessments. Time was handled as a classification variable, thus the primary test of the hypothesis (the time by treatment interaction) assessed whether the pattern of means over time differed across the two treatment groups. Prior to testing the primary hypothesis of the impact of treatment on days of substance use, we examined the appropriateness of combining results across sites (Feaster, Mikulich-Gilbertson, & Brinka, 2012) and by whether alcohol was the primary substance of abuse because the original METS trial had found different results for alcohol users. To accomplish this, models were run with classification variables for time, treatment assignment and either site or alcohol as primary substance of abuse, each with two-way and three-way interactions. If the higher order interactions were significant, results of treatment by subgroup are reported; otherwise the primary test did not include these interactions. To assess counselors’ adherence and competence, we conducted mixed models with Site, Treatment Assignment and Treatment Assignment by Site included as fixed effects. Models were estimated using Proc Mixed in SAS 9.2. These models included random effects for both patient and counselors to account for the nesting of repeated ratings within a participant and nesting of participants within counselors. Degrees of freedom were calculated using Satterthwaite's method (Satterthwaite, 1946). For all outcomes, Cohen's d was calculated to provide an estimate of effect size for each condition across and within each site for the outcome variables.
Results
Study performance and retention
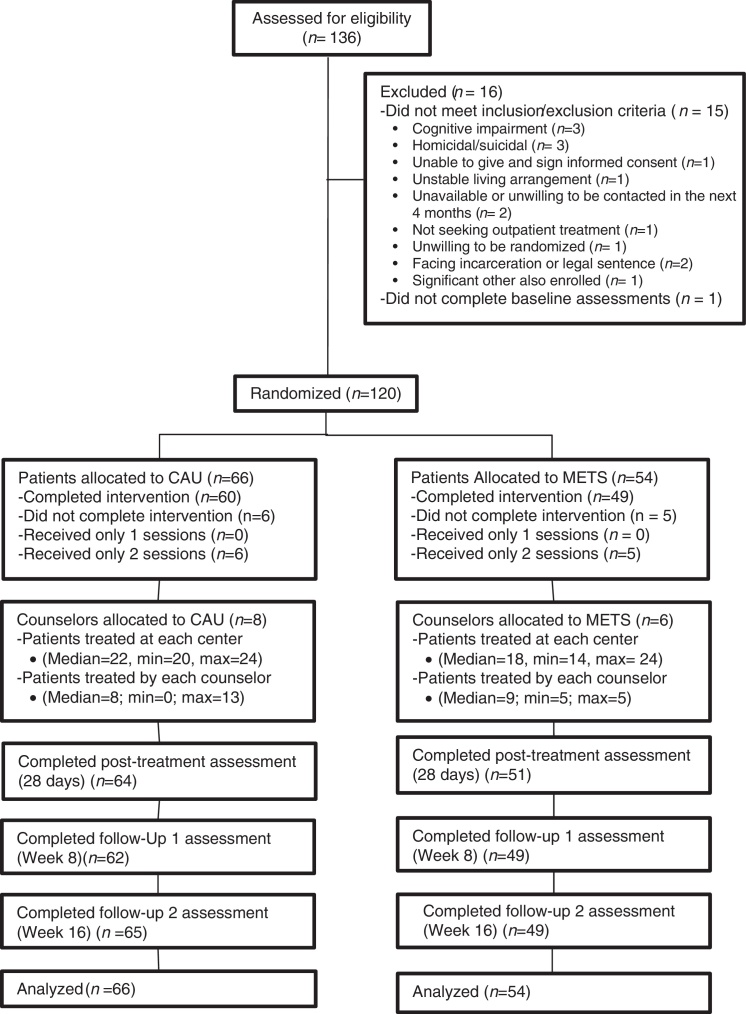
A total of 136 participants were enrolled in the study. Of these, only 16 did not meet the inclusion/exclusion criteria resulting in 120 randomized participants of whom 91% completed all study treatment sessions, and 93% were retained throughout all study phases (see Figure 1).
Figure 1.
CONSORT Diagram. Participant recruitment and randomization.
Note. CAU = Conuselling as Usual; METS = Motivational Enhancement Treatment (Spanish).
Participant safety
Three SAEs were identified during the study and none was related to study procedures or interventions. Only one non-serious event was related to study procedures (an attempted assault linked to delivery of study assessment compensation). All events were resolved before study termination.
