Abstract
Extracellular vesicles comprise a heterogenous population of exosomes and microvesicles that have critical roles in intercellular signalling and tissue development. These complex particles have been implicated as mediators of the therapeutic effects of stem cells via the transfer of an assorted cargo of proteins and nucleic acids, which can modulate inflammation and enhance endogenous regeneration in a range of tissues. In addition, extracellular vesicles have the capacity to be loaded with therapeutic molecules for targeted delivery of pharmaceuticals. The versatility, biostability and biocompatibility of extracellular vesicles make them appealing for regenerative medicine and may endow considerable advantages over single molecule approaches. Furthermore, since production can be optimised and assessed ex vivo, extracellular vesicles present a decreased risk of neoplastic transformation when compared with cell-based methods. To date, the contribution of vesicles to tissue development has perhaps been most comprehensively defined within hard tissues, such as endochondral bone, where they were first identified in 1969 and henceforth referred to as matrix vesicles. Within developing bone, vesicles function as vehicles for the delivery of pro-osteogenic factors and initiate early nucleational events necessary for matrix mineralisation. However, advancement in our understanding of the biogenesis and characterisation of matrix vesicles has occurred largely in parallel to associated developments in wider extracellular vesicle biology. As such, there is a requirement to align current understanding of matrix vesicle–mediated mineralisation within the context of an evolving literature surrounding exosomes and microvesicles. In this review, we present an overview of current progress and opinion surrounding the application of vesicles in regenerative medicine with a primary focus on their potential as an acellular approach for enhancing hard tissue regeneration. This is balanced with an assessment of areas where further development is required to maximise their application for regenerative medicine.
Keywords: Extracellular vesicles, exosomes, mineralisation, matrix vesicles, pathological calcification, regenerative medicine
Introduction
Stem-cell therapies have shown great promise for the regeneration of a wide range of tissues lost to trauma or disease. Several approved stem-cell therapies are currently undergoing clinical trials, and routine procedures such as blood transfusions and bone marrow transplantation have now been applied in clinical practice for decades. However, despite the significant promise offered by these approaches, there are considerable limitations restricting their wider application. These restrictions primarily relate to the low availability of stem cells within donor tissues, loss of potency following expansion, regulatory issues surrounding translation and even questions surrounding their precise mode of action (MoA).1 Significantly, there is evidence to suggest that only a small percentage (1%–3%) of stem cells actually engraft to the host tissue and that the majority may not reach their target sites, instead becoming trapped in the lungs, spleen and liver.2 Following a recent paradigm shift, it has been proposed that the therapeutic effects of stem cells are largely evoked through paracrine activity rather than through engraftment and differentiation. A growing body of evidence suggests that paracrine activity is exerted through the action of trophic factors as well as nano-sized bioactive particles termed extracellular vesicles (EVs).3–6
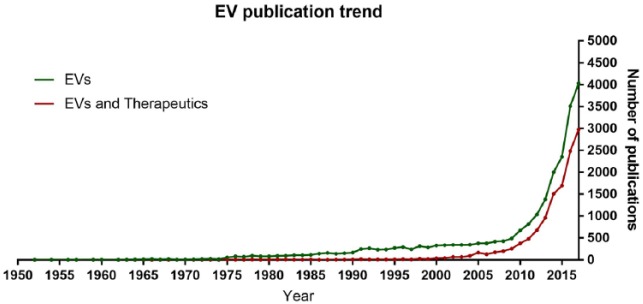
Over the last decade, EVs have demonstrated considerable promise as naturally derived nanoparticles that can be utilised for a number of therapeutic applications, which include predictive and regenerative medicine (Figure 1). EVs are ubiquitous within biological fluids (e.g. blood, urine, semen, milk and amniotic fluid), contributing to critical physiological and pathological processes such as tissue development, regeneration, inflammation and cancer metastasis.7 Knowledge of the biological contribution of EVs to health and disease dates back to the 1960s.8–10 However, only in the last decade has the potential of these multifaceted particles become fully appreciated, with an exponential increase in the number of publications featuring ‘extracellular vesicles’ and/or ‘exosomes’ (Figure 1). Interest has aligned with the establishment of the International Society for Extracellular Vesicles (ISEV) and its affiliated journal, the Journal of Extracellular Vesicles. In line with growing research activity, position statements have been published outlining guidelines for the definition and analysis of EVs.11,12 These developments are reminiscent of events in the field of stem-cell biology less than a decade earlier when the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy outlined the minimal criteria for defining human mesenchymal stem cells (MSCs).13 Mirroring progress previously observed with the expansion and translation of stem-cell therapies, a framework is now beginning to be established through which EVs may have a pronounced influence on the future direction of medicine.14 Much like stem cells, these complex bioactive particles can be combined with a bioactive scaffold or delivery system to enhance endogenous tissue regeneration and resolve inflammation in a host of tissues, including the skeletal system.15 In this review, we will outline current knowledge surrounding EVs, summarising their critical roles in skeletal development, and highlight the advantages and challenges of an EV-based approach to regenerative medicine.
Figure 1.
Publication trend of extracellular vesicles. Data were exported from Web of Science using the following criteria: (1) all databases, keywords (exosomes OR extracellular vesicle) and year range (1930–2017); (2) all databases, keywords (exosomes OR extracellular vesicle AND therapy) and year range (1930–2017).
