Abstract
Substance use and addiction are disproportionately experienced by individuals with a history of exposure to early life stress (ELS), such as maltreatment, domestic violence and parent psychopathology. Unfortunately, extant interventions have mixed effectiveness at improving outcome trajectories for ELS-exposed children, who are often underserved by evidenced-based programs. Here, we employ a translational neuroscience framework to delineate how neuroscience can deepen our understanding of ELS-linked alterations in children's function to inform the development of more targeted, effective early intervention and addiction prevention programs. Candidate neural pathways altered by ELS and linked to addiction are described across sensory, affective, motivational, and executive function domains. Next, we provide an example of the application of translational neuroscience principles in a family of early interventions (i.e. Multidimensional Treatment Foster Care – Preschool, Kids in Transition to School) focused on improving self-regulation in ELS-exposed children. Future directions and areas of unmet need in intervention research detail the significant potential of translational neuroscience to advance interventionists' ability to support positive adjustment in ELS-exposed children and prevent harmful addiction outcomes.
Keywords: Translational neuroscience, Prevention, Intervention, Adversity, Early life stress, Addiction
Children exposed to early life stress (ELS) are at risk for a range of early mental health and behavior problems that can lead to long-term negative outcomes across risk-taking and substance use domains. Although definitions can vary, it is generally agreed upon that experiences that induce repetitive or prolonged activation of children's stress response systems constitute ELS, with relevant research drawing from samples of children exposed to a variety of experiences including abuse, neglect, domestic violence, parent psychopathology (Pechtel and Pizzagalli, 2011; McLaughlin et al., 2012). Exposure to such stressors has considerable cost to society with an estimated 21.0% of adult and 37.0% of adolescent substance use disorders in the United States, attributed to individuals with histories of ELS (Green et al., 2010; McLaughlin et al., 2012). This represents a 40–160% increased risk in adults and an 80–380% increased risk in adolescents, based on the type of stressor experienced (Green et al., 2010; McLaughlin et al., 2012).
Despite the high burden of addiction-related illness incurred by individuals with such histories, extant family-based intervention programs have had mixed effectiveness at improving trajectories for at-risk children (Shonkoff and Fisher, 2013; Shonkoff, 2016). Further, children with a history of ELS, such as maternal psychopathology or family violence, are often underserved by extant interventions (Maliken and Katz, 2013; Shonkoff, 2016). One impediment to progress in the field is a lack of understanding of the neurobiological mechanisms by which stress influences mental and physical wellbeing. Translational neuroscience offers substantial promise of increasing the efficacy of early intervention and prevention efforts by informing them with detailed knowledge about the neurobiological pathways through which ELS alters development.
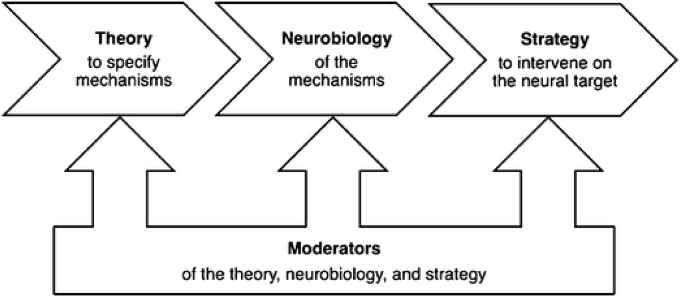
Translational neuroscience specifically refers to the process of using theory about the mechanisms that underlie the relationship between an intervention and a given outcome to directly measure the extent to which individuals’ underlying neurobiological processes change in response to an intervention (Fisher and Berkman, 2015, Fig. 1). This allows for precise testing of how a given intervention strategy is linked to neurobiological and behavioral change.
Fig. 1.
A Translational Neuroscience Framework for Program Development.
Note: From Fisher & Berkman (2015), with permission.
Further, this framework offers the potential to update, replicate, or emphasize certain strategies to increase program efficacy. Finally, it permits the systematic investigation of moderators to understand individual differences in improvements and gain insight into which pathways are more or less amenable to change. Because substance use and addiction occur many years after experiences of ELS, preventative approaches typically seek to intervene on more proximal targets (e.g. mental health, behavior problems, and self-regulation) that support children's development on prosocial trajectories and buffer them from later substance use risk. Examining neural processes altered by ELS that are linked to both these proximal targets and addiction can inform interventions efforts in this area.
Two challenges exist in the prevention field for leveraging translational neuroscience strategies to improve addiction-related outcomes in at-risk children. First, there is limited integrated knowledge about the multiple causal pathways through which ELS may affect neurobiological vulnerabilities to addiction. For example, it is possible that exposure to excess glucocorticoids early in life, such as is often experienced by infants exposed to neglect or maternal depression, initiates altered development of key brain areas important for emotion processing thus increasing individuals' risk for using substance as a maladaptive coping mechanism. Alternatively, it is possible that harsh parenting practices that co-occur with stressors, such as abuse or domestic violence, result in the insufficient development of brain regions important to behavioral regulation skills, which increases children's likelihood of engaging in risk-taking behavior, such as substance use. Although many such associations have been demonstrated [i.e., early life emotional neglect predicts blunted striatum reactivity to rewards (Hanson et al., 2015), and blunted striatum reactivity to positive stimuli is associated with cocaine dependency (Asensio et al., 2010)], there is minimal longitudinal research demonstrating that biological alterations mediate links between adversity and behavioral outcomes or inferential research demonstrating that intervention-linked changes in biology mediate changes in behavior.
Second, extant neuroscience knowledge has only been minimally incorporated into current intervention theory or strategies and is rarely measured as part of systematic tests of interventions (and moreover, when neurobiological measures have been included their specified role as mediators, moderators, or mechanisms is often imprecise). These barriers are cumulative in that each limits advances in the other; without the basic science knowledge of precise causal pathways, it may be difficult to theorize which mechanisms are altered by a given intervention. Without interventions directly testing the extent to which certain neurobiological processes are amenable to change, it may be difficult to target basic science efforts to more systematically narrow-in on causal pathways of influence.
With a focus on candidate neural pathways linked to addiction-related processes, in this manuscript we review the initial neuroimaging evidence, including magnetic resonance imaging (MRI) and electroencephalogram (EEG) techniques, for differences between ELS-exposed and non-exposed children. We specifically investigate the key domains of sensory, affective, motivational, and executive function processes. Within each domain, we emphasize how such neural changes have potential relevance to children's vulnerability for addiction or related risk-taking behaviors. Next, we provide an example of how translational neuroscience principles have been applied to a family of early interventions focused on improving self-regulation for children exposed to ELS. Finally, we delineate promising pathways forward for clinical researchers to employ these principles in developing more targeted and efficacious interventions to prevent addiction related outcomes for vulnerable children.
1. Neuroimaging evidence documenting neurodevelopmental differences amongst ELS exposed and non-exposed children: relevance to substance abuse
Though critical for highlighting the significance of ELS to children's long-term function, the simple identification of heterogeneous constructs (e.g. self-regulation, externalizing behavior) linked to substance abuse does not lend to precise predictions about altered neurobiological development because the target constructs and putative neural networks are so vast. Owing to improvements in neuroimaging technology and theory, it has become possible to make far more nuanced predictions about which neural systems are affected by ELS and thus might be promising targets for intervention and prevention efforts.
Developmental programming theory emphasizes the principle that, during times of rapid growth, biological systems are highly susceptible to both positive and negative environmental influences (Weiss and Wagner, 1998; Heim and Binder, 2012). Consistent with this perspective, the brain undergoes rapid development from birth to ∼5 years of age, with ongoing changes through adolescence and early adulthood. This begins with an overproduction of cellular growth, followed by neural “pruning” and myelination processes (Knickmeyer et al., 2008; Tau and Peterson, 2009). These changes increase both the efficiency and fidelity of neural signaling within and between neural regions, which is critical to the complex functional integration needed for mature higher-level cognitive processes (Padmanabhan et al., 2011). From ages ∼7 to ∼25, increases in communication between brain regions represent the largest changes in functional activation, with increased long-range connectivity strength and decreased intraregion, short-range strength (Dosenbach et al., 2010). Importantly, these processes have different time courses in terms of both onset and duration, with primary sensory and basal ganglia areas maturing earliest, and higher association and goal-directed areas (including frontal, temporal, and parietal regions) maturing later (Rubia, 2013). This protracted course of development results in critical periods for not only experience-dependent learning but also associated skill vulnerability. Accordingly, adversities experienced at specific developmental time periods may induce differential vulnerabilities to addiction-related neural alterations.
