Abstract
The CNTNAP2 gene has been proposed to be one of the major susceptibility genes for neurodevelopmental disorders, in which numerous heterozygous missense variants have been identified in patients with autism spectrum disorder (ASD). The contribution of these variants to the manifestations of ASD is however highly controversial because numerous heterozygous missense variants have also been identified in control subjects. In a recent study, we set up a sensitive developmental in vitro cell assay to clarify the potential functional impact of these variants in a heterozygous Cntnap2 background relevant for CNTNAP2 heterozygosity in patients with ASD. We showed that the cell adhesion glycoprotein Caspr2 encoded by CNTNAP2 plays a dose-dependent role in cortical neuron axon growth and provided a proof of principle that some variants have functional consequences, either a loss of function or a dominant-negative effect. This indicates that phenotypes mimicking CNTNAP2 heterozygous and homozygous null mutation may exist in humans. Our observations further suggest that more variants than originally expected could be functionally deleterious and induce a high heterogeneity of phenotypes at the scale of the whole brain. This raises the interesting possibility that CNTNAP2 heterozygous missense variants could define an overall endophenotype shaping a risk for ASD and questions whether, beyond ASD, the variants could contribute to the development of other neurodevelopmental disorders and/or genetically less complex pathologies.
Keywords: Autism spectrum disorder, CNTNAP2, heterozygous missense variants, Caspr2, axon growth, loss-of-function, dominant-negative effect
Comment on: Canali G, Garcia M, Hivert B, Pinatel D, Goullancourt A, Oguievetskaia K, Saint-Martin M, Girault J-A, Faivre-Sarrailh C, Goutebroze L. Genetic variants in autism-related CNTNAP2 impair axonal growth of cortical neurons. Hum Mol Genet. 2018;27(11):1941–1954. doi: 10.1093/hmg/ddy102. PubMed PMID: 29788201. https://www.ncbi.nlm.nih.gov/pubmed/29788201.
Commentary
The CNTNAP2 gene, encoding the neuronal cell adhesion transmembrane glycoprotein Caspr2, has been proposed to be one of the major susceptibility genes for neurodevelopmental disorders, including autism spectrum disorder (ASD), Gilles de la Tourette syndrome, intellectual disability, obsessive-compulsive disorder, cortical dysplasia-focal epilepsy (CDFE) syndrome, schizophrenia, and attention-deficit hyperactivity disorder (ADHD).1 CNTNAP2 genetic alterations identified in patients with ASD include complex genomic rearrangements, heterozygous intragenic deletions, and especially a large number of heterozygous missense variants distributed over the entire extracellular domain of Caspr2.1–3 Cntnap2 knock-out mice display ASD-related behavioral deficits supporting human genetic data.4 However, a large sequencing study did not find evidence for statistical differences between CNTNAP2 heterozygous missense variants in ASD and control subjects,3 and more than 500 missense variants have been identified in individuals of the global population reported in the Exome Aggregation Consortium (http://exac.broadinstitute.org), thus questioning the clinical significance of the variants identified in patients with ASD. In a recent study, we attempted to unravel this question by developing biochemical approaches and a sensitive developmental in vitro cell assay to clarify the potential functional impact of various CNTNAP2 missense variants in a heterozygous Cntnap2 background relevant for CNTNAP2 heterozygosity in patients with ASD.
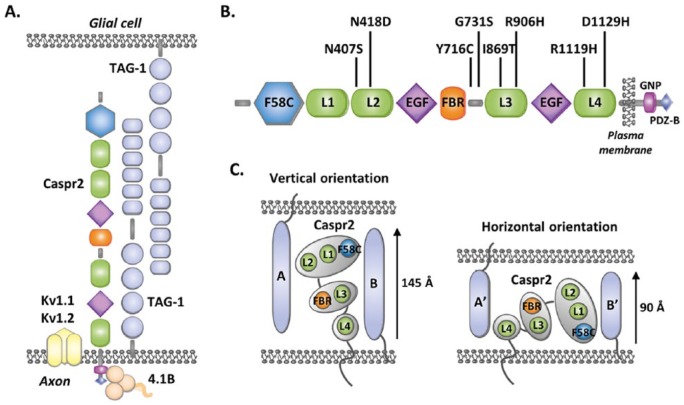
Caspr2 was originally identified as a component of axo-glial contacts in matured myelinated axons, forming a cytoskeleton-associated complex in cis and in trans with the immunoglobulin cell-adhesion molecule CNTN2/Contactin2/TAG-1 at the juxtaparanodal regions of the nodes of Ranvier, where it is required for the clustering of Shaker-type voltage-gated potassium channels (Kv1) (Figure 1A).5,6 Since Cntnap2 is expressed in mouse brain as early as embryonic day 14.5, we asked whether Caspr2 could play additional roles in the axons during early development. Performing cortical neuron cultures from mouse embryos, we demonstrated that Caspr2 plays a dose-dependent role in axon growth in vitro. Loss of one Cntnap2 allele is sufficient to elicit axonal growth decrease, revealing a situation that may be relevant for CNTNAP2 heterozygosity in patients with ASD. This offered a reliable assay to identify the effects of the variants.