EVs
History
Wolf and colleagues first acknowledged the presence of phospholipid-rich particulates within platelets, originally classifying the material as ‘platelet dust’ (Figure 1). It was noted that these particulates could be separated by ultracentrifugation (UC) and displayed coagulant properties.8 In 1967, Anderson and Bonucci observed electron-dense ‘leaf-like’ particles with ‘needle-like’ projections that were attached to collagen fibrils within ossifying cartilaginous matrix, which they subsequently defined as matrix vesicles (MVs).9,10 In the 1980s, the physiological contribution of EVs was found to be more widespread, with these nano-sized messengers shown to mediate important immunological processes,16 such as antigen presentation17 and anti-tumour activity. Today EVs are known to play a wide role in intercellular communication and development within a number of hard and soft tissues, where they deliver a cargo of nucleic acids, peptides and lipids to neighbouring cells and tissues. The provision of these heterogeneous biological cargos has been observed to have a downstream effect on a variety of molecular functions,18 including the signalling and regulation of gene expression in target cells, such as the upregulation of proteoglycan and type-II collagen by osteoarthritic chondrocytes.19 More recently, the delivery of both osteoblast- and MSC-derived EVs has been shown to promote osteoblast differentiation and mineralisation both in vitro and in vivo.20 Thus, suggesting these bioactive particles are capable of promoting de novo mineralisation and may have considerable potential for driving skeletal tissue regeneration.
Current definition
EVs are defined as phospholipid-enclosed nanoparticles (30–2000 nm) that carry a complex and variable cargo of biological contents including proteins and nucleic acids. In the context of bone formation, vesicles will also contain elements required to direct early mineralisation such as and inorganic phosphate (Pi). These elements are thought to be derived from the cytoplasm or organelles such as mitochondria. The precise content and membrane composition of EVs are largely heterogeneous and dependent on cell type, location and condition of the local microenvironment.
To date, three different subtypes of EVs have been identified that are typically classified based on diameter and biogenesis (Table 1). Exosomes (30–150 nm) are formed within the endosomal network and are released when multivesicular bodies fuse with the plasma membrane. Microvesicles (50–1000 nm) are generated by outward budding (blebbing) of the plasma membrane and will contain only local cytosolic proteins and nucleic acids. Larger vesicles termed apoptotic bodies (500–2000 nm) are released as fragments of dying cells and can often be distinguished by the presence of nuclear particulates as a consequence of karyorrhexis.21 Notably, there is some discrepancy in the literature regarding the size range of each EV subtype, with considerable overlap noted in their size and content. As a consequence, studies that define exosomes purely by size are likely more representative of a heterogeneous population comprising exosomes, small microvesicles and some additional non-vesicular extracellular material – depending on the efficiency of isolation, this may include small membrane fragments and large extracellular proteins. It is of considerable importance that vesicles derived from different tissue and biofluid sources are defined in accordance with published guidelines presented by the ISEV.11 To further our understanding of the molecular composition of these EV subtypes, online resources are now available that provide a comprehensive and continually evolving database of protein, lipid and RNA analyses of EVs derived from a wide variety of cell and sample types. These can be found freely available online and include the databases Vesiclepedia (http://www.microvesicles.org), EVpedia (http://student4.postech.ac.kr/evpedia2_xe/xe) and ExoCarta (http://www.exocarta.org). Only by adhering to implemented standards and contributing to the developing body of resources can we continue to generate rigorous and reproducible research as well as develop EV therapeutics with translational potential.
Table 1.
Definition and features of extracellular vesicles.
| Features | Exosomes | Microvesicles | Apoptotic bodies | Matrix vesicles |
|---|---|---|---|---|
| Size (nm) | 30–150 | 50–1000 | 500–2000 | 50–400 |
| Other names | Prostasomes Tolerosomes Dexosomes Nanovesicles Exosome-like vesicles |
Microparticles Blebbing vesicles Shedding vesicles Ectosomes |
Microparticles Microvesicles Exosome-like vesicles Exosomes Ectosomes |
|
| Lipid composition | Enriched in phosphatidylserine, cholesterol, ceramide and other sphingolipids, LBPA and lipid rafts | Enriched in phosphatidylserine, phosphatidylethanolamine and sphingolipids | ND | Enriched in phosphatidylserine, phosphatidylethanolamine and sphingolipids |
| Main protein markers | CD9, CD63, CD81, Alix, TSG101, Flotilin, Rab and ESCRT | CK18, MMP2, integrins, selectins and CD40 | ND | CD9, CD81, Flotilin, Rab, MMPs, HSPs, integrins, annexins and TNAP |
| Other cargo | MicroRNA and other non-coding RNAs, mRNA, HSP70, HSP90, syntenin, ubiquitin, clathrin, VPS32, VPS4, protein kinases, β catenin, 14-3-3, G proteins, peroxidases, pyruvate kinase, enolase, GAPDH, histones and ribosomal proteins | Other non-coding RNAs, mRNA, CD9, CD81, CD82, integrin, PECAM1, fibronectin, RAB, GTPases, annexins, GAPDH, ALIX, TSG101, ERK, PLD, VPS4, ALIX, TSG101, ERK, PLD, VPS4, actin, tubulin, histones and ribosomal proteins | Cell debris and organelles | MicroRNA and other non-coding RNAs, mRNA, actins, cofilin, moesin, myosin, heat shock proteins and chaperones, 14-3-3, GTPases, histones and ribosomal proteins |
| Biogenesis | Endosomal system as ILVs and secreted when MVBs fuse with the PM | Outward budding from the PM | Fragments of apoptotic cells | Population of exosomes and microvesicles |
| References | 7, 16, 20 and 21 | 7, 16, 20 and 21 | 7, 16, 20 and 21 | 21 and 31 |
Biological functions
The precise biological function of EVs is a reflection of the parental cell from which they were derived and the local microenvironment (e.g. inflammatory or hypoxic). Divergence in biological function can be demonstrated by the fact that they have been implicated in critical processes such as tissue formation as well as in pathological conditions, including vascular calcification and many forms of cancer such as osteosarcoma.18,22 There have been studies showing that MSC-derived EVs have an anti-inflammatory and immunomodulatory capacity, promoting macrophage polarisation and proliferation.23 Furthermore, cardiac-derived EVs have been shown to be powerful stimulators of angiogenesis in endothelial cells during a myocardial infarction so they potentially hold promise for applications such as cardiac vessel regeneration.24 EVs also play a prominent role within the nervous system, regulating myelin formation, neuronal outgrowth and survival, receptor recycling and the removal of pathological proteins, which makes them promising candidates for neuronal regeneration and the treatment of neuro-degenerative diseases.25 However, the critical and long-defined contribution of these nanoparticles to early osteogenesis will be the focus of this review.