Participant characteristics
Regarding participant demographics, mean age was 30.1 (SD = 9.2) years; most of the participants were male (82.5%) (see Table 1). The most frequently reported main substance of abuse was alcohol (37.5%), followed by cannabis (28.3%) and cocaine (24.2%). In terms of baseline levels of substance use, 51.7% of the population reported between 1-10 days of substance use, 21.7% between 11-20 days, and 26.7% between 21-28 days. Baseline characteristics of the sample, including the description of the association with comorbid conditions have been published by Marín-Navarrete et al. (2014).
Table 1.
Participant demographics by site.
| Site 1 (n = 44) |
Site 2 (n = 38) |
Site 3 (n = 38) |
Total (n = 120) |
Significance between groups |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean or % | SD | Mean or % | SD | Mean or % | SD | Mean or % | SD | F or χ2 | df | p |
| Age | 31.4 | 9.8 | 28.5 | 9.05 | 30.1 | 8.6 | 30.1 | 9.2 | 1.02 | 2 | .36 |
| Years or Education | 10.8 | 3.6 | 11.5 | 2.44 | 9.6 | 2.5 | 10.6 | 3.0 | 4.04 | 2 | .02 |
| Gender | |||||||||||
| Male | 86.4 | 89.5 | 71.1 | 82.5 | 5.18 | 2 | .08 | ||||
| Female | 13.6 | 10.5 | 28.9 | 17.5 | |||||||
| Marital Status | |||||||||||
| Married/Cohabiting | 27.3 | 21.1 | 23.7 | 24.2 | 4.67 | 8 | .79 | ||||
| Separated/Divorced | 18.2 | 21.1 | 18.4 | 19.2 | |||||||
| Never Married | 54.5 | 57.9 | 57.9 | 56.7 | |||||||
| Employment (Past 3 years)a | |||||||||||
| Paid Activity | 81.8 | 63.9 | 73.0 | 73.5 | 3.27 | 2 | .19 | ||||
| Unpaid Activity | 18.2 | 36.1 | 27.0 | 26.5 | |||||||
| Employment (Past 30 days)a | |||||||||||
| Paid Activity | 47.7 | 61.1 | 54.1 | 53.8 | 1.42 | 2 | .49 | ||||
| Unpaid Activity | 52.3 | 38.9 | 45.9 | 46.2 | |||||||
Note.
3 missing values
Hypotheses testing
Substance Use. There was no difference in the relative changes in the days of substance use over time in the two treatment conditions by either site [χ2 (8) = 11.27, p = .188] or by alcohol as main substance of abuse [χ2 (4) = 2.74, p = .602]. We therefore present the test of the primary hypothesis aggregated across site and across major substance used. There was a significant difference in the patterns of means over time in level of substance use [χ2 (4) = 11.58, p = .021] (see Figure 2). Examination of the coefficients associated with the difference in treatments at each individual time showed that at no-time were the mean days of drug use different between the two conditions (see Table 2).
Figure 2.
Mean days of any substance use by time Treatment condition by Time (as phase) interaction for 28 days of any substance use at 28 days (Active Phase) (1), 8-weeks (2), 12 weeks (3) and 16 weeks (4) after randomization.Note. CAU = Counseling as Usual, METS = Motivational Enhancement Treatment (Spanish).
Table 2.
Substance Use and Service Utilization effect size (Cohen's d) by treatment condition and site.