1.1. Sensory processing
Recent advances in neuroimaging techniques have provided evidence that exposure to ELS creates alterations in sensory development, at the earliest stages of processing. Such changes may be particularly meaningful because they hold the potential to bias all incoming information that is built upon by more complex cognitive processes, creating multiple points of vulnerability for ELS-exposed children. As discussed, below, ELS experiences linked to altered sensory development tend to occur early in life, when these systems are particularly plastic.
Children and early adolescents with severe neglect experiences in the first two years of life (e.g., adopted from institutions with minimal responsive caregiving or stimulation) exhibit specific reductions in white matter tracts that are critical for the execution of complex goal-directed behavior, such as the internal capsule, which integrates auditory and visual information with prefrontal cortex and motor processes (Hanson et al., 2013). In non-human primates, infant maltreatment predicts reduced white matter in visual processing regions (i.e., occipital cortex), with greater reductions associated with higher infant cortisol and greater adolescent aggression (Howell et al., 2013). A recent review of the effects of child maltreatment further highlights that the modalities of ELS can be linked to domain-specific reductions in white matter structure later in life (e.g., verbal abuse: auditory cortex, observing domestic violence: visual cortex, sexual abuse: somatosensory cortex; Teicher et al., 2016). Interestingly, maltreatment-linked white matter visual cortex reductions are linked to poorer visual memory, which may contribute to difficulty in engaging in effective goal-directed behavior (Teicher et al., 2016).
Volumetric grey matter differences in sensory regions have also been linked to ELS. For example, maltreated children with reactive attachment disorders (versus community controls) exhibited reduced left visual cortex volume which predicted internalizing problems and were conceptualized as underlying visual emotional regulation impairments (Shimada et al., 2015). Notably, poor working memory increases children's risk for adolescent substance use (Wiers et al., 2015). There is mixed evidence for the links between internalizing and adolescent substance use, with research documenting that internalizing problems may immediately precede the onset of substance use or predict a transition to dependence, but, when assessed in early childhood, does not robustly predict adolescent substance use (reviewed in Dodge et al., 2009).
Functional magnetic resonance imaging (fMRI) measures of young children's brain activity lend further insight into sensory processing differences. As assessed during infant sleep, babies exposed to high levels of interparental conflict show exaggerated processing (i.e., greater neural activity) of angry, versus neutral, tones of voice in the thalamus region, which serves as a sensory relay center (Graham et al., 2017).
Such sensory processing differences may reflect once-evolutionary adaptive biology approaches to coping with early life stress or preparing children to grow up in harsh and unpredictable environments (Del Giudice & Ellis, 2016). For example, the combination of maltreatment-linked structural white matter reductions in sensory areas and the heightened affective processing may allow children to feel less distress while maintaining vigilance to potentially threatening cues (Teicher et al., 2016). However, such differences may also have far reaching consequences on brain organization and developmental processes, which later increase individuals’ addiction-related risk.
1.1.1. Candidate sensory system links to addiction
Alterations in sensory processes are increasingly highlighted as a potential aspect of neural alterations linked to substance dependence (Yalachkov et al., 2010). In particular, individuals who are substance-dependent or at-risk for addiction exhibit exaggerated processing of addiction-relevant stimuli (e.g., drugs, alcohol, cigarettes) in visual and auditory cortices (Hanlon et al., 2014; Yalachkov et al., 2010). Further, a meta-analysis of fMRI studies across addictive substances (i.e., alcohol, cigarettes, opiates, marijuana, cocaine) emphasized links between substance-related reactivity and increased craving, with notable effects in sensory processing regions (Yalachkov et al., 2012).
Importantly, amongst individuals with substance dependence, heightened occipital cortex cue reactivity was particularly exaggerated for individuals with a history of childhood maltreatment (Elton et al., 2015). This reactivity may be a pathway influencing relapse vulnerability amongst maltreatment-exposed individuals. ELS-exposed children with alterations in sensory processes may have a difficult time filtering out unwanted information in their environment, making them more likely to attend to unwanted cues linked to risk-taking (and later to substance use) and show greater sensory processing of any such cues present in the environment. Further, such alterations could be hypothesized to decrease the effectiveness of meta-cognitive, reason based therapeutic approaches (e.g. cognitive behavioral therapy) for ELS-exposed individuals, due to challenges in disengaging from craving-related cues. Should this be the case, translational neuroscience informed interventions that offer ELS-exposed individuals access to therapeutic techniques that target attentional control could be a potential avenue to enhancing addiction treatment.
1.2. Affective processing
One of the most consistently documented ELS-linked alterations in brain function is compromised development of affective neural networks, particularly in their responsivity to negative or threatening environmental cues. These networks are defined by a range of interconnected brain regions linked to the processing of emotion and associated memories, including amygdala and hippocampus regions (Phelps, 2004). Given that early responsive caregiving is important for individuals’ ability to regulate their own negative emotions and take in information about the safety and resource availability of their environment, affective neural networks are prime candidates for early biological programming. In particular, it has been theorized that both prenatal and post-natal stressors provide key inputs that bias neurobiological development towards phenotypes that facilitate survival in stressful environments, such as increased sensitivity towards potentially threatening stimuli (McLaughlin et al., 2014).
Both ELS-exposed rodents and humans demonstrate exaggerated early development in the amygdala, including increases in volume and dendritic arborization (Cohen et al., 2013; Lupien et al., 2009; Tottenham et al., 2010). Over time, chronic exposure to stress may reduce structural amgydala growth and/or increase cell death (Hanson et al., 2015; Edmiston et al., 2011; Luby at al., 2013). These changes fit into a nonlinear model in which early excitation and exaggerated growth results in eventual downregulation of amygdala development (Hanson et al., 2015a, Hanson et al., 2015b, Hanson et al., 2015c). In human models, fMRI evidence links ELS experiences such as maltreatment and institutional neglect to heightened amygdala reactivity to negative or threat-related stimuli in late childhood through adulthood (McLaughlin et al., 2015; Fareri and Tottenham, 2016; See Hein and Monk, 2017 for meta-analysis). Even in infancy, ELS (i.e., interparental conflict) predicts altered amygdala connectivity with prefrontal regions (Graham et al., 2017). In spite of these findings, only a small number of studies have found that changes in amygdala volume or reactivity mediate links between early life stress and mental health difficulties (Tottenham et al., 2010).
Relatively consistent findings link ELS to subsequent reductions in hippocampal size, particularly when volume is examined in older children, adolescents, and adults, as the downregulation of hippocampal growth is understood to emerge over time (reviewed in Teicher et al., 2012; Riem et al., 2015). Research documents that maltreatment and cumulative life stress predict reduced hippocampal volume in late childhood to early adolescence (Luby et al., 2013; Hanson et al., 2015a, Hanson et al., 2015b, Hanson et al., 2015c; Teicher et al., 2012; Teicher et al., 2016). Research on resting state connectivity also shows lower hippocampal connectivity to a range of regions in the default mode and limbic networks (reviewed in Teicher et al., 2016). However, similar to the amygdala, the hippocampus also exhibits elevated responses to negative stimuli and closely related regions, with a recent meta-analysis of linking maltreatment to activation of the parahippocampal region (which surrounds the hippocampus and is key to memory storage and retrieval; Hein and Monk, 2017). A recent aversive-learning study demonstrated the potential significance of this effect by showing that, in severely neglected post-institutionalized youth, stronger hippocampal activation predicted aversive learning, which was linked to concurrent anxiety (Silvers et al., 2016). Stress-associated reductions in hippocampal volume have also been shown to mediate links between early life stress and a range of behavioral outcomes including disruptive school behaviors and depression, both of which are established risk factors for later substance use (Hanson et al., 2015a, Hanson et al., 2015b, Hanson et al., 2015c; Rao et al., 2010).