Role of EVs in hard tissue mineralisation
Matrix vesicles as sites of early mineral nucleation
Bone is the most ubiquitous mineralised tissue within vertebrates and is composed of an inorganic multi-substituted hydroxyapatite. Although the biological and physicochemical properties of bone are well described, many of the processes governing mineral formation, transport and deposition within the extracellular matrix (ECM) remain unclear. The pioneering studies of Anderson and Bonucci in 1967 were the first to identify the presence of what they referred to as matrix vesicles (MVs) within hypertrophic cartilage. These vesicles were described as electron-dense bodies that became anchored within the developing ECM. The studies were the first to suggest that matrix mineralisation was not restricted to collagen fibrils.26 Since these initial findings, subsequent data have consolidated MVs as sites of initial calcification leading to the formation of early inorganic apatite crystals in epiphyseal cartilage in a range of species, including mice and guinea pigs.27 Within the evolving body of literature, MVs (Figure 2) refer to vesicles of varied size (50–400 nm) that reside in the pre-mineralised matrix of dentin, cartilage and bone.28 Although not comprehensively defined, MVs likely represent a mixture of exosomes and microvesicles containing specialised components required to direct ECM mineralisation (Table 1).29–31 Currently, no study has sought to define the independent contribution of distinct vesicle subsets during hard tissue mineralisation and few have begun to align MV theory within the context of the burgeoning field of EV biology.32 Perhaps, our most advanced understanding of the biogenesis of MVs derives from a study by Boonrungsiman et al.,33 where calcium phosphate–containing vesicles were found to associate with the mitochondria, thereby implicating specific intracellular transport pathways rather than simple membrane blebbing, characteristic of microvesicle biogenesis, as a mechanism for MV-mediated mineralisation. Even though the contribution of MVs to early mineralisation events in tissues such as calcifying cartilage, bone and dentine can no longer be disputed, the underlying mechanisms by which this is achieved remain unclear. Here, we will present an overview of current opinion surrounding the role of MVs in physiological and pathological mineralisation.
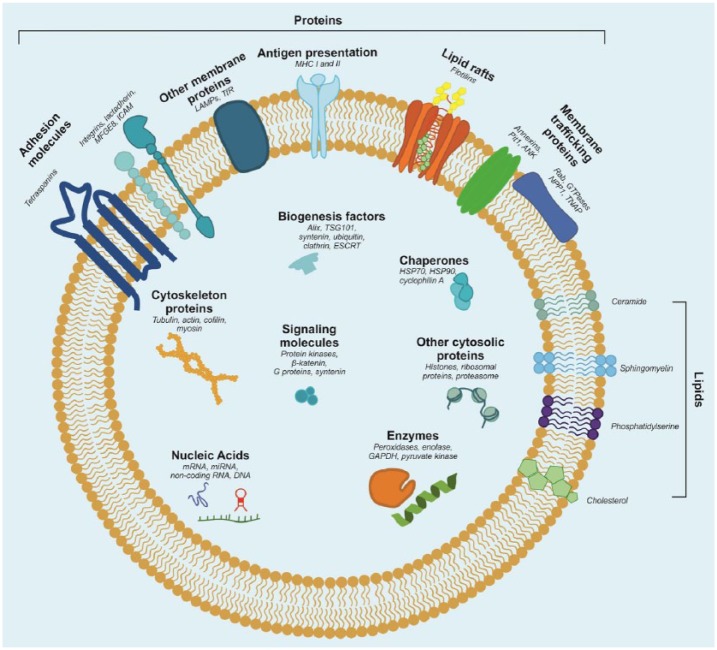
Figure 2.
Matrix vesicle (MV) cargo. Graphical representation of the matrix vesicle contents and membrane orientation of proteins, lipids and nucleic acids. Some of the listed components may be present in some matrix vesicles but not in others. For instance, in our previous study, we did not detect MHC complexes.
