Abstract
Adolescence represents a developmental stage in which initiation of drug use typically occurs and is marked by dynamic neurobiological changes. These changes present a sensitive window during which perturbations to normative development lead to alterations in brain circuits critical for stress and emotional regulation as well as reward processing, potentially resulting in an increased susceptibility to psychopathologies. The occurrence of early life stress (ELS) is related to a greater risk for the development of substance use disorders (SUD) during adolescence. Studies using nonhuman primates (NHP) are ideally suited to examine how ELS may alter the development of neurobiological systems modulating the reinforcing effects of drugs, given their remarkable neurobiological, behavioral, and developmental homologies to humans. This review examines NHP models of ELS that have been used to characterize its effects on sensitivity to drug reinforcement, and proposes future directions using NHP models of ELS and drug abuse in an effort to develop more targeted intervention and prevention strategies for at risk clinical populations.
Keywords: Adolescence, Drug abuse, Monkeys, Stress, Sex, Self-administration
Highlights
-
•
ELS has long-lasting neurobiological and behavioral consequences.
-
•
ELS is a major risk factor for the initiation of adolescent drug use.
-
•
Sex differences are apparent in the consequences of ELS, including drug use.
-
•
Nonhuman primate models of ELS are critical for understanding ELS effects on neurobiology and risk for adolescent drug use.
1. Introduction
Drug addiction is a chronic relapsing disorder, with a complex etiology and a limited number of approved pharmacotherapeutics to combat it. Additionally, stress can exacerbate the reinforcing properties of multiple drugs of abuse, leading to an increased likelihood for individuals with stress-related disorders, such as anxiety, to develop substance use disorders (SUDs). The experience of adverse events early in life, defined broadly as early life stress (ELS), is a major risk factor for the emergence of SUDs. Especially troubling is the relationship between ELS, the development of SUDs during adolescence, and the risk to relapse after a period of abstinence (Hyman et al., 2007; Sinha, 2009). Given that initial experimentation with recreational drugs during adolescence has been linked to problematic drug use later in life (Kandel et al., 1992; Wong et al., 2013), it is especially concerning for a SUD to develop at such a young age. Furthermore, that chronic drug use may dramatically alter the normative neurobiological development that occurs in adolescence is also disconcerting. Finally, women who have experienced ELS tend to have worse drug use outcomes as a function of ELS in comparison to men.
The impetus for this review is the fact that the neurobiological mechanisms underlying this increased vulnerability to adolescent SUDs following a history of ELS remain unclear. However recent research suggests that deficits in emotional regulation as a result of neurobiological alterations that accompany ELS may be a mechanism of interest. Deficits in emotional regulation also accompany anxiety and other stress-related disorders that are not only co-morbid with SUDs but also more prevalent in women (Hyman et al., 2006; Hyman et al., 2007; Simpson and Miller, 2002). Moreover, a hyperactive stress-response system as a consequence of ELS may drive alterations in reward-related circuitry, creating a primed neurobiological system that may be uniquely sensitive to the reinforcing effects of drugs of abuse and to relapse.
These questions are very difficult to address in human populations, as they require prospective, longitudinal studies beginning in early childhood. Thus, we turn to animal models of ELS, given that stress also increases vulnerability to drug self-administration in animal models and is similarly reflected in increases in anxiety and emotionality (Dilleen et al., 2012; Goeders, 2003; Morgan et al., 2002; Nader and Czoty, 2005). Given that ELS results in alterations in neural circuits involved in reward and inhibitory control of behavior in adult humans (Cicchetti and Toth, 2005; Hein et al., 2005), examining the functional consequences of ELS on neurobiological targets using NHP models is critical due to the high homology of these brain regions with humans. We will also explore the possibility that alterations in areas of the brain relevant to drug use happen in parallel to disruptions in stress and emotional regulatory systems occurring during adolescence.
The purpose of this review is to examine the literature linking the occurrence of ELS and adolescent SUDs in NHP models of ELS and drug abuse. In doing so, our goals are threefold: 1) to elucidate potential mechanisms linking a history of ELS to adolescent SUD, 2) to highlight sex differences where they occur, despite the general paucity of studies examining sex as a biological variable in models of ELS and drug abuse, and 3) given the scarce literature, to encourage researchers working with NHP models of ELS or drug abuse to collaborate across laboratories to bridge both scientific fields. To accomplish these goals, we will start by examining the current landscape of adolescent drug use in the United States as well as the clinical literature reporting ELS as a major risk for the development of SUDs later in life. We will then highlight the critical importance of NHP models of ELS and drug abuse as an opportunity to expand on what is currently known in the rodent literature as a translational bridge to humans. Finally, we will touch on the few studies explicitly studying NHP models of ELS that have also examined the contribution of ELS to adolescent drug self-administration.
2. Current adolescent drug use trends
Adolescence is widely viewed as the developmental stage whereby drug use is initiated. Based on the findings of the latest Monitoring the Future national survey on drug use in 2017 (Johnston et al., 2018) alcohol remained the most used substance in high-school teenagers (8th, 10th, and 12th grades), with around 60% of teenagers reporting that they had consumed alcohol ever and 20% consuming alcohol by 8th grade. Additionally, the annual prevalence of marijuana use in teenagers also rose in 2017, reaching approximately 23.9% combined across school grades, and annual prevalence of cocaine use increased marginally to approximately 1.6% combined across school grades. Interestingly, the same study documented specific sex differences in adolescent drug use as well as period-specific drug use that differed as a function of sex (see Table 1; Johnston et al., 2018). For example, although boys start using alcohol earlier than girls, the opposite is true for initiation of other drug classes, such as amphetamines, sedatives and inhalants. These findings highlight that both the adolescent stage (early versus late) and the drug used are necessary variables to consider when examining unique susceptibilities of male and female adolescents to drug use.
Table 1.
Early versus late adolescent alcohol, tobacco, amphetamine, sedative, and inhalant drug use. X indicates greater usage. – Indicates no difference between males and females.
| Age | Drug | Males | Females |
|---|---|---|---|
| 8th Grade (Early Adolescence) | Alcohol | x | |
| Tobacco | – | – | |
| Amphetamines | x | ||
| Sedatives | x | ||
| Inhalants | x | ||
| 12th Grade (Late Adolescence) | Alcohol | x | |
| Tobacco | x | ||
| Amphetamines | – | – | |
| Sedatives | – | – | |
| Inhalants | – | – |
These sex and age-specific differences in drug use may have consequences for later drug use in life. In fact, considerable sex differences are evident in the progression from initiation of drug use (or experimentation) to the onset of drug dependence, a phenomenon termed “telescoping,” (Greenfield et al., 2010; Hernandez-Avila et al., 2004). In a study examining substance-dependent men and women, women showed higher rates of telescoping for opiates, marijuana, and alcohol, and also reported experiencing greater psychiatric, medical, and social consequences of their dependence in comparison to men (Hernandez-Avila et al., 2004). Importantly, there were no differences in initiation of regular drug use in this study, corroborating other reports that sex differences in SUDs typically do not emerge until late adolescence or early adulthood (for a review, see Kuhn, 2015). That there are no sex differences in the onset of adolescent SUD until late adolescence suggests important neurobiological, hormonal, social, and cultural factors that may modulate period-specific differences in drug use. Of the factors that may be critical in determining susceptibility to drug abuse, this review will focus on how early adverse experiences (i.e. ELS) may be a risk factor in both the onset and severity of drug use.
3. ELS as a risk factor in the etiology of drug abuse
While many genetic, social, and environmental factors are critical in determining an individual's risk to SUDs, the occurrence of ELS represents a significant vulnerability factor in the etiology of addiction (Enoch, 2011; Hyman et al., 2006; Hyman et al., 2008; Sinha, 2008). ELS can encompass a wide range of adverse experiences, including childhood maltreatment and exposure to trauma, the effects of which are robust and long lasting (for a review, see Enoch, 2011). SUD outcomes show dramatic differences based on the sex of the individual as well as the severity and type of childhood maltreatment experienced (i.e. physical, emotional, or sexual abuse; physical or emotional neglect). For example, greater severity of emotional abuse, sexual abuse, and overall maltreatment predicted a younger age of first alcohol use in women, whereas only severity of emotional abuse predicted age of first alcohol use in men (Hyman et al., 2006). In the same study, severity of emotional abuse, emotional neglect, and overall maltreatment predicted lifetime substance use severity in women, whereas emotional abuse severity predicted lifetime substance use severity in men. Interestingly, age of first alcohol use predicted age of first cocaine use in men and women, but only predicted age at which cocaine was used regularly in women. Furthermore, studies have shown that greater severity of childhood emotional abuse is associated with an increased risk of relapse and number of days where cocaine is used during relapse in women but not men (Hyman et al., 2008). Collectively, these data suggest that the occurrence of ELS leads to worse outcomes in women in comparison to men, including severity of drug use as well as propensity to relapse following abstinence. They also demonstrate that emotional abuse and neglect may be better predictors for measurements of drug addiction than physical abuse, suggesting specific forms of ELS may better predict different psychopathologies and that these effects vary based on the sex of the victim.