Figure 1.
Structure and cell-adhesion function of Caspr2. (A) Axo-glial complexes at the juxtaparanodal regions of the nodes of Ranvier in matured myelinated axons. The protein 4.1B mediates the interaction of Caspr2 with the axonal cytoskeleton. (B) Domain organization of Caspr2 and position of the variants studied. F58C, discoidin domain; L1-L4, laminin G domains; FBR, fibrinogen-like domain; EGF, EGF-like domains; GNP, “Glycophorin C-Neurexin IV-Paranodin” motif mediating the interaction with the cytoskeleton-associated proteins 4.1B; PDZ-B, PDZ-binding motif. (C) Vertical and horizontal orientations of the ectodomain organized into 3 lobes, which could influence the ability of Caspr2 to recruit different cis (B, B′) and trans (A, A′) interacting partners in the extracellular space between the cells at contact sites.
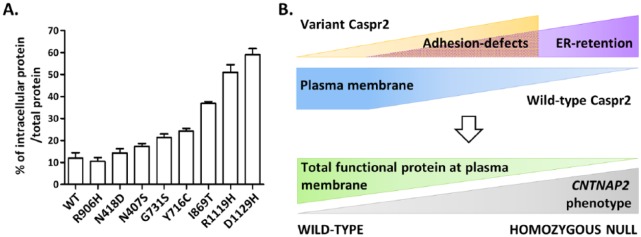
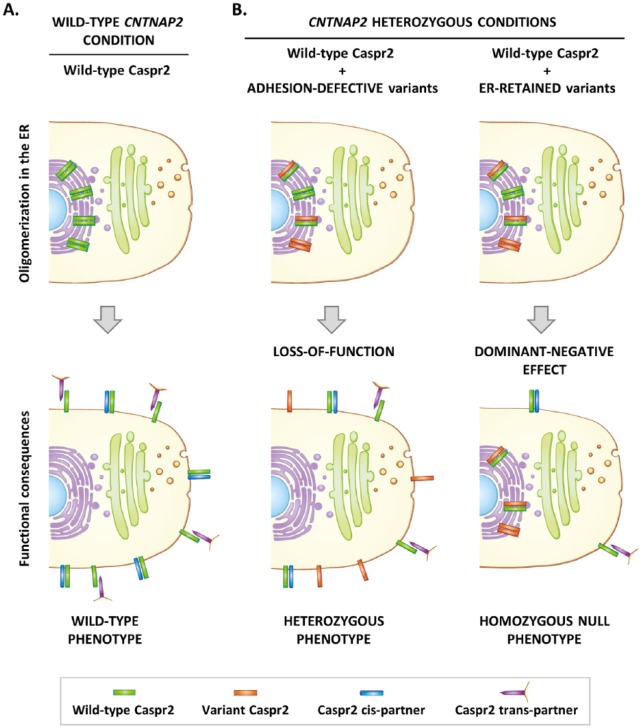
The extracellular domain of Caspr2, where all missense variants have been detected, is composed of 8 defined structural domains: a discoidin (F58C) domain, 4 laminin G (L1-L4) domains, 2 epidermal growth factor (EGF)-like domains, and a fibrinogen (FBR)-like domain (Figure 1B). Electron microscopy studies indicate that this ectodomain probably displays a very compact F-shaped monomeric architecture organized into 3 major lobes, which could harbor 2 main orientations, vertical or horizontal (Figure 1C).7 The 3 lobes are composed of the L4 domain, L3 and FBR domains, and L1, L2, and F58C domains, respectively, while the EGF-like domains may serve as molecular hinges permitting the lobes to flex with respect to each other. Such a complex architecture implies that Caspr2 has to pass multiple biosynthetic quality-control checkpoints during its maturation in the endoplasmic reticulum (ER) and the Golgi apparatus to make sure that only properly folded and glycosylated membrane molecules reach their sites of action in cells. Initial studies showed that some CNTNAP2 missense variants lead to Caspr2 misfolding and retention in the ER, but the impact of other variants on protein maturation was not clear.8 Showing that the transport of Caspr2 from the ER to the plasma membrane in transfected COS-7 cells can be monitored by a change in migration on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), we re-evaluated the impact of 8 ASD missense variants identified by Bakkaloglu et al2 (Figures 1B and 3A). We confirmed that some variants indeed induce strong protein retention in the ER (R1119H, D1129H). Because oligomerization in the ER is one of the mechanisms known to ensure protein maturation controls, we then asked whether Caspr2 variant proteins could interact with wild-type (WT) Caspr2, impair its trafficking, subcellular localization, and/or function at the plasma membrane. Using multiple biochemical approaches, we showed that Caspr2 is able to self-associate through its extracellular domain during its intracellular processing but not at the plasma membrane (Figure 2A), an observation consistent with the monomeric status of the extracellular domain observed by electron microscopy, indicating that Caspr2 probably interacts with its extracellular partners in its monomeric form. Co-expressing variant proteins with WT Caspr2 to mimic CNTNAP2 heterozygosity in