MVs are enriched in membrane proteins with well-defined roles in critical processes required for ECM mineralisation. These include the calcium-dependent phospholipid-binding annexin proteins (annexins II, V and VI) and enzymes including ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) and alkaline phosphatase (ALP), which are required for maintaining the ratio of pyrophosphate (PPi) and inorganic phosphate (Pi) to regulate hydroxyapatite crystal formation26 and drive matrix remodelling (Figure 3).27 Under physiological conditions, mineralisation is inhibited by PPi generated through the cleavage of nucleotide triphosphates by ENPP1.34 Inorganic Pi, derived through ALP-mediated hydrolysis of PPi, which drives mineralisation in both physiological and pathological conditions including vascular calcification, is loaded into MVs via transmembrane phosphate transporter proteins, such as Pit1, where these ions associate with calcium localised to the phospholipid-rich bilayer. Phospholipids comprising the MV membrane, including phosphatidylserine (PS), act in conjunction with membrane-binding proteins (e.g. annexins) to sequester and which drives the nucleation of immature mineral35 along with a less well-characterised pool of and Pi thought to be bound to luminal proteins.36 This association between PS and Pi forms the nucleational core complex, which has been hypothesised to function as an intra-vesicular niche for the formation of mature apatite. It is proposed that as mineral matures and becomes increasingly crystalline, it ruptures the EV membrane and associates with the underlying cartilaginous matrix,37 thereby driving the early mineralisation events required for bone development and regeneration.
Figure 3.

Schematic diagram of the mineralisation process. NPP1 inhibits mineralisation by generating PPi by catalysing extracellular ATP. TNAP promotes mineralisation by hydrolysing PPi into inorganic phosphate ions, which are in turn transported to the matrix vesicle (MV) through phosphate transporters such as Pit1. Conversely, ANK transports PPi from the MV into the developing ECM. Annexins function as calcium channels, transporting inside the MV and localise and in a nucleational core complex, which facilitates mineral nucleation and transition to a crystalline hydroxyapatite. This is hypothesised to eventually rupture the vesicle membrane and propagate within the collagenous extracellular matrix.
As stated, MV calcification is tightly linked with the presence of acidic membrane phospholipids.35 The presence of acidic phospholipids is significant in conferring membrane fluidity to promote fusion34 and uptake of vesicles for intercellular communication, and in the transfer of signalling molecules between cells that comprise the skeletal niche.11 It also has significant implications when one considers current opinions surrounding the formation of mature and physiologically stable hydroxyapatite from an amorphous precursor.38 This transition is hypothesised to initiate from an amorphous precursor and proceed via a series of transient intermediate phases that include dicalcium phosphate dihydrate (DCPD) and octacalcium phosphate (OCP) that are only stable at a sub-neutral pH. Based on our published findings, we hypothesise the presence of acidic phospholipids acts to generate a localised reduction in pH, thereby facilitating the stable formation of intermediate phases such as OCP that can be detected analytically.39 Intriguingly, the presence of DCPD and OCP has also been associated with pathological calcification in which an acidic pH is frequently encountered. Furthermore, chemical analysis of the mineral deposited during pathological calcification, particularly in medial and intimal sites of coronary arteries, has led researchers to suggest an active vesicle-mediated mechanism similar to that underpinning physiological early bone formation.22 Vesicles localised to sites of vascular calcification derive from atrophying smooth muscle cells and recruited macrophages. The process is inflammation-dependent and associated with the rupture of vulnerable plaques.40 Significantly, there is evidence to suggest that not all plaque-associated vesicles are harmful, with non-calcifying fetuin-A+ and matrix Gla protein+ vesicles also identified. The presence of vesicles containing these calcification inhibitors is likely to be important for maintaining vascular health, and methods for enhancing their presence in patients at risk of atherosclerosis could have significant clinical implications.
Clinical perspective
A growing body of evidence has accumulated implicating EVs as complex biological mediators of tissue development and regeneration. As such, there exists the potential to harness the regenerative capacity of these natural particles and exploit them to develop acellular yet complex biological approaches for driving tissue regeneration. At present, research into the regenerative application of EVs is in its infancy and the majority of documented research remains pre-clinical, with few clinical trials registered (Table 2). However, interest is rapidly growing with a number of recent publications outlining the potential therapeutic utility of EVs for the regeneration of a wide range of tissues, including bone and cartilage. In this section, we shall discuss current research into EV-mediated tissue regeneration and highlight the promise and current limitations of this approach.
Table 2.
Registered clinical trials in clinicaltrials.gov.
| Row | Status | Study title | Conditions | Interventions | Locations |
|---|---|---|---|---|---|
| 1 | Completed | Effect of exosomes derived from red blood cell units on platelet function and blood coagulation | Blood coagulation and platelet function | Other: in vitro study | University Hospital Frankfurt, Frankfurt am Main, Hessen, Germany |
| 2 | Completed | Phosphate in blood pressure regulation | Hypertension | Dietary supplement: sodium phosphate Drug: sevelamer, sodium bicarbonate and sodium chloride |
University Hospital Zurich, Nephrology, Zurich, ZH, Switzerland |
| 3 | Completed | Pilot immunotherapy trial for recurrent malignant gliomas | Malignant glioma of brain | Drug: IGF-1R/AS ODN Device: biodiffusion chamber |
Thomas Jefferson University Hospital; Jefferson Hospital for Neurosciences, Philadelphia, PA, USA |
| 4 | Completed | Influence of high and low salt on exosomes in the urine | Healthy | Dietary supplement: high-salt diet followed by low-salt
diet Dietary supplement: low-salt diet followed by high-salt diet |
University of Southern Denmark, Odense, Denmark |
| 5 | Completed | Influence of rosiglitazone on the diuretic effect of furosemide and amiloride | Insulin resistance | Drug: rosiglitazone versus placebo Drug: response (sodium excretion) to amiloride infusion Drug: response (sodium excretion) to furosemide infusion |
Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands |
Keyword: exosomes. The inclusion criteria were the following: recruitment status (completed), age (all), sex (all) and study type (interventional).