4. The need for translational preclinical models of human disease: WHY NHPs?
4.1. Development of socioemotional behavior
NHPs provide valuable translational animal models of ELS in relation to risk for drug abuse, based on their close social, behavioral, and neurobiological similarity to humans (Howell and Murnane, 2008; Howell and Sanchez, 2011; Sanchez et al., 2001; Sanchez, 2006). NHP models of ELS can also employ experimental manipulations not feasible in humans, and allow for in-depth examinations of developmental psychopathology (Gibbs et al., 2007). Monkeys, including rhesus macaques, demonstrate cortical complexity and phylogenetic similarity to humans (Barbas, 2000; Croxson et al., 2005; Reep, 1984; Thiebaut de Schotten et al., 2012). This includes the prefrontal cortex (PFC), which has a high degree of homology between humans and NHP species, in comparison with rodents (Fuster, 1997; Haber et al., 2006; Ongur and Price, 2000; Preuss and Goldman-Rakic, 1991b, Preuss and Goldman-Rakic, 1991a; Preuss, 1995). NHPs also have additional advantages when modeling aspects of stress neuroendocrine function (e.g. hypothalamic-pituitary-adrenal (HPA) axis) due to differences between rodent and primate brain stress systems, which is particularly important in relation to ELS and risk for drug addiction (Patel et al., 2000; Pryce et al., 2005; Sanchez et al., 1999; Sanchez et al., 2000; Sanchez, 2006). Brain development and maturation in NHPs also follows trajectories and anatomical patterns comparable to humans, albeit monkeys are precocious at birth and develop at about 4 times the speed of human children (Hayashi, 1992; Huttenlocher and Dabholkar, 1997; Kilb, 2012).
Like humans, NHPs have an extended postnatal period during which maternal caregiving is critical for physiological growth, socioemotional maturation, and neurodevelopment sensitivity to environmental experiences. Rhesus monkeys, in particular, have been a species widely used to study the impact of social environment because they experience complex social relationships and form strong social alliances and bonds (Hinde and Spencer-Booth, 1967; Suomi, 2005), beginning with the strong and enduring mother-infant bond, similar to that seen in humans (Bowlby, 1969; Hinde, 1974). The mother provides nourishment, protection, and social lessons while also regulating the infant's fear and stress responses (Coe et al., 1983; Gunnar et al., 2015; Hennessy, 1986; Hinde and McGinnis, 1977; Sanchez et al., 2001; Sanchez et al., 2015). Even as the offspring becomes more independent and interact more with their peers and other troop members they will maintain contact with their mother, particularly for comfort and support during stressful events (Hinde and Spencer-Booth, 1967; Suomi, 2005). In doing so, the mother still exerts a buffering effect on her offspring's stress responses, protecting them from the deleterious effects of stress on neurobiological and neuroendocrine development (Sanchez et al., 2015). Altogether, this evidence demonstrates that competent maternal care is necessary for the proper development of emotional behavior and stress neuroendocrine function (including HPA axis), and predicts better emotional and stress regulation later in life (Sanchez, 2006; Sanchez et al., 2010).
4.2. Development of neurobiological systems regulating stres/emotion and reward
Areas of the brain related to emotional regulation and inhibitory control of behavior, such as the PFC, follow a region-specific developmental progression (Lenroot and Giedd, 2006; Malkova et al., 2006). These higher-order association cortices reach cortical maturation later in life in comparison to lower-order cortices, while white matter tracts allow these regions to be functionally connected along a similar maturational pattern (Giedd et al., 1999; Giedd, 2004; Gogtay et al., 2004; Reiss et al., 1996). This developmental pattern suggests that the more frontal regions such as the PFC that have a protracted or prolonged maturation period may be the most impacted by ELS (Andersen, 2003; Crews et al., 2007). In addition to their vulnerability to ELS due to the protracted development of these regions, these brain areas play a critical role in modulating stress and emotional reactivity, with the PFC exerting critical top-down control of amygdala activation in response to threats and stressors (Kim et al., 2011). Higher emotional reactivity and risky behavior that is typical of adolescence may be occurring because this time period occurs in the interim between earlier/faster maturation of subcortical regions and the protracted development of cortical regulatory regions such as the PFC (Somerville et al., 2010). Therefore, the increased sensitivity of these regions to ELS during adolescence may lead to experience-based alterations in PFC-amygdala functional connectivity and contribute to early initiation of drug abuse (O'Connor and Cameron, 2006).
In addition to the development of top-down inhibitory control of the amygdala by the PFC, there is also considerable remodeling within the PFC itself that occurs until early adulthood, but particularly during adolescence (Giedd et al., 1999; Gogtay et al., 2004; Shaw et al., 2008; Sowell et al., 1999). Dopamine (DA) innervation to the medial PFC (mPFC) increases throughout this developmental period with marked refinement and organization that occurs during adolescence (Benes et al., 2000; Kalsbeek et al., 1988; Manitt et al., 2011; Naneix et al., 2012; Rosenberg and Lewis, 1995). For example, synapses with these DA afferents in the PFC, especially with GABAergic interneurons (Benes et al., 1993; Sesack et al., 1995; Verney et al., 1990) become more sensitive to modulation by DA (Gonzalez-Islas and Hablitz, 2001; Seamans et al., 2001; Zhou and Hablitz, 1999). Additionally, DA 1 (D1) receptor expression increases during adolescence, compared to the juvenile period and adulthood, modulates glutamatergic projections from PFC to subcortical regions like the nucleus accumbens (NAc), which plays a prominent role in drug-seeking behaviors (Kalivas et al., 2003; Seamans and Yang, 2004). Altogether, the important developmental changes in DA innervation and receptors in the PFC and NAc during adolescence can explain, at least in part, the unique vulnerability of these systems to drug exposure, which can result in enduring behavioral effects (Reynolds et al., 2015).
4.3. Development and dysregulation of neuroendocrine stress systems
Additional outcomes of ELS associated with drug abuse include alterations in neuroendocrine stress response systems such as the HPA axis (for a review, see Enoch, 2011). ELS can result in the development of a hyperreactive HPA axis through different mechanisms, including: (1) a hyperactive amygdala that increases stress neuroendocrine responses through downstream projections to the HPA axis and sympathetic systems, and (2) local changes in HPA axis function. These local changes include impaired glucocorticoid negative feedback as a result of downregulation/desensitization of glucocorticoid receptors (GRs) in areas of the brain such as the paraventricular nucleus (PVN), pituitary, hippocampus, and the PFC (Howell and Sanchez, 2011; Sanchez et al., 2001; Sanchez, 2006). Altogether, this can result in persistently elevated levels of cortisol and activation of the stress neuropeptide corticotropin-releasing factor (CRF) within the NAc (Koob and Le Moal, 2005; Koob, 2008; Koob and Volkow, 2016; Kreek and Koob, 1998; Lemos et al., 2012) which can lead to altered dopaminergic transmission in reward circuits linked to stress-induced increases in the reinforcing properties of drugs of abuse as measured by self-administration (Kalivas and Stewart, 1991).
Stressful events increase the acquisition of a number of drugs of abuse (Goeders, 2003; Haney et al., 1995; Kosten et al., 2000; Piazza et al., 1990), and chronic stress has long-term effects on the dopaminergic system that may impose an increased risk for drug addiction (Enoch, 2011). Specifically, DA regulates motivational, emotional, and reinforced behavior through mesolimbic dopaminergic projections from the ventral tegmental area (VTA) to the NAc and PFC. Stress-induced increases in the reinforcing properties of drugs of abuse may be explained by the fact that stress and drugs of abuse (such as psychostimulants) increase extracellular DA levels in the NAc on their own. Repeated stress exposure increases both the magnitude and duration of NAc DA release in response to psychostimulants (Kalivas, 1993). This is mediated in part by DA 2 (D2) receptor upregulated-mediated effects in synaptic function (Sim et al., 2013) and by glucocorticoid action on GRs in neurons with DA afferents (Ambroggi et al., 2009). These data suggest that elevations in glucocorticoids and alterations in the mesolimbic DA system constitute two pathways whereby chronic stress primes neurobiological systems to be more sensitive to the reinforcing effects of drugs of abuse. This is particularly true for the ventral striatum (NAc) as well as the PFC, which provides top-down control in the execution of inhibitory control, decision-making, and receives innervation from both the VTA and ventral striatum (for reviews, see Koob and Volkow, 2016; Volkow and Baler, 2014).
Stress and emotional regulatory circuits, such as the extended amygdala (which includes the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and the shell of the NAc) have strong connections to the ventral striatum, and these connections may play a prominent role in stress-induced increases in the reinforcing effects of drugs (Koob and Le Moal, 2008). Thus, chronic stress and drug self-administration both increase activation of the HPA axis, leading to persistent elevations of circulating cortisol and activation of the stress neuropeptide and neuromodulator CRF within the NAc and the extended amygdala (Koob and Le Moal, 2005; Koob, 2008; Koob and Volkow, 2016; Kreek and Koob, 1998; Lemos et al., 2012). Recent findings from an ELS rodent model suggest that increased CRF in NAc could also originate in increased CRF projections from the CeA, where CRF is overexpressed in ELS animals (Bolton et al., 2018). The same study also shows alterations in amygdala connectivity with the mPFC that affect top-down control of anxiety/fear responses. Altogether, the studies summarized here highlight that connections between the amygdala, PFC, and ventral striatum constitute a pathophysiological circuit by which ELS can impact the development of both stress and reward systems that modulate the effects of drugs of abuse.
