Abstract
In this review, we will discuss the safety of repeated treatments with ketamine for patients with treatment-resistant depression (TRD), a condition in which patients with major depression do not show any clinical improvements following treatments with at least two antidepressant drugs. We will discuss the effects of these treatments in both sexes at different developmental periods. Numerous small clinical studies have shown that a single, low-dose ketamine infusion can rapidly alleviate depressive symptoms and thoughts of suicidality in patients with TRD, and these effects can last for about one week. Interestingly, the antidepressant effects of ketamine can be prolonged with intermittent, repeated infusion regimens and produce more robust therapeutic effects when compared to a single infusion. The safety of such repeated treatments with ketamine has not been thoroughly investigated. Although more studies are needed, some clinical and preclinical reports indicated that repeated infusions of low doses of ketamine may have addictive properties, and suggested that adolescent and adult female subjects may be more sensitive to ketamine's addictive effects. Additionally, during ketamine infusions, many TRD patients report hallucinations and feelings of dissociation and depersonalization, and therefore the effects of repeated treatments of ketamine on cognition must be further examined. Some clinical reports indicated that, compared to women, men are more sensitive to the psychomimetic effects of ketamine. Preclinical studies extended these findings to both adolescent and adult male rodents and showed that male rodents at both developmental periods are more sensitive to ketamine's cognitive-altering effects. Accordingly, in this review we shall focus our discussion on the potential addictive and cognitive-impairing effects of repeated ketamine infusions in both sexes at two important developmental periods: adolescence and adulthood. Although more work about the safety of ketamine is warranted, we hope this review will bring some answers about the safety of treating TRD with repeated ketamine infusions.
1. Introduction
Treatment-resistant depression (TRD) occurs in a subset of individuals suffering from major depressive disorder. TRD is diagnosed following an insufficient treatment response to at least two classes of typical antidepressants (Berlim and Turecki, 2007). Since over half of TRD patients report thoughts of suicidality (Papakostas et al., 2003), there is a crucial need for more effective and faster treatment options. Recently, the N-methyl D-aspartate receptor (NMDAR) antagonist, ketamine, has shown considerable promise as a fast-acting antidepressant in patients with TRD, alleviating depressive symptoms and reversing suicidal ideation within hours following a single, low-dose (0.5 mg/kg) intravenous (i.v.) infusion, and having effects lasting up to one week, on average (Ballard et al., 2014; Berman et al., 2000; Phelps et al., 2009; Zarate et al., 2006). Meta-analyses examining the acute effects of 0.5 mg/kg ketamine (i.v.) also report a significant reduction in suicidality 4 h post-infusion with effects maintained for up to one week (Bartoli et al., 2017; Wilkinson et al., 2018). However, following acute ketamine treatment, most patients remitted (DiazGranados et al., 2010; Zarate et al., 2006), suggesting a need for repeated infusions to maintain long-term therapeutic efficacy. Thus, recent studies have shown that TRD patients treated repeatedly with ketamine have superior therapeutic outcome when compared to those treated with single infusions. For example, ketamine administration every 2–3 days over a two-week period, maintains the antidepressant response, increases the percentage of patients responding to ketamine, and lengthens the duration of antidepressant response after cessation of treatment (aan het Rot et al., 2010; Murrough et al., 2013; Shiroma et al., 2014a).
Following the excitement and hype surrounding ketamine's fast-acting antidepressant effects, numerous private clinics started offering ketamine, sometimes in a repeated manner, as an off-label treatment for TRD. The safety of such repeated ketamine infusions has not been fully investigated in large clinical and well-powered studies. It is important to keep in mind that ketamine is a schedule III drug, and has great potential for abuse and dependence in humans (DEA, 2018; Chang et al., 2016; Narendran et al., 2005; Schak et al., 2016). Also, ketamine produces psychomimetic effects such as hallucinations and delusions that can induce long-term cognitive deficits (D'Souza et al., 2012; Stefanovic et al., 2009). However, ketamine is recreationally abused at doses higher than what is used in the clinic, and it remains unclear whether the effects observed at high doses would manifest at a clinically relevant dose. For these reasons, administration of low-dose ketamine repeatedly over a defined or undefined period to treat depression may pose a risk for both addiction and cognitive impairments, and thus it is important for more studies to delve into the safety of such treatments. Importantly, little is known regarding sex and age differences in sensitivity to ketamine's effects. Some animal studies indicated an increased sensitivity in female rodents to ketamine's antidepressant effects (Carrier and Kabbaj, 2013; Franceschelli et al., 2015; Saland et al., 2017; Zanos et al., 2016), and in humans, ketamine abuse is most common among adolescents and young adults (WHO, 2012). As such, the purpose of this review is to discuss, in both rodents and humans, sex differences in the addictive and cognitive effects of ketamine at two developmental periods: adolescence and adulthood. We hope through this review to gain some understanding of the safety of repeated, low-dose ketamine infusions for the treatment of TRD.
2. The addictive potential of repeated low-dose ketamine infusions
2.1. Clinical evidence
Double-blind, placebo controlled clinical studies have reported a greater number of TRD patients responding to low-dose ketamine infusions (0.5 mg/kg i.v. over 40 min) as well as sustained low-depressive scores in repeated infusion as compared to single infusion paradigms (Murrough et al., 2013; Shiroma et al., 2014a). Indeed, ketamine infusions thrice weekly for two weeks decreased scores in both the Montgomery-Asberg Depression Rating Scale (MADRS) (Murrough et al., 2013), and the Visual Analog Scale (VAS) (Shiroma et al., 2014a). Furthermore, the duration of antidepressant response after treatment cessation was longer, and the rate of remission higher, in patients receiving repeated infusions (Shiroma et al., 2014a) versus those receiving a single infusion (Zarate et al., 2006). These data demonstrate superior antidepressant efficacy of repeated ketamine infusions when compared to single infusions.
The enhancement of antidepressant efficacy of low-dose ketamine treatment under repeated infusion paradigms could be indicative of behavioral sensitization to ketamine's antidepressant effects. Sensitization occurs when the response induced by a drug increases over time and is considered to reflect mesocorticolimbic reorganization which occurs in people with addiction (Scofield et al., 2016; Wise and Koob, 2014). However, a meta-analysis study comparing depression scores from 11 separate studies in which ketamine was repeatedly infused found no evidence of behavioral sensitization (Cho et al., 2005). While this analysis seems to suggest that repeated low dose ketamine infusions are safe, it is important to note that the studies described above were conducted in non-depressed subjects in which the plasticity of the reward circuitry has been shown to be very different from depressed subjects (Admon and Pizzagalli, 2015; Felger et al., 2016; Quevedo et al., 2017). Furthermore, other baseline alterations in TRD patients as compared to healthy subjects, such as elevated levels of inflammatory cytokines and adipokines, have been reported and indicate different metabolism profiles which may impact sensitivity to a drug over time (Kiraly et al., 2017; Machado-Vieira et al., 2017). A large, well-controlled study examining both behavioral and neurophysiological differences in TRD patients receiving repeated infusions compared to a single ketamine infusion across doses, other than the standard clinically-prescribed dose of 0.5 mg/kg, i.v., is necessary to determine whether or not sensitization to the antidepressant effects of ketamine truly occur.
Neuroimaging studies have provided useful insights into the neurocircuitry mediating ketamine's antidepressant effects. Reviewed in Lener et al. (2017), the anterior cingulate cortex, in communication with the medial prefrontal cortex (mPFC) and hippocampus, appear to be keycircuitry mediating the acute antidepressant effects of ketamine in healthy and depressed subjects. However, ketamine-induced brain activation is more widespread, and includes regions of the reward-circuitry, which is involved in the pathogenesis of depression as well as addiction. Pharmacological magnetic resonance imaging (phMRI) acquired during a single infusion of low-dose ketamine in healthy male subjects revealed activation of the caudate and putamen regions as well as the mPFC and dorsolateral PFC (dlPFC), all of which play important roles in mediating the rewarding and/or reinforcing effects of drugs such as cocaine, morphine, and alcohol (Doyle et al., 2013; Scofield et al., 2016). High doses of ketamine have also been shown to alter mPFC and dlPFC function since chronic ketamine abusers showed increased dopamine 1 receptor binding due potentially to a reduction in dopamine signaling in both regions (Narendran et al., 2005).
It is possible that reward-circuitry activation is partly responsible for mediating the antidepressant actions of ketamine. Indeed, ventral striatum activation during positron emission tomography (PET) imaging was increased following two ketamine infusions (0.5 mg/kg, i.v.) in depressed subjects and correlated with the score of antidepressant response (Nugent et al., 2014). Along the same line, using magnetoencephalography (MEG), subjects’ ratings of a “blissful state” during a low-dose ketamine infusion (0.25 mg/kg bolus, 0.375 mg/kg/1 h, i.v.) correlated positively with increased frontoparietal activation (Muthukumaraswamy et al., 2015). Given that human cocaine addicts also show frontoparietal activation when exposed to drug-related stimuli (Costumero et al., 2017), it is possible the same neurocircuitry mediating the antidepressant effects of ketamine could also contribute to its abuse potential. However, it should be noted that the neuroimaging studies, to date, have only examined the acute effects of ketamine; thus, neuroimaging studies combined with chronic ketamine treatment are necessary to elucidate the mechanism mediating the long-term antidepressant effects of ketamine as well as its abuse potential.
In recent years, few case reports have documented the development of a full-blown ketamine addiction following clinical treatment of depression with repeated low-dose ketamine (Bonnet, 2015; Schak et al., 2016). While definitive conclusions about these few cases cannot be made, they highlight the need for more thorough research in humans to address the addictive potential of repeated treatments with ketamine. Statistics on ketamine abuse in the United States indicate that the majority of ketamine abusers are young adults (WHO, 2012), while ketamine abusers in East and Southeast Asia comprise in majority of adolescents and young adults as well (UNODC, 2013). Given that depression and addiction are the two most prevalent mental disorders observed among adolescents and because of the high comorbidity rate between them (Andrews et al., 2002; Kaminer et al., 2007), it is crucial to explore the safety of repeated ketamine infusions at this critical developmental period.