| Outcome measure | Site 1 (n = 44) |
Site 2 (n = 38) |
Site 3 (n = 38) |
Total (n = 120) |
||||
|---|---|---|---|---|---|---|---|---|
| CAU | METS | CAU | METS | CAU | METS | CAU | METS | |
| Days of primary substance use (active phasea) | ||||||||
| Mean | 9.1 | 13.92 | 6.33 | 10.14 | 9.77 | 7.5 | 8.32 | 11.04 |
| SD | 7.97 | 9.63 | 6.66 | 8.50 | 8.08 | 10.49 | 7.60 | 9.84 |
| Effect size (95% CI) | −0.55 (−1.16, 0.08) | −0.50 (−1.21, 0.18) | 0.24 (−0.92, 0.42) | −0.31 (−0.68, 0.05) | ||||
| Days of primary substance use (follow-upb) | ||||||||
| Mean | 30.5 | 25.96 | 17.21 | 23.71 | 21.59 | 19.63 | 22.70 | 23.5 |
| SD | 26.74 | 22.20 | 16.12 | 19.77 | 20.44 | 22.28 | 21.59 | 21.39 |
| Effect size (95% CI) | 0.19 (−0.43, 0.8) | −0.36 (−1.06, 0.31) | 0.09 (−0.76, 0.57) | −0.04 (−0.37, 0.36) | ||||
| Days of service utilization (from active phaseathrough follow-upb) | ||||||||
| Mean | 31.55 | 12.21 | 10.04 | 10.5 | 8.27 | 16.56 | 15.97 | 13.06 |
| SD | 63.13 | 10.97 | 7.46 | 17.48 | 4.70 | 20.28 | 36.05 | 15.79 |
| Effect size (95% CI) | −0.43 (−1.07, 0.17) | 0.03 (−0.72, 0.64) | 0.56 (−0.07, 1.29) | −0.11 (−0.26, 0.46) | ||||
| Days of counseling services utilization (from active phaseathrough follow-upb) | ||||||||
| Mean | 3.3 | 3.13 | 3.08 | 1.93 | 2.23 | 3 | 2.86 | 2.78 |
| SD | 6.67 | 6.32 | 4.06 | 2.40 | 1.90 | 3.35 | 4.50 | 4.71 |
| Effect size (95% CI) | −0.03 (−0.64, 0.58) | −0.35 (−1.01, 0.36) | 0.28 (−0.37, 0.97) | −0.02 (−0.34, 0.38) | ||||
Note.
Active phase = Weeks 1-3.
Follow-up = Weeks 5-16.
Service utilization. There was no difference in the relative changes in service utilization over time in the two treatment conditions by either site [χ2 (6) = 6.59, p = .361] or by alcohol as main substance of abuse [χ2 (6) = 3.67, p = .300]. There was, however, a difference in the pattern of service utilization over time across sites, which did not differ by treatment assignment [χ2 (6) = 12.62, p = .049]. The impact of treatment condition on service utilization over time was therefore tested across site and main substance of abuse, but allowing for differences in levels over time by site. There was not a significant difference in service utilization over time across treatment conditions [χ2 (3) = .65, p = .885] (see Table 2).
Because of differences in counseling services offered between sites (e.g., number of additional counseling services available would differ), it was deemed adequate to perform an analysis regarding counseling services utilization separately. There was no difference in the relative changes in counseling service utilization over time in the two treatment conditions by either site [χ2 (6) = 1.74, p = .942] or by alcohol as main substance of abuse [χ2 (3) = 3.97, p = .266]. There was no significant difference across conditions in the pattern of means over time in counseling services utilization [χ2 (3) = .82, p = .844]. There was a significant difference in the patterns of means over time in level of counseling services utilization by site that was not related to treatment condition [χ2 (6) = 17.24, p = .008].
METS versus CAU fidelity
Analysis of independent ratings with STR showed that METS counselors demonstrated significantly higher means than CAU counselors in METS consistent fundamental strategies in adherence [F (1, 14.1) = 127.55, p < .0001] and competence [F (1, 12.9) = 94.78, p < .0001]. In METS consistent advanced strategies, METS counselors also had significantly higher scores than CAU counselors in adherence [F (1, 13) = 168.49, p < .0001] and competence [F (1,346) = 33.82, p < .0001]. CAU counselors had significantly higher scores in METS inconsistent strategies than METS counselors in adherence [F (1, 10.2) = 163.9, p < .0001] and competence [F (1,214) = 35.18, p < .0001]. In addition, a significant treatment by site interaction was found in METS inconsistent strategies [F (2, 10.2) = 10.77, p = .003] where differences among conditions were larger in Site 1 (see Table 3).
Table 3.