5. NHP models of ELS and drug abuse
5.1. NHP models of ELS
The development and utilization of NHP models of ELS has substantially increased our understanding of both healthy and pathological disease states. While this is not an exhaustive literature review of NHP models of ELS, this section covers several relevant models of ELS that have also been used to examine its link to drug use later in life. Sex differences will be discussed within each model if data are available, as well as the potential neurobiological, neuroendocrine, and/or behavioral consequences of each specific model of ELS. It is important to point out that there is a dearth of literature on NHP models of ELS and drug use in adolescence.
Peer-rearing versus Maternal Rearing: One of the most devastating ELS experiences is the disruption of the mother-infant bond. Decades of research have demonstrated the powerful role of the mother on primate infant development beyond nutrition, protection, and comfort. Specifically, they have examined how alterations in the early social environment can lead to the development of different psychopathologies (for extensive reviews, see Harlow et al., 1971; Howell et al., 2016; Sanchez et al., 2001; Seay and Gottfried, 1975; Suomi et al., 1971). From these pioneering studies, one specific form of ELS (peer-rearing; PR) has been used extensively to examine multiple consequences of ELS. In PR procedures, neonates are removed from their mothers at birth, cared for in a nursery for 30 days postnatal, and then placed in a cage with age-matched peers for 6 months, and they are compared with infants maternally-reared (MR) during the first 6 months of their life. In both groups, animals do not have access to other adults. At approximately 7 months, MR animals are removed from their mothers and placed with PR animals, so that all animals are treated identically thereafter. In this model, PR animals consistently show elevated levels of plasma cortisol in comparison to MR animals (Fahlke et al., 2000; Higley et al., 1991; Howell et al., 2016). As a result of this early adversity, decreases in cerebrospinal fluid (CSF) concentrations of the serotonin (5HT) metabolite 5-Hydroxyindoleacetic acid (5-HIAA) have been reported in PR animals, suggesting lower brain 5HT function (turnover), an effect that has been widely associated with higher incidence of risk-taking and anxiety.
Repeated Maternal Separation: Repeated maternal separation (RMS) is another model of ELS which involves the intermittent and unpredictable separation of the mother from the infant, who remains in the social group, for periods ranging from 0.5 to 6 h. During weaning (3–6 months postpartum), these experimental manipulations have profound effects on the mother-infant bond, the tempo of social development, anxiety, and altered HPA axis function (Sanchez et al., 2005). Maternal separation at earlier ages (less than 3 months of age) causes even more severe developmental effects (O'Connor and Cameron, 2006). RMS also results in alterations in brain function, such that left dorsolateral PFC activation is blunted and a compensatory increase in right dorsolateral and right ventral temporal/occipital lobe is observed during separations (Howell et al., 2016; Rilling et al., 2001). Regarding sex differences in vulnerability to this type of ELS, female infants seem more vulnerable than males both in terms of their higher cortisol responses to brief maternal separations and the long-term impact on diurnal cortisol rhythms (Sanchez et al., 2005).
Social Subordination: Social subordination represents another translational model of ELS-induced risk for substance abuse. Because rhesus monkeys live in complex social troops with matrilineal hierarchies, an animal's place within that hierarchy determines its access to many resources (Bernstein, 1976; Howell et al., 2016; Sade, 1967). This hierarchy is strictly maintained through the use of aggression from dominant to subordinate animals, and as a result of these unprovoked and unpredictable agonistic interactions, subordinate animals show greater instances of stress and anxiety. Because infants acquire the relative rank of their mothers and families within the social group, infants from subordinate mothers are subject to significant pressure, aggression, and stress as a result of their social status beginning early in life (Holekamp and Smale, 1991; Howell et al., 2016; Sade, 1967). Consequences of social subordination include exposure to higher rates of aggression, lower rates of affiliation (Howell et al., 2016; Michopoulos et al., 2012a), and chronic hyperactivity of the HPA axis demonstrated by hypercortisolemia and impaired glucocorticoid negative feedback (Howell et al., 2016; Jarrell et al., 2008; Kaplan et al., 2010; Michopoulos et al., 2012a) that is evident during juvenile and prepubertal developmental stages (Howell et al., 2014a).
Infant Maltreatment: Finally, our laboratory has utilized an infant maltreatment model of ELS, which displays high translational relevance to humans. Infant maltreatment is not unique to humans, as it is reported to spontaneously occur in NHP species, such as macaques, baboons and marmosets, both in wild and captive populations (Brent et al., 2002; Carroll and Maestripieri, 1998; Johnson et al., 1996; Maestripieri et al., 1997; Maestripieri, 1998; Troisi et al., 1982; Troisi and D'Amato, 1984). In macaques, infant maltreatment by the mother is operationalized by two, often comorbid, behaviors: physical abuse and rejection, for which the highest rates occur in the first two to three months of life (Maestripieri, 1998; Maestripieri and Carroll, 1998; McCormack et al., 2006; McCormack et al., 2009). Physical abuse includes aberrant, violent behaviors that the mother exhibits towards the infant, leading to pain and distress in the infant (Howell et al., 2017; Maestripieri, 1998; McCormack et al., 2006; McCormack et al., 2009). Although infant rejection is a developmentally typical behavior around the time of weaning, the mother pushing away the infant when it tries to make contact with her is abnormal earlier in life and results in similar distress behaviors as abuse (Maestripieri, 1998; McCormack et al., 2006). In addition to intense infant distress, these adverse experiences also lead to elevations in stress hormones (Drury et al., 2017; Howell et al., 2013; Howell et al., 2014b), long-term HPA axis hyperreactivity (Koch et al., 2014; Sanchez et al., 2010), increased emotional reactivity (Sanchez and Pollak, 2009), decreased brain DA and 5HT function that is associated with increased anxiety (Maestripieri et al., 2006a, Maestripieri et al., 2006b), and alterations in the structure and connectivity of PFC-amygdala tracts important for emotional/stress regulation and reward processes (Howell et al., 2013, Howell et al., 2014a, Howell et al., 2017). Sex differences are also present well before puberty, with females showing heightened vulnerability to emotional and stress alterations in comparison to males (Drury et al., 2017).
5.2. NHP models of adolescent drug abuse
NHPs represent an invaluable tool in examining the progression of drug use from initiation to dependence, as well as for carefully dissecting how other variables may play a role in the etiology of drug dependence. Through the use of operant schedules of reinforcement, NHPs are trained to perform a set of response requirements that, once met, results in the presentation of a reinforcing stimulus (Brady et al., 1987; Collins et al., 1983). Validated extensively, drug self-administration has been widely characterized and regarded as the most translationally-relevant behavioral model to examine multiple facets of human drug use and abuse such as initiation, maintenance, escalation, and relapse of drug-taking (for extensive reviews, see Ator and Griffiths, 2003; Banks et al., 2017; Haney and Spealman, 2008; Howell and Murnane, 2008; Mello and Negus, 1996). As demonstrated in the clinical literature, the ability to thoroughly examine the developmental etiology of drug dependence requires a longitudinal approach, whereby initiation of drug use and its progression to dependence can be studied over a significant period of time. The use of drug self-administration procedures also provides excellent control over parametric conditions and variables (such as sex, age, and early environmental history) that are known to influence not only drug-taking (Yokel, 1987), but that may also be inherently critical to our understanding of potential mechanisms responsible for individual susceptibility to the reinforcing effects of drugs.
Sex differences in NHP models of drug self-administration often depend on the drug examined, the schedule of reinforcement used, as well as the phase of drug self-administration examined (i.e., acquisition versus maintenance; for a review, see Roth et al., 2004). For example, while no sex differences were reported in the acquisition of ethanol self-administration in adult rhesus macaques (Grant and Johanson, 1988), females do show a greater frequency of alcohol intake during maintenance conditions in comparison to males (Juarez et al., 1993). Similarly, adult female rhesus macaques show higher break points for cocaine across multiple doses in comparison to males (Mello et al., 2007). These data suggest that both the drug and phase of drug self-administration are critical variables in examining potential sex differences in drug-maintained responding. Unfortunately, there is a general lack of studies examining adolescent self-administration in NHPs. Furthermore, there are no studies to the authors’ knowledge that directly compare adolescent versus adult self-administration in NHPs. While a review of rodent models of adolescent self-administration is beyond the scope of this review, multiple studies have demonstrated age differences in drug-reinforced behavior using rodents. Specifically, adolescent rats self-administer more nicotine (Levin et al., 2003; Levin et al., 2007), escalate methamphetamine self-administration (Anker et al., 2012), acquire amphetamine self-administration faster and show greater amphetamine intake (Shahbazi et al., 2008), and consume greater amounts of ethanol (Doremus et al., 2005) in comparison to adults. To date, the vast majority of adolescent rodent self-administration work has suggested that females show a unique susceptibility to psychostimulants as well as other drugs of abuse, and that measures of adolescent drug self-administration typically exceed adult drug self-administration. These data demonstrate the utility and versatility of the drug self-administration procedure, and reveal the ability to adequately examine factors that contribute to drug abuse liability, such as age and sex.