Additionally, depression is twice as prevalent in women as compared to men (Whiteford et al., 2013), is highly comorbid with addiction (Ng et al., 2017), and women who recreationally abuse ketamine report more severe symptoms during withdrawal from ketamine, concomitant substance use, and more cognitive impairments when compared to men (Chen et al., 2014). As we will discuss later, animal studies have also reported a heightened sensitivity to antidepressant and addictive effects of ketamine in females compared to males (Carrier and Kabbaj, 2013; Franceschelli et al., 2015; Schoepfer et al., 2017; Strong et al., 2017; Zanos et al., 2016). The meta-analysis mentioned above analyzed gender and age as factors across the 11 studies and found no effect of either (Cho et al., 2005), and a recent report found no evidence of gender nor age being significant predictors of ketamine's antidepressant efficacy (Niciu et al., 2014). Rather, Niciu et al., reported that previous suicide attempts, body mass index, and a family history of alcoholism to be significant predictors of antidepressant response. It should be noted, however, that most clinical ketamine studies examined the antidepressant effects of ketamine at one dose (0.5 mg/kg i.v.), and therefore a dose-response study is necessary to further elucidate whether there are sex differences in ketamine's antidepressant effects in humans. In fact, clinical trials are underway to examine the efficacy of different doses of ketamine in adult men and women suffering from TRD (doses ranging from 0.1 mg/kg to 0.5 mg/kg i.v.) (ClinicalTrials.gov, Identifier: NCT01558063). Another clinical trial is also underway to examine the effects of low-dose ketamine in adolescents with TRD (ClinicalTrials.gov, Identifier: NCT02078817). These clinical studies could clarify whether an interaction between sex and any of the three aforementioned predictors of antidepressant response exists.
2.2. Preclinical evidence
In line with clinical studies, a single intraperitoneal (i.p.) injection of low-dose ketamine (5–10 mg/kg) elicits rapid antidepressant-like effects in adult male rodents, demonstrated by areduction in forced swim test (FST) immobility time, an antidepressant effect that is sustained for 3–7 days (Li et al., 2010; Autry et al., 2011). Furthermore, our lab was the first to show that adult female rodents are more sensitive to the acute antidepressant effects of ketamine, responding to a dose (2.5 mg/kg, i.p.) that was sub-threshold in male rats (Carrier and Kabbaj, 2013). These findings have since been replicated in rats and mice Franceschelli et al., 2015; Zanos et al., 2016) and expanded upon, as recent evidence suggests this increased sensitivity is mediated by the presence of the ovarian hormones estrogen and progesterone (Dossat et al., 2018; Saland et al., 2017) as well as sex differences in ketamine metabolism (Zanos et al., 2016).
When compared to adult males, juvenile male rats are less sensitive to ketamine's antidepressant effects. Indeed, they required a higher dose of a single ketamine injection to elicit an antidepressant-like response measured in the FST (20 mg/kg, i.p.) (Parise et al., 2013). Under chronic treatment (20 mg/kg, i.p. bi-daily, 15 days) however, both juvenile and adult male rats showed an anti-depressant response measured in the FST, and anxiolytic response measured in the elevated plus maze (EPM) (Parise et al., 2013). It remains unclear, however, whether juvenile female rats also have a heightened sensitivity to ketamine's antidepressant effects.
In C57BL/6J adult mice however, repeated ketamine treatment (10 mg/kg; 21days) induced an antidepressant-like effect in male mice, but induced anxiety and depressive-like behaviors in female mice (Thelen et al., 2016), suggesting that the effects of repeated effects of ketamine on depression like-behaviors might be species and sex specific.These findings also indicate a change in response to low-dose ketamine following repeated administration in both sexes. No studies so far have examined the effects of such treatments in juvenile male and female mice.
It should be noted that the studies mentioned above assessed the effects of repeated ketamine only in stress-naïve rodents. The examination of ketamine's effects in Wistar Kyoto (WKY) rats, which are known to display putative depressive-like behaviors (Pare and Redei, 1993), revealed that 2-weeks of daily ketamine (0.5–2.5 mg/kg, i.p.) injections to adult females resulted in a dose-dependent and prolonged antidepressant effect (Tizabi et al., 2012), suggesting that the effects of repeated ketamine administration produce differential antidepressant effects depending on genetic predisposition and vulnerability to stress. Recently, studies have reported the use of ketamine, albeit at higher doses (30 mg/kg, i.p.), as prophylactic to prevent negative outcomes of stressful situations. This was demonstrated in male mice where prophylactic ketamine prior to stress reduced freezing in a fear conditioning paradigm (McGowan et al., 2017). It will be thus interesting to determine whether such effects seen in male adult mice would be replicated in adult female and juvenile male and female mice.
Repeated administration of ketamine, which is necessary to sustain an antidepressant response in humans, may have an abuse potential. Ketamine, like most drugs of abuse, can induce sensitization to its locomotor activating effects. In both sexes of rats, daily administration of ketamine induces sensitization to its locomotor-activating effects (Table 1) (Botanas et al., 2015; Wiley et al., 2011). Intermittent, every other day administration of ketamine induced locomotor sensitization in both sexes, but females were more sensitive as locomotor sensitization occurred at lower doses (2.5 mg/kg, i.p.) when compared to males (5 mg/kg, i.p.) (Schoepfer et al., 2017). Interestingly, behavioral sensitization in both sexes was positively correlated with ΔfosB –a marker of long-term plasticity-expression in the NAc (Schoepfer et al., 2017).
Table 1.
Summary of the addictive properties of low-dose ketamine in rodents and molecular correlates. Female adolescent and adult rodents are more sensitive to the locomotor-activating effects of ketamine. The reinforcing properties of ketamine in females are mediated by the presence of ovarian hormones.
| Sex | Age | Dose | R.O.A. | Treatment Regimen | Behavioral Response | Molecular Change | References |
|---|---|---|---|---|---|---|---|
| Male | Adult | 100 μg per inf | i.v. | 1x/4 days | ↑ FR1 self-administration | n/a | (Wright et al., 2017) |
| ↑ cue-induced reinstatement | |||||||
| 2.5 mg/kg | i.p. | 1x/2 days | no change | ↑NAc ΔfosB | (Schoepfer et al., 2017) | ||
| Weekly | no change | ↑ NAc shell spines | (Strong et al., 2017) | ||||
| 5 mg/kg | i.p. | Daily | ↑ locomotor sensitization | n/a | (Botanas et al., 2015) | ||
| ↑ CPP | |||||||
| 1x/2 days | ↑ locomotor sensitization | ↑NAc ΔfosB | (Schoepfer et al., 2017) | ||||
| Weekly | ↑ locomotor sensitization | ↑ NAc ΔfosB | (Strong et al., 2017) | ||||
| ↑ NAc GluA1 | |||||||
| ↑ NAc BDNF | |||||||
| ↑ NAc CaMKIIα | |||||||
| ↑ NAc shell spines | |||||||
| 10 mg/kg | i.p. | Daily | ↑ locomotor sensitization | n/a | (Wiley et al., 2008) | ||
| ↑ CPP | n/a | (Li et al., 2008) | |||||
| 1x/2 days | ↑ locomotor sensitization | ↑ NAc ΔfosB | (Schoepfer et al., 2017) | ||||
| ↑ CPP | |||||||
| Female | Adult | 100 μg per inf | i.v. | 1x/4 days | ↑ FR1 self-administration (proestrous) | n/a | (Wright et al., 2017) |
| ↑cue-induced reinstatement (proestrous) | |||||||
| ↓FR1 self-administration (diestrous) | |||||||
| ↓ cue-induced reinstatement (diestrous) | |||||||
| 2.5 mg/kg | i.p. | 1x/2 days | no change | ↑NAc ΔfosB | (Schoepfer et al., 2017) | ||
| Weekly | ↑ locomotor sensitization (diestrous) | ↑ NAc shell spines | (Strong et al., 2017) | ||||
| 5 mg/kg | i.p. | 1x/2 days | ↑ locomotor sensitization | ↑NAc ΔfosB | (Schoepfer et al., 2017) | ||
| ↓ CPP | |||||||
| Weekly | ↑ locomotor sensitization | ↑ NAc GluA1 | (Strong et al., 2017) | ||||
| ↓ CPP (diestrous) | ↑ NAc core spines | ||||||
| ↑ NAc shell spines | |||||||
| 10 mg/kg | i.p. | Daily | ↑ locomotor sensitization | n/a | (Wiley et al., 2011) | ||
| 1x/2 days | ↑ locomotor sensitization | ↑NAc ΔfosB | (Schoepfer et al., 2017) | ||||
| Male | Adolescent | 10 mg/kg | i.p. | Daily | no change | n/a | (Wiley et al., 2007) |
| 20 mg/kg | i.p. | Daily | ↑ locomotor sensitization no change in CPP | n/a | (Rocha et al., 2017) | ||
| n/a | (Parise et al., 2013) | ||||||
| Female | Adolescent | 10 mg/kg | i.p. | Daily | ↑ locomotor sensitization | n/a | (Wiley et al., 2011) |
(inf = infusion; i.p. = intraperitoneal; CPP = conditioned place preference; NAc = nucleus accumbens; GluA1 = AMPA receptor 1; CaMKIIα = calcium calmodulin kinase II alpha).
When compared to male rats, the increased sensitivity of female rats to the locomotor-activating effects of low-dose ketamine (2.5 mg/kg, i.p.) was also maintained when ketamine was administered at longer intervals (i.e. once weekly). Increased NAc shell dendritic spine density and NAc protein expression of GluA1 was associated with the heightened locomotor response in both sexes, while other targets such as ΔfosB, CaMKIIα, and BDNF were increased only in sensitized males but not females (Strong et al., 2017). It is therefore clear that both male and female rats develop sensitization to the locomotor activating effects of ketamine, but with differing sensitivities.