Reflecting the summative neural activity of multiple task-relevant brain-regions, event-related potential (ERP) electroencephalogram research further demonstrates negative affective processing biases in ELS-exposed children. For example, one ERP study found that neural reactivity to angry faces during an emotion identification task mediated links between maltreatment and physical aggression in boys (Shackman and Pollak, 2014). Similarly, 15-month-old maltreated infants exhibit greater attentional discrimination ERPs to angry faces (Curtis and Cicchetti, 2013).
It is important to note that recent research suggests that some alterations in neurodevelopmental processes may reflect ontogenetic adaptation to support children's function following adversity. Multiple research groups link severe neglect to the earlier development of amygdala to prefrontal cortex functional connectivity, which, in turn, predicts reduced emotional/internalizing problems amongst ELS-exposed children (Herringa et al., 2016; Gee et al., 2013). Similarly, post-institutionalized youth exhibited greater prefrontal to hippocampal functional connectivity, which predicted lower anxiety at 2-year follow-up (Silvers et al., 2016). Here, it is theorized that greater prefrontal connectivity to these lower-level regions, perhaps spurned by early over-development, may be important for the down-regulation of emotional distress. More research is needed to determine if profiles of early onset adult-like prefrontal connectivity has long-term consequences related to the shortening of developmental windows of plasticity.
1.2.1. Candidate affective links to addiction
Neural processing of affectively negative stimuli may be one pathway through which ELS-linked alterations in amgydala and hippocampal development increase risk for addiction. For example, exaggerated processing of negative stimuli may lead ELS-exposed children to have a difficult time disengaging from anxious or depressive feelings, resulting in a vulnerability to self-medicate with alcohol and other substances. Other children may process social stimuli as particularly negative and develop reactive aggression and impulsivity, a relatively common behavioral phenotype amongst stress-exposed children (Shields and Cicchetti, 1998). In contrast, among previously institutionalized children, attentional biases towards positive stimuli have been linked to fewer internalizing problems and more positive coping strategies (Troller-Renfree et al., 2017).In child and adolescent samples, both externalizing psychopathology and internalizing psychopathology (particularly depression) predict early substance use onset (O'Neil et al., 2011; King et al., 2004).
Profiles of heightened amygdala reactivity to negative cues are also implicated in substance use and addiction risk. The combination of heightened amygdala reactivity to threat and lower ventral striatum reactivity to rewards (discussed below) predicts problematic substance use in young adults (Nikolova et al., 2016), and heightened amygdala reactivity to threat alone predicts early onset of alcohol use (and subsequent addiction). Notably, substances of abuse have been found to dampen profiles of heightened amygdala reactivity, potentially creating a vulnerability towards self-medication for ELS-exposed individuals. In one double-blind study, heroin (versus saline) administration acutely reduced amygdala reactivity to fearful faces, which was associated with concurrent levels of state anxiety (Schmidt et al., 2014). Building on these affective system vulnerabilities, interventions that target emotion regulation skills (e.g. dialectical behavior therapy) or shift attentional biases towards more positive (versus negative/threatening bias) stimuli could be candidate targets of future translational neuroscience research intervening on addiction risk and maintenance factors amongst ELS-exposed individuals.
1.3. Motivational processing
Within the domain of motivational processing, there is substantial evidence that ELS affects neural networks linked to reward. Motivational networks are conceptualized as being based in the mesolimbic dopamine system and involving the basal ganglia projecting to the striatum, which also receives inputs from the amygdala, hippocampus, and medial prefrontal cortex (Pechtel and Pizzagalli, 2011). Alterations in motivational processing have been found in ELS-exposed individuals in both the anticipation and receipt of rewards and punishments. This research has been informed by clinical theory noting that ELS-exposed individuals show both a lack of motivation (e.g., anhedonia, depression) and increased hedonic-seeking behavior (e.g., substance use, risk-taking; Pechtel and Pizzagalli, 2011; Fareri and Tottenham, 2016).
Regarding the anticipatory processing of rewards, individuals exposed to a range of ELS experiences (e.g., maltreatment, institutionalized neglect, family adversity, cumulative life stress) consistently demonstrate either diminished neural activation of basal ganglia and striatum regions to the anticipation of rewards or reduced neural differentiation to rewards of different values (reviewed in Pechtel and Pizzagalli, 2011; Mehta et al., 2010; Boecker et al., 2014). Multiple studies also link ELS to reduced neural activation to reward receipt across monetary rewards (Hanson et al., 2015a, Hanson et al., 2015b, Hanson et al., 2015c and positive social-affective cues (e.g., happy faces; Goff et al., 2013). However, links between early life stress and diminished neural activation to receipt of reward are not uniformly discovered, with some studies reporting no association between ELS and receipt (reviewed in Pechtel and Pizzagalli, 2011) and another study reporting exaggerated activation of other reward-related brain regions to reward receipt (e.g., putamen, insula; Boecker et al., 2014). The developmental timing of ELS may help account for inconsistencies in the extant literature. One prospective longitudinal study found that stress experienced before grade 3, but not afterwards, predicted reduced activation to reward (Hanson et al., 2015a). Another study in young adults similarly found that stress experienced before age 10, but not current life stress, predicted reduced activation to rewards cues (Birn et al., 2017).
Particularly promising for its translational implications, the diminished neural processing of both reward cue and receipt has been found to predict clinical symptoms amongst ELS-exposed individuals. Notably this includes both indices of low motivation (e.g., anhedonia, depressive symptoms; Goff et al., 2013; reviewed in Pechtel and Pizzagalli, 2011) as well as indices of impulsivity and risk-taking behavior (e.g., ADHD symptoms, Birn et al., 2017; Boecker et al., 2014). A critical next step for the clinical intervention area is determining the extent to which such processes are malleable.
1.3.1. Candidate motivational links to addiction
Diminished neural activity to reward cues related to ELS might reflect one side of the asymmetry often found in addiction, characterized by decreased reactivity to non-drug cues and increased reactivity to drug cues (Berkman, 2015). This is especially likely because the stimuli used in the studies reviewed above are normatively rewarding (e.g., money) but are often not personalized to the substance use patterns of the individual participant. One future direction, is to examine the effects of ELS on reactivity to both non-drug and drug cues among participants who use them. It is possible that adolescents with ELS who use substances, or who are at risk of developing substance use disorders, would exhibit the drug/non-drug asymmetry in reward response to an even greater degree than non-exposed adolescents.
Interestingly, ELS also may increase the reactivity of neural systems to substances of abuse (Oswald et al., 2014). A positron emission tomography (PET) study recently demonstrated higher ventral striatum dopamine responses to amphetamine administration amongst participants with a history of childhood trauma, an effect that was partially mediated by higher levels of perceived stress (Oswald et al., 2014). Research suggests that similar links between ELS and addiction may be at play with drug-related cues. For example, among cocaine-dependent individuals (abstinent in an inpatient unit), a history of physical emotional or sexual abuse predicted heightened limbic activity, which included the striatum, to cocaine-related cues (Regier et al., 2017). Taken together, these results suggest that adolescents and adults exposed to ELS may have neural-based vulnerabilities such that they have higher motivation towards greater risk-taking behavior and greater reward-related reactivity to pharmacological effects of substances of abuse.
1.4. Executive function
Executive function (EF) refers to an individual's ability to engage in effective goal-directed behavior across domains of working memory, response inhibition, and attention shifting (Miyake and Friedman, 2012). The term EF originates from the neurocognitive literature and evidence that the coordination of complex goal-directed is closely linked to the effective function of the prefrontal cortex (or “frontal executive”). Notably, successful EF skills require both the effective function of the prefrontal cortex to recruit, coordinate, and execute appropriate behavior as well as the multitude of ‘lower-level’ brain regions related to processes such as sensory, affective, reward, memory, and motor control, to provide information and carry out the actions directed by prefrontal areas. EF is closely related to, and shares overlap with other effortful cognitive terms such as self-regulation, emotion regulation, and effortful control. A wide body of literature has demonstrated associations between ELS and poor executive function (Pechtel and Pizzagalli, 2011). ELS-exposed children may have altered executive function for a multitude of reasons, including lower caregiver scaffolding of complex skills, less consistent monitoring and reinforcement of self-regulatory skills, and the vulnerability of key prefrontal brain regions to excessive cortisol exposure (Blair et al., 2016).