5.3. NHP models of ELS as a predictive risk factor in the etiology of drug abuse
While this section will focus on NHP models of ELS that have examined risk to drug self-administration, we were able to find only one study that met the three criteria as originally set out for this review. Those criteria included 1) the utilization of a NHP model of ELS, 2) adolescent drug self-administration, and 3) the utilization of both male and female subjects. Therefore, the following section will cover NHP models of ELS that examine risk to drug self-administration in adulthood, while reporting on sex differences where available.
The contribution of ELS to susceptibility to the reinforcing effects of drugs in rhesus monkeys has been investigated in several studies (Barr et al., 2004c; Fahlke et al., 2000; Higley et al., 1991; Schwandt et al., 2010). The focus of these studies was on alcohol consumption and only one explicitly examined differences between adolescent males and females (Fahlke et al., 2000), highlighting the general paucity of data surrounding NHP models of ELS, sex, and adolescent vulnerability to drug intake, despite their high translational value. Utilizing a well-characterized PR model of early adversity, infant rhesus monkeys were subjected to either PR or normal MR early in life (Higley et al., 1991). In late adolescence (50 months), animals were given access to a choice of an aspartame-sweetened 7% ethanol solution as well as an aspartame-sweetened non-caloric vehicle for 1 h a day, 4 days a week, for 8 consecutive weeks. Alcohol consumption was measured at baseline (two-week home cage period with their cage-mates), as well as during social separations (four 4-day periods whereby animals were isolated from their cage-mates and characterized as a period of intense stress). At baseline and during the recovery period following separation, PR animals consumed alcohol at nearly double the rate in comparison to MR animals. During social separations, MR animals significantly increased their alcohol consumption in comparison to their baseline levels and to levels similar to the PR animals. Interestingly, PR animals did not show significantly different consumption compared to their baseline during these separation phases, nor did significant differences in consumption between MR and PR animals emerge following separations. In both rearing conditions, alcohol consumption resulted in blood-alcohol (BAC) levels sufficient to produce intoxication. Finally, plasma cortisol was higher in PR animals at baseline as well as during separation in comparison to MR animals, and this positively correlated with alcohol consumption during the separation period in all animals regardless of rearing condition.
The findings described above were replicated using a near identical procedure where both PR and MR animals underwent 4 sequential weeks of a 4-day social separation, starting at 6 months of age (Fahlke et al., 2000). The same animals were tested for voluntary alcohol intake over several weeks during young adulthood. Social separation increased plasma cortisol levels in all infants, though PR infants showed a larger increase than MR infants. Cortisol response to social separation in infancy positively correlated with alcohol consumption in adulthood, such that animals with higher cortisol responses to the separation procedure consumed more alcohol as adults independent of rearing condition. Finally, male and PR animals consumed significantly more alcohol than female and MR animals, respectively (Fahlke et al., 2000).
Low levels of brain 5HT have been consistently reported in PR macaques which, as has previously been demonstrated in humans, are associated with impaired impulse control, and this hypo-serotonergic tone was also related to greater voluntary alcohol consumption when animals were given free access to alcohol (Barr et al., 2004a, Barr et al., 2004b, Barr et al., 2004c; Mehlman et al., 1994). Interestingly, decreased 5HT neurotransmission has also been linked to genetic variants in the 5HT transporter (5HTT) gene promoter region of rhesus monkeys (rh5-HTTLPR) that reduce its expression, so that adolescent and young macaques that are carriers of the short (s) allele (which results in decreased 5HTT mRNA levels), show higher levels of alcohol preference than animals homozygous for the long (l) allele (l/l) (Barr et al., 2004a, Barr et al., 2004b; Barr et al., 2004a, Barr et al., 2004b, Barr et al., 2004c; Lopez and Higley, 2002). Finally, while PR females did not show differences in alcohol consumption during initial exposures in comparison to MR subjects, they did show marked increases in their levels of alcohol consumption through successive exposure to alcohol than seen in males. These two studies demonstrate that early adversity in the form of PR was related to greater vulnerability to alcohol consumption in late adolescence/early adulthood particularly in response to stress in comparison to MR animals, and females showed greater increases in their alcohol consumption with successive exposures in comparison to males.
As seen throughout this section very limited data exist on the effects of PR and RMS on drug self-administration in NHPs. However, one study examined the effects of RMS on sensitivity to the reinforcing effects of cocaine in a group of adult females that experienced RMS as infants (Corcoran and Howell, 2010). At approximately 5–6 years of age both control and RMS animals were examined under a second-order schedule of reinforcement, where completion of a fixed-ratio (FR) requirement resulted in the presentation of a light stimulus and a drug infusion was delivered upon completing the first FR 20 lever responses after a fixed interval of 10 min had elapsed. Interestingly, maternally separated animals showed significantly lower response rates in comparison to control animals during acquisition as well as maintenance of self-administration, suggesting that maternally separated animals were less sensitive to the reinforcing effects of cocaine. The authors suggested that lower response rates may be related to decreases in motivation to obtain a highly reinforcing stimulus in the maternally separated animals, especially in light of the fact that response rates across a full dose-response function in these animals resulted in significantly lower response rates in comparison to control animals at almost all doses. These findings raise the possibility that ELS, and specifically RMS, may result in a decreased sensitivity to the reinforcing effects of psychostimulants.
The effects of social subordination on risk for drug abuse have been explored in laboratory environments using cocaine self-administration, although it is important to note that the impact of social subordination was studied based on social ranks established during adulthood, and may not be synonymous with early life stress (Morgan et al., 2002; Nader et al., 2012a, Nader et al., 2012b). Thus, when male adult cynomolgus monkeys were socially housed after previously living under individual housing procedures, a social hierarchy was formed whereby dominance rank was determined by a number of dyadic agonistic encounters, such that the first-ranking (dominant) monkey aggressed toward and usually elicited submission from all other monkeys (Morgan et al., 2002). Following the formation of these hierarchies, monkeys were tested under a fixed-ratio schedule of reinforcement, whereby 30 responses resulted in the intravenous delivery of cocaine. Under these conditions cocaine functioned as a potent reinforcer in subordinate but not dominant males, and these effects were accompanied by changes in D2 receptor availability, such that social housing increased the availability of D2 receptors in dominant monkeys which remained unchanged in subordinate monkeys. When the same effects were examined in females, the opposite effect was found (Nader et al., 2012a). Specifically, while dominant females also showed greater increases in D2 receptor availability in comparison to subordinate females, dominant females acquired cocaine reinforcement at significantly lower doses in comparison to subordinate females. These results suggest that while dominance in a social hierarchy may confer an increase in D2 receptor availability in both males and females, this increased D2 receptor availability results in differential sensitivity to the reinforcing properties of cocaine, such that subordinate males and dominant females demonstrate greater sensitivity to the reinforcing effects of cocaine in comparison to dominant males and subordinate females. These apparently conflicting data offer researchers a rare glimpse into the impact of including sex as a biological variable when examining the effects of ELS on drug self-administration. Furthermore, future research examining how social subordination early in life may change adolescent cocaine self-administration would provide a needed comparison to the studies mentioned above.
Recent studies have examined the potential role of subordination stress on emotional feeding as a proxy measure for dysregulated appetitive behavior (Arce et al., 2010; Godfrey et al., 2018; Michopoulos et al, 2012b; Michopoulos et al., 2016). Specifically, exposure to subordinate stress in group-living females results in a greater consumption of a calorically dense diet in subordinates in comparison to dominants (Arce et al., 2010; Michopoulos et al., 2016). These behavioral differences are correlated with decreases in lower D2 receptor binding potentials in the orbitofrontal cortex (oPFC) as well as lower functional connectivity between the NAc and the PFC (Godfrey et al., 2018; Michopoulos et al., 2016). Given that these hypodopaminergic systems are evident in areas of the brain related to drug addiction, the extension of the application of NHP models of ELS to dysregulated eating may help elucidate pathological behavior and its neurobiological mechanisms, leading to the development of therapeutics that may be useful in both drug addiction as well as obesity (Volkow et al., 2011; Volkow and Baler, 2015).
Our group has extensively characterized the behavioral, neuroendocrine and neurobiological impact of the ELS macaque model of infant maltreatment. Importantly, studies are ongoing in our lab to examine the contribution of infant maltreatment to sensitivity to the reinforcing effects of cocaine in male and female adolescent rhesus monkeys. Combined with both the neurobiological and neuroendocrine effects previously reported by our lab, we believe these studies will further contribute to our knowledge and understanding of how ELS impacts adolescent drug use and abuse. Our laboratory is currently addressing these questions using infant maltreatment to investigate if this specific form of ELS leads to differential sensitivities to the reinforcing effects of cocaine during adolescence and whether increased stress/emotional reactivity resulting from infant maltreatment may mediate vulnerability to cocaine use, particularly in females.
