Abstract
Background.
Soil contamination from heavy metals and polycyclic aromatic hydrocarbons (PAHs) released during informal e-waste processing and disposal poses human and ecological health risks in Nigeria.
Objectives.
This study assesses the levels of heavy metals and PAHs in soils of e-waste dumpsites in Lagos and Ibadan, Nigeria.
Methods.
Composite soil samples were collected at depths of 0–15 cm, 15–30 cm and 30–45 cm from major e-waste dumpsites in Lagos and Ibadan and analyzed for lead (Pb), cadmium (Cd), copper (Cu), nickel (Ni), zinc (Zn), chromium (Cr) and PAHs to evaluate the potential contaminant contribution from e-waste activities. Control samples were collected at the Botanical Garden, University of Ibadan. Samples were analyzed for heavy metals after acid digestion using atomic absorption spectrophotometry, while PAHs were extracted using cold solvent extraction and quantified by gas chromatography-mass spectrometry. Blank determination and recovery studies were carried out for each metal. Contamination and ecological risks were assessed using soil contamination indices such as contamination factor, geo-accumulation and pollution load indices, and potential ecological risk index to categorize contaminant concentrations and associated impacts. Soil physico-chemical characteristics such as pH and total organic matter were also determined.
Results.
Metals concentrations in the dumpsite soils ranged from 114–2,840 mg/kg and not detectable - 6.50 mg/kg for Pb and Cd, and 42.8–5,390 mg/kg, 27.5–3,420 mg/kg, 11.0–128 mg/kg and 94.0–325 mg/kg for Cu, Zn, Ni and Cr, respectively. Serious metals accumulation was observed at every e-waste dumpsite, as shown by the pollution load index. The potential ecological risk values were between 584 and 10,402 at all of the dumpsites, signifying very high ecological risk. The total PAHs ranged from 1,756–2,224 μg/kg at the 0–15 cm level, 1,664–2,152 μg/kg at 15–30 cm and 278 μg/kg in the top- and sub-soil of the control site.
Discussion.
The total PAHs in the soil of e-waste dumpsites was significantly higher than in the control soil.
Conclusions.
The results of this study indicate that indiscriminate dumping and open burning of e-waste are potential sources of PAH and toxic metal emissions, which can pose serious human health and ecological risks.
Keywords: e-waste, soil contamination, heavy metals, PAHs, ecological risk, waste management
Introduction
Soil contamination resulting from uncontrolled dumping of municipal, industrial, and agricultural solid waste, as well as hazardous waste such as e-waste, has become a public health concern in Nigeria.1–6 Of particular concern is soil contamination at informal electronic waste recycling and disposal sites. In Nigeria, domestic and imported e-waste streams are growing steadily due to the increased availability of secondhand computers used in computer training centers, printing houses, cyber cafes, business centres and homes. Researchers have estimated that, on average, 500 shipping containers, with 400,000 computer monitors or 175,000 large TV sets enter the port of Lagos, Nigeria per year. As much as 75% of this waste is unserviceable and unable to be refurbished, and thus becomes e-waste.7–10
In addition to precious metals such as gold, silver, and platinum, e-waste contains toxic metals such as lead (Pb) and cadmium (Cd), arsenic (As), and mercury (Hg).11 Informal e-waste recycling and disposal practices such as open burning and dumping can lead to leaching of these toxic metals into the soil. Humans can be exposed to soil contaminants from e-waste dumpsites through accidental soil ingestion or direct dermal exposure.12–21 Lead levels in dust have been significantly associated with Pb levels in children's blood, and a blood lead level greater than 10 μg Pb/dL has been associated with a decrease in intelligence quotient (IQ).22,23 Exposure to high levels of heavy metals such as Pb, Cd, and Hg through ingestion and dermal contact can result in acute and chronic toxicity. These metals can damage the central and peripheral nervous systems, result in blood abnormalities, impair the lungs, kidneys, and liver, and even lead to death. The health and environmental effects of individual metals vary from toxic to endocrine disruption.24–27 Elevated metals concentrations in surface soils can pose a risk to human health.28 Heavy metals can migrate from surface soil to subsoil and contaminate ground water. They can also bio-accumulate in the food chain, posing health risks at high concentrations.
Polycyclic aromatic hydrocarbons, another class of toxic chemicals, are released by low-temperature combustion of e-waste.19,29,30 Although limited data exist on the distribution and transport of polycyclic aromatic hydrocarbons (PAHs) from e-waste dumpsites in Nigeria, PAHs are known to be lipophilic and accumulate in the food chain near contaminated sites.20,31–37 Their lipophilicity also makes dermal absorption possible. Epidemiological studies on occupational exposure to PAHs indicate that they can contribute to induction of skin and lung cancers. It has been reported that certain PAH metabolites interact with deoxyribonucleic acid (DNA) and are genotoxic, causing malignancies and heritable genetic damage in humans.38 The lower molecular weight PAHs (e.g., 2–3 rings) such as naphthalene, fluorene, phenanthrene and anthracene have significant acute toxicity to aquatic organisms, while higher molecular weight PAHs (4–7 rings) such as chrysene and coronene do not, but are carcinogenic.