Determining the pathways through which stress alters executive function is an area of particular interest given the compelling links between strong executive function and positive outcomes for ELS-exposed children. For example, executive function skills have been found to mediate links between maltreatment exposure and both academic and socio-emotional competence in young children, in addition to moderating links between exposure to multiple types of maltreatment and subsequent externalizing problems, within maltreated samples (Pears et al., 2010; Horn et al., 2018).
At the structural level, atypical white matter is associated with maltreatment-linked post-traumatic stress disorder in early childhood, with specific reductions in prefrontal cortical regions and the right temporal lobe (De Bellis et al., 2002). Amongst a sample of post-institutionalized children, reductions in prefrontal white matter tracts, and those linking the prefrontal cortex to temporal (e.g., auditory processing regions) predicted lower cognitive performance on tests of spatial planning and visual learning/memory (Hanson et al., 2013). Consistent with such white matter reductions, research employing ERP techniques has found that ELS predicts a longer latency for attention-related ERP components (e.g., the N200; McDermott et al., 2012) and slower reaction times (McDermott et al., 2012; Mueller et al., 2010). The aforementioned white matter reductions could underlie slower processing speeds and electrophysiological processes and are likely to negatively affect a range of executive function processes.
Much of the neuroimaging research on executive function amongst ELS-exposed samples focuses on tasks requiring attentional control or inhibition. This work demonstrates altered neural processing in a range of prefrontal and sensory processing areas. In adolescent post-institutionalized adoptees (versus controls), prior institutionalization has been linked to greater activation to inhibit trials on a Go/NoGo task in a range of sensory processing, conflict monitoring (anterior cingulate cortex) and inhibitory control (inferior frontal cortex) regions (Mueller et al., 2010). Somewhat in contrast, maltreated youth in foster care (versus controls) have been found to exhibit less activation in similar brain regions (e.g. anterior cingulate cortex; Bruce et al., 2009; Jankowski et al., 2017) in other Go/NoGo task studies. Such inconsistencies should be resolved in future work but differences may be influenced by developmental timing, types of ELS, or subtle differences in task demands. Using ERP techniques, early adversity has also been associated with reduced magnitude of neural responses related to attention and inhibitory control (e.g., N200 component; P300 component), which are theorized to contribute to self-regulatory difficulties (Loman et al., 2013; McDermott et al., 2012).
Performance monitoring skills (i.e. the ability to process when one has made an error via internal or external feedback) are also highlighted as key neural vulnerabilities for ELS-exposed children, with implications for goal-directed behavior. This includes research on both children's response monitoring (e.g., the error-related negativity (ERN), though to reflect awareness of a behavioral error) and feedback monitoring (e.g., the feedback-related negativity (FRN), thought to reflect processing of feedback about performance). For example, during executive function tests of selective attention/inhibition (i.e., the Flanker task), both children in regular foster care and those exposed to institutionalized neglect exhibited a lack of exaggerated performance monitoring ERPs in childhood (Bruce et al., 2009; McDermott et al., 2012). Notably, more exaggerated performance monitoring ERPs have been documented to be protective amongst ELS-exposed samples, with larger feedback monitoring ERPs predicting better inhibitory control performance amongst maltreated children with high impulsivity (Roos et al., 2015). Other research shows that larger response monitoring ERPs buffer post-institutionalized children from the development of externalizing behavior problems (Troller-Renfree et al., 2016).
1.4.1. Candidate executive function links to addiction
Substantial literature links childhood executive function broadly, and inhibition deficits, specifically, to substance use and addiction risk amongst ELS-exposed individuals (reviewed in Edalati and Krank, 2016). Executive function has also been shown to mediate links between factors closely linked to ELS (e.g., poor discipline and parent substance use) and the early onset of substance use (Pears et al., 2007). At the neural level, systematic reviews of inhibition tasks note lower activation amongst individuals with substance dependence in both prefrontal neural networks (i.e., dorsolateral prefrontal cortex, inferior frontal gyrus) during fMRI tasks and blunted inhibition-linked ERPs (i.e., N200; Luijten et al., 2014). Importantly, in adolescence, lower prefrontal activation during inhibition tasks has also predicted the onset of substance use, suggesting that these neural alterations can precede addiction (Mahmood et al., 2013).
The performance monitoring literature similarly highlights the relevance of alterations in neural markers of feedback and response monitoring amongst individuals at-risk for, or with, addiction-related challenges. The aforementioned systematic review of inhibition and addiction documented key alterations in response-monitoring neural processes (Luijten et al., 2014). This includes both blunted activation of the anterior cingulate and blunting of the ERN components associated with response monitoring amongst individuals with substance use disorders compared to those without. This work has been paralleled in adolescents, with smaller performance monitoring ERPs (i.e., ERN and FRN) linked to problematic internet use (conceptualized as behavioral addiction) and addiction-related risk (e.g., family history of addiction; Yau et al., 2015; Euser et al., 2013).
Taken together, challenges in the recruitment of neural systems supportive of goal-directed behavior in ELS-exposed individuals, combined with limited performance monitoring skills, likely contribute to the increased susceptibility to substance use in these individuals. ELS-exposed people might experience difficulty in inhibiting impulsive response tendencies, monitoring the consequences of their addiction-related behaviors, and re-aligning their actions to promote long-term goals. Such challenges may be both influenced and compounded by the noted alterations sensory, affective, and motivational neural systems (Pechtel and Pizzagalli, 2011).
Very few studies have yet directly examined the extent to which alterations in the neural correlates of executive function prospectively contribute to substance use and addiction-related risk in ELS-exposed individuals. However, given results suggesting that behavioral and neural markers of executive function predict resiliency from mental health and behavioral phenotypes associated with later addiction (e.g., externalizing problems), directly targeting these neural systems represents a promising opportunity for translational neuroscience efforts. Because of the prolonged developmental plasticity of prefrontal regions supportive of planning and executing goal-directed behavior, interventions targeted at supporting skills may be of particular value.
2. A translational neuroscience approach to addiction prevention for ELS-exposed children
To illustrate the potential value of a translational neuroscience approach, in this section we describe how this approach has been applied in the context of several early intervention programs that aim to increase children's self-regulation, broadly conceptualized as a child's ability to effortfully control their attention, emotion, and behavior (Blair and Diamond, 2008). These programs, developed at the Oregon Social Learning Center by authors Pears and Fisher of the present paper, have been adapted to support positive outcomes for young children (ages 3–5 years) from a range of backgrounds (e.g. children living in foster care, children with identified developmental and/or behavioral difficulties, and children living in high-poverty, high-crime neighborhoods) who experience self-regulatory difficulties and associated negative outcomes including school failure, antisocial behavior, substance misuse and addiction. It is notable that other early intervention programs (e.g. Tools of the Mind, Attachment and Biobehavioral Catch-up for Toddlers) also show promise for improving at-risk children's self-regulation through school and home-based techniques (Blair and Raver, 2014; Lind et al., 2017). Here, we discuss how translational neuroscience principles have complemented and informed our early intervention/prevention research in addition to delineating how translational neuroscience approaches could be used in the future to understand individual differences in outcomes and clarify mechanisms of change.
These interventions (i.e., Multidimensional Treatment Foster Care – Preschool, MTFC-P; Kids In Transition to School, KITS) are grounded in social learning theory about the etiology of childhood behavior problems that are subsequently linked to adjustment problems such substance use in adolescence and adulthood (Gilliam and Fisher, 2014; Pears et al., 2015). Although the specific techniques of MTFC-P and KITS Programs have been described in detail, elsewhere, a few key tenets are noted here that are conceptualized as particularly important to the subsequently discussed areas of relevance to translational neuroscience (Fisher and Gilliam, 2012; Pears et al., 2018). The premise of both is that improving young children's self-regulation and lower household stress can bend negative long-term trajectories towards more positive outcomes.