humans, we then found that all variant proteins are able to interact with WT Caspr2 and that the interaction with the variants strongly retained in the ER leads to WT Caspr2 retention in the ER and consequently to a decrease in functional protein at the plasma membrane (Figure 2B). Testing one of these ER-retained variants (R1119H) in heterozygous Cntnap2 cortical neurons in culture, we found that it has a dominant-negative effect on axon growth, arguing that phenotypes mimicking homozygous CNTNAP2 null mutation may exist in humans (Figure 2B). We next take advantage that Caspr2 is able to interact with Contactin2/TAG-1 in vitro to evaluate whether variant proteins could present adhesion defects. We identified 2 variants (I869T and G731S) which likely present structural changes preventing their interactions with Contactin2/TAG-1 and showed that they do not rescue the heterozygous axon growth phenotype in cortical neurons, indicating a loss of function and arguing that phenotypes related to CNTNAP2 heterozygosity may also exist in humans (Figure 2B). Thus, our observations provided a proof of principle that some variants have functional consequences in a genetic background relevant for CNTNAP2 heterozygosity in ASD. Of interest, experiments in COS-7 cells further showed that variant proteins studied display a gradient of intracellular retention rather than being either strictly retained in the ER or strictly addressed at the plasma membrane (Figure 3A). This indicates that Caspr2 is highly sensitive to missense mutations, likely because of the complex architecture of its extracellular domain. It also strongly suggests that the phenotypic consequences of a large number of variants could result from the combination of a dominant-negative effect on WT Caspr2 function through retention in the ER and a loss of function at the plasma membrane because of structural changes. Therefore, one can speculate that ASD variants could induce a continuum of phenotypes depending on the total level of functional protein at the plasma membrane, rather than discrete phenotypes strictly mimicking CNTNAP2 heterozygous or homozygous null mutation (Figure 3B). Remarkably, some additional mechanisms could lead to Caspr2 function impairments and increase the impact spectrum of the variants since we also identified a variant (N407S) which is well localized at the membrane and properly binds to Contactin2/TAG-1 but plays a dominant-negative effect on axon growth.
Figure 3.
CNTNAP2 heterozygous missense variants could induce a continuum of phenotypes when Caspr2 functions are dose-dependent. (A) Variant proteins display a gradient of intracellular retention in transfected COS-7 cells. Percentage of intracellular form for each variant protein normalized to its total expression, evaluated by immunoblot. Data are means ± SEM of 3 independent experiments (see original manuscript). (B) The phenotypic consequences of each variant could result from the combination of a dominant-negative effect on wild-type Caspr2 function through retention in the endoplasmic reticulum (ER) and a loss of function at the plasma membrane (adhesion defects) because of structural changes, leading to variable level of functional protein at the plasma membrane, and as a whole to a continuum of phenotypes.
Figure 2.