METS and CAU adherence and competence scores for three groups of counselor skills by treatment condition and site.
| Scale | Total (n = 13) |
Site 1 (n = 4) |
Site 2 (n = 4) |
Site 3 (n = 5) |
Treatment condition |
Site |
Treatment condition x Site interaction |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| METS | CAU | METS | CAU | METS | CAU | METS | CAU | F | df | p | F | df | p | F | df | p | |
| METS-Consistent Basic skills | |||||||||||||||||
| Adherence | |||||||||||||||||
| M | 4.84 | 3.77 | 4.90 | 3.87 | 4.52 | 3.74 | 5.03 | 3.70 | 127.55 | 1,14.1 | < .0001 | 2.77 | 2,13.9 | .097 | 2.79 | 2,13.9 | .095 |
| SD | 0.57 | 0.74 | 0.55 | 0.88 | 0.55 | 0.62 | 0.52 | 0.73 | |||||||||
| n | 157 | 191 | 71 | 59 | 39 | 67 | 47 | 65 | |||||||||
| Competence | |||||||||||||||||
| M | 4.20 | 3.70 | 4.26 | 3.64 | 4.15 | 3.88 | 4.17 | 3.57 | 94.78 | 1,12.9 | < .0001 | 2.81 | 2,12.8 | .098 | 4.70 | 2,12.8 | .030 |
| SD | 0.41 | 0.32 | 0.41 | 0.31 | 0.43 | 0.28 | 0.40 | 0.29 | |||||||||
| n | 157 | 191 | 71 | 59 | 39 | 67 | 47 | 65 | |||||||||
| METS-Consistent Advanced skills | |||||||||||||||||
| Adherence | |||||||||||||||||
| M | 3.17 | 2.00 | 3.36 | 2.04 | 2.77 | 1.86 | 3.21 | 2.11 | 168.49 | 1,13 | < .0001 | 7.32 | 2,12.9 | .008 | 1.84 | 2,12.9 | .198 |
| SD | 0.67 | 0.57 | 0.62 | 0.59 | 0.69 | 0.51 | 0.60 | 0.59 | |||||||||
| n | 157 | 191 | 71 | 59 | 39 | 67 | 47 | 65 | |||||||||
| Competence | |||||||||||||||||
| M | 4.05 | 3.85 | 4.04 | 3.78 | 4.00 | 3.92 | 4.09 | 3.85 | 33.82 | 3,346 | < .0001 | 1.36 | 2,346 | .257 | 2.73 | 2,346 | .067 |
| SD | 0.30 | 0.31 | 0.26 | 0.32 | 0.36 | 0.26 | 0.30 | 0.34 | |||||||||
| n | 157 | 189 | 71 | 59 | 39 | 65 | 47 | 65 | |||||||||
| METS–Inconsistent skills | |||||||||||||||||
| Adherence | |||||||||||||||||
| M | 1.04 | 1.81 | 1.03 | 2.21 | 1.03 | 1.57 | 1.07 | 1.69 | 163.9 | 1,10.2 | < .0001 | 9.3 | 2,10.2 | .005 | 10.77 | 2,10.2 | .003 |
| SD | 0.10 | 0.50 | 0.07 | 0.48 | 0.12 | 0.36 | 0.12 | 0.41 | |||||||||
| n | 157 | 191 | 71 | 59 | 39 | 67 | 47 | 65 | |||||||||
| Competence | |||||||||||||||||
| M | 3.63 | 4.10 | 3.58 | 4.07 | 3.38 | 4.09 | 3.75 | 4.12 | 35.18 | 1,214 | < .0001 | 1.77 | 2,214 | .173 | 1.07 | 2,214 | .344 |
| SD | 0.45 | 0.40 | 0.45 | 0.32 | 0.48 | 0.44 | 0.43 | 0.42 | |||||||||
| n | 31 | 183 | 13 | 59 | 4 | 62 | 14 | 62 | |||||||||
| Standard Counseling | |||||||||||||||||
| Adherence | |||||||||||||||||
| M | 1.59 | 2.12 | 1.71 | 2.37 | 1.53 | 1.92 | 1.47 | 2.10 | 91.28 | 1,13.4 | < .0001 | 10.36 | 2,13.3 | .002 | 2.30 | 2,13.3 | .139 |
| SD | 0.29 | 0.50 | 0.30 | 0.46 | 0.29 | 0.42 | 0.22 | 0.50 | |||||||||
| n | 157 | 191 | 71 | 59 | 39 | 67 | 47 | 65 | |||||||||
| Competence | |||||||||||||||||
| M | 3.99 | 3.94 | 4.03 | 3.98 | 3.93 | 3.98 | 3.97 | 3.87 | 1.24 | 1,14.4 | .283 | 2.71 | 2,14 | .10 | 1.81 | 2,14 | .20 |
| SD | 0.33 | 0.25 | 0.32 | 0.21 | 0.35 | 0.29 | 0.34 | 0.22 | |||||||||
| n | 154 | 190 | 71 | 59 | 37 | 67 | 46 | 64 | |||||||||
Discussion
This study compared the effect of METS and CAU in three outpatient addiction care centers in Mexico. Results did not support the hypothesis that METS is more effective than CAU in increasing treatment engagement and reducing days of substance use. While there was a significant time effect for reduction in days of substance use, there were no time points in which the two conditions had significantly different days of substance use. Therefore, our findings suggest that both METS and CAU had an effect in reducing days of substance use in the whole study sample, without returning to baseline levels, even after finishing the active phase of treatment. This lack of significant differences in study outcomes between conditions should be interpreted in the context of the high study retention and study treatment exposure observed in the sample. The majority of participants in both METS (90.7%) and CAU (90.9%) were exposed to their three study treatment sessions, and were constantly contacted by the site RA to attend their study assessments at the treatment center throughout the 4 months of study duration. Results from the original METS study also showed no significant differences between conditions with a pattern of decrease from baseline substance use in both conditions, and also achieved high retention rates in a population hard to retain in treatment (Carroll et al., 2009). Overall, findings from both trials may show that, regardless of the therapeutic approach, individual counseling delivered at the beginning of treatment along with the implementation of constant contact efforts, might boost the effect of treatment in the Latino and Mexican populations