In order to promote adaptive function, these programs were developed based on the assumption that outcomes would be most positive when children are cared for in the context of their family and community. Accordingly, these programs work to strengthen children's skills in naturalistic settings that include home, school, and community contexts, as opposed to residential settings. The programs aim to employ similar strategies across caregiver contexts (i.e. parents, teachers, individual therapists) that increase consistency in the lives of children who are likely to have previously experienced low consistency of both support and discipline within and across multiple caregivers. Specific strategies include exposing children to supportive, consistent and contingent environments in which positive and prosocial behavior is increased through targeted reinforcement (e.g., caregiver feedback about a child's behavior). Such positive feedback can include direct and specific praise, caregiver attention, immediate rewards such as stickers, or the use of token economies. Negative parenting strategies (e.g., harsh discipline, inconsistent monitoring) are targeted for reduction, through the use of alternate strategies (e.g. ignoring, age-appropriate time outs, privilege removal) to manage unwanted behaviors in a calm manner. Positive parenting strategies (e.g., creating predictable routines, enjoyable family activities) are promoted to increase caregiver sensitivity and predictability. Principles from applied behavior analysis are also incorporated to identify the function of children's dysregulated behaviors and alter the environmental consequences to support a child's ability to effectively meet prosocial expectations (e.g., focusing attention, following caregiver's directions, and inhibiting impulsive or aggressive responses). Taken together, children's self-regulatory skills are scaffolded, in that expectations are set to enable a high frequency of success while gradually increasing in difficulty. Prosocial behavior is consistently reinforced in ways that build the caregiver-child relationship. Simultaneously, dysregulated behaviors are not reinforced by caregiver attention and coercive caregiver-child interactions, which contribute to family stress and harm the caregiver-child relationship, are dramatically reduced or eliminated. By intervening on children's environments in ways that improve the caregiver-child relationship and reduce caregiver stress, it is theorized that the programs enable caregivers to more effectively buffer children from stressful events that do occur supporting normalized trajectories of stress-system function (Hostinar et al., 2014).
These key reinforcement-based tenets are shared across programs, although there are also program-specific strategies of varying intensity unique to the populations of children and families they serve (e.g., one-on-one phone support for foster parents, workshops focused on school engagement for parents in at-risk neighborhoods). The KITS Program intended dosage is 24 2-h sessions for children and 12 2-h sessions for the parents. KITS also involves teaching children explicit strategies for behavioral and emotion regulation in the classroom (Pears et al., 2018). The MTFC-P dosage is somewhat more variable based on caregiver outreach to clinicians, but typically involves 6–12 months of services. The caregivers receive intensive training along with weekly meetings, daily telephone contact and 24-h support availability while the children receive individual skills-based training with a therapist alongside a therapeutic playgroup (Fisher and Stoolmiller, 2008). It is notable that these interventions include key components identified as important to improving executive function, including strategies that change children's environments and allow for opportunities of increasing difficulty to practice skills gains (Diamond and Lee, 2011).
2.1. Evidence for changes in self-regulation and stress-physiology
Early program research demonstrated that children in foster care participating in MTFC-P exhibited fewer behavior problems from pre-to-post intervention, while children in regular foster care exhibited an increase in behavior problems (Fisher et al., 2000). Notably, these changes in child behavior were accompanied by improvements in caregiver parenting (e.g., more consistent discipline, monitoring, and positive reinforcement; Fisher et al., 2000). Similarly, children who participated in the KITS intervention have exhibit improvements in behavior problems and self-regulation (i.e. lower disinhibited behavior problems, better emotion regulation, better executive function) and parents demonstrate concurrent parenting improvements (Pears et al., 2012; Pears et al., 2013, 2015).
Results across programs have demonstrated the potential for these types of interventions to restore vulnerable children's stress-physiology to more normative profiles across time and specific to the school entry context (reviewed in Gilliam and Fisher, 2014; Roos et al., 2018; Graham et al., 2017).
2.2. Evidence for changes in neural indices of response monitoring
Prior research (detailed above) highlighted young children's impaired performance monitoring as a key neural process linked to multiple types of ELS (maltreatment, institutionalized deprivation; Bruce et al., 2009; McDermott et al., 2012). Further, previously institutionalized children who were placed in foster care, versus experiencing continued institutionalization, also exhibited changes in neural indices of response monitoring towards more normative profiles, suggesting the plasticity of these neural systems (McDermott et al., 2012). A key hypothesis was whether MTFC-P and KITS would change children's ability to process feedback about their behavior given the focus on creating consistent and predictable environmental contingencies.
For MTFC-P, a subset of children participating in a larger randomized efficacy trial were recruited post-intervention to examine indices of response monitoring. ERP techniques were used to assess neural activity in response to children's own behavior (e.g., ERN) and feedback about their behavior (e.g., FRN). Children included those in the MTFC-P intervention, those in regular foster care, and community controls. Results indicated that children in MTFC-P and the community control exhibited significant neural differentiation based on feedback about their performance in incorrect, versus correct, trials. However, children in regular foster care did not have differentiation between feedback types, suggesting difficulty at the neural level for processing important environmental feedback about their behavior. Notably, children (across groups) at this young age did not exhibit strong response-monitoring ERNs, which was consistent with the late maturing ACC brain regions and prior research using the flanker task.
A subset of children participating in a larger randomized efficacy trial of KITS that focused on children with developmental disabilities and delays and significant behavior problems participated in an ERP study at both pre-and post-intervention assessment. Consistent with the prior results, children who had received the KITS intervention exhibited significant enhancement in neural indices of feedback monitoring, while children who received services as usual did not exhibit changes in the FRN and had a significantly smaller post-intervention FRN, versus KITS intervention children (McDermott et al., 2017).
Interestingly, despite the aforementioned longitudinal evidence for improvements in self-regulation and behavior amongst children in these interventions, the children did not exhibit specific improvements in the flanker task measures of behavioral performance. However, larger FRNs were associated with better performance overall, suggesting that the ability to understand feedback may be importantly linked to regulate behavior on task. One interpretation is that the neural indices of response monitoring may change prior to observable changes in the subsequent behavioral outcomes. It is also possible that such changes in response monitoring are relevant to inhibitory control for some, but not all children. In a separate sample of children in foster care we documented that the FRN was linked to both higher inhibitory control performance and a behavioral index of response monitoring (i.e., post-error slowing), but only for children with high impulsivity (Roos et al., 2015). Future moderation analyses that include sufficiently powered sample sizes may be particularly valuable to understanding how interventions support positive development for children with varying developmental profiles and vulnerabilities to addiction (Del Giudice and Ellis, 2016).
3. Ongoing opportunities and challenges for translational neuroscience program research
Results to date provide exciting evidence for the potential of interventions focused on the promotion of self-regulatory skills to improve a key aspect of neural processing supportive of regulated behavioral function. Notably, this is just one measure, but it is closely linked to the theory of intervention change (e.g., supporting a more consistent and contingent environment and child regulation strategies) and a candidate neural alteration to later life substance use and addiction-related problems.
Recent 4-year follow-up findings of the KITS randomized control trial for children in foster care have key relevance to addiction (Pears et al., 2016). Specifically, the KITS intervention was shown to decrease positive attitudes towards alcohol use and antisocial behaviors. Furthermore, there was an indirect effect of the intervention on reducing children's association with deviant peers, through increased self-competence. Each of these outcomes (i.e., self-competence, positive attitudes towards alcohol, antisocial behaviors, and associations with deviant peers) are important risk factors for early onset substance use and addiction. Children in these interventions have not had time for sufficient follow-up through adolescence to directly determine the extent to which substance abuse is prevented; however other programs based on the same approaches with older children and adolescents do demonstrate longitudinal reductions in substance use (i.e., Multi-Dimensional Treatment Foster Care; Middle School Success; Kim and Leve, 2011; Smith et al., 2010).
Ongoing plans include following up the present findings to links improvements in neural indices of response monitoring to later life self-regulation. This research may be particularly helpful for linking the FRN to specific risk-taking behaviors or its role as a buffer for high-impulsivity children to prevent the development of serious externalizing and addiction-related problems.