There are several significant advantages in applying EVs for regenerative medicine when compared to conventional biological approaches that typically incorporate a cell or growth factor to encourage endogenous tissue formation. Firstly, vesicles may be superior to single approaches since they allow the delivery of multiple physiologically relevant factors and, as they are naturally derived, confer enhanced biocompatibility and biostability.41 The fact that EVs deliver multiple biomolecules to recipient tissues may also enable them to target several therapeutic pathways simultaneously, thus enhancing their therapeutic efficacy. Another advantage is that EVs do not contain replicative machinery, which is significant in that it may allow for genetic modification of the parent cell line without the transmission of manipulated DNA into the vesicle. This can be applied to confer advantages over primary cell-based approaches, such as decreased population doubling times and phenotypic stability, which have significant implications when one considers the limited therapeutic window of primary cell therapies. The third benefit derives from the fact that EVs are not dynamic and cannot change phenotype, which has significant advantages when predicting therapeutic outcomes, ensuring reproducibility and reducing the risk of neoplastic transformation. In addition, the relatively small diameter of EVs means that they are less likely to become trapped inside the lungs, liver and spleen if administered intravenously and their innate homing ability increases the likelihood of these drugs reaching the target site. This makes EVs appealing as vehicles for loading pharmaceuticals and/or other therapeutic compounds to reduce drug clearance rates, with the aim of enhancing or extending their efficacy. In addition, by tailoring vesicle properties such as lipid composition, there may be the opportunity to further improve the stability of the nanoparticle while in circulation and increase their half-life when delivered locally or intravenously. Overall, EVs manufactured ex vivo may offer many of the benefits of a cell-based approach while avoiding the inherent pitfalls. The benefits of EVs as naturally derived particles for enhancing tissue regeneration have been documented in a wide range of tissues. We shall next explore current literature surrounding the application of EVs in soft and hard tissue regeneration.
The majority of studies applying EVs for regenerative applications have utilised vesicles derived from stem cells, including MSCs, embryonic stem cells (ESCs) and other tissue-specific stem cells due to their anticipated immuno-compatibility and documented roles as paracrine mediators of tissue repair.42 To date, MSC-derived EVs have been shown to enhance proliferation, inhibit apoptosis, decrease inflammation and promote processes such as angiogenesis and cellular reprogramming.43 In animal studies of myocardial ischaemia, the delivery of MSC-derived EVs has cardioprotective effects, promoting angiogenesis that improved recovery and reduced infarct site in laboratory animals with experimental myocardial infarction.44 Similarly, delivery of ESC vesicles enriched in miRNA-290-295 was found to promote neovascularisation and enhance cadiomyocyte survival and function following myocardial infarction.45 In a separate study, cutaneous burns treated with MSC-derived exosomes demonstrated accelerated cell proliferation and re-epithelialisation through the delivery of Wnt4 and downstream β-catenin nuclear translocation.46 These promising particles have also been shown to promote myogenesis and muscle regeneration in an in vivo model of skeletal muscle injury. Interestingly, in this study, MSC-derived vesicles were found to contain a relatively low concentration of muscle-repair proteins, such as vascular endothelial growth factor (VEGF) and interleukin (IL)-6, with the regenerative effects primarily attributed to the presence of small RNAs, including miRNA-494.5 Finally, in an in vitro model of inflammatory osteoarthritis, bone marrow–derived and adipose-derived MSC EVs were found to inhibit the action of pro-inflammatory mediators including ILs and tumor necrosis factor-alpha (TNFα)-induced collagenase activity.19,47 This list presents several significant outcomes to date but is by no means comprehensive with additional therapeutic effects observed in a variety of other tissues including the kidneys, liver and nervous system. For a comprehensive overview of the potential of EVs for soft tissue regeneration, we recommend the following articles by Chen et al.48 and Lamichhane et al.49
To date, the considerable potential of an EV-based approach to regenerative medicine has perhaps been most comprehensively demonstrated in the skeletal system with prospective applications for enhancing fracture repair and in treatment of degenerative conditions, including osteoporosis. Osteoblast-derived vesicles isolated from actively differentiating cultures are enriched in metalloproteinases conducive to matrix remodelling and calcification.50 These vesicles have defined roles in regulating osteoclastogenesis through the delivery of the pro-osteoclastic cytokine, receptor activator of nuclear factor κ-B ligand (RANKL). Stimulation of osteoblasts with parathyroid hormone has been shown to further enrich EVs with RANKL.51 As such, there exists the potential to manipulate culture conditions ex vivo to generate biocompatible and therapeutically enhanced vesicles that are biologically programmed to target osteoclasts for the treatment of osteoporosis. Alternatively, EVs can be modified to modulate osteoclast activity post-isolation through the active loading of anti-osteoclast drugs, such as zoledronate or dasatinib. In addition to osteoclast modulation, we have previously shown that when administered within MSC cultures, osteoblast-derived EVs act as sites for extracellular nucleation of calcium phosphate, enhancing mineralisation when compared to a current gold standard, BMP-2.33 MSC-derived EVs have been shown to stimulate osteoblastic activity and differentiation through miR-196a, miR-27a, miR206 expression and bone regeneration in vivo.20 In addition, the injection of MSC-derived exosomes has been shown to promote healing in a murine CD9−/− femur fracture model – a model known to produce reduced levels of exosomes.52 Evolving the concept of EV-based regenerative medicine even further, a recent study combined MSC-derived vesicles with a scaffold composed of decalcified bone matrix. The regenerative effects of EVs were monitored following subcutaneous implantation in mice and represented the first time that EVs had been delivered in combination with a biological scaffold. Outcomes were positive with enhanced angiogenesis and mineralisation observed in vivo.53 A subsequent study combined EVs with a poly(lactic-co-glycolic acid) (PLGA) scaffold by immobilising them in polydopamine coating. The scaffold was subsequently implanted in a critical size calvarial defect with sustained release of EVs observed and immunohistochemical evidence of bone regeneration.54 The aforementioned studies highlight the considerable advances made in EV-focused regenerative medicine. However, these approaches are the first to be published in this novel therapeutic area. As such, there is considerable scope for development and refinement, as we shall now discuss.