6. Potential mechanisms linking ELS to adolescent drug abuse
Despite the known risks of adolescent initiation of drug use and the development of drug dependence later in life (Kandel et al., 1992; Wong et al., 2013), the neurobiological mechanisms underlying the increased risk during this developmental period are poorly understood. It may be the case that a combination of factors creates a perfect storm by which the adolescent brain is uniquely susceptible to both the consequences of ELS as well as the reinforcing effects of drugs. First, adolescence is a critical transition period of drastic social, emotional, endocrine, cognitive, and brain changes. These changes result in increased stress and emotional reactivity (Dahl and Gunnar, 2009; Romeo and McEwen, 2006; Romeo, 2010a, Romeo, 2010b; Spielberg et al., 2014). Developmental changes in and between limbic and prefrontal circuits involved in stress, emotional control, and reward have been proposed to play a role in vulnerability of the adolescent brain to psychopathology (Bramen et al., 2011; Casey et al., 2010; Gee et al., 2013; Hare et al., 2008). In Fig. 1, we outline a schematic that incorporates these areas of the brain, as well as multiple neurotransmitter systems involved to outline ways the brain's stress-response system and the mesolimbic dopaminergic pathway interact. ELS results in a hyperactive amygdala (i.e. increased stress/emotional reactivity) and HPA system (i.e. increased stress neuroendocrine reactivity), whereby cortisol released from the adrenal cortex may show impaired negative feedback to the hypothalamus and anterior pituitary, as well as extrahypothalamic structures, such as the PFC and hippocampus to shutdown secretion of cortisol. CRF is a stress neuropeptide and a critical neuromodulator of NAc DA; some CRF input to the NAc could originate in the CeA, where CRF expression has been reported to be increased as a consequence of ELS (Bolton et al., 2018). Because cortisol directly modulates DA release in the PFC, NAc, and VTA, cortisol-induced increases in DA release occurring in the NAc can increase the magnitude and duration of DA release in response to a drug reinforcer. Glutamatergic projections (from the PFC to the NAc and VTA) as well as serotonergic and GABAergic systems also serve as potent modulators of DA release throughout these major structures. The intense remodeling of the PFC characteristic during adolescence, combined with ELS-induced amygdala and HPA axis hyperreactivity (which may include impaired glucocorticoid negative feedback), and the self-administration of reinforcers that increase DA release in the NAc may create a perfect set of circumstances through which the adolescent brain becomes uniquely vulnerable to the consequences of ELS and drug abuse.
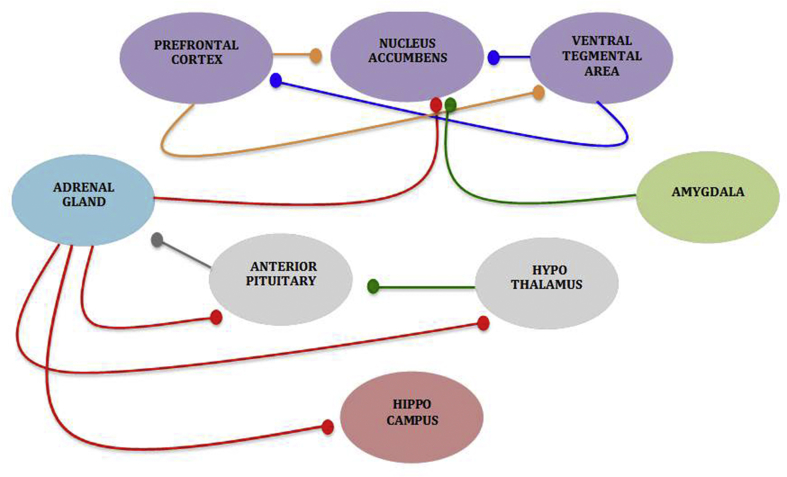
Fig. 1.
Schematic of the dynamic neurocircuitry and neurochemistry related to ELS and adolescent drug vulnerability. In response to a stressor, CRF (green line) is secreted from the hypothalamus (gray oval), triggering the release of adrenocorticotropic hormone (ACTH; dark gray line) from the anterior pituitary (gray oval), which initiates the release of cortisol (red line) from the adrenal gland (blue oval). ELS, however, results in a hyperactive HPA system whereby cortisol may show impaired negative feedback to the hypothalamus and anterior pituitary (both gray ovals), as well as extrahypothalamic structures, such as the PFC (purple oval; part of mesolimbic DA system) and the hippocampus (red oval) to shutdown secretion of cortisol. With increases in cortisol release, DA release may also increase in other areas of the mesolimbic system, such as the NAc, and directly affect the ability of drug reinforcers to stimulate DA release. Glutamate (orange line), as well as the GABAergic and serotonergic systems (not pictured) also serve as potent modulators of DA release throughout these major structures. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
7. Conclusions and future directions
Similar to the effects reported in the clinical literature, in cases where ELS risk has been examined specifically in the context of drug self-administration, sensitivity to the reinforcing effects of drugs is specific to the model used, the drug in question, and the sex of the individual. However, several limitations should be discussed in light of this review. First and foremost, a general paucity of data exists regarding the biological mechanisms that may underlie ELS-increased risk for drug addiction. This lack of information is due in part to the fact that understanding the long-term risks of ELS requires an experimental design that allows researchers to characterize and quantify ELS as well as the initiation of drug use during adolescence. In many ways, the prospective/longitudinal experimental designs required to address these questions are very difficult to implement in humans, and highlights the enormous translational utility preclinical models can provide in answering these questions. This is especially true regarding NHP models, given that NHPs show a remarkable neurobiological, neuroendocrine, and socioemotional similarity to humans. However, there are very few neurodevelopmental studies in NHPs that have examined the normative and pathological neurobiological and neuroendocrine development as a function of ELS. If anything, the lack of developmental studies in NHP models of ELS to study adolescent drug use in male and female subjects represents a critical need for institutions to support NHP biomedical research.
Secondly, the analysis of sex as a biological variable is tremendously valuable especially in the context of developing novel therapeutics for the treatment of drug use. Given the enormous wealth of data demonstrating sex differences in drug use as well as in consequences of drug use (in both clinical and preclinical subjects) and ELS, the comparison of male and female subjects with regards to sensitivity to drug self-administration is necessary. This is also true for studying drug self-administration in adolescence, instead of exclusively studying these outcomes in adulthood. Because intense neurobiological, neuroendocrine, and socio-emotional changes occur both as a result of ELS and during adolescence, examining how ELS affects adolescent drug use in NHP models is of critical importance.
Thirdly, NHP studies that have characterized the effects of ELS on drug abuse outcomes have focused almost exclusively on two drugs (alcohol and cocaine). Decades of research have demonstrated that while almost all drugs of abuse produce their euphoric effects through the mesolimbic dopaminergic system, different drugs of abuse accomplish this with help from the glutamatergic, GABAergic, and brain stress neuromodulators such as CRF (for extensive reviews, see Koob, 2008; Koob and Volkow, 2016; Volkow and Baler, 2014). Furthermore, most drug use is marked by polysubstance abuse and not just the use of one specific substance. Finally, it will be critical for future studies to evaluate whether or not changes in sensitivity to the reinforcing effects of drugs in one classification generalize to other drugs in the same classification. Examining the effects of ELS on drug self-administration using drug reinforcers other than alcohol and cocaine may lead to the development of novel effective therapeutics for a unique at risk population.
Although different ELS experiences likely lead to different neurobehavioral outcomes, we believe that naturalistic models such as social subordination and infant maltreatment bring unique translational value for human at-risk populations and need to be further explored. While PR and RMS offer experimenters the ability to examine how disruptions or deprivation of maternal care can result in different behavioral outcomes in comparison to healthy controls, these ELS paradigms are artificial in that the experience of the juvenile to maternal deprivation or separation would not normally happen in the wild (for a review, see Howell et al., 2017; Insel, 2007). Because of these artificial manipulations, interpretations of consequences associated with these forms of ELS can be confounded and run the risk of generalizing to human populations. Both social subordination and infant maltreatment result in species-specific adverse experiences. These experiences result in relevant developmental adaptations to the species and, therefore, are translationally valuable to human clinical conditions. Examining how ELS induces its wide array of consequences in a social environment is valuable in that ELS in humans also occurs within a social environment that shapes the individual's resilience or susceptibility to drug abuse. It is our belief that an ethologically valid NHP model of ELS that examines adolescent self-administration of drugs of abuse in males and females would contribute a wealth of knowledge to multiple scientific fields, and may aid in developing treatments and interventions for at risk populations.
Declarations of interest
None.