Another translational neuroscience application of the present findings is to test the utility of brief biologically based measurements, such as the FRN, for identifying endophenotypes of children who exhibit self-regulatory difficulties due to underlying response monitoring impairment. Individual differences in these measurements might serve as indicators of specific deficits and be predictive of response to treatment. Autism research has made notable progress in the use of translational neuroscience for developing endophenotypic models of altered neural responsivity to language and social stimuli (e.g. Lloyd-Fox et al., 2013; Edwards et al., 2017), but there are limited such marker in ELS-exposed children. Ongoing research could examine the potential utility of the FRN as a screener to help identify children in particular need of early self-regulatory focused interventions, such as KITS or MTFC-P to support positive development. In combination with clinical judgement, blunted FRNs could be used as a potential biomarker to identify children, post-intervention, who are in need of more intensive services to support response monitoring skills.
Finally, we note the conceptualization of the KITS and MTFC-P programs as both early intervention and prevention-based programs. The goal is for such intervention efforts to promote positive function for children presenting with, or at-risk for, the early development of mental health and behavior problems while also preventing the onset of more serious problems (e.g. addiction) later in life. From a translational neuroscience lens, these programs aim to alter trajectories of individuals' neurobiological function during the highly plastic developmental window from 3 to 5 years of age. Theoretically, intervening during this time period should minimize the further neurobiological embedding of high-stress environments and dysregulated behaviors, thus preventing the onset of children's future mental health and behavioral problems. substantially reducing long-term mental health, achievement, and economic costs. Indeed, economic analysis of MTFC-P indicated significant cost-savings for children in foster care through increases in permanent placements for two-years post randomization (Lynch et al., 2014). KITS has also been shown to be a cost-effective program for reducing clinically significant mental health and behavioral problems (both internalizing and externalizing) in the first-year post intervention (Lynch et al., 2017). However, the extent to which effects are preventive and sustainable is an empirical question that needs to be followed up with longer term research.
Future iterations of KITS and MTFC-P may benefit from empirically assessing the ‘active ingredients’ of the programs so as to determine how to most effectively and efficiently serve the largest number of families, given limited budgets across funding environments. As noted by leading experts in intervention science, we should move beyond questions of ‘can we move the bar’ to more precise questions about the mechanisms of change including what works in what contexts, and why (Center on the Developing Child, 2018). There may also be value to applying innovative approaches beyond RCTs to determine the active components of interventions, as is advocated for by precision medicine approaches such as the Multiphase Optimization Strategy (MOST) approach (Collins, 2014). Examining person-level moderators of program efficacy, including both caregiver (e.g. mental health, executive function) and child qualities (e.g. gender or sex, co-morbidities) is also important to understand for whom programs work. Attention to child sex and gender may be particularly important in addiction prevention given that males tend to use substances and develop substance use disorders at higher rates than females, with research highlighting biochemical, neuroanatomical and socialization pathways (Thatcher et al., 2010; Kuhn, 2015). Given emerging work suggesting sex-based differences in brain development following early life stress, using translational neuroscience approaches to delineate differential vulnerability and sensitivity to intervention may be particularly valuable (Clarke et al., 2013; Bale and Epperson, 2015; Chen and Baram, 2016).
4. Conclusion
Neuroscience evidence highlights a range of differences between ELS-exposed and non-exposed individuals in sensory, affective, motivational, and executive function processes that are linked to addiction. At the same time, there is much to learn in this area such as clarity regarding how each cognitive domain may confer specific, versus additive, risk for addiction-related outcomes. Further, the relative malleability of each cognitive domain to intervention across developmental periods is not well-understood. Subsequent research might also examine the relative roles of early life versus proximal stressors in contributing neurocognitive vulnerability to substance abuse given that many ELS experiences are established risk factors for later life trauma and stress exposure (Whitfield et al., 2003). Similarly, there is an increasingly well-described separate body of research linking stress exposure during the prenatal period to altered neurobiological development with candidate pathways for addiction (see Horn et al., 2018 in this journal issue).
With the potential for alternations in such a range of cognitive processes contributing to psychopathology and addiction, the translational neuroscience approach of using theory-driven investigations of ELS-affected neural systems that are amenable to intervention can be particularly powerful for developing targeted programs (Shonkoff, 2016). For both the KITS and MTFC-P programs, evidence for stronger performance monitoring amongst intervention-randomized children provided important support for the theories of change regarding the emphasis on consistent, contingent feedback in low-stress environments. Moving forwards, increased precision about the relevance of stressor timing, type and windows of neural plasticity may be key for understanding what works and for whom in intervention research. Such work undoubtedly requires resources including both funding and non-traditional partnerships between researchers with expertise in neuroscience and intervention science. At the same time, there is substantial untapped potential for employing such translational neuroscience techniques for developing innovative interventions that can better mitigate addiction-related outcomes for vulnerable ELS-exposed children.
Funding
Leslie E. Roos received support from HHS-2014-ACF-ACYF-CA-0803 and P50 DA035763.
Sarah R. Horn received support from the National Science Foundation Graduate Research Fellowship Program.
Elliot T. Berkman received support from NIH grant P50 DA035763 and CA211224.
Katherine Pears received support from NIH grant P50 DA035763.
Philip A. Fisher received support from NIH grants R01 HD075716 and P50 DA035763.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.10.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Asensio S., Romero M.J., Palau C., Sanchez A., Senabre I., Morales J.L., Romero F.J. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict. Biol. 2010;15(4):504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T. Functional neural predictors of addiction outcomes. In: Wilson S., editor. The Wiley-Blackwell Handbook on the Neuroscience of Addiction. Wiley; New York: 2015. pp. 503–526. [Google Scholar]
- Birn R.M., Roeber B.J., Pollak S.D. Early childhood stress exposure, reward pathways, and adult decision making. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114(51):13549–13554. doi: 10.1073/pnas.1708791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Diamond A. Biological processes in prevention and intervention: the promotion of self-regulation as a means of preventing school failure. Dev. Psychopathol. 2008;20(3):899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Raver C.C. Closing the achievement gap through modification of neurocognitive and neuroendocrine function: results from a cluster randomized controlled trial of an innovative approach to the education of children in kindergarten. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Raver C.C., Finegood E.D. Self-regulation and developmental psychopathology: experiential canalization of brain and behavior. In: Cicchetti, editor. Developmental Psychopathology. 3rd. III. John Wiley & Sons, Inc; New York: 2016. pp. 484–522. 2016. [Google Scholar]
- Boecker R., Holz N.E., Buchmann A.F., Blomeyer D., Plichta M.M., Wolf I., Baumeister S., Meyer-Lindenberg A., Banaschewski T., Brandeis D., Laucht M. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., McDermott J.M., Fisher P.A., Fox N.A. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: a preliminary study with preschool-aged foster children. Prev. Sci. 2009;10(2):129–140. doi: 10.1007/s11121-008-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center on the Developing Child . 2018. Frontiers of Innovation.https://developingchild.harvard.edu/innovation-application/frontiers-of-innovation/ Retrieved from. [Google Scholar]
- Chen Y., Baram T.Z. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41(1):197. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatr. 2013;18(6):666. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Cohen M.M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L.M. In Emerging Methods in Family Research. Springer; Cham: 2014. Optimizing family intervention programs: the multiphase optimization strategy (MOST) pp. 231–244. [Google Scholar]