Despite the considerable benefits offered by an EV-based approach to regenerative medicine, there are several limitations that need to be overcome before this method can become a clinical reality. For a comprehensive overview of current barriers to translation, the authors recommend a previous publication by Davies and Rafiq.55 At present, the most limiting factors restricting the advancement of EV therapies can be grouped into the following categories:
Devising efficient methods for the scaling and harvest of EVs;
Identifying markers of therapeutic potency;
Generating a consistent and homogeneous EV product;
Storage, biosafety, biodistribution and pharma-cokinetics;
Positioning regenerative EV therapies within the current regulatory framework.
Manufacturing large numbers of therapeutically viable EVs at scale is essential if we are to meet clinical and commercial demand. This represents a challenging task, particularly when using a primary cell source with a limited window of passage. The task becomes more demanding when we consider the complex media formulations that are typically employed in EV research and the commercial requirement for chemically defined and serum-free systems that maintain EV yield and function. To date, hollow fibre bioreactors have been most applied for this purpose. These reactors apply a fibre-based cartridge with a molecular weight cut off to retain EVs while allowing the free diffusion of nutrients and waste. It has been postulated that the application of hollow fibre reactors could also address issues surrounding the need for serum-free media, since any vesicles contained within the serum fraction will exceed the molecular cut off and, as such, be excluded from the condition medium.56 We anticipate that other reactor technologies, including stirred tank bioreactors, will also demonstrate utility in EV manufacture given their legacy in the production of numerous biologics, as well as the potential to combine these systems with microcarriers.57 Furthermore, these reactors can be operated with spin filters to allow for the retention of cells and small particles including EVs. For a comprehensive overview of the application of bioreactors for EV manufacture, we recommend the recent publication by Colao et al.58 as well as the aforementioned publication by Davies and Rafiq.55 At present, there is limited knowledge of how the cell culture microenvironment impacts EV production and content. As such, there will be a need to comprehensively evaluate the effects of bioreactor systems designed for cell biomanufacturing on EV production.59
In addition to manufacturing large concentrations of EVs, it is essential that methods are in place for their efficient isolation. At present, several approaches have been applied to isolate EVs from cell culture media as well as a variety of biofluids. These include differential UC, ultrafiltration, sucrose density gradient sedimentation, size exclusion chromatography, tangential flow filtration, kit-based precipitation and affinity-based selection. A critical overview of several of these techniques for the isolation of EVs from serum can be found in the publication of Buschmann et al.60 All these methods have advantages and disadvantages and are highly dependent on the availability of specialised laboratory equipment and relevant in-house technical expertise. Perhaps, the most ubiquitously applied, and certainly the one most frequently utilised for the isolation of EVs from hard tissues such as bone, is differential UC. Unlike the other methods listed, UC can be applied to isolate EVs from large volumes of conditioned culture medium. As such, it currently represents the most viable option for the direct isolation of high concentrations required for regenerative applications. However, UC is a specialised, time consuming, laborious and inefficient process. Furthermore, variation can be introduced depending on the type of centrifuge and rotor applied. For example, a shorter sedimentation path length in the fixed angle compared to the swinging bucket rotor will result in a faster sedimentation rate for peripheral vesicles. As a consequence, it is necessary to adjust the duration and force of centrifugation depending on the model and type of centrifuge used.61 Consequently, different isolation methods will yield EVs of variable heterogeneity, making it difficult to compare results between studies.55 As interest in therapeutic EVs continues to increase, it is critical that we implement consistent and readily available methods for generating large concentrations of EVs that retain maximal therapeutic efficacy. Recently, a promising method has been developed at the National University of Singapore, which applies centrifugal microfluidics for the label-free isolation of microvesicles.62 This approach is appealing in that can be applied to efficiently isolate microvesicles over short time periods (typically minutes) using only a standard bench-top centrifuge. However, at present, the technology can only been applied for the isolation of EVs from small sample volumes (µL). As such, further development will be required if this approach is to be used for high-volume samples required for regenerative applications.