Acknowledgements
The authors would like to thank Drs Mark Wilson, Brittany Howell, Vasiliki Michopoulos, Jodi Godfrey, as well as Anne Glenn, Christine Marsteller, Dora Guzman, Erin Siebert and other members of the Sanchez's lab for conceptual discussions, technical assistance and expertise in characterizing some of the NHP models of ELS described herein. We also thank the staff at the Yerkes National Primate Research Center (YNPRC) for the excellent technical support and animal care provided during those studies. Funding for this research was provided by NIH grants MH078105, DA038588, HD077623 and Office of Research Infrastructure Programs/OD grant OD11132 (Yerkes National Primate Research Center Base grant, formerly RR000165). The YNPRC is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), International.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.09.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ambroggi F., Turiault M., Milet A., Deroche-Gamonet V., Parnaudeau S., Balado E., Barik J., Van Der Veen R., Maroteaux G., Lemberger T. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat. Neurosci. 2009;12(3):247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anker J.J., Baron T.R., Zlebnik N.E., Carroll M.E. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012;124(1–2):149–153. doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce M., Michopoulos V., Shepard K.N., Ha Q.-C., Wilson M.E. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol. Behav. 2010;101(4):446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator N.A., Griffiths R.R. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70(3):S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Banks M.L., Czoty P.W., Negus S.S. Utility of nonhuman primates in substance use disorders research. ILAR J. 2017;58(2):202–215. doi: 10.1093/ilar/ilx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 2000;52(5):319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barr C.S., Newman T.K., Lindell S., Shannon C., Champoux M., Lesch K.P., Suomi S.J., Goldman D., Higley J.D. Interaction between serotonin transporter gene variation and rearingcondition in alcohol preference and consumption in female primates. Arch. Gen. Psychiatr. 2004;61(11):1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr C.S., Newman T.K., Shannon C., Parker C., Dvoskin R.L., Becker M.L., Schwandt M., Champoux M., Lesch K.P., Goldman D. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatr. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Barr C.S., Schwandt M.L., Newman T.K., Higley J.D. The use of adolescent nonhuman primates to model human alcohol intake: neurobiological, genetic, and psychological variables. Ann. N. Y. Acad. Sci. 2004;1021(1):221–233. doi: 10.1196/annals.1308.027. [DOI] [PubMed] [Google Scholar]
- Benes F.M., Taylor J.B., Cunningham M.C. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cerebr. Cortex. 2000;10(10):1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Benes F.M., Vincent S.L., Molloy R. Dopamine‐Immunoreactive axon varicosities form nonrandom contacts with GABA‐immunoreactive neurons of rat medial prefrontal cortex. Synapse. 1993;15(4):285–295. doi: 10.1002/syn.890150405. [DOI] [PubMed] [Google Scholar]
- Bernstein I.S. Dominance, aggression and reproduction in primate societies. J. Theor. Biol. 1976;60(2):459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Regev L., Chen Y., Rismanchi N., Haddad E., Yang D.Z., Obenaus A., Baram T.Z. Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol. Psychiatr. 2018;83(2):137–147. doi: 10.1016/j.biopsych.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss v. 3 (Vol. 1)." Random House. Furman, W., & Buhrmester, D.(2009). Methods and measures: the network of relationships inventory: behavioral systems version. IJBD (Int. J. Behav. Dev.) 1969;33:470–478. doi: 10.1177/0165025409342634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Griffiths R., Hienz R., Ator N., Lukas S., Lamb R. Springer; 1987. Assessing Drugs for Abuse Liability and Dependence Potential in Laboratory Primates. Methods of Assessing the Reinforcing Properties of Abused Drugs; pp. 45–85. [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Forbes E.E., Chen J., Toga A.W., Dinov I.D., Worthman C.M., Sowell E.R. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebr. Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent L., Koban T., Ramirez S. Abnormal, abusive, and stress-related behaviors in baboon mothers. Biol. Psychiatr. 2002;52(11):1047–1056. doi: 10.1016/s0006-3223(02)01540-8. [DOI] [PubMed] [Google Scholar]
- Carroll K.A., Maestripieri D. Infant abuse and neglect in monkeys--a discussion of definitions, epidemiology, etiology, and implications for child maltreatment: reply to Cicchetti (1998) and Mason (1998) Psychol. Bull. 1998;123(3):234–237. doi: 10.1037/0033-2909.123.3.234. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Duhoux S., Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D., Toth S.L. Child maltreatment. Annu. Rev. Clin. Psychol. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Coe C.L., Glass J.C., Wiener S.G., Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8(4):401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Collins R.J., Weeks J.R., Cooper M.M., Good P.I., Russell R.R. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology. 1983;82(1–2):6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Corcoran S.B.E., Howell L.L. Impact of early life stress on the reinforcing and behavioral-stimulant effects of psychostimulants in rhesus monkeys. Behav. Pharmacol. 2010;21(1):69–76. doi: 10.1097/FBP.0b013e3283359f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F., He J., Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Croxson P.L., Johansen-Berg H., Behrens T.E., Robson M.D., Pinsk M.A., Gross C.G., Richter W., Richter M.C., Kastner S., Rushworth M.F. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J. Neurosci. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E., Gunnar M.R. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev. Psychopathol. 2009;21(1):1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dilleen R., Pelloux Y., Mar A.C., Molander A., Robbins T.W., Everitt B.J., Dalley J.W., Belin D. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology. 2012;222(1):89–97. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Doremus T.L., Burnell S.C., Rajendran P., Spear L.P. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin. Exp. Res. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Drury S.S., Howell B.R., Jones C., Esteves K., Morin E., Schlesinger R., Meyer J.S., Baker K., Sanchez M.M. Shaping long-term primate development: telomere length trajectory as an indicator of early maternal maltreatment and predictor of future physiologic regulation. Dev. Psychopathol. 2017;29(5):1539–1551. doi: 10.1017/S0954579417001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M.A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C., Lorenz J.G., Long J., Champoux M., Suomi S.J., Higley J.D. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin. Exp. Res. 2000;24(5):644–650. [PubMed] [Google Scholar]
- Fuster J. Lippincott-Raven; Philadelphia: 1997. Anatomy, Physiology and Neuropsychology. The Prefrontal Cortex. [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Hare T.A., Bookheimer S.Y., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R.A., Rogers J., Katze M.G., Bumgarner R., Weinstock G.M., Mardis E.R., Remington K.A., Strausberg R.L., Venter J.C., Wilson R.K., Batzer M.A., Bustamante C.D., Eichler E.E., Hahn M.W., Hardison R.C., Makova K.D., Miller W., Milosavljevic A., Palermo R.E., Siepel A., Sikela J.M., Attaway T., Bell S., Bernard K.E., Buhay C.J., Chandrabose M.N., Dao M., Davis C., Delehaunty K.D., Ding Y., Dinh H.H., Dugan-Rocha S., Fulton L.A., Gabisi R.A., Garner T.T., Godfrey J., Hawes A.C., Hernandez J., Hines S., Holder M., Hume J., Jhangiani S.N., Joshi V., Khan Z.M., Kirkness E.F., Cree A., Fowler R.G., Lee S., Lewis L.R., Li Z., Liu Y.S., Moore S.M., Muzny D., Nazareth L.V., Ngo D.N., Okwuonu G.O., Pai G., Parker D., Paul H.A., Pfannkoch C., Pohl C.S., Rogers Y.H., Ruiz S.J., Sabo A., Santibanez J., Schneider B.W., Smith S.M., Sodergren E., Svatek A.F., Utterback T.R., Vattathil S., Warren W., White C.S., Chinwalla A.T., Feng Y., Halpern A.L., Hillier L.W., Huang X., Minx P., Nelson J.O., Pepin K.H., Qin X., Sutton G.G., Venter E., Walenz B.P., Wallis J.W., Worley K.C., Yang S.P., Jones S.M., Marra M.A., Rocchi M., Schein J.E., Baertsch R., Clarke L., Csuros M., Glasscock J., Harris R.A., Havlak P., Jackson A.R., Jiang H., Liu Y., Messina D.N., Shen Y., Song H.X., Wylie T., Zhang L., Birney E., Han K., Konkel M.K., Lee J., Smit A.F., Ullmer B., Wang H., Xing J., Burhans R., Cheng Z., Karro J.E., Ma J., Raney B., She X., Cox M.J., Demuth J.P., Dumas L.J., Han S.G., Hopkins J., Karimpour-Fard A., Kim Y.H., Pollack J.R., Vinar T., Addo-Quaye C., Degenhardt J., Denby A., Hubisz M.J., Indap A., Kosiol C., Lahn B.T., Lawson H.A., Marklein A., Nielsen R., Vallender E.J., Clark A.G., Ferguson B., Hernandez R.D., Hirani K., Kehrer-Sawatzki H., Kolb J., Patil S., Pu L.L., Ren Y., Smith D.G., Wheeler D.A., Schenck I., Ball E.V., Chen R., Cooper D.N., Giardine B., Hsu F., Kent W.J., Lesk A., Nelson D.L., O'Brien W E., Prufer K., Stenson P.D., Wallace J.C., Ke H., Liu X.M., Wang P., Xiang A.P., Yang F., Barber G.P., Haussler D., Karolchik D., Kern A.D., Kuhn R.M., Smith K.E., Zwieg A.S. "Evolutionary and biomedical insights from the rhesus macaque genome.". Science. 2007;316(5822):222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Godfrey J.R., Diaz M.P., Pincus M., Kovacs-Balint Z., Feczko E., Earl E., Miranda-Dominguez O., Fair D., Sanchez M.M., Wilson M.E., Michopoulos V. Diet matters: glucocorticoid-related neuroadaptations associated with calorie intake in female rhesus monkeys. Psychoneuroendocrinology. 2018;91:169–178. doi: 10.1016/j.psyneuen.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders N.E. The impact of stress on addiction. Eur. Neuropsychopharmacol. 2003;13(6):435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C., Hablitz J.J. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J. Neurophysiol. 2001;86(6):2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Grant K.A., Johanson C.E. Oral ethanol self-administration in free-feeding rhesus monkeys. Alcohol Clin. Exp. Res. 1988;12(6):780–784. doi: 10.1111/j.1530-0277.1988.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Greenfield S.F., Back S.E., Lawson K., Brady K.T. Substance abuse in women. Psychiatr. Clin. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Hostinar C., Sanchez M.M., Tottenham N., Sullivan R. Parental buffering of fear and stress neurobiology: reviewing parallels across rodent, monkey and human models. Soc. Neurosci. 2015;10:474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Kim K.-S., Mailly P., Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M., Maccari S., Le Moal M., Simon H., Piazza P.V. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698(1–2):46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Haney M., Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199(3):403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatr. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow H.F., Harlow M.K., Suomi S.J. From thought to therapy: lessons from a primate laboratory. Am. Sci. 1971;59(5):538–549. [PubMed] [Google Scholar]