Furthermore, the molecular mechanisms associated with behavioral plasticity are sex-dependent. These studies highlight the importance of potential sensitizing effects of repeated, low-dose ketamine administration as well as the sex-specific mechanisms that may mediate ketamine sensitization.
Juvenile male rats injected with an effective antidepressant dose of ketamine (20 mg/kg, i.p.) were more sensitive to its acute locomotor-activating effects as compared to adults (Parise et al., 2013; Rocha et al., 2017). However, sensitization to the locomotor activating effects of ketamine occurred in both adolescent and adult male rats (Rocha et al., 2017). At lower doses of ketamine (3–10 mg/kg, i.p.), which is an ineffective antidepressant dose in male juvenile rats, adult, but not juvenile male rats, developed ketamine-induced sensitization to its locomotor activating effects (Wiley et al., 2008). Interestingly, at these lower doses (3–10 mg/kg, i.p.), female juvenile and adult rats developed sensitization to ketamine locomotor activating effects (Wiley et al., 2011). Together, these data indicate that, compared to juvenile male rodents, juvenile female rodents are likely more sensitive to the locomotor-activating effects of ketamine.
This differential sensitivity to ketamine in both sexes at the juvenile period could be explained by levels of circulating gonadal hormones. Indeed, estrogen and progesterone are detected in the bloodstream around PND 28, albeit at lower concentrations when compared to adults (Vetter- O’Hagen and Spear, 2012), and given the potential role ovarian hormones play in enhancing ketamine's reinforcing properties in adult rodents (Wright et al., 2017), the same could be true for juvenile rodents. In support of this idea is the fact that the locomotor-activating effects of low-dose ketamine in preadolescent animals (<PND28) was similar between males and females (McDougall et al., 2017). It is also possible that ketamine metabolism is different between juveniles and adults as well as males and females since there are sex-divergent changes in various metabolic enzymes across the rodent lifespan. In fact, the P450 family of enzymes, which make up the first phase of drug metabolism for many drugs including ketamine (Santamaria et al., 2014; Zheng et al., 2017), decrease across the lifespan in both sexes of mouse (Fu et al., 2012).
Conditioned place preference (CPP) and conditioned place aversion (CPA) are models of Pavlovian conditioning that pairs a conditioned stimulus (a previously neutral stimulus, i.e. novel environment) with an unconditioned stimulus (i.e. drug injection) to ascertain positive (CPP) or negative (CPA) associations between the two stimuli (Tzschentke, 2007; Scofield et al., 2016). It is well established that low doses of ketamine (5–10 mg/kg, i.p.) induce CPP in male rodents (Table 1) (Botanas et al., 2015; Li et al., 2008; Schoepfer et al., 2017; Suzuki et al., 2000). However, low-dose ketamine (5 mg/kg, i.p.) induced CPA in female rats, suggesting that the “rewarding” effects of ketamine at low doses may be sex-specific (Schoepfer et al., 2017). Furthermore, weekly administration of ketamine during the conditioning phase did not induce CPP or CPA in males and, as shown earlier with shorter conditioning sessions, produced a CPA in female rats (Strong et al., 2017). These studies suggest that, in male subjects, the “rewarding” properties of ketamine at low doses may be more prevalent when the conditioning sessions are shorter time intervals apart (daily versus weekly), and that female rodents may be more sensitive to the aversive effects of low-dose ketamine. It should be noted, though, that drugs inducing aversion can still induce locomotor sensitization and have reinforcing properties, as is the case with alcohol (Rustay et al., 2001; Camarini and Pautassi, 2016). In fact, ketamine at low doses induces CPA in female rats, but the same doses cause sensitization to their locomotor activating effects and as we will see later have reinforcing effects.
The rewarding properties of low-dose ketamine in juvenile rodents have not been thoroughly investigated. In the one study that has examined this topic, juvenile male rats chronically administered low doses of ketamine (5, 10, and 20 mg/kg, i.p.) failed to form a place preference at any dose tested, indicating that ketamine may not be rewarding in this age group (Parise et al., 2013). More work is therefore necessary, in both sexes, to fully elucidate the rewarding properties of repeated exposure to low-dose ketamine in younger animals. The reinforcing properties of ketamine at high doses is evident as demonstrated by adult male rodents’ propensity to self-administer ketamine (0.5 mg/kg/infusion, i.v.) under various schedules of reinforcement (Caffino et al., 2016, 2017; DeLuca and Badiani, 2011; van der Kam et al., 2007; Venniro et al., 2015). Escalation of ketamine self-administration is observed as the schedule of reinforcement is increased from fixed ratio 1 (FR1) to FR5 and occurs in a dose-dependent manner, as demonstrated by increased number of ketamine infusions at the highest dose (0.5 mg/kg, i.v.) compared to the lowest dose tested (0.125 mg/kg, i.v.) (DeLuca and Badiani, 2011). While DeLuca and Badiani tested low-dose ketamine self-administration in male rats, there is only one study to date to investigate this in both sexes, which assessed intermittent, low-dose ketamine self-administration under an FR1 schedule of reinforcement (Wright et al., 2017) (Table 1). Male rats and female rats in proestrous acquired ketamine self-administration, while females in diestrous did not, implicating a potential role for ovarian hormones in mediating the reinforcing effects of low-dose ketamine. Furthermore, males and females in proestrous, but not diestrous, reinstated to cues, but not to ketamine, indicating that ketamine-related cues play a crucial role in mediating ketamine craving and relapse behaviors in both sexes (Wright et al., 2017). Based on the data presented above, it is clear that ketamine, at low doses, even if administered intermittently, can elicit abuse potential in both sexes, though female rodents are more sensitive to these effects. Additionally, the enhanced sensitivity of female rodents to the reinforcing properties of low-dose ketamine might be mediated by ovarian hormones. No studies to date have examined ketamine self-administration in juvenile rodents, making it difficult to assess the reinforcing and motivational properties of ketamine within this subpopulation. While locomotor sensitization suggests a similar abuse potential profile in juvenile rodents as compared to adults, where females are more sensitive to the locomotor-activating effects of ketamine as compared to males, more CPP and self-administration studies are necessary to further our understanding of the safety of repeated ketamine infusions among the adolescent population.
Studies examining ketamine self-administration at low doses have not yet examined the molecular mechanisms underlying ketamine's reinforcing effects. At high doses, ketamine self-administration in adult rats induces phosphorylation of calcium calmodulin kinase II alpha (CaMKIIα) in the nucleus accumbens (NAc), which leads to increased transcription and facilitation of AMPAR trafficking to the membrane (Kristensen et al., 2011) and increased phosphorylation of the GluN2B subunit of the NMDAR (Caffino et al., 2017), which has a higher sensitivity to calcium (Ca2+) than other NMDA subunits (Evans et al., 2012). These molecular changes induce a hyperexcitable state in the NAc, which is also observed following chronic exposure to other drugs of abuse such as alcohol, another NMDAR-antagonist (Ron and Barak, 2016). It is therefore important to examine whether these molecular changes will also occur at therapeutic low doses of ketamine, and it is particularly important to further delve into the molecular mechanisms mediating the reinforcing properties of chronic exposure to low-dose ketamine as a treatment for depression.
Examination into the consequences of repeated treatment with ketamine, an NMDAR-antagonist, during adolescence is still lacking but is extremely important given the large-scale reorganization and receptor pruning occurring in the brain at this time-sensitive period (Spear, 2000). For instance, expression of the obligatory GluN1 subunit of the NMDAR fluctuates in a brain-region specific manner such that PFC GluN1 expression peaks in early postnatal days (PNDs) and steadily declines throughout adulthood (Ontl et al., 2004) whereas hippocampal GluN1 expression rapidly increases from PND2-10 and remains stable into adulthood (Sans et al., 2000). Interestingly, female rodents show a more robust GluN1 enhancement in the PFC compared to males, though this has yet to be examined in the hippocampus (Ontl et al., 2004).
The GluN2 subunit also peaks in early PNDs throughout the brain. A “switch” in expression levels of GluN2A and GluN2B occurs such that GluN2B expression peaks prenatally and declines over time whereas GluN2A expression gradually increases over time (Sans et al., 2000; Laurie et al., 1997). Given the enhanced Ca2+-permeability of GluN2B (Monyer et al., 1994; Paul and Connor, 2010) and its role in mediating the addictive properties of ketamine (Caffino et al., 2017), repeated treatment with ketamine across different developmental periods could have differential effects given that its putative mechanism of action is through the blockade of NMDARs. As such, it is particularly important to examine the effects of repeated, low-dose ketamine can have on the developing brain.
3. Cognitive deficits induced by repeated ketamine infusions
3.1. Clinical evidence
Ketamine induces schizophrenia-like symptoms and leads to deficits in memory recall and executive functions (Liang et al., 2013, 2015; Newcomer et al., 1999; Tang et al., 2015). The severity of these deficits varies with the individual, the dose, and the chronicity of intake. Most available data examining the effects of ketamine on cognition was collected in ketamine users. While infrequent ketamine users (∼1x/month) show elevated levels of dissociative symptoms, frequent ketamine users (>4x/week) display feelings of dissociation along with long-lasting deficits in recognition memory and working memory (Morgan et al., 2010). In addition, frequent users continue to experience memory deficits three days after drug use as well as after prolonged abstinence periods (>30 days) (Curran and Monaghan, 2001; Liang et al., 2013). Furthermore, about a third of ketamine-dependent subjects suffered from substance-induced psychotic disorder (Liang et al., 2015). In TRD patients administered a single, low-dose infusion of ketamine, patients experience dissociative effects and transient deficits in memory recall that dissipate within two hours (Adler et al., 1998; Malhotra et al., 1996; Murrough et al., 2015). This has since been extended to studies examining the effects of repeated, low-dose ketamine infusions and suggests that a portion of patients experience transient dissociative effects that do not seem to be long-lasting (Cusin et al., 2017; Diamond et al., 2014; Shiroma et al., 2014b). Despite this, low-dose ketamine still leads to delusions and mild hallucinations and it remains unknown whether longer treatment timeframes will lead to cognitive deficits. For these reasons, it is tremendously important to further examine the cognitive side effects of receiving repeated, low-dose ketamine infusions for the treatment of depression.