Other factors that need to be clarified before EV therapies can begin to be successfully translated related to nomenclature and the comprehensiveness of methods applied for EV characterisation and quantification of batch homogeneity. Current standards have been published in a position statement outlining the minimal experimental criteria required for defining EVs.11 However, such a definition does not serve to provide a quantitative measurement of therapeutic efficacy, which will no doubt depend on the cell from which the EVs are derived and the purpose of their application. Such a measure can perhaps only be achieved through the identification of specific surface or intra-vesicular markers that can be measured during or post-isolation. In a previous study, we identified a correlation between certain calcium-binding annexin proteins (annexins I, II and VI) and EV-mediated mineralisation in human stem-cell cultures.39 However, further work will be necessary to determine whether selecting for annexin-enriched EV populations can enhance hard tissue regeneration in vivo. In addition, the application of antibody-based selection methods will pose further challenges when applied for the isolation of large concentrations of EV product at scale. Reflecting on this last comment, it may be advantageous to employ label-free methods that provide non-specific biochemical fingerprints that align with known measures of therapeutic potency. Such methods could include Raman spectroscopy, which has shown successful application for the biochemical profiling of tissue-specific differences in MSC-derived EVs.63 Once the MoA has been identified and accurate markers of potency defined, we can begin to evaluate critical pre-clinical parameters such as dosage, toxicity and biodistribution. It will also be pertinent to determine how EVs can be stored to maximise biological potency and minimise operational burden in the clinic. There is evidence to indicate sustained viability and functionality of human urinary exosomes when stored at −80°C in the presence of a cocktail of protease inhibitors.64 This evidence is encouraging given that it may reduce costs associated with more complex cryo-preservation procedures that are a requirement for cell-based therapies. However, studies will be required to determine the effects of long-term storage on EVs isolated from other biofluids and cell sources, including osteoblasts.
Finally, regulation surrounding the application of EV therapeutics is at present unclear. It has been proposed that EV-based therapies will likely fall under the category of biological medicines unless delivering trans-gene products derived from genetically manipulated cells in which case they will fall under the category of Advanced Therapy Medicinal Products (ATMPs).65 However, regulatory classification will remain challenging until the MoA by which EVs exert their therapeutic effects is established. It is likely that this will vary between applications and may require assessment on a case-by-case basis. For a comprehensive review of current limitations surrounding the advancement of vesicle-based therapeutics in regenerative medicine and beyond, we recommend the article by Gimona et al.65
Summary and conclusion
EVs offer considerable value as a biological yet acellular approach for driving endogenous tissue regeneration. These complex particles offer significant advantages over growth factor and cell-based approaches and, to date, positive outcomes have been demonstrated in a variety of soft and hard tissues. Within the last 3 years, several groups have shown that these EVs can be combined with a variety of biocompatible scaffolds and implanted in vivo to drive de novo ossification and tissue vascularisation. However, before the considerable potential of EVs can become a clinical reality, inherent variability relating to methods of EV isolation and characterisation need to be standardised.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article Owen G Davies was supported by an EPSRC E-TERM Landscape fellowship. Ioannis Azoidis was funded by a School of Chemical Engineering PhD Studentship.
ORCID iD: Ioannis Azoidis  https://orcid.org/0000-0001-5953-1808
https://orcid.org/0000-0001-5953-1808
References
- 1. McHeik JN, Barrault C, Levard G, et al. Epidermal healing in burns: autologous keratinocyte transplantation as a standard procedure: update and perspective. Plast Reconstr Surg Global Open 2014; 2: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009; 18: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009; 20: 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan CY, Lai RC, Wong W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 2014; 5: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura Y, Miyaki S, Ishitobi H, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 2015; 589: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 6. Camussi G, Deregibus Maria C, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans 2013; 41: 283–287. [DOI] [PubMed] [Google Scholar]
- 7. Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesic 2015; 4: 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967; 13: 269–288. [DOI] [PubMed] [Google Scholar]
- 9. Anderson HC. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol 1967; 35: 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonucci E. Fine structure of early cartilage calcification. J Ultrastruc Res 1967; 20: 33–50. [DOI] [PubMed] [Google Scholar]
- 11. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesic. Epub ahead of print 22 December 2014. DOI: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill AF, Pegtel DM, Lambertz U, et al. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesic. Epub ahead of print 23 December 2013. DOI: 10.3402/jev.v2i0.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 14. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesic 2015; 4: 30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellavia D, Raimondi L, Costa V, et al. Engineered exosomes: a new promise for the management of musculoskeletal diseases. Biochim Biophys Acta Gen Subj 2018; 1862: 1893–1901. [DOI] [PubMed] [Google Scholar]
- 16. Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta Gen Subj 1985; 822: 203–218. [DOI] [PubMed] [Google Scholar]
- 17. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579. [DOI] [PubMed] [Google Scholar]
- 18. Morhayim J, Baroncelli M, van Leeuwen JP. Extracellular vesicles: specialized bone messengers. Arch Biochem Biophys 2014; 561: 38–45. [DOI] [PubMed] [Google Scholar]
- 19. Vonk LA, van Dooremalen SFJ, Liv N, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018; 8: 906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin Y, Wang L, Gao Z, et al. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep 2016; 6: 21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Revi Molec Cell Biol 2018; 19: 213–228. [DOI] [PubMed] [Google Scholar]