Hypothetic model showing how CNTNAP2 heterozygous missense variants could impact Caspr2 functions when dose-dependent. (A) In wild-type condition, Caspr2 self-associates during its intracellular processing but is expressed in a monomeric form at the plasma membrane where it interacts with its cis and trans extracellular partners. (B) CNTNAP2 heterozygous conditions. After oligomerization during intracellular processing, adhesion-defective variant proteins are correctly addressed to the plasma membrane but do not interact with their extracellular partners (loss-of-function), a situation leading to phenotypes mimicking CNTNAP2 heterozygosity. On the other hand, misfolded variant proteins retain wild-type Caspr2 in the endoplasmic reticulum (ER), leading to a decrease in functional protein at the plasma membrane (dominant-negative effect) and phenotypes mimicking homozygous CNTNAP2 null mutation.
Our observations predict that CNTNAP2 variants may lead to a high heterogeneity of phenotypes at the scale of the whole brain. Recent data provide evidence that Caspr2 may have a broad range of functions during embryonic and early post-natal neurodevelopment and contribute to normal neuronal network assembly and activity, being notably required for the normal development of cortical neuron dendritic arborization and spines, synaptic strength, AMPA receptors trafficking, and myelination.9–11 The ability of adhesion molecules to recruit partners is influenced by how these molecules are positioned in the extracellular space between the cells at the contact sites, notably how their overall dimensions, domains, and molecular hinges fit in the cleft. Electron microscopy studies suggest that the extracellular domain of Caspr2 harboring a horizontal orientation (~90 Å) could fit in the narrow cleft of the juxtaparanodes (~74-150 Å) and the inhibitory synapses (~100-120 Å), while the extracellular domain harboring a vertical orientation (~145 Å) could be accommodated at the excitatory synaptic contacts (~160-240 Å) (Figure 1C).7 As lobes can flex with respect to each other, they may also change their conformation upon protein partner binding so that the molecule could fit in alternative way in the clefts. Thus, it is likely that each variant which does not strongly impair protein trafficking could have specific impacts on the structure of Caspr2 at the plasma membrane and on its ability to interact with its partners depending on the sites of cell contacts. In addition, it has been shown that Cntnap2+/– fast-spiking PV+ cortical interneurons also exhibit an intermediate electrophysiological phenotype between WT and Cntnap2–/– interneurons, while the maturation of PV+ GABAergic cortical interneurons is only altered in Cntnap2–/– mice,10 indicating that several functions of Caspr2 are probably dose-sensitive but not all. In the latter case, adhesion-defective variants are not expected to have a functional impact in a CNTNAP2 heterozygous background. Thus, Caspr2 functions may be differentially affected by each individual variant, depending both on their sensitivity to Caspr2 level and the direct impact of the variants on the trafficking, structure, and/or interactions of the protein.
Considering these complex structure-function relationships, one can expect that the consequences of CNTNAP2 variants are hardly predictable by bioinformatics analyses and that more variants than originally predicted are functionally deleterious. Accordingly, the variants N407S and I869T were predicted to be “tolerated” and “benign” (SIFT software)3 and therefore not expected to induce major functional impairments such as we observed. There is thus no rational reason to think that the variants found in control subjects, which are also distributed over the entire extracellular domain of Caspr2, could have strictly different or no biological consequences when compared with variants identified in patients with ASD. It does not however exclude the possibility that CNTNAP2 missense variants could contribute to ASD, but rather highlights the interesting hypothesis that CNTNAP2 variants could define an overall endophenotype shaping a risk for ASD, the development of ASD-related phenotypes being dependent on the interaction with additional genetic and/or environmental risk factors. This hypothesis is supported by the fact that the CNTNAP2 variants identified in patients with ASD are inherited from a non-affected parent (Bakkaloglu et al2 and our unpublished observations in French ASD families).