- Curtis W.J., Cicchetti D. Affective facial expression processing in 15-month-old infants who have experienced maltreatment: an event-related potential study. Child. Maltreat. 2013;18(3):140–154. doi: 10.1177/1077559513487944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Shifflett H., Iyengar S., Beers S.R., Hall J., Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol. Psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Del Giudice M.D., Ellis B.J. Evolutionary foundations of developmental psychopathology. In: Cichetti D., editor. Developmental Psychopathology Vol. 1: Theory and Method. third ed. Wiley & Sons; New York: 2016. [Google Scholar]
- Diamond A., Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge K.A., Malone P.S., Lansford J.E., Miller S., Pettit G.S., Bates J.E. A dynamic cascade model of the development of substance-use onset. Monogr. Soc. Res. Child Dev. 2009;74(3):1–120. doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edalati H., Krank M.D. Childhood maltreatment and development of substance use disorders: a review and a model of cognitive pathways. Trauma Violence Abuse. 2016;17(5):454–467. doi: 10.1177/1524838015584370. [DOI] [PubMed] [Google Scholar]
- Edmiston E.E., Wang F., Mazure C.M., Guiney J., Sinha R., Mayes L.C., Blumberg H.P. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediatr. Adolesc. Med. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L.A., Wagner J.B., Tager-Flusberg H., Nelson C.A. Differences in neural correlates of speech perception in 3 Month olds at high and low risk for autism spectrum disorder. J. Autism Dev. Disord. 2017;47(10):3125–3138. doi: 10.1007/s10803-017-3222-1. [DOI] [PubMed] [Google Scholar]
- Elton A., Smitherman S., Young J., Kilts C.D. Effects of childhood maltreatment on the neural correlates of stress‐and drug cue‐induced cocaine craving. Addict. Biol. 2015;20(4):820–831. doi: 10.1111/adb.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser A.S., Greaves-Lord K., Crowley M.J., Evans B.E., Huizink A.C., Franken I.H. Blunted feedback processing during risky decision making in adolescents with a parental history of substance use disorders. Dev. Psychopathol. 2013;25(4pt1):1119–1136. doi: 10.1017/S0954579413000412. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Tottenham N. Effects of early life stress on amygdala and striatal development. Developmental Cognitive Neuroscience. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Berkman E.T. Designing interventions informed by scientific knowledge about effects of early adversity: a translational neuroscience agenda for next-generation addictions research. Current Addiction Reports. 2015;2(4):347–353. doi: 10.1007/s40429-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Gilliam K.S. Multidimensional treatment foster care: an alternative to residential treatment for high risk children and adolescents. Intervencion psicosocial. 2012;21(2):195. doi: 10.5093/in2012a20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Stoolmiller M. Intervention effects on foster parent stress: associations with child cortisol levels. Dev. Psychopathol. 2008;20(3):1003–1021. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P.A., Gunnar M.R., Chamberlain P., Reid J.B. Preventive intervention for maltreated preschool children: impact on children's behavior, neuroendocrine activity, and foster parent functioning. J. Am. Acad. Child Adolesc. Psychiatr. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam K.S., Fisher P.A. Evidence-based Approaches for the Treatment of Maltreated Children. Springer; Dordrecht: 2014. Multidimensional treatment foster care for preschoolers: a program for maltreated children in the child welfare system; pp. 145–162. [Google Scholar]
- Goff B., Gee D.G., Telzer E.H., Humphreys K.L., Gabard-Durnam L., Flannery J., Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Pears K., Kim H., Bruce J., Fisher P.A. Effects of a school readiness intervention on hypothalamus-pituitary-adrenal axis functioning and school adjustment for children in foster care. Dev. Psychopathol. 2017:1–14. doi: 10.1017/S0954579417001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatr. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C.A., Dowdle L.T., Naselaris T., Canterberry M., Cortese B.M. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. 2014;143:206–212. doi: 10.1016/j.drugalcdep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Adluru N., Chung M.K., Alexander A.L., Davidson R.J., Pollak S.D. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 2013;84(5):1566–1578. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Albert D., Iselin A.M.R., Carre J.M., Dodge K.A., Hariri A.R. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc. Cognit. Affect Neurosci. 2015;11(3):405–412. doi: 10.1093/scan/nsv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hariri A.R., Williamson D.E. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry. 2015;78(9):598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015;27(4pt2):1611–1619. doi: 10.1017/S0954579415000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Binder E.B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp. Neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hein T.C., Monk C.S. Research Review: neural response to threat in children, adolescents, and adults after child maltreatment–a quantitative meta‐analysis. JCPP (J. Child Psychol. Psychiatry) 2017;58(3):222–230. doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- Herringa R.J., Burghy C.A., Stodola D.E., Fox M.E., Davidson R.J., Essex M.J. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol. Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(4):326–334. doi: 10.1016/j.bpsc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S.R., Roos L.E., Beauchamp K.G., Flannery J.E., Fisher P.A. Polyvictimization and externalizing symptoms in foster care children: the moderating role of attention/executive functioning. J. Trauma & Dissociation. 2018;19(3):307–324. doi: 10.1080/15299732.2018.1441353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Sullivan R.M., Gunnar M.R. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: a review of animal models and human studies across development. Psychol. Bull. 2014;140(1):256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., McCormack K.M., Grand A.P., Sawyer N.T., Zhang X., Maestripieri D., Hu X., Sanchez M.M. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol. Mood Anxiety Disord. 2013;3(1):21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski K.F., Bruce J., Beauchamp K.G., Roos L.E., Moore W.E., Fisher P.A. Preliminary evidence of the impact of early childhood maltreatment and a preventive intervention on neural patterns of response inhibition in early adolescence. Dev. Sci. 2017;20(4) doi: 10.1111/desc.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Leve L.D. Substance use and delinquency among middle school girls in foster care: a three-year follow-up of a randomized controlled trial. J. Consult. Clin. Psychol. 2011;79(6):740–750. doi: 10.1037/a0025949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.M., Iacono W.G., McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99(12):1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol. Ther. 2015;153:55–78. doi: 10.1016/j.pharmthera.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind T., Raby K.L., Caron E.B., Roben C.K., Dozier M. Enhancing executive functioning among toddlers in foster care with an attachment-based intervention. Dev. Psychopathol. 2017;29(2):575–586. doi: 10.1017/S0954579417000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E., Charman T., Murphy D., Johnson M.H. Reduced neural sensitivity to social stimuli in infants at risk for autism. Proc. R. Soc. Ser. B. 2013;280 doi: 10.1098/rspb.2012.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman M.M., Johnson A.E., Westerlund A., Pollak S.D., Nelson C.A., Gunnar M.R. The effect of early deprivation on executive attention in middle childhood. JCPP (J. Child Psychol. Psychiatry) 2013;54(1):37–45. doi: 10.1111/j.1469-7610.2012.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Nishino T., Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M., Machielsen M.W., Veltman D.J., Hester R., de Haan L., Franken I.H. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J. Psychiatry Neurosci. 2014;39(3):149–169. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch F.L., Dickerson J.F., Pears K.C., Fisher P.A. Cost effectiveness of a school readiness intervention for foster children. Child. Youth Serv. Rev. 2017;81:63–71. doi: 10.1016/j.childyouth.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch F.L., Dickerson J.F., Saldana L., Fisher P.A. Incremental net benefit of early intervention for preschool-aged children with emotional and behavioral problems in foster care. Child. Youth Serv. Rev. 2014;36:213–219. doi: 10.1016/j.childyouth.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood O.M., Goldenberg D., Thayer R., Migliorini R., Simmons A.N., Tapert S.F. Adolescents' fMRI activation to a response inhibition task predicts future substance use. Addict. Behav. 2013;38(1):1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliken A.C., Katz L.F. Exploring the impact of parental psychopathology and emotion regulation on evidence-based parenting interventions: a transdiagnostic approach to improving treatment effectiveness. Clin. Child Fam. Psychol. Rev. 2013;16(2):173–186. doi: 10.1007/s10567-013-0132-4. [DOI] [PubMed] [Google Scholar]