- Hayashi M. Ontogeny of some neuropeptides in the primate brain. Prog. Neurobiol. 1992;38(3):231–260. doi: 10.1016/0301-0082(92)90021-6. [DOI] [PubMed] [Google Scholar]
- Hein D., Cohen L., Campbell A. Is traumatic stress a vulnerability factor for women with substance use disorders? Clin. Psychol. Rev. 2005;(25):813–823. doi: 10.1016/j.cpr.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy M.B. Multiple, brief maternal separations in the squirrel monkey: changes in hormonal and behavioral responsiveness. Physiol. Behav. 1986;36(2):245–250. doi: 10.1016/0031-9384(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila C.A., Rounsaville B.J., Kranzler H.R. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Higley J., Hasert M., Suomi S., Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc. Natl. Acad. Sci. Unit. States Am. 1991;88(16):7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde R.A. McGraw-Hill; 1974. Biological Bases of Human Social Behaviour. [Google Scholar]
- Hinde R.A., McGinnis L. Some factors influencing the effects of temporary mother-infant separation: some experiments with rhesus monkeys. Psychol. Med. 1977;7(2):197–212. doi: 10.1017/s0033291700029275. [DOI] [PubMed] [Google Scholar]
- Hinde R.A., Spencer-Booth Y. The behaviour of socially living rhesus monkeys in their first two and a half years. Anim. Behav. 1967;15(1):169–196. doi: 10.1016/s0003-3472(67)80029-0. [DOI] [PubMed] [Google Scholar]
- Holekamp K.E., Smale L. Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. Am. Zool. 1991;31(2):306–317. [Google Scholar]
- Howell B.R., Godfrey J., Gutman D.A., Michopoulos V., Zhang X., Nair G., Hu X., Wilson M.E., Sanchez M.M. Social subordination stress and serotonin transporter polymorphisms: associations with brain white matter tract integrity and behavior in juvenile female macaques. Cerebr. Cortex. 2014;24(12):3334–3349. doi: 10.1093/cercor/bht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Grand A.P., McCormack K.M., Shi Y., LaPrarie J.L., Maestripieri D., Styner M.A., Sanchez M.M. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev. Psychobiol. 2014;56(8):1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., McCormack K.M., Grand A.P., Sawyer N.T., Zhang X., Maestripieri D., Hu X., Sanchez M.M. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol. Mood Anxiety Disord. 2013;3(1):21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., McMurray M.S., Guzman D.B., Nair G., Shi Y., McCormack K.M., Hu X., Styner M.A., Sanchez M.M. Maternal buffering beyond glucocorticoids: impact of early life stress on corticolimbic circuits that control infant responses to novelty. Soc. Neurosci. 2017;12(1):50–64. doi: 10.1080/17470919.2016.1200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Neigh G.N., Sanchez M. John Wiley; New York, NY: 2016. Animal Models of Developmental Psychopathology. [Google Scholar]
- Howell B.R., Sanchez M.M. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Dev. Psychopathol. 2011;23(4):1001–1016. doi: 10.1017/S0954579411000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell L.L., Murnane K.S. Nonhuman primate neuroimaging and the neurobiology of psychostimulant addiction. Ann. N. Y. Acad. Sci. 2008;1141(1):176–194. doi: 10.1196/annals.1441.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyman S.M., Garcia M., Sinha R. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am. J. Drug Alcohol Abuse. 2006;32(4):655–664. doi: 10.1080/10623320600919193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Paliwal P., Chaplin T.M., Mazure C.M., Rounsaville B.J., Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92(1):208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.M., Paliwal P., Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychol. Addict. Behav. 2007;21(2):233–238. doi: 10.1037/0893-164X.21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. From animal models to model animals. Biol. Psychiatr. 2007;62:1337–1339. doi: 10.1016/j.biopsych.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Jarrell H., Hoffman J.B., Kaplan J.R., Berga S., Kinkead B., Wilson M.E. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol. Behav. 2008;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.O., Kamilaris T.C., Calogero A.E., Gold P.W., Chrousos G.P. Effects of early parenting on growth and development in a small primate. Pediatr. Res. 1996;39(6):999–1005. doi: 10.1203/00006450-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Johnston L.D., Miech R.A., O'Malley P.M., Bachman J.G., Schulenberg J.E., Patrick M.E. 2018. Monitoring the Future National Survey Results On Drug Use, 1975-2017: Overview, Key Findings On Adolescent Drug Use. [Google Scholar]
- Juarez J., Guzman-Flores C., Ervin F.R., Palmour R.M. 1993. Voluntary Alcohol Consumption in Vervet Monkeys: Individual, Sex, and Age Differences; pp. 985–988. 46(4) [DOI] [PubMed] [Google Scholar]
- Kalivas P.W. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res. Rev. 1993;18(1):75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., McFarland K., Bowers S., Szumlinski K., XI Z.X., Baker D. Glutamate transmission and addiction to cocaine. Ann. N. Y. Acad. Sci. 2003;1003(1):169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Stewart J. Dopamine transmission in the initiation and expression of drug-and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., Voorn P., Buijs R., Pool C., Uylings H. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J. Comp. Neurol. 1988;269(1):58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kandel D.B., Yamaguchi K., Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J. Stud. Alcohol. 1992;53(5):447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Chen H., Appt S., Lees C., Franke A., Berga S., Wilson M., Manuck S., Clarkson T. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum. Reprod. 2010;25(12):3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist. 2012;18(6):613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H., McCormack K., Sanchez M.M., Maestripieri D. The development of the hypothalamic–pituitary–adrenal axis in rhesus monkeys: effects of age, sex, and early experience. Dev. Psychobiol. 2014;56(1):86–95. doi: 10.1002/dev.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Plasticity of reward neurocircuitry and the'dark side'of drug addiction. Nat. Neurosci. 2005;8(11):1442. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatr. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.A., Miserendino M.J., Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875(1–2):44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kreek M.J., Koob G.F. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kuhn C. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol. Therapeut. 2015;153:55–78. doi: 10.1016/j.pharmthera.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J.C., Wanat M.J., Smith J.S., Reyes B.A.S., Hollon N.G., Van Bockstaele E.J., Chavkin C., Phillips P.E.M. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490(7420):402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levin E.D., Rezvani A.H., Montoya D., Rose J.E., Swartzwelder H.S. Adolescent-onset nicotine self-administration modeled in female rates. Psychopharmacology. 2003;169(2):141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin E.D., Lawrence S.S., Petro A., Horton K., Rezvani A.H., Seidler F.J., Slotkin T.A. Adolescent vs adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol. Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Higley J. The effect of early experience on brain corticosteroid and serotonin receptors in rhesus monkeys. Biol. Psychiatr. 2002;51(8) 100S-100S. [Google Scholar]
- Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim. Behav. 1998;55(1):1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Carroll K.A. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychol. Sci. 1998;9(2):143–145. [Google Scholar]