Our observations further question whether, beyond ASD, CNTNAP2 missense variants could confer susceptibility and/or contribute to the development of additional pathologies including neurodevelopmental disorders but also genetically less complex disorders. Epilepsy is frequently associated with the core manifestations of ASD. CNTNAP2 genomic alterations have been identified in patients with epilepsy and Cntnap2 knock-out mice display epileptic seizures.1,4 It is therefore possible that CNTNAP2 variants contribute to epileptic symptoms independently of ASD. The implication of Caspr2 in axon outgrowth, myelination, and Ranvier node organization also questions whether CNTNAP2 variants could contribute to disorders such as peripheral neuropathies or axon recovery and remyelination impairments after injury or demyelination, notably in patients with multiple sclerosis who show a large interindividual heterogeneity of myelin repair capacity. Besides, one can question whether CNTNAP2 variants could be implicated in the development and/or malignancy progression of neuroblastoma and glioma, where Caspr2 has been proposed to function as a tumor suppressor protein.12,13 CNTNAP2 methylation and heterozygous genomic rearrangements, but also a heterozygous missense mutation (A25T), have been detected in glioma, where Caspr2 levels are strongly decreased as compared to controls. Finally, using chromosome substitution strain-based mapping strategy in mouse, Buchner et al14 identified a Cntnap2 missense variant (H538Q) which regulates diet-induced obesity, the effects being either obesity-promoting or obesity-resistant, depending on the genetic background. This raises the intriguing possibility that CNTNAP2 variants constitute susceptibility factors for obesity,14 which interestingly presents a positive association with ASD.15
In conclusion, our study provides evidence that CNTNAP2 heterozygous missense variants could contribute to the pathophysiology of ASD and possibly to the development of other pathologies. It also indicates that, in non-pathological conditions, the variants could act as modulators of multiple functions of Caspr2 by modifying the total level of functional protein at the plasma membrane of the cells, maybe contributing to interindividual phenotypic diversity. Further research in vivo is now required to strengthen these hypotheses.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LG designed and wrote the manuscript; GC contributed to manuscript design and figure drawing.
ORCID iD: Laurence Goutebroze  https://orcid.org/0000-0002-8712-000X
https://orcid.org/0000-0002-8712-000X
References
- 1. Poot M. Connecting the CNTNAP2 networks with neurodevelopmental disorders. Mol Syndromol. 2015;6:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakkaloglu B, O’Roak BJ, Louvi A, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murdoch JD, Gupta AR, Sanders SJ, et al. No evidence for association of autism with rare heterozygous point mutations in contactin-associated protein-like 2 (CNTNAP2), or in other contactin-associated proteins or contactins. PLoS Genet. 2015;11:e1004852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penagarikano O, Abrahams BS, Herman EI, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poliak S, Salomon D, Elhanany H, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scott R, Sanchez-Aguilera A, van Elst K, et al. Loss of Cntnap2 causes axonal excitability deficits, developmental delay in cortical myelination, and abnormal stereotyped motor behavior [published online ahead of print December 28, 2017]. Cereb Cortex. doi: 10.1093/cercor/bhx341. [DOI] [PubMed] [Google Scholar]
- 7. Lu Z, Reddy MV, Liu J, et al. Molecular architecture of contactin-associated protein-like 2 (CNTNAP2) and its interaction with contactin 2 (CNTN2). J Biol Chem. 2016;291:24133–24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falivelli G, De Jaco A, Favaloro FL, et al. Inherited genetic variants in autism-related CNTNAP2 show perturbed trafficking and ATF6 activation. Hum Mol Genet. 2012;21:4761–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varea O, Martin-de-Saavedra MD, Kopeikina KJ, et al. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci U S A. 2015;112:6176–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogt D, Cho KKA, Shelton SM, et al. Mouse Cntnap2 and human CNTNAP2 ASD alleles cell autonomously regulate PV+ cortical interneurons. Cereb Cortex. 2017;28:3868–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Sudhof TC. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci U S A. 2012;109:18120–18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thorell K, Bergman A, Caren H, et al. Verification of genes differentially expressed in neuroblastoma tumours: a study of potential tumour suppressor genes. BMC Med Genomics. 2009;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bralten LB, Gravendeel AM, Kloosterhof NK, et al. The CASPR2 cell adhesion molecule functions as a tumor suppressor gene in glioma. Oncogene. 2010;29:6138–6148. [DOI] [PubMed] [Google Scholar]
- 14. Buchner DA, Geisinger JM, Glazebrook PA, et al. The juxtaparanodal proteins CNTNAP2 and TAG1 regulate diet-induced obesity. Mamm Genome. 2012;23:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Healy S, Aigner CJ, Haegele JA. Prevalence of overweight and obesity among US youth with autism spectrum disorder [published online ahead of print August 13, 2018]. Autism. doi: 10.1177/1362361318791817. [DOI] [PubMed] [Google Scholar]