Another possible interpretation of these findings is that equivalent results between interventions in the whole sample could be attributed to trial participation. It has been documented that clinical trial participation has an impact on patient outcomes as an effect of the heightened attention toward participating-patients, commonly referred as “trial effect” or “research participation effect” (McCambridge, Witton, & Elbourne, 2014). Unfortunately, evidence is needed in order to assess the size of such effects in behavioral trials and how to control for this possible source of bias (McCambridge at al., 2014).
For the second hypothesis regarding improved outcomes in alcohol users, when comparing the effect of METS against CAU in primary alcohol users, results did not show any added benefit from METS, either in alcohol use or service utilization outcomes compared with the whole sample; differing with findings from the U.S. METS study (Carroll et al., 2009). While the sample in the U.S. trial was larger and comparisons should be taken with caution, this finding stresses the possible difference in effect of tested EBPs when transferred to other populations (Patel & Saxena, 2014).
The fact that this study was implemented in three different outpatient clinical settings that provide services to a heterogeneous population of treatment seekers (Marín-Navarrete et al., 2014) may have also contributed to the lack of significant differences between conditions. Results showed that there were differences between sites (independent of treatment condition) in service utilization, which may suggest that many variables in the patients (e.g. severity of substance use, presence or absence of co-occurring disorders, individual and system level barriers to treatment, etc.), as well as the variability of services offered within sites (e.g. group drug counseling, psychiatric treatment, etc.) might be moderators of the effect observed at the sites (Carroll et al., 2009). More complex sub-group analyses could help identify which kind of patients in which settings received more therapeutic benefit from METS and could inform its dissemination (Carroll, 2012). However, such analysis would be difficult considering sample size limitations.
This study has various limitations. First, despite its statistical power, sample size and number of sites were limited in this study. As mentioned above, a larger sample may have permitted more complex analysis of subgroup observations and other variable interactions. Second, service utilization measurement in this study may have been impacted by the fact that in Mexico, mental health care services are underutilized regardless of level of addiction severity or perceived need for treatment (Berenzon Gorn et al., 2003, Borges et al., 2006), so an alternative approach that considers measuring engagement in other help-seeking behaviors idiosyncratic to the Mexican population (e.g. seeking support in the family or within the community) at follow-up should be considered for future trials. Third, we did not perform any viability, acceptability or cultural adequateness analyses of METS with Mexican patients and counselors before trial implementation.
Finally, another possible limitation of the study is the capacity of the STR to fully characterize what happened during intervention sessions, since it is focused on rating counselor adherence to METS consistent and inconsistent strategies, but does not assess patient speech and behaviors. A systematic review by Magill et al. (2014) on MI process supports that better outcomes rely on a dynamic relationship between MI consistent strategies and increased fluctuations in the patient's language between willingness to change behavior or to maintain the status quo; unfortunately, the STR form does not allow testing this technical hypothesis. Additionally, a more detailed process analysis may help establish counselor-patient interactions that may be linked to better outcomes in the Mexican population, and therefore help identify common factors between the two interventions that could explain why both were equally effective (Laska, Gurman & Wampold, 2014).