- 22. New SEP, Aikawa E. Role of extracellular vesicles in de novo mineralization. An additional novel mechanism of cardiovascular calcification. Arterioscler Thromb Vasc Biol 2013; 33: 1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Translat Med 2017; 6: 1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deddens JC, Vrijsen KR, Girao H, et al. Cardiac-released extracellular vesicles can activate endothelial cells. Ann Translat Med 2017; 5: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer-Albers EM, Hill AF. Extracellular vesicles: interneural shuttles of complex messages. Curr Opin Neurobiol 2016; 39: 101–107. [DOI] [PubMed] [Google Scholar]
- 26. Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Relat Res 1995; 314: 266–280. [PubMed] [Google Scholar]
- 27. Bonucci E, Cascio VL, Adami S, et al. The ultrastructure of bone cells and bone matrix in human primary hyperparathyroidism. Virchows Arch A Pathol Anat Histol 1978; 379: 11–23. [DOI] [PubMed] [Google Scholar]
- 28. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 29. Hale JE, Wuthier RE. The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem 1987; 262: 1916–1925. [PubMed] [Google Scholar]
- 30. Akisaka T, Gay CV. The plasma membrane and matrix vesicles of mouse growth plate chondrocytes during differentiation as revealed in freeze-fracture replicas. Am J Anat 1985; 173: 269–286. [DOI] [PubMed] [Google Scholar]
- 31. Cui Y, Luan J, Li H, et al. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett 2016; 590: 185–192. [DOI] [PubMed] [Google Scholar]
- 32. Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: are they anchored exosomes? Bone 2015; 79: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boonrungsiman S, Gentleman E, Carzaniga R, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Nat Acad Sci 2012; 109: 14170–14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang MS, Sage AP, Lu J, et al. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Comm 2008; 374: 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Genge BR, Wu LNY, Wuthier RE. Mineralization of annexin-5-containing lipid-calcium-phosphate complexes: modulation by varying lipid composition and incubation with cartilage collagens. J Biol Chem 2008; 283: 9737–9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu LN, Yoshimori T, Genge BR, et al. Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem 1993; 268: 25084–25094. [PubMed] [Google Scholar]
- 37. Skrtic D, Eanes ED. Membrane-mediated precipitation of calcium-phosphate in model liposomes with matrix vesicle-like lipid-composition. Bone Miner 1992; 16: 109–119. [DOI] [PubMed] [Google Scholar]
- 38. Nitiputri K, Ramasse QM, Autefage H, et al. Nanoanalytical electron microscopy reveals a sequential mineralization process involving carbonate-containing amorphous precursors. ACS Nano 2016; 10: 6826–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Davies OG, Cox SC, Williams RL, et al. Annexin-enriched osteoblast-derived vesicles act as an extracellular site of mineral nucleation within developing stem cell cultures. Sci Rep 2017; 7: 12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bobryshev YV, Killingsworth MC, Lord RS, et al. Matrix vesicles in the fibrous cap of atherosclerotic plaque: possible contribution to plaque rupture. J Cell Molec Med 2008; 12: 2073–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riazifar M, Pone EJ, Lötvall J, et al. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol 2017; 57: 125–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 2018; 9: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006; 20: 847. [DOI] [PubMed] [Google Scholar]
- 44. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010; 4: 214–222. [DOI] [PubMed] [Google Scholar]
- 45. Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulat Res 2015; 117: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bin Z, Mei W, Aihua G, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015; 33: 2158–2168. [DOI] [PubMed] [Google Scholar]
- 47. Tofiño-Vian M, Guillén MI, Pérez del Caz MD, et al. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem 2018; 47: 11–25. [DOI] [PubMed] [Google Scholar]
- 48. Chen B, Li Q, Zhao B, et al. Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Translat Med 2017; 6: 1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamichhane TN, Sokic S, Schardt JS, et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 2015; 21: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dean DD, Schwartz Z, Bonewald L, et al. Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of β-glycerophosphate and ascorbic acid. Calc Tissue Int 1994; 54: 399–408. [DOI] [PubMed] [Google Scholar]
- 51. Alfredo C, Alexander L, Maurizio M, et al. Osteoblast-derived extracellular vesicles are biological tools for the delivery of active molecules to bone. J Bone Miner Res 2018; 33: 517–533. [DOI] [PubMed] [Google Scholar]
- 52. Taisuke F, Shigeru M, Hiroyuki I, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Translat Med 2016; 5: 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xie H, Wang Z, Zhang L, et al. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep 2017; 7: 45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li W, Liu Y, Zhang P, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces 2018; 10: 5240–5254. [DOI] [PubMed] [Google Scholar]
- 55. Davies OG, Rafiq QA. Considerations for the bioprocessing, manufacture and translation of extracellular vesicles for therapeutic applications. Cell Gene Ther Insights 2017; 3: 683–694. [Google Scholar]
- 56. Yan IK, Shukla N, Borrelli DA, et al. Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. In: Patel T. (ed.) Extracellular RNA: methods and protocols. New York: Springer, 2018, pp. 35–41. [DOI] [PubMed] [Google Scholar]
- 57. Nienow AW. Reactor engineering in large scale animal cell culture. Cytotechnology 2006; 50: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Colao IL, Corteling R, Bracewell D, et al. Manufacturing exosomes: a promising therapeutic platform. Trends Molec Med 2018; 24: 242–256. [DOI] [PubMed] [Google Scholar]
- 59. Patel DB, Santoro M, Born LJ, et al. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. Epub ahead of print 12 September 2018. DOI: 10.1016/j.biotechadv.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buschmann D, Kirchner B, Hermann S, et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J Extracell Vesic 2018; 7: 1481321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Livshits MA, Khomyakova E, Evtushenko EG, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep 2015; 5: 17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yeo JC, Kenry ZZ, Zhang P, et al. Label-free extraction of extracellular vesicles using centrifugal microfluidics. Biomicrofluidics 2018; 12: 024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gualerzi A, Niada S, Giannasi C, et al. Raman spectroscopy uncovers biochemical tissue-related features of extracellular vesicles from mesenchymal stromal cells. Sci Rep 2017; 7: 9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhou H, Yuen PST, Pisitkun T, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 2006; 69: 1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gimona M, Pachler K, Laner-Plamberger S, et al. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Molec Sci 2017; 18: 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]