- McDermott J.M., Pears K.C., Bruce J., Kim H.K., Roos L., Yoerger K.L., Fisher P.A. Improving kindergarten readiness in children with developmental disabilities: changes in neural correlates of response monitoring. Appl. Neuropsychol.: Child. 2017:1–13. doi: 10.1080/21622965.2017.1286239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J.M., Westerlund A., Zeanah C.H., Nelson C.A., Fox N.A. Early adversity and neural correlates of executive function: implications for academic adjustment. Developmental Cognitive Neuroscience. 2012;2:S59–S66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch. Gen. Psychiatr. 2012;69(11):1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatr. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M.A., Gore-Langton E., Golembo N., Colvert E., Williams S.C.R., Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cognit. Neurosci. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P. The nature and organization of individual differences in executive functions: four general conclusions. Curr. Dir. Psychol. Sci. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C., Maheu F.S., Dozier M., Peloso E., Mandell D., Leibenluft E. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova Y.S., Knodt A.R., Radtke S.R., Hariri A.R. Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol. Psychiatr. 2016;21(3):348–356. doi: 10.1038/mp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K.A., Conner B.T., Kendall P.C. Internalizing disorders and substance use disorders in youth: comorbidity, risk, temporal order, and implications for intervention. Clin. Psychol. Rev. 2011;31(1):104–112. doi: 10.1016/j.cpr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Oswald L.M., Wand G.S., Kuwabara H., Wong D.F., Zhu S., Brasic J.R. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology. 2014;231(12):2417–2433. doi: 10.1007/s00213-013-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A., Geier C.F., Ordaz S.J., Teslovich T., Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Developmental Cognitive Neuroscience. 2011;1(4):517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K.C., Carpenter L., Kim H.K., Peterson E., Fisher P.A. The kids in transition to school program. In: Mashburn A.J., LoCasale-Crouch J., Pears K.C., editors. Kindergarten Transition and Readiness: Promoting Cognitive, Social-emotional, and Self-regulatory Development (283-302) Springer; New York: 2018. [Google Scholar]
- Pears K.C., Fisher P.A., Bruce J., Kim H.K., Yoerger K. Early elementary school adjustment of maltreated children in foster care: the roles of inhibitory control and caregiver involvement. Child Dev. 2010;81(5):1550–1564. doi: 10.1111/j.1467-8624.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K.C., Fisher P.A., Kim H.K., Bruce J., Healey C.V., Yoerger K. Immediate effects of a school readiness intervention for children in foster care. Early Educ. Dev. 2013;24(6):771–791. doi: 10.1080/10409289.2013.736037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K.C., Kim H.K., Fisher P.A. Effects of a school readiness intervention for children in foster care on oppositional and aggressive behaviors in kindergarten. Child. Youth Serv. Rev. 2012;34(12):2361–2366. doi: 10.1016/j.childyouth.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K.C., Kim H.K., Fisher P.A. Decreasing risk factors for later alcohol use and antisocial behaviors in children in foster care by increasing early promotive factors. Child. Youth Serv. Rev. 2016;65:156–165. doi: 10.1016/j.childyouth.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K.C., Kim H.K., Healey C.V., Yoerger K., Fisher P.A. Improving child self-regulation and parenting in families of pre-kindergarten children with developmental disabilities and behavioral difficulties. Prev. Sci. 2015;16(2):222–232. doi: 10.1007/s11121-014-0482-2. [DOI] [PubMed] [Google Scholar]
- Pears K., Capaldi D.M., Owen L.D. Substance use risk across three generations: the roles of parent discipline practices and inhibitory control. Psychol. Addict. Behav. 2007;21(3):373. doi: 10.1037/0893-164X.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Rao U., Chen L.A., Bidesi A.S., Shad M.U., Thomas M.A., Hammen C.L. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol. Psychiatry. 2010;67(4):357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier P.S., Monge Z.A., Franklin T.R., Wetherill R.R., Teitelman A., Jagannathan K., Suh J.J., Wang Z., Young K.A., Gawrysiak M., Langleben D.D., Kampman K.M., O'Brien C.P., Childress A.R. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict. Biol. 2017;22(6):1768–1777. doi: 10.1111/adb.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem M.M., Alink L.R., Out D., Van Ijzendoorn M.H., Bakermans-Kranenburg M.J. Beating the brain about abuse: empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 2015;27(2):507–520. doi: 10.1017/S0954579415000127. [DOI] [PubMed] [Google Scholar]
- Roos L.E., Pears K., Bruce J., Kim H.K., Fisher P.A. Impulsivity and the association between the feedback‐related negativity and performance on an inhibitory control task in young at‐risk children. Psychophysiology. 2015;52(5):704–713. doi: 10.1111/psyp.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos L.E., Beauchamp K.G., Flannery J.E., Horn S.R., Fisher P.A. Interventions, stress during development, and psychosocial adjustment. In: Schultheiss O.C., Mehta & P.H., editors. The Routledge Handbook of Social Endocrinology. Routledge; New York, NY: 2018. (pp. 586–208) [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatr. 2013;22(12):719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Borgwardt S., Gerber H., Wiesbeck G.A., Schmid O., Riecher-Rössler A., Smieskova R., Lang U.E., Walter M. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol. Psychiatry. 2014;76(4):289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Shackman J.E., Pollak S.D. Impact of physical maltreatment on the regulation of negative affect and aggression. Dev. Psychopathol. 2014;26(4pt1):1021–1033. doi: 10.1017/S0954579414000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Cicchetti D. Reactive aggression among maltreated children: the contributions of attention and emotion dysregulation. J. Clin. Child Psychol. 1998;27(4):381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Shimada K., Takiguchi S., Mizushima S., Fujisawa T.X., Saito D.N., Kosaka H., Okazawa H., Tomoda A. Reduced visual cortex grey matter volume in children and adolescents with reactive attachment disorder. Neuroimage: Clinica. 2015;9:13–19. doi: 10.1016/j.nicl.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J.P. Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatrics. 2016;170(10):1003–1007. doi: 10.1001/jamapediatrics.2016.1559. [DOI] [PubMed] [Google Scholar]
- Shonkoff J.P., Fisher P.A. Rethinking evidence-based practice and two-generation programs to create the future of early childhood policy. Dev. Psychopathol. 2013;25(4pt2):1635–1653. doi: 10.1017/S0954579413000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S., Caldera C., Flannery J., Telzer E.H., Humphrey K.L., Tottenham N. Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci. 2016;36(24):6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.K., Chamberlain P., Eddy J.M. Preliminary support for multidimensional treatment foster care in reducing substance use in delinquent boys. J. Child Adolesc. Subst. Abuse. 2010;19(4):343–358. doi: 10.1080/1067828X.2010.511986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau G.Z., Peterson B.S. Normal development of brain circuits. Neuropsychopharmacology. 2009;35(1):147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thatcher D.L., Pajtek S., Chung T., Terwilliger R.A., Clark D.B. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug Alcohol Depend. 2010;110(1–2):55–61. doi: 10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M.C., Eigsti I.-M., Thomas K.M., Freed P.J., Booma E.S., Gunnar M.R., Altemus M., Aronson J., Casey B.J. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S., McLaughlin K.A., Sheridan M.A., Nelson C.A., Zeanah C.H., Fox N.A. The beneficial effects of a positive attention bias amongst children with a history of psychosocial deprivation. Biol. Psychol. 2017;122:110–120. doi: 10.1016/j.biopsycho.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S., Nelson C.A., Zeanah C.H., Fox N.A. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. JCPP (J. Child Psychol. Psychiatry) 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M.J.S., Wagner S.H. What explains the negative consequences of adverse childhood experiences on adult health. Am. J. Prev. Med. 1998;14(4):356–360. doi: 10.1016/s0749-3797(98)00011-7. [DOI] [PubMed] [Google Scholar]
- Whitfield C.L., Anda R.F., Dube S.R., Felitti V.J. Violent childhood experiences and the risk of intimate partner violence in adults: assessment in a large health maintenance organization. J. Interpers Violence. 2003;18(2):166–185. [Google Scholar]
- Wiers R.W., Boelema S.R., Nikolaou K., Gladwin T.E. On the development of implicit and control processes in relation to substance use in adolescence. Current Addiction Reports. 2015;2(2):141–155. doi: 10.1007/s40429-015-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y., Kaiser J., Naumer M.J. Sensory and motor aspects of addiction. Behav. Brain Res. 2010;207(2):215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y., Kaiser J., Naumer M.J. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci. Biobehav. Rev. 2012;36(2):825–835. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Yau Y.H., Potenza M.N., Mayes L.C., Crowley M.J. Blunted feedback processing during risk-taking in adolescents with features of problematic Internet use. Addict. Behav. 2015;45:156–163. doi: 10.1016/j.addbeh.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.