Cognitive impairments in attention, memory, and executive function are usually associated with depressive disorders (reviewed in Roca et al., 2015). Interestingly, TRD patients with lower baseline levels of neurocognitive performance respond better to acute antidepressant effects of ketamine and do not exhibit cognitive impairments. Contrastingly, TRD patients with higher baseline neurocognitive performance were more likely to not benefit from ketamine's antidepressant effects and they experience cognitive deficits in working memory and processing speed (Murrough et al., 2015). A follow-up study reported that reduced baseline cognition predicted ketamine's antidepressant efficacy in patients administered six infusions thrice weekly (Shiroma et al., 2014b). Supporting the idea that ketamine-induced cognitive deficits occur in only a subset of TRD patients, it was recently reported that six low-dose ketamine infusions over two weeks caused amnesia and depersonalization in one third of patients while two thirds reported thoughts of derealization (Cusin et al., 2017). Accordingly, these studies suggest that baseline cognition could be used as a predictor of ketamine antidepressant efficacy and highlight the importance of individual differences in the cognitive-impairing effects of ketamine, further supporting the need for more studies examining the long-term consequences on cognition of repeated exposures to low doses ketamine to treat depression. Given that baseline neurocognitive performance predicts the antidepressant efficacy of ketamine, it is possible that ketamine is rescuing the homeostatic imbalance in cognition that some TRD patients experience. While the common ketamine dose administered to TRD patients (0.5 mg/kg, i.v.) may be necessary for these individuals, patients with higher baseline cognition may require a much lower dose of ketamine.
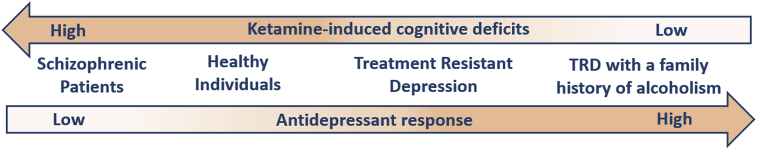
Interestingly, patients with a family history of alcoholism report no instances of dissociative symptoms when receiving low-dose ketamine (0.5 mg/kg, i.v.) infusions (Fig. 1) (Petrakis et al., 2004). In fact, subjects with a family history of alcoholism are less sensitive to the effects of the NMDAR-antagonist, memantine, likely because of increased NMDAR-function (Jamadar et al., 2012). Furthermore, subjects with a family history of alcoholism show baseline deficits in executive function and working memory, as demonstrated by their low performance scores on the Wisconsin Card Sorting Task (Gierski et al., 2013), and postmortem human studies showed increased expression of Grin2B mRNA in the PFC and hippocampus of alcohol-dependent subjects (Farris and Mayfield, 2014; Zhou et al., 2011), suggesting that the level of baseline cognitive performance may be mediated by NMDAR-function and could be altered by ketamine.
Fig. 1.
Schematic representing the inverse relationship between the level of ketamine-induced antidepressant response and cognitive deficits experienced during a ketamine infusion. When administered a single, low-dose ketamine infusion (0.5 mg/kg, i.v.), patients exhibiting fewer cognitive impairments experience an increased antidepressant response. (TRD = treatment resistant depression).
Contrastingly, patients with schizophrenia, which is associated with NMDAR-hypofunction (Seshadri et al., 2018), are more sensitive to ketamine's cognitive-impairing effects, compared to healthy human subjects (Lahti et al., 2001). Indeed, acute infusion of ketamine (0.5 mg/kg, i.v.) re-activated states of psychosis in many schizophrenic patients tested (Lahti et al., 1995).
Though the effects of ketamine at doses lower than 0.5 mg/kg, i.v. have not yet been examined in the context of cognition in TRD patients, healthy volunteers still experience dissociative effects when administered 0.3 mg/kg, i.v., but not 0.1 mg/kg, i.v. while schizophrenic patients experience these symptoms across all doses (Lahti et al., 1995, 2001).
These data suggest that baseline cognition may predict sensitivity to the cognitive-altering effects of ketamine. Future studies should consider screening for baseline cognitive performance prior to treating TRD patients with repeated ketamine infusions.
Gender and gonadal hormones seem to be important factors when examining ketamine's effects on cognition. A meta-analysis examining studies administering acute ketamine infusions with doses ranging from 0.5 to 1.3 mg/kg, i.v., reported that men are more sensitive to ketamine-induced deficits in memory and verbal recall (Morgan et al., 2006). Specifically, men administered a single ketamine infusion displayed enhanced verbal and subjective memory impairments, as measured by the Hopkins Verbal Learning Task and Clinician-Administered Dissociative States Scale (CADSS) (Morgan et al., 2006). At baseline conditions, women show better verbal recall memory when compared to men (Sundermann et al., 2016), an effect likely associated with circulating levels of estrogen (Hampson, 1990). Estrogen enhances NMDAR function and increases synaptogenesis in the hippocampus (Gazzaley et al., 1996; Tuscher et al., 2016a; b), thus it is possible that fluctuating levels of ovarian hormones in women, especially estrogen, can be protective against deficits in cognitive function induced by low-dose ketamine.
Healthy volunteers subjected to phMRI testing during an acute infusion with low-dose ketamine displayed increased BOLD activation throughout the PFC that was attenuated by pre-treatment with either the antipsychotic, risperidone, or the anticonvulsant, lamotrigine (Doyle et al., 2013). Given that these drugs act on a variety of receptors such as dopamine and serotonin receptors as well as GABA and glutamate receptors, respectively, it is possible that the cognitive-impairing effects of ketamine are mediated by a wide variety of neurotransmitter systems. It should be noted, though, that the brain circuits mediating ketamine responses could be different in depressed patients compared to healthy controls. The PFC plays a critical role in executive function and memory processing and does not reach full maturation until around 25 years of age in humans (Tsujimoto, 2008). For this reason, and because the cognitive deficits induced by ketamine likely implicate a circuit including the PFC, it is important to further explore the consequences of repeated ketamine treatments in TRD adolescents. Though the first clinical trial for treating adolescents with TRD is still underway, the study description indicates that adolescents will be administered repeated, low-dose ketamine infusions and subjected to both the BPRS and CADSS to examine the effects of ketamine on cognition within this age group (ClinicalTrials.gov, Identifier: NCT02078817), which will hopefully provide insight into these effects in a younger population.
3.2. Preclinical evidence
In animal models, the effects of ketamine on cognition depend upon dose, whether the drug is administered acutely or chronically, as well as stress conditions. For example, stress-naïve male rats subjected to the five-choice serial reaction time task (5-CSRTT), which measures sustained attention and memory processing speed by testing the time it takes a rodent to detect and respond to a brief cue light that is randomly associated with a particular hole, exhibit deficits when administered both low and high doses of ketamine. Indeed, an acute, low-dose ketamine (10 mg/kg, i.p.) injection attenuated both attention and processing speed on the 5-CSRTT in male rats, and chronic exposure to a high dose of ketamine (30 mg/kg, i.p.) also induced deficits in attention and processing speed, though the deficits induced by chronic, high-dose ketamine were greater and sustained for a longer time period than the deficits observed following acute, low-dose ketamine (Nikiforuk and Popik, 2014a). The cognitive deficits induced by ketamine appear to be heavily time-dependent as well, since one report indicated deficits on an attentional set-shifting task (ASST), which measures executive function by testing for food reward via odor discrimination, when low-dose ketamine (10 mg/kg, i.p.) was administered 50 min prior to testing but not when ketamine was administered 3 and 24 h prior to testing, suggesting that the cognitive-impairing effects of low-dose ketamine are transient (Kos et al., 2011). Interestingly, though, chronically stressed male rats exposed to ASST, displayed baseline deficits in the ASST that were rescued by acute, low-dose (10 mg/kg, i.p.) administration of etamine (Nikiforuk and Popik, 2014b). These data suggest that while acute, low-dose ketamine may enhance cognitive deficits in stress-naïve rodents, it actually rescues stress-induced cognitive deficits in chronically stressed male rodents.
Chronic administration of high doses of ketamine (30 mg/kg, i.p.) to stress-naïve male rats also induced deficits in a novel object recognition (NOR) task, which measures recognition memory by testing the amount of time a rodent spends with a novel compared to a familiar object (reviewed in Neill et al., 2010). These behavioral deficits were associated with decreases in the number of hippocampal and PFC GABAergic parvalbumin neurons (Hauser et al., 2017; Keilhoff et al., 2004) and increased glutamate receptor binding sites and dopamine 2 receptor (D2R) binding sites (Becker et al., 2003), suggesting that chronic exposure to a high dose of ketamine induces a hyperexcitable state.
Repeated administration of ketamine at low doses (5 mg/kg, i.p.) to male mice induced hippocampal CA3 cell death, demonstrated by caspace-3 labeling, suggesting that repeated exposure to low doses of ketamine induces apoptotic effects in stress-naïve male rodents (Majewski-Tiedeken et al., 2008) (Table 2). It is therefore important to examine whether similar effect would be observed in mice that were chronically stressed.
Table 2.