In spite of these limitations, this study has important strengths. This study is the first in Mexico to test the effect of a behavioral intervention for substance use treatment following clinical trial standards. Therefore, its strengths include recruitment and randomization of a diverse sample of treatment-seekers, delivery of interventions by randomized counselors currently working in real-world treatment settings, choice of an active control intervention, independent ratings to ensure discriminability between interventions and intensive training, certification and supervision efforts on the study intervention.
Overall, despite the lack of conclusive findings on the effect of METS against CAU, this study adds a trial to the existing evidence that supports the need to study how brief interventions for substance use work (Hingson & Compton, 2014) by analyzing treatment effects moderators and mediators (Beutler, Someah, Kimpara, & Miller, 2016) and raises important questions for further treatment research with this population. Finally, this project constituted an unprecedented collaboration in Mexico between researchers and community service providers, proving that rigorous trials are feasible in Mexico, thus opening the door to future studies of this nature in the country.
Acknowledgements and funding
The authors wish to acknowledge the following institutions for their key support during the conduction of this study: National Institute on Drug Abuse–Clinical Trials Network (NIDA-CTN), Consejo Nacional Contra las Adicciones (CONADIC), and Centro Nacional para la Prevención y el Control de las Adicciones (CENADIC). They also wish to extend a special acknowledgment to the following clinical sites for their contributions to the implementation of this study: Clínica de Trastornos Adictivos of the INPRFM, Centros de Integración Juvenil A. C. (CIJ), Consejo Estatal contra las Adicciones of Puebla (CECAP); as well as the sites’ clinical staff and research assistants for their help during all phases of study implementation. Finally, the authors also wish to thank the following research collaborators and consultants: Luis Villalobos-Gallegos (INPRFM), Liliana Templos-Nuñez (INPRFM), Angélica Eliosa-Hernández (INPRFM), Javier Graue-Moreno (INPRFM), Ximena Tiscareño-Osorno (INPRFM), Carla Fernández de la Fuente (IMPRFM), Lorena Larios Chavéz (INPRFM), María Pérez (FNA-UM), Ingrid Usaga (FNA-UM), Chris Farentinos (FNA-UM), Thelma Vega (FNA-UM), Manual Paris (Yale University) Luis Anez (Yale University), Erick Ponce (FNA-UM), Patrick Shironoshita (Infotech Soft), Jerónimo Blanco (INPRFM) and Alejandro Ortíz (INPRFM).
This study was funded by a grant from the U.S. Department of State (Grants No. SINLEC11GR0015/A001/A002) awarded to the Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz in Mexico. The U.S. Department of State had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit this paper for publication.
References
- Ball S.A., Martino S., Nich C., Frankforter T., Van Horn D., Crits-Cristoph P., Woody G.E., Obert J.L., Farentinos C., Carroll K.M. Site matters: Multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. Journal Consulting and Clinical Psychology. 2007;75:556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenzon Gorn S., Medina-Mora M.E., Lara Cantú M.A. Servicios de salud mental: veinticinco años de investigación. Salud Mental. 2003;26:61–72. [Google Scholar]
- Beutler L.E., Someah K., Kimpara S., Miller K. Selecting the most appropriate treatment for each patient. International Journal of Clinical and Health Psychology. 2016;16:99–108. doi: 10.1016/j.ijchp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G., Medina-Mora M.E., Wang P.S., Lara C., Berglund P., Walters E. Treatment and adequacy of treatment of mental disorders among respondents to the Mexico National Comorbidity Survey. American Journal of Psychiatry. 2006;163:1371–1378. doi: 10.1176/ajp.2006.163.8.1371. [DOI] [PubMed] [Google Scholar]
- Carroll K.M. Dissemination of evidence-based practices: How far we’ve come, and how much further we’ve got to go. Journal of Addiction. 2012;107:1031–1033. doi: 10.1111/j.1360-0443.2011.03755.x. [DOI] [PubMed] [Google Scholar]