- Maestripieri D., Higley J.D., Lindell S.G., Newman T.K., McCormack K.M., Sanchez M.M. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta) Behav. Neurosci. 2006;120(5):1017. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., McCormack K., Lindell S.G., Higley J.D., Sanchez M.M. Influence of parenting style on the offspring's behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav. Brain Res. 2006;175(1):90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Wallen K., Carroll K.A. Genealogical and demographic influences on infant abuse and neglect in group-living sooty mangabeys (Cercocebus atys) Dev. Psychobiol. 1997;31(3):175–180. doi: 10.1002/(sici)1098-2302(199711)31:3<175::aid-dev2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Malkova L., Heuer E., Saunders R.C. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur. J. Neurosci. 2006;24(11):3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- Manitt C., Mimee A., Eng C., Pokinko M., Stroh T., Cooper H.M., Kolb B., Flores C. The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J. Neurosci. 2011;31(23):8381–8394. doi: 10.1523/JNEUROSCI.0606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K., Newman T., Higley J., Maestripieri D., Sanchez M. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm. Behav. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K., Sanchez M.M., Bardi M., Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Dev. Psychobiol. 2006;48(7):537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- Mehlman P., Higley J., Faucher I., Lilly A., Taub D., Vickers J., Suomi S., Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am. J. Psychiatr. 1994;151(10):1485. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Mello N.K., Knudson I.M., Mendelson J.H. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello N.K., Negus S.S. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Michopoulos V., Diaz M.P., Wilson M.E. Social change and access to a palatable diet produces differences in reward neurochemistry and appetite in female monkeys. Physiol. Behav. 2016;162:102–111. doi: 10.1016/j.physbeh.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Higgins M., Toufexis D., Wilson M.E. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Toufexis D., Wilson M.E. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012;37(9):1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Grant K.A., Gage H.D., Mach R.H., Kaplan J.R., Prioleau O., Nader S.H., Buchheimer N., Ehrenkaufer R.L., Nader M.A. Social dominance in monkeys: dopamine D 2 receptors and cocaine self-administration. Nat. Neurosci. 2002;5(2):169. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader M.A., Czoty P.W., Nader S.H., Morgan D. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology. 2012;224(1):57–67. doi: 10.1007/s00213-012-2843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader M.A., Nader S.H., Czoty P.W., Riddick N.V., Gage H.D., Gould R.W., Blaylock B.L., Kaplan J.R., Garg P.K., Davies H.M. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol. Psychiatr. 2012;72(5):414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader M.A., Czoty P.W. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am. J. Psychiatr. 2005;162(8):1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Naneix F., Marchand A.R., Di Scala G., Pape J.-R., Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J. Neurosci. 2012;32(46):16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T.G., Cameron J.L. Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. Am. J. Prev. Med. 2006;31(6,1):S175–S181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebr. Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Patel P.D., Lopez J.F., Lyons D.M., Burke S., Wallace M., Schatzberg A.F. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatr. Res. 2000;34(6):383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Piazza P.V., Deminiere J.M., Le Moal M., Simon H. Stress-and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514(1):22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Preuss T.M. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J. Cognit. Neurosci. 1995;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Preuss T.M., Goldman-Rakic P.S. Ipsilateral cortical connections of granular frontal cortex in the strepsirhine primate Galago, with comparative comments on anthropoid primates. J. Comp. Neurol. 1991;310(4):507–549. doi: 10.1002/cne.903100404. [DOI] [PubMed] [Google Scholar]
- Preuss T.M., Goldman-Rakic P.S. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J. Comp. Neurol. 1991;310(4):429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Ruedi-Bettschen D., Dettling A.C., Weston A., Russig H., Ferger B., Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neurosci. Biobehav. Rev. 2005;29(4–5):649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Reep R. Relationship between prefrontal and limbic cortex: a comparative anatomical review. Brain Behav. Evol. 1984;25(1):5–80. doi: 10.1159/000118849. [DOI] [PubMed] [Google Scholar]
- Reiss A.L., Abrams M.T., Singer H.S., Ross J.L., Denckla M.B. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reynolds L.M., Makowski C.S., Yogendran S.V., Kiessling S., Cermakian N., Flores C. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a dcc-dependent manner. Neuropsychopharmacology. 2015;40:1101–1112. doi: 10.1038/npp.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Winslow J.T., O'Brien D., Gutman D.A., Hoffman J.M., Kilts C.D. Neural correlates of maternal separation in rhesus monkeys. Biol. Psychiatr. 2001;49(2):146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. Adolescence: a central event in shaping stress reactivity. Dev. Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo R.D. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., McEwen B.S. Stress and the adolescent brain. Ann. N. Y. Acad. Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.R., Lewis D.A. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J. Comp. Neurol. 1995;358(3):383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Roth M.E., Cosgrove K.P., Carroll M.E. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci. Biobehav. Rev. 2004;28(6):533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Sade D.S. Univ. of Chicago Press; Chicago: 1967. Determinants of Dominance in a Group of Free-ranging Rhesus Monkeys. [Google Scholar]
- Sanchez M.M. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm. Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez M.M., Ladd C.O., Plotsky P.M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez M.M., McCormack K., Grand A.P., Fulks R., Graff A., Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev. Psychopathol. 2010;22(1):45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.M., McCormack K.M., Howell B.R. Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci. 2015;10(5):512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.M., Noble P.M., Lyon C.K., Plotsky P.M., Davis M., Nemeroff C.B., Winslow J.T. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatr. 2005;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Pollak S. 2009. Socio-emotional development following early abuse and neglect: challenges and insights from translational research. Handbook of developmental social neuroscience; pp. 497–520. [Google Scholar]
- Sanchez M.M., Young L.J., Plotsky P.M., Insel T.R. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J. Neurosci. 2000;20(12):4657. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.M., Young L.J., Plotsky P.M., Insel T.R. Autoradiographic and in situ hybridization localization of CRF1 and CRF2 receptors in non-human primate brain. J. Comp. Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Schwandt M.L., Lindell S.G., Chen S., Higley J.D., Suomi S.J., Heilig M., Barr C.S. Alcohol response and consumption in adolescent rhesus macaques: life history and genetic influences. Alcohol. 2010;44(1):67–80. doi: 10.1016/j.alcohol.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Gorelova N., Durstewitz D., Yang C.R. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J. Neurosci. 2001;21(10):3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Yang C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seay B., Gottfried N.W. A phylogenetic perspective for social behavior in primates. J. Gen. Psychol. 1975;92(1):5–17. doi: 10.1080/00221309.1975.9711323. [DOI] [PubMed] [Google Scholar]
- Sesack S.R., Snyder C.L., Lewis D.A. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA‐immunoreactive dendrites in rat and monkey cortex. J. Comp. Neurol. 1995;363(2):264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Shahbazi M., Moffett A.M., Williams B.F., Frantz K.J. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology. 2008;196(1):71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim H.-R., Choi T.-Y., Lee H.J., Kang E.Y., Yoon S., Han P.-L., Choi S.-Y., Baik J.-H. Role of dopamine D2 receptors in plasticity of stress-induced addictive behaviours. Nat. Commun. 2013;4:1579. doi: 10.1038/ncomms2598. [DOI] [PubMed] [Google Scholar]
- Simpson T.L., Miller W.R. Concomitance between childhood sexual and physical abuse and substance use problems. A review. Clin. Psychol. Rev. 2002;22(1):27–77. doi: 10.1016/s0272-7358(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biol. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cognit. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spielberg J.M., Olino T.M., Forbes E.E., Dahl R.E. Exciting fear in adolescence: does pubertal development alter threat processing? Dev. Cognit. Neurosci. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi S.J. Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Hum. Dev. 2005;48(1–2):67–79. [Google Scholar]
- Suomi S.J., Harlow H.F., Kimball S.D. Behavioral effects of prolonged partial social isolation in the rhesus monkey. Psychol. Rep. 1971;29(3):1171–1177. doi: 10.2466/pr0.1971.29.3f.1171. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell'Acqua F., Valabregue R., Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Troisi A., D'Amato F.R. Ambivalence in monkey mothering. Infant abuse combined with maternal possessiveness. J. Nerv. Ment. Dis. 1984;172(2):105–108. doi: 10.1097/00005053-198402000-00007. [DOI] [PubMed] [Google Scholar]
- Troisi A., D'Amato F.R., Fuccillo R., Scucchi S. Infant abuse by a wild-born group-living Japanese macaque mother. J. Abnorm. Psychol. 1982;91(6):451–456. doi: 10.1037//0021-843x.91.6.451. [DOI] [PubMed] [Google Scholar]
- Verney C., Alvarez C., Geffard M., Berger B. Ultrastructural double‐labelling study of dopamine terminals and GABA‐containing neurons in rat anteromedial cerebral cortex. Eur. J. Neurosci. 1990;2(11):960–972. doi: 10.1111/j.1460-9568.1990.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Baler R. Addiction science: uncovering neurobiological complexity. Neuropharmacology. 2014;76:235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Baler R.D. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38(6):345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Baler R.D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cognit. Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W.C., Ford K.A., Pagels N.E., McCutcheon J.E., Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J. Neurosci. 2013;33(11):4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel R.A. Springer; 1987. Intravenous Self-administration: Response Rates, the Effects of Pharmacological Challenges, and Drug Preference. Methods of Assessing the Reinforcing Properties of Abused Drugs; pp. 1–33. [Google Scholar]
- Zhou F.-M., Hablitz J.J. Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. J. Neurophysiol. 1999;81(3):967–976. doi: 10.1152/jn.1999.81.3.967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.