Summary of the cognitive-impairing effects of ketamine and their molecular correlates. Male adolescent and adult rodents are more sensitive to ketamine-induced impairments in memory. Ovarian hormones protect against ketamine-induced cognitive impairments.
| Sex | Age | Dose | Treatment Regimen | Stress | Behavioral response | Molecular effect | References |
|---|---|---|---|---|---|---|---|
| Male | Adult | 5 mg/kg | sub-chronic | NS | n/a | ↑ CA3 caspace 3 activation | (Majewski-Tiedeken et al., 2008) |
| ↓ PPI | ↑ HPC oxidative stress | (Celia Moreira Borella et al., 2016) | |||||
| ↓ y-maze | |||||||
| 10 mg/kg | acute | NS | ↓ 5-CSRTT time | n/a | (Nikiforuk and Popik, 2014a, Nikiforuk and Popik, 2014b) | ||
| CUS | rescued ASST deficit | n/a | (Nikiforuk and Popik, 2014a, Nikiforuk and Popik, 2014b) | ||||
| 20 mg/kg | chronic | NS | ↑ radial arm maze latency | ↓ HPC EAAT2 expression | (Featherstone et al., 2012) | ||
| Female | Adult | 5 mg/kg | sub-chronic | NS | ↑ PPI in proestrous | n/a | (Celia Moreira Borella et al., 2016) |
| ↓ PPI in diestrous | |||||||
| ↓ y-maze in proestrous | |||||||
| ↓ y-maze in diestrous | |||||||
| 10 mg/kg | acute | NS | ↓ PPI in OVX females | n/a | (van den Buuse et al., 2015) | ||
| ↑ PPI in E2+P4 females | |||||||
| Male | Adolescent | 5 mg/kg | sub-chronic | NS | no effect on PPI | n/a | (Celia Moreira Borella et al., 2016) |
| ↓ y-maze | |||||||
| 20 mg/kg | chronic | NS | ↑ radial arm maze latency | ↓ HPC EAAT2 expression in adulthood | (Nagy et al., 2015; Dodman et al., 2015) | ||
| ↑ SIT duration in adulthood | rescued with EAAT2 antagonist | ||||||
| 25 mg/kg | acute | NS | ↓ NOR | ↓ HPC LTD | (Silvestre de Ferron et al., 2015) | ||
| Female | Adolescent | 5 mg/kg | sub-chronic | NS | no effect (PPI) | n/a | (Celia Moreira Borella et al., 2016) |
| no effect (y-maze) |
(NS= non-stressed; CUS = chronic unpredictable stress; PPI = pre-pulse inhibition; 5-CSRTT = 5 choice serial reaction time test; ASST = attentional set-shifting task; OVX = ovariectomized; E2 = estrogen; P4 = progesterone; SIT = social interaction test; NOR = novel object recognition; HPC = hippocampus; EAAT2 = excitatory amino acid transporter 2; LTD = long-term depression.
Given that the age and sex can influence sensitivity to ketamine's antidepressant and addictive effects, it is imperative to understand the effects of repeated, low-dose ketamine treatments have on cognitive performance and the molecular changes associated with it in both sexes as well as during different developmental periods. Sub-chronic exposure to a high dose of ketamine (30 mg/kg, i.p.), used as a putative animal model of schizophrenia, induces cognitive behavioral deficits in a sex-dependent manner. When assessing short- and long-term memory function with the hole-board test, which measures the number of times a previously trained rodent visits food-reward associated holes, male rats were more sensitive to ketamine-induced deficits in working and long-term memory compared to female rats. However, when tested for pre-pulse inhibition (PPI), which assesses sensory gating by measuring a startle reflex following an auditory cue, ketamine induced deficits in female but not male rats (Kekesi et al., 2015). Deficits in PPI are associated with an inability to filter irrelevant sensory inputs (Neill et al., 2010), and animal models of schizophrenia reliably reduce pre-pulse inhibition of the acoustic startle reflex (reviewed in Neill et al., 2010). Evidence from female rodents suggests that the presence of ovarian hormones may protect against deficits in PPI since ovariectomy (OVX) reduced while estrogen rescued PPI deficits in a dose-dependent manner (Van den Buuse and Eikelis, 2001). Furthermore, OVX female rats displayed a reduction in PPI following acute, low-dose administration of ketamine (10 mg/kg, i.p.) that was subsequently rescued following treatment with estrogen and progesterone, but not estrogen on its own (van den Buuse et al., 2015).
Similarly, repeated administration of low-dose ketamine (5 mg/kg, i.p.) to neonate rat pups tested for PPI in adulthood led to reductions in the percentage of PPI in males and females in diestrous while actually improving percentage of PPI in female rats in proestrous, the estrus phase when circulating estrogen and progesterone are at their highest levels. Interestingly, neither male nor female rats tested for PPI in adolescence displayed deficits, suggesting that adolescent rats of both sexes may be less susceptible to impairments in sensory gating (Celia Moreira Borella et al., 2016). In adults, changes in PPI behavior was associated with enhancement of hippocampal oxidative stress pathways in males and females in diestrous, but not in proestrous females, as demonstrated by reduced levels of glutathione (GSH) and increased levels of malondialdehyde (MDA) and nitrite (Celia Moreira Borella et al., 2016) (Table 2). These results suggest that sensory gating deficits induced by low-dose ketamine are mediated, at least in part, by oxidative stress pathways in a sex- and cycle dependent manner.
While male rodents were more sensitive to the memory-impairing effects of ketamine at high doses, neonatal administration of low-dose ketamine (5 mg/kg, i.p.) induced spatial working memory deficits in adult males and females (Celia Moreira Borella et al., 2016). Furthermore, adolescent male rodents administered ketamine neonatally display impairments in spatial working memory, tested by the y-maze, whereas adolescent females were unaffected, suggesting that adolescent, but not adult, male rats are more sensitive to the memory-impairing effects of ketamine (Celia Moreira Borella et al., 2016). Given that this is the only study to date to examine the effects of repeated, low-dose ketamine on cognition in both sexes and ketamine administration occurred neonatally, it is difficult to interpret whether the observed effects would be the same if ketamine administration occurred during adulthood. Furthermore, all of the above-mentioned studies occurred in stress-naïve rodents, and given that stress itself can induce cognitive deficits, it remains unclear what the effect of repeated treatment with low-dose ketamine would be on cognition in a chronically stressed state in both sexes.
Acute ketamine administration (25 mg/kg, i.p.) to adolescent male rats (∼4 weeks old) led to deficits in the NOR task, suggesting deficits in long-term memory retrieval (Silvestre de Ferron et al., 2015). The observed memory impairments in juvenile male rats were associated with a loss of NMDA-dependent long-term depression (LTD) in the hippocampus that was mediated by enhanced function of the GluN2B subunit (Silvestre de Ferron et al., 2015). Since the NOR is a hippocampus-dependent task (reviewed in Cohen and Stackman, 2015), this suggests that the loss of LTD induced a hyperexcitable state in the hippocampus that may have mediated the impairments in memory retrieval of juvenile male mice (Silvestre de Ferron et al., 2015).
Chronic exposure to a higher dose of ketamine (20 mg/kg, i.p.) is necessary to elicit an antidepressant-like response in juvenile male mice (Parise et al., 2013). This treatment regimen not only impairs memory but also induces a delayed onset of aberrant social behavior in juvenile mice. In the radial arm water maze test, which assesses spatial working memory, chronic administration of ketamine (20 mg/kg, i.p.) to juvenile male mice, from PND weeks 4–7, increased latency as well as the number of errors that occurred to find a hidden platform, a deficit that was normalized when the mice were tested later in adulthood (Nagy et al., 2015). In the social interaction test, repeated exposure to ketamine (20 mg/kg, i.p.) during adolescence, from PND weeks 4–7, did not alter any measures of social interaction in juvenile mice, but social interaction was disturbed when mice became adults (Nagy et al., 2015).
Repeated administration of ketamine (20 mg/kg, i.p.) to adult male mice leads to impairments in working memory, tested by the radial arm maze, and a reduction in hippocampal excitatory amino acid transporter-2 (EAAT2) (Featherstone et al., 2012). EAAT2 is an astrocytic transporter that regulates glutamate clearance, and a reduction in EAAT2 expression can lead to increased extracellular glutamate overflow that can bind to glutamate receptors on nearby neurons and enhance long-term potentiation (Shen et al., 2011). Repeated ketamine (20 mg/kg, i.p.) administration to juvenile male mice, from PND weeks 4–7, does not affect hippocampal EAAT2 expression when measured during the same period, but leads to a reduction in its expression when measured during adulthood (Dodman et al., 2015). Furthermore, juvenile male mice exposed to ceftriaxone, an EAAT2-antagonist, did not exhibit ketamine-induced reduction in hippocampal EAAT2 expression during adulthood. These results suggest that repeated, low-dose ketamine administration restricted to the period of adolescence leads to cognitive impairments that are mediated by increased extracellular glutamate as a result of reduced glutamate clearance, and can have long-lasting behavioral and molecular effects in adulthood.
Altogether, the data suggest that adolescent male rodents are more sensitive to the memory-impairing effects of repeated, low-dose ketamine administration, an effect likely mediated by a loss of hippocampal LTD and an enhancement in glutamatergic signaling. However, no studies to date have examined the effects of repeated, low-dose ketamine in chronically stressed adolescents, which may have different effects on cognition and neural circuitry. Thus, while studies are beginning to shed light on the effects of low-dose ketamine during adolescence, it is difficult to interpret in the context of depression.
4. Conclusion
Both preclinical and clinical studies indicate that repeated treatment with low-dose ketamine infusions can have addictive properties and induce cognitive deficits. Animal studies suggest that adolescent and adult female rodents are more sensitive to ketamine's abuse potential, while adolescent and adult male rodents are more sensitive to the memory-impairing effects following repeated ketamine exposure. Furthermore, the presence of ovarian hormones enhances sensitivity to the abuse potential of low-dose ketamine. In humans, it remains unclear whether women are more sensitive to the antidepressant and addictive potential of ketamine since most studies only examine one dose (0.5 mg/kg, i.v.). A clinical trial examining the effects of ketamine across a wide range of doses is currently underway, though, and should provide insight on any effect of gender on antidepressant response in the near future (ClinicalTrials.gov, Identifier: NCT01558063). Animal studies examining the effect of repeated, low-dose ketamine exposure suggest that adolescent and adult male rodents are more sensitive to cognitive deficits induced by ketamine. The presence of ovarian hormones protects against ketamine-induced cognitive deficits since female rodents with high levels of estrogen and progesterone are unaffected by these adverse symptoms. In humans, it has been reported that men are more sensitive to the impairments ketamine causes on both episodic and semantic memory following repeated, low-dose infusions. While no studies to date have reported on the effects of ketamine in human adolescents with TRD, a clinical trial administering repeated, low-dose ketamine infusions is currently underway (ClinicalTrials.gov, Identifier: NCT02078817), which should provide insight on both the antidepressant and cognitive effects within this subpopulation.