- Carroll K.M., Ball S.A., Nich C., Martino S., Frankforter T.L., Farentinos C., Kunkel L.E., Mikulich-Gilbertson S.K., Morgenstern J., Obert J., Polcin D., Snead N., Woody G.E. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug and Alcohol Dependence Journal. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K.M., Martino S., Ball S.A., Nich C., Frankforter T.L., Anez L.M., Paris M., Suarez-Morales L., Szapocznik J., Miller W.R., Rosa C., Mathews J., Farentinos C. A multi-site randomized effectiveness trial of motivational enhancement therapy for Spanish-speaking substance users. Journal of Consulting and Clinical Psychology. 2009;77:993–999. doi: 10.1037/a0016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrol K.M., Nich C., Sifry R.L., Nuro K.F., Frankforter T.L., Ball S.A., Fenton L., Rounsaville B.J. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence Journal. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Collins P.Y., Patel V., Joestl S.S., March D., Insel T.R., Daar A.S. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan D.M., Bigelow G.E., Brigham G.S., Carroll K.M., Cohen A.J., Gardin J.G., Hamilton J.A., Huestis M.A., Hughes J.R., Lindbald R., Marlatt G.A., Preston K.L., Selzer J.A., Somoza E.C., Wakin P.G., Wells E.A. Primary outcome indices in illicit drug dependence treatment research: Systematic approach to selection and measurement of drug use end-points in clinical trials. Journal of Addiction. 2012;107:694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W., O’Farrell T.J., Freitas T.T., McFarlin S.K., Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Farentinos C., Obert J.L. CTN Motivational Enhancement Treatment Manual. Unpublished manuscript. 2000 [Google Scholar]
- Feaster D.J., Mikulich-Gilbertson S., Brinks A. Modeling site effects in the design and analysis of multisite trials. American Journal of Drug and Alcohol Abuse. 2012;37:383–391. doi: 10.3109/00952990.2011.600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R., Compton W.M. Screening and brief intervention and referral to treatment for drug use in primary care: Back to the drawing board. The Journal of the American Medical Association. 2014;312:488–489. doi: 10.1001/jama.2014.7863. [DOI] [PubMed] [Google Scholar]
- Hintze J. NCSS, LLC; Kaysville, Utah: 2013. PASS (Version 12) [Computer software] [Google Scholar]
- Horigian V.E., Espinal P.S., Alonso E., Verdeja R.E., Duan R., Usaga I.M., Pérez-López A., Marín-Navarrete R., Feaster D.J. Readiness and barriers to adopt evidence-based practices for substance abuse treatment in Mexico. Salud Mental. 2016;39:77–84. [Google Scholar]
- Horigian V.E., Marín-Navarrete R., Verdeja R.E., Alonso E., Perez M.A., Fernández-Mondragón J., Berlanga C., Medina-Mora M.E., Szapocnik J. Technology transfer for the implementation of a clinical trials network on drug abuse and mental health treatment in Mexico. Revista Panamericana de Salud Pública. 2015;38:233–242. [PMC free article] [PubMed] [Google Scholar]
- Laska K.M., Gurman A.S., Wampold B.E. Expanding the lens of evidence-based practice in psychotherapy: A common factors perspective. Psychotherapy. 2014;51:467. doi: 10.1037/a0034332. [DOI] [PubMed] [Google Scholar]
- Lundahl B., Burke B.L. The effectiveness and applicability of motivational interviewing: A practice-friendly review of four meta-analyses. Journal of Clinical Psychology. 2009;65:1232–1245. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- Magill M., Gaume J., Apodaca T.R., Walthers J., Mastroleo N.R., Borsari B., Longabaugh R. The technical hypothesis of motivational interviewing: A meta-analysis of MI's key causal model. Journal of Consulting and Clinical Psychology. 2014;82:973–983. doi: 10.1037/a0036833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Navarrete R., Templos-Nuñez L., Eliosa-Hernández A., Villalobos-Gallegos L., Fernández-Mondragón J., Pérez-López A., Galván-Sosa D., Verdeja R.E., Alonzo A., Feaster D.J., Horigian V.E. Characteristics of a Treatment-Seeking Population in Outpatient Addiction Treatment Centers in Mexico. Substance Use and Misuse. 2014;49:1784–1794. doi: 10.3109/10826084.2014.931972. [DOI] [PubMed] [Google Scholar]