5. Unanswered questions
Regarding the safety of repeated ketamine infusions, it remains unclear whether ketamine treatment for TRD further increases risk for the development of addiction. While it is important to further examine the addictive properties of ketamine, repeated low-dose infusions have had groundbreaking therapeutic value in reducing suicidal ideation in TRD individuals. From the literature reviewed above, it is clear that ketamine, at low doses, has addictive properties. It remains unclear, though, if repeated infusions of ketamine will increase propensity for ketamine abuse or relapse to other addictive drugs following antidepressant treatment. To answer this, studies examining the effects of slow, low-dose ketamine infusions on subsequent ketamine self-administration are necessary. Additionally, there is evidence that (R)-ketamine has less addictive properties compared to (S)-ketamine, but (S)-ketamine is currently being tested in clinical trials because of its reduced psychotomimetic features and increased antidepressant efficacy (Andrade, 2017), despite the fact that the psychotomimetic effects of ketamine may actually improve antidepressant efficacy (Luckenbaugh et al., 2014; Sos et al., 2013). Studies examining the antidepressant effects of repeated (R)-ketamine infusions across different doses are also necessary to potentially reduce risk for abuse and cognitive deficits.
Conflicts of interest
None
Acknowledgements
This work was supported by the National Institute of Mental Health R01 MH099085 and MH190450 to M.K.
References
- aan het Rot M., Collins K.A., Murrough J.W., Perez A.M., Reich D.L., Charney D.S., Mathew S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatr. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Adler C.M., Goldberg T.E., Malhotra A.K., Pickar D., Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol. Psychiatr. 1998;43(11):811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Admon R., Pizzagalli D.A. Dysfunctional reward processing in depression. Curr. Opin. Psychol. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C. Ketamine for depression, 3: does chirality matter? J. Clin. Psychiatr. 2017;78(6):e674–e677. doi: 10.4088/JCP.17f11681. [DOI] [PubMed] [Google Scholar]
- Andrews G., Szabo M., Burns J. Preventing major depression in young people. Br. J. Psychiatry. 2002;181:460–462. doi: 10.1192/bjp.181.6.460. [DOI] [PubMed] [Google Scholar]
- Autry A.E., Adachi M., Nosyreva E., Na E.S., Los M.F., Cheng P.F., Kavalali E.T., Monteggia L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard E.D., Ionescu D.F., Vande Voort J.L., Niciu M.J., Richards E.M., Luckenbaugh D.A., Brutsche N.E., Ameli R., Furey M.L., Zarate C.A., Jr. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J. Psychiatr. Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli F., Riboldi I., Crocamo C., Di Brita C., Clerici M., Carrà G. Ketamine as a rapid-acting agent for suicidal ideation: a meta-analysis. Neurosci. Biobehav. Rev. 2017;77:232–236. doi: 10.1016/j.neubiorev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Becker A., Peters B., Schroeder H., Mann T., Huether G., Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2003;27(4):687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Berlim M.T., Turecki G. Definition, assessment, and staging of treatment- resistant refractory major depression: a review of current concepts and methods. Can. J. Psychiatr. 2007;52(1):46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatr. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine's antidepressant response and the development of ketamine addiction. J. Psychoact. Drugs. 2015;47(4):276–285. doi: 10.1080/02791072.2015.1072653. [DOI] [PubMed] [Google Scholar]
- Botanas C.J., de la Pena J.B., Dela Pena I.J., Tampus R., Yoon R., Kim H.J., Lee Y.S., Jang C.G., Cheong J.H. Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: evidence of its abuse potential. Pharmacol. Biochem. Behav. 2015;133:31–36. doi: 10.1016/j.pbb.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Caffino L., Di Chio M., Giannotti G., Venniro M., Mutti A., Padovani L., Cheung D., Fumagalli G.F., Yew D.T., Fumagalli F., Chiamulera C. The modulation of BDNF expression and signalling dissects the antidepressant from the reinforcing properties of ketamine: effects of single infusion vs. chronic self-administration in rats. Pharmacol. Res. 2016;104:22–30. doi: 10.1016/j.phrs.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Caffino L., Piva A., Mottarlini F., Di Chio M., Giannotti G., Chiamulera C., Fumagalli F. Ketamine self-administration elevates alphaCaMKII autophosphorylation in mood and reward-related brain regions in rats. Mol. Neurobiol. 2017;55(7):5453–5461. doi: 10.1007/s12035-017-0772-3. [DOI] [PubMed] [Google Scholar]
- Camarini R., Pautassi R.M. Behavioral sensitization to ethanol: neural basis and factors that influence its acquisition and expression. Brain Res. Bull. 2016;125:53–78. doi: 10.1016/j.brainresbull.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Carrier N., Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Celia Moreira Borella V., Seeman M.V., Carneiro Cordeiro R., Vieira dos Santos J., Romario Matos de Souza M., Nunes de Sousa Fernandes E., Santos Monte A., Maria Mendes Vasconcelos S., Quinn J.P., de Lucena D.F., Carvalho A.F., Macedo D. Gender and estrous cycle influences on behavioral and neurochemical alterations in adult rats neonatally administered ketamine. Dev. Neurobiol. 2016;76(5):519–532. doi: 10.1002/dneu.22329. [DOI] [PubMed] [Google Scholar]
- Chang H., Huang M.C., Chen L.Y. Major depressive disorder induced by chronic ketamine abuse: a case report. Prim. Care Companion CNS Disord. 2016;18(3) doi: 10.4088/PCC.15l01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.Y., Huang M.C., Lin S.K. Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst. Abuse Treat. Prev. Pol. 2014;9:39. doi: 10.1186/1747-597X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.S., D'Souza D.C., Gueorguieva R., Perry E.B., Madonick S., Karper L.P., Abi-Dargham A., Belger A., Abi-Saab W., Lipschitz D., Bennet A., Seibyl J.P., Krystal J.H. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005;179(1):136–143. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- Cohen S.J., Stackman R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costumero V., Rosell-Negre P., Bustamante J.C., Fuentes-Claramonte P., Llopis J.J., Avila C., Barros-Loscertales A. Left frontoparietal network activity is modulated by drug stimuli in cocaine addiction. Brain Imag. Behav. 2017 Nov 20 doi: 10.1007/s11682-017-9799-3. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Curran H.V., Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction. 2001;96(5):749–760. doi: 10.1046/j.1360-0443.2001.96574910.x. [DOI] [PubMed] [Google Scholar]
- Cusin C., Ionescu D.F., Pavone K.J., Akeju O., Cassano P., Taylor N., Eikermann M., Durham K., Swee M.B., Chang T., Dording C., Soskin D., Kelley J., Mischoulon D., Brown E.N., Fava M. Ketamine augmentation for outpatients with treatment-resistant depression: preliminary evidence for two-step intravenous dose escalation. Aust. N. Z. J. Psychiatr. 2017;51(1):55–64. doi: 10.1177/0004867416631828. [DOI] [PubMed] [Google Scholar]
- D'Souza D.C., Ahn K., Bhakta S., Elander J., Singh N., Nadim H., Jatlow P., Suckow R.F., Pittman B., Ranganathan M. Nicotine fails to attenuate ketamine-induced cognitive deficits and negative and positive symptoms in humans: implications for schizophrenia. Biol. Psychiatr. 2012;72(9):785–794. doi: 10.1016/j.biopsych.2012.05.009. [DOI] [PubMed] [Google Scholar]
- De Luca M.T., Badiani A. Ketamine self-administration in the rat: evidence for a critical role of setting. Psychopharmacology (Berl) 2011;214(2):549–556. doi: 10.1007/s00213-010-2062-x. [DOI] [PubMed] [Google Scholar]
- Diamond P.R., Farmery A.D., Atkinson S., Haldar J., Williams N., Cowen P.J., Geddes J.R., McShane R. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J. Psychopharmacol. 2014;28(6):536–544. doi: 10.1177/0269881114527361. [DOI] [PubMed] [Google Scholar]
- DiazGranados N., Ibrahim L.A., Brutsche N.E., Ameli R., Henter I.D., Luckenbaugh D.A., Machado-Vieira R., Zarate C.A., Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment- resistant major depressive disorder. J. Clin. Psychiatr. 2010;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodman K., Featherstone R.E., Bang J., Liang Y., Siegel S.J. Ceftriaxone reverses ketamine-induced lasting EEG and astrocyte alterations in juvenile mice. Drug Alcohol Depend. 2015;156:14–20. doi: 10.1016/j.drugalcdep.2015.07.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat A.M., Wright K.N., Strong C.E., Kabbaj M. Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57BL/6 mice. Neuropharmacology. 2018;130:30–41. doi: 10.1016/j.neuropharm.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle O.M., De Simoni S., Schwarz A.J., Brittain C., O'Daly O.G., Williams S.C., Mehta M.A. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J. Pharmacol. Exp. Therapeut. 2013;345(1):151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) Diversion Control Division. Drug & Chemical Evaluation Section. March, 2018. Scheduling actions, controlled substances, regulated chemicals. DEA. [Google Scholar]
- Evans R.C., Morera-Herreras T., Cui Y., Du K., Sheehan T., Kotaleski J.H., Venance L., Blackwell K.T. The effects of NMDA subunit composition on calcium influx and spike timing-dependent plasticity in striatal medium spiny neurons. PLoS Comput. Biol. 2012;8(4) doi: 10.1371/journal.pcbi.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S.P., Mayfield R.D. RNA-Seq reveals novel transcriptional reorganization in human alcoholic brain. Int. Rev. Neurobiol. 2014;116:275–300. doi: 10.1016/B978-0-12-801105-8.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone R.E., Liang Y., Saunders J.A., Tatard-Leitman V.M., Ehrlichman R.S., Siegel S.J. Subchronic ketamine treatment leads to permanent changes in EEG, cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol. Dis. 2012;47(3):338–346. doi: 10.1016/j.nbd.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X., Miller A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatr. 2016;21(10):1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A., Sens J., Herchick S., Thelen C., Pitychoutis P.M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and "depressed" mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Fu Z.D., Csanaky I.L., Klaassen C.D. Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab. Dispos. 2012;40(6):1216–1225. doi: 10.1124/dmd.111.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A.H., Weiland N.G., McEwen B.S., Morrison J.H. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16(21):6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierski F., Hubsch B., Stefaniak N., Benzerouk F., Cuervo-Lombard C., Bera-Potelle C., Cohen R., Kahn J.P., Limosin F. Executive functions in adult offspring of alcohol- dependent probands: toward a cognitive endophenotype? Alcohol Clin. Exp. Res. 2013;37(Suppl. 1):E356–E363. doi: 10.1111/j.1530-0277.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15(2):97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hauser M.J., Isbrandt D., Roeper J. Disturbances of novel object exploration and recognition in a chronic ketamine mouse model of schizophrenia. Behav. Brain Res. 2017;332:316. doi: 10.1016/j.bbr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Jamadar S., DeVito E.E., Jiantonio R.E., Meda S.A., Stevens M.C., Potenza M.N., Krystal J.H., Pearlson G.D. Memantine, an NMDA receptor antagonist, differentially influences Go/No-Go performance and fMRI activity in individuals with and without a family history of alcoholism. Psychopharmacology (Berl) 2012;222(1):129–140. doi: 10.1007/s00213-011-2628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer Y., Connor D.F., Curry J.F. Comorbid adolescent substance use and major depressive disorders: a review. Psychiatry (Edgmont) 2007;4(12):32–43. [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G., Becker A., Grecksch G., Wolf G., Bernstein H.G. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126(3):591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Kekesi G., Petrovszki Z., Benedek G., Horvath G. Sex-specific alterations in behavioral and cognitive functions in a "three hit" animal model of schizophrenia. Behav. Brain Res. 2015;284:85–93. doi: 10.1016/j.bbr.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Kiraly D.D., Horn S.R., Van Dam N.T., Costi S., Schwartz J., Kim-Schulze S. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl. Psychiatry. 2017;7(3):e1065. doi: 10.1038/tp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos T., Nikiforuk A., Rafa D., Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl) 2011;214(4):911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen A.S., Jenkins M.A., Banke T.G., Schousboe A., Makino Y., Johnson R.C., Huganir R., Traynelis S.F. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat. Neurosci. 2011;14(6):727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti A.C., Holcomb H.H., Medoff D.R., Tamminga C.A. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6(6):869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- Lahti A.C., Weiler M.A., Tamara Michaelidis B.A., Parwani A., Tamminga C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Laurie D.J., Bartke I., Schoepfer R., Naujoks K., Seeburg P.H. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res. Mol. Brain Res. 1997;51(1–2):23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- Lener M.S., Kadriu B., Zarate C.A., Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381–401. doi: 10.1007/s40265-017-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Fang Q., Liu Y., Zhao M., Li D., Wang J., Lu L. Cannabinoid CB(1) receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats. Eur. J. Pharmacol. 2008;589(1–3):122–126. doi: 10.1016/j.ejphar.2008.04.051. [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H.J., Lau C.G., Tang A., Chan F., Ungvari G.S., Tang W.K. Cognitive impairments in poly-drug ketamine users. Addict. Behav. 2013;38(11):2661–2666. doi: 10.1016/j.addbeh.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Liang H.J., Tang K.L., Chan F., Ungvari G.S., Tang W.K. Ketamine users have high rates of psychosis and/or depression. J. Addict. Nurs. 2015;26(1):8–13. doi: 10.1097/JAN.0000000000000060. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh D.A., Niciu M.J., Ionescu D.F., Nolan N.M., Richards E.M., Brutsche N.E. Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R., Gold P.W., Luckenbaugh D.A., Ballard E.D., Richards E.M., Henter I.D. The role of adipokines in the rapid antidepressant effects of ketamine. Mol. Psychiatr. 2017;22(1):127–133. doi: 10.1038/mp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski-Tiedeken C.R., Rabin C.R., Siegel S.J. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 2008;92(1–3):217–227. doi: 10.1016/j.drugalcdep.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A.K., Pinals D.A., Weingartner H., Sirocco K., Missar C.D., Pickar D., Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14(5):301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- McDougall S.A., Moran A.E., Baum T.J., Apodaca M.G., Real V. Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose. Psychopharmacology (Berl) 2017;234(18):2683–2696. doi: 10.1007/s00213-017-4660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J.C., LaGamma C.T., Lim S.C., Tsitsiklis M., Neria Y., Brachman R.A. Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology. 2017;42(8):1577–1589. doi: 10.1038/npp.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D.J., Sakmann B., Seeburg P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morgan C.J., Muetzelfeldt L., Curran H.V. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105(1):121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Morgan C.J., Perry E.B., Cho H.S., Krystal J.H., D'Souza D.C. Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology (Berl) 2006;187(4):405–414. doi: 10.1007/s00213-006-0409-0. [DOI] [PubMed] [Google Scholar]
- Murrough J.W., Burdick K.E., Levitch C.F., Perez A.M., Brallier J.W., Chang L.C., Foulkes A., Charney D.S., Mathew S.J., Iosifescu D.V. Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment- resistant depression: a randomized controlled trial. Neuropsychopharmacology. 2015;40(5):1084–1090. doi: 10.1038/npp.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J.W., Perez A.M., Pillemer S., Stern J., Parides M.K., aan het Rot M., Collins K.A., Mathew S.J., Charney D.S., Iosifescu D.V. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatr. 2013;74(4):250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Shaw A.D., Jackson L.E., Hall J., Moran R., Saxena N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 2015;35(33):11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L.R., Featherstone R.E., Hahn C.G., Siegel S.J. Delayed emergence of behavioral and electrophysiological effects following juvenile ketamine exposure in mice. Transl. Psychiatry. 2015;5:e635. doi: 10.1038/tp.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R., Frankle W.G., Keefe R., Gil R., Martinez D., Slifstein M., Kegeles L.S., Talbot P.S., Huang Y., Hwang D.R., Khenissi L., Cooper T.B., Laruelle M., Abi- Dargham A. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am. J. Psychiatr. 2005;162(12):2352–2359. doi: 10.1176/appi.ajp.162.12.2352. [DOI] [PubMed] [Google Scholar]
- Neill J.C., Barnes S., Cook S., Grayson B., Idris N.F., McLean S.L., Snigdha S., Rajagopal L., Harte M.K. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol. Ther. 2010;128(3):419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Newcomer J.W., Farber N.B., Jevtovic-Todorovic V., Selke G., Melson A.K., Hershey T., Craft S., Olney J.W. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20(2):106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Ng E., Browne C.J., Samsom J.N., Wong A.H.C. Depression and substance use comorbidity: what we have learned from animal studies. Am. J. Drug Alcohol Abuse. 2017;43(4):456–474. doi: 10.1080/00952990.2016.1183020. [DOI] [PubMed] [Google Scholar]
- Niciu M.J., Luckenbaugh D.A., Ionescu D.F., Guevara S., Machado-Vieira R., Richards E.M., Brutsche N.E., Nolan N.M., Zarate C.A., Jr. Clinical predictors of ketamine response in treatment-resistant major depression. J. Clin. Psychiatr. 2014;75(5):e417–423. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A., Popik P. The effects of acute and repeated administration of ketamine on attentional performance in the five-choice serial reaction time task in rats. Eur. Neuropsychopharmacol. 2014;24(8):1381–1393. doi: 10.1016/j.euroneuro.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A., Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology. 2014;40:119–122. doi: 10.1016/j.psyneuen.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Nugent A.C., Diazgranados N., Carlson P.J., Ibrahim L., Luckenbaugh D.A., Brutsche N., Herscovitch P., Drevets W.C., Zarate C.A., Jr. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2014;16(2):119–128. doi: 10.1111/bdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontl T., Xing Y., Bai L., Kennedy E., Nelson S., Wakeman M., Magnusson K. Development and aging of N-methyl-D-aspartate receptor expression in the prefrontal/frontal cortex of mice. Neuroscience. 2004;123(2):467–479. doi: 10.1016/j.neuroscience.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Papakostas G.I., Petersen T.J., Farabaugh A.H., Murakami J.L., Pava J.A., Alpert J.E., Fava M., Nierenberg A.A. Psychiatric comorbidity as a predictor of clinical response to nortriptyline in treatment-resistant major depressive disorder. J. Clin. Psychiatr. 2003;64(11):1357–1361. doi: 10.4088/jcp.v64n1112. [DOI] [PubMed] [Google Scholar]