- Martino S., Ball S.A., Nich C., Frankforter T.L., Carroll K.M. Community program therapist adherence and competence in motivational enhancement therapy. Drug and Alcohol Dependence. 2008;96:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino S., Ball S.A., Nich C., Frankforter T.L., Carroll K.M. Correspondence of motivational enhancement treatment integrity ratings among counselors, supervisors, and observers. Psychotherapy Research. 2009;19:181–193. doi: 10.1080/10503300802688460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J., Witton J., Elbourne D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. Journal of Clinical Epidemiology. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.R., Rollnick S. Guilford Press; New York: 2002. Motivational interviewing. [Google Scholar]
- Miller W.R., Rose G.S. Toward a theory of Motivational Interviewing. American Journal of Psychology. 2009;64:527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin M.J., Chandler K. Department of Education. National Center for Education Statistics; Washington, D.C.: 1996. Use of Cognitive Laboratories and Recorded Interviews in the National Household Education Survey. Retrieved from: http://files.eric.ed.gov/fulltext/ED401337.pdf in July 2014. [Google Scholar]
- Organización Panamericana de la Salud. (2005). Buenas prácticas clínicas: documento de las Américas. IV Conferencia Panamericana para la Armonización de la Reglamentación Farmacéutica. Retrieved from: http://apps.who.int/medicinedocs/documents/s18627es/s18627es.pdf in July 2014.
- Patel V., Saxena S. Transforming lives, enhancing communities—innovations in global mental health. New England Journal of Medicine. 2014;370:498–501. doi: 10.1056/NEJMp1315214. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group Project MATCH secondary a priori hypotheses. Journal of Addiction. 1997;92:1671–1698. [PubMed] [Google Scholar]
- Ramada-Rodilla J.M., Serra-Pujadas C., Delclós-Clanchet G.L. Adaptación cultural y validación de cuestionarios de salud: revisión y recomendaciones metodológicas. Salud Pública de México. 2013;55:57–66. doi: 10.1590/s0036-36342013000100009. [DOI] [PubMed] [Google Scholar]
- Rojas E., Real T., García S., Medina-Mora M.E. Revisión sistemática sobre tratamiento de adicciones en México. Salud Mental. 2011;34:351–365. [Google Scholar]
- Rubak S., Sandbaek A., Lauritzen T., Christensen B. Motivational interviewing: A systematic review and meta-analysis. British Journal of General Practice. 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- Santa Ana E.J., Carroll K.M., Añez L., Paris M., Ball S.A., Nich C., Frankforter T.L., Suárez-Morales L., Szapocnik J., Martino S. Evaluating motivational enhancement therapy adherence and competence among Spanish-speaking therapists. Drug and Alcohol Dependence. 2009;103:44–51. doi: 10.1016/j.drugalcdep.2009.03.006. doi: 0.1016/j.drugalcdep.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite F.E. An approximate distribution of estimates of variance components. Biometric Bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- Secretaría de Salud (2012a). Encuesta Nacional de Adicciones 2011: Reporte de Alcohol. Retrieved from: http://www.conadic.salud.gob.mx/pdfs/ENA_2011_ALCOHOL.pdf in july 2014.
- Secretaría de Salud. (2012b). Encuesta Nacional de Adicciones 2011: Reporte de Drogas. Retrieved from: http://www.conadic.salud.gob.mx/pdfs/ENA_2011_DROGAS_ILICITAS_.pdf in July 2014.
- Smedslund G., Berg R.C., Hammerstrøm K.T., Steiro A., Leiknes K.A., Dahl H.M., Karlsen K. Motivational interviewing for substance abuse. Cochrane Database Systematic Review. 2011;11(5):CD008063. doi: 10.1002/14651858.CD008063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.C. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten R.Z., Allen J.P., editors. Measuring alcohol consumption. Humana Press; Totowa: 1992. pp. 41–72. doi: 10.1007/978-1-4612-0357-5_3. [Google Scholar]
- Simpson D.D., Joe G.W. A longitudinal evaluation of treatment engagement and recovery stages. Journal of Substance Abuse Treatment. 2004;27:89–97. doi: 10.1016/j.jsat.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Vasilaki E.I., Hosier S.G., Cox W.M. The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol and Alcoholism. 2006;41:328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Wei L.J., Lachin J.M. Properties of the Urn Randomization in Clinical Trials. Controlled Clinical Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]