- Pare W.P., Redei E. Sex differences and stress response of WKY rats. Physiol. Behav. 1993;54(6):1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Parise E.M., Alcantara L.F., Warren B.L., Wright K.N., Hadad R., Sial O.K., Kroeck K.G., Iniguez S.D., Bolanos-Guzman C.A. Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol. Psychiatr. 2013;74(10):750–759. doi: 10.1016/j.biopsych.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Connor J.A. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J. Neurochem. 2010;114(4):1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis I.L., Limoncelli D., Gueorguieva R., Jatlow P., Boutros N.N., Trevisan L., Gelernter J., Krystal J.H. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am. J. Psychiatr. 2004;161(10):1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Phelps L.E., Brutsche N., Moral J.R., Luckenbaugh D.A., Manji H.K., Zarate C.A., Jr. Family history of alcohol dependence and initial antidepressant response to an N- methyl-D-aspartate antagonist. Biol. Psychiatr. 2009;65(2):181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K., Ng R., Scott H., Kodavaganti S., Smyda G., Diwadkar V., Phillips M. Ventral striatum functional connectivity during rewards and losses and symptomatology in depressed patients. Biol. Psychol. 2017;123:62–73. doi: 10.1016/j.biopsycho.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M., Vives M., Lopez-Navarro E., Garcia-Campayo J., Gili M. Cognitive impairments and depression: a critical review. Actas Esp. Psiquiatr. 2015;43(5):187–193. [PubMed] [Google Scholar]
- Rocha A., Hart N., Trujillo K.A. Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration. Pharmacol. Biochem. Behav. 2017;157:24–34. doi: 10.1016/j.pbb.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat. Rev. Neurosci. 2016;17(9):576–591. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay N.R., Boehm S.L., 2nd, Schafer G.L., Browman K.E., Erwin V.G., Crabbe J.C. Sensitivity and tolerance to ethanol-induced incoordination and hypothermia in HAFT and LAFT mice. Pharmacol. Biochem. Behav. 2001;70(1):167–174. doi: 10.1016/s0091-3057(01)00595-0. [DOI] [PubMed] [Google Scholar]
- Saland S.K., Duclot F., Kabbaj M. Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr. Opin. Behav. Sci. 2017;14:19–26. doi: 10.1016/j.cobeha.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N.A., Montcouquiol M.E., Raymond J. Postnatal developmental changes in AMPA and NMDA receptors in the rat vestibular nuclei. Brain Res. Dev. Brain Res. 2000;123(1):41–52. doi: 10.1016/s0165-3806(00)00082-1. [DOI] [PubMed] [Google Scholar]
- Santamaria R., Pailleux F., Beaudry F. In vitro ketamine CYP3A-mediated metabolism study using mammalian liver S9 fractions, cDNA expressed enzymes and liquid chromatography tandem mass spectrometry. Biomed. Chromatogr. 2014;28(12):1660–1669. doi: 10.1002/bmc.3199. [DOI] [PubMed] [Google Scholar]
- Schak K.M., Vande Voort J.L., Johnson E.K., Kung S., Leung J.G., Rasmussen K.G., Palmer B.A., Frye M.A. Potential risks of poorly monitored ketamine use in depression treatment. Am. J. Psychiatr. 2016;173(3):215–218. doi: 10.1176/appi.ajp.2015.15081082. [DOI] [PubMed] [Google Scholar]
- Schoepfer K.J., Strong C.E., Saland S.K., Wright K.N., Kabbaj M. Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats. Physiol. Behav. 2017 Oct 18 doi: 10.1016/j.physbeh.2017.10.021. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield M.D., Heinsbroek J.A., Gipson C.D., Kupchik Y.M., Spencer S., Smith A.C., Roberts-Wolfe D., Kalivas P.W. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol. Rev. 2016;68(3):816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S., Klaus A., Winkowski D.E., Kanold P.O., Plenz D. Altered avalanche dynamics in a developmental NMDAR hypofunction model of cognitive impairment. Transl. Psychiatry. 2018;8(1):3. doi: 10.1038/s41398-017-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Moussawi K., Zhou W., Toda S., Kalivas P.W. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc. Natl. Acad. Sci. U. S. A. 2011;108(48):19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroma P.R., Albott C.S., Johns B., Thuras P., Wels J., Lim K.O. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment- resistant depression. Int. J. Neuropsychopharmacol. 2014;17(11):1805–1813. doi: 10.1017/S1461145714001011. [DOI] [PubMed] [Google Scholar]
- Shiroma P.R., Johns B., Kuskowski M., Wels J., Thuras P., Albott C.S., Lim K.O. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J. Affect. Disord. 2014;155:123–129. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Silvestre de Ferron B., Bennouar K.E., Kervern M., Alaux-Cantin S., Robert A., Rabiant K., Antol J., Naassila M., Pierrefiche O. Two binges of ethanol a day keep the memory away in adolescent rats: key role for GLUN2B subunit. Int. J. Neuropsychopharmacol. 2015;19(1) doi: 10.1093/ijnp/pyv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sos P., Klirova M., Novak T., Kohutova B., Horacek J., Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol. Lett. 2013;34(4):287–293. [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stefanovic A., Brandner B., Klaassen E., Cregg R., Nagaratnam M., Bromley L.M., Das R.K., Rossell S.L., Morgan C.J., Curran H.V. Acute and chronic effects of ketamine on semantic priming: modeling schizophrenia? J. Clin. Psychopharmacol. 2009;29(2):124–133. doi: 10.1097/JCP.0b013e31819a4b91. [DOI] [PubMed] [Google Scholar]
- Strong C.E., Schoepfer K.J., Dossat A.M., Saland S.K., Wright K.N., Kabbaj M. Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology. 2017;121:195–203. doi: 10.1016/j.neuropharm.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann E.E., Maki P.M., Rubin L.H., Lipton R.B., Landau S., Biegon A., Alzheimer's Disease Neuroimaging I. Female advantage in verbal memory: evidence of sex-specific cognitive reserve. Neurology. 2016;87(18):1916–1924. doi: 10.1212/WNL.0000000000003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kato H., Aoki T., Tsuda M., Narita M., Misawa M. Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice. Life Sci. 2000;67(4):383–389. doi: 10.1016/s0024-3205(00)00639-1. [DOI] [PubMed] [Google Scholar]
- Tang J., Morgan H.L., Liao Y., Corlett P.R., Wang D., Li H., Tang Y., Chen J., Liu T., Hao W., Fletcher P.C., Chen X. Chronic administration of ketamine mimics the perturbed sense of body ownership associated with schizophrenia. Psychopharmacology (Berl) 2015;232(9):1515–1526. doi: 10.1007/s00213-014-3782-0. [DOI] [PubMed] [Google Scholar]
- Thelen C., Sens J., Mauch J., Pandit R., Pitychoutis P.M. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav. Brain Res. 2016;312:305–312. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- Tizabi Y., Bhatti B.H., Manaye K.F., Das J.R., Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Tuscher J.J., Luine V., Frankfurt M., Frick K.M. Estradiol-mediated spine changes in the dorsal Hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal Hippocampus. J. Neurosci. 2016;36(5):1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher J.J., Szinte J.S., Starrett J.R., Krentzel A.A., Fortress A.M., Remage-Healey L., Frick K.M. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav. 2016;83:60–67. doi: 10.1016/j.yhbeh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- UNODC . United National Publications; New York, NY: 2013. Patterns and Trends of Amphetamine-type Stimulants and Other Drugs: Challenges for Asia and the Pacific. [Google Scholar]
- Van den Buuse M., Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur. J. Pharmacol. 2001;425(1):33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- van den Buuse M., Mingon R.L., Gogos A. Chronic estrogen and progesterone treatment inhibits ketamine-induced disruption of prepulse inhibition in rats. Neurosci. Lett. 2015;607:72–76. doi: 10.1016/j.neulet.2015.09.019. [DOI] [PubMed] [Google Scholar]
- van der Kam E.L., de Vry J., Tzschentke T.M. Effect of 2-methyl-6- (phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav. Pharmacol. 2007;18(8):717–724. doi: 10.1097/FBP.0b013e3282f18d58. [DOI] [PubMed] [Google Scholar]
- Venniro M., Mutti A., Chiamulera C. Pharmacological and non-pharmacological factors that regulate the acquisition of ketamine self-administration in rats. Psychopharmacology (Berl) 2015;232(24):4505–4514. doi: 10.1007/s00213-015-4077-9. [DOI] [PubMed] [Google Scholar]
- Vetter-O'Hagen C.S., Spear L.P. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol. 2012;54(5):523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford H.A., Degenhardt L., Rehm J., Baxter A.J., Ferrari A.J., Erskine H.E., Charlson F.J., Norman R.E., Flaxman A.D., Johns N., Burstein R., Murray C.J., Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 35th ECDD Agenda Item 422012. 2012. Ketamine: expert peer review on critical review report. WHO: hammamet, Tunisia, June 4-8. [Google Scholar]
- Wiley J.L., Evans R.L., Grainger D.B., Nicholson K.L. Age-dependent differences in sensitivity and sensitization to cannabinoids and 'club drugs' in male adolescent and adult rats. Addict. Biol. 2008;13(3–4):277–286. doi: 10.1111/j.1369-1600.2007.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J.L., Evans R.L., Grainger D.B., Nicholson K.L. Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug. Pharmacol. Rep. 2011;63(5):1085–1092. doi: 10.1016/s1734-1140(11)70627-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson S.T., Ballard E.D., Bloch M.H., Mathew S.J., Murrough J.W., Feder A. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatr. 2018;175(2):150–158. doi: 10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A., Koob G.F. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.N., Strong C.E., Addonizio M.N., Brownstein N.C., Kabbaj M. Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology (Berl) 2017;234(3):393–401. doi: 10.1007/s00213-016-4470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S., Fang Y., Huang X.P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Jr., Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C.A., Jr., Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatr. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zheng X., Fang P., Bao S.S., Lin D., Cai J.P., Hu G.X. Function of 38 variants CYP2C9 polymorphism on ketamine metabolism in vitro. J. Pharmacol. Sci. 2017;135(1):8–13. doi: 10.1016/j.jphs.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Yuan Q., Mash D.C., Goldman D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc. Natl. Acad. Sci. U. S. A. 2011;108(16):6626–6631. doi: 10.1073/pnas.1018514108. [DOI] [PMC free article] [PubMed] [Google Scholar]