This study assesses the distribution and levels of toxic metals and PAHs in the soil of selected e-waste dumpsites in Lagos and Ibadan, Nigeria, where open burning is prevalent. Data of this nature are currently lacking for Nigeria, and understanding local contaminant levels is important for effective health risk assessment. We also estimated human and ecological health risks using the pollution load index and ecological risk index, using our soil concentration data as inputs.39,40 A secondary objective was to determine contaminant origin (lithogenic versus anthropogenic) using the index of geo-accumulation and contamination factors.39,41
Methods
Study Area
Lagos and Ibadan are located in southwestern Nigeria. Alaba international market, Ojo (LLS1) and Chinatown, Ojota (LLS2) are the locations of the two e-waste dumpsites selected for the present study in Lagos. The Alaba market sampling site is a large expanse of land adjacent to the market shopping complex. The major wastes observed on this site were e-waste, followed by polythene bags, cartons, cardboards and cans. The Chinatown dumpsite is located on a small plot of land adjacent to the Chinese building at Ojota, a suburb in Lagos. Wastes observed there included broken monitor glass, plastics, cans, polythene bags and paper. The three Ibadan dumpsites include along Iwo road/Ile-pupa, located behind an electronics shopping complex (ISS1), the Ogunpa dumpsite, adjacent to the Ogunpa River channel (ISS2), and the Dugbe dumpsite, adjacent to residential buildings (ISS3). At every site except for Dugbe (ISS3), open burning to recover copper and other valuable materials is commonly practiced. At Dugbe, no traces of burning were apparent among the e-waste piles. Control samples were also collected at the Botanical Garden, University of Ibadan, Ibadan.
Soil Sample Collection
Samples for PAH determination were collected with a stainless steel hand trowel, while plastic was used for collection of samples for heavy metal determination. The stainless hand trowel and plastic were cleaned thoroughly to prevent cross contamination. Samples were collected randomly at almost 5 m distance from five different points and combined to form a composite sample, with this process repeated at three different depths (0–15 cm, 15–30 cm and 30–45 cm) for heavy metal determination and two depths (0–15 cm and 15–30 cm) for PAH determination. Samples for PAHs were packed in pre-cleaned aluminum foil, which was previously solvent rinsed and dried at 80°C. Polyethylene bags were used for packing soils for heavy metal determination. Samples for metals and soil characteristics determination were air-dried in the laboratory after manual removal of stones, twigs and other large materials then ground in a porcelain mortar and passed through a 2-mm sieve. PAH samples were preserved on ice and kept in the refrigerator prior to extraction and analyses.
Abbreviations
- Cfi
Contamination factor
- Eir
Ecological risk factors
- HMW
High molecular weight
- Igeo
Geo-accumulation index
- LMW
Low molecular weight
- PAH
Polycyclic aromatic hydrocarbon
- PLI
Pollution load index
- RI
Ecological risk index
- VROM
Dutch Ministry of Housing, Spatial Planning and the Environment
Analytical Procedures
Samples were analyzed for PAHs, heavy metals and soil characteristics. For the metals analysis, approximately 1 g each of the sieved samples were weighed into digestion tubes and 10 ml aqua regia (concentrated hydrogen chloride and nitric acid, ratio 3:1 vol/vol) added (United States Environmental Protection Agency (USEPA) method 3050b).42 The tubes were covered, heated in a water bath to 100°C for 2 hours with intermittent shaking, cooled to room temperature, and then filtered using filter papers (pore size 110 mm). The filtrate was diluted with distilled water to 25 mL and analyzed for total Pb, chromium (Cr), Cd, nickel (Ni), zinc (Zn) and copper (Cu) using atomic absorption spectrophotometry (Buck Scientific Model 205A). Metal recovery was carried out by spiking 1 g of the soil sample with known concentrations of each metal. The concentrations of the metals were determined after taking the spiked sample through the entire procedure. The concentrations of each metal in the unspiked sample was deducted from that of the spiked sample and divided by the concentrations of the metals used for spiking, then multiplied by 100. The recovery was between 93.2 -100.4% for all the metals.
Sixteen target PAHs were analyzed using gas chromatography-mass spectrometry (GS/MS) following modified USEPA methods (method 827°C).43 Approximately 5 g of each sample and 5 g of anhydrous sodium sulphate were weighed and homogenized to a complete mixture. The mixtures were transferred to pre-cleaned extraction tubes, and 25 mL dichloromethane added. The tubes were tightly capped, allowed to stand for 30 minutes, and then shaken vigorously for 30 minutes. The solids were allowed to settle and solvent layers were filtered using filter papers. The procedure was repeated with 25 mL dichloromethane. The two extracts were combined, concentrated on a rotary evaporator (Büchi Rotavapor R-114), exchanged with 5 mL of n-hexane and re-concentrated to 1 mL for clean-up. The extracts were then eluted with 25 mL dichloromethane/hexane (20:80 v/v) on a silica gel column. The extracts were evaporated and re-dissolved in 1 mL n-hexane. The cleaned extracts were analyzed for the 16 representative PAHs using a Shimadzu GS/MS QP 2010 model. Helium gas was used as the carrier gas with a constant flow rate of 1 mL/min, HP-1 ms column (30 m × 0.25 μm 0.25 mm ID), injection mode was pulsed splitless, volume of extract injected was 1 μL, injection port temperature was 290°C, pulse pressure and flow were 35 psi (0.5 min) and 20 mL/min (2 min), respectively; solvent delay was 5 min, initial oven temperature and hold time was 50°C (1 min), ramped at 30°C/min to 280°C and 15°C/min to 310°C with final hold time of 4 min. External calibration using PAHs standard was used for analytes quantification, while identification was based on retention time. The quantification limit of the PAHs in the standard and the samples was 0.001 ppm. The average response factor for the weight ranges were calculated and used for sample quantification. The concentration of each analyte was determined by calculating the amount of analyte injected from the peak response in area ratio as shown below:
- Calibration factor for each priority PAH =
AC/Mc
- Average calibration factor for each priority PAH, CFav =
(ΣCF)/N
- The amount of analyte injected, Xs =
AS/CFav
- Actual concentration of the analyte in the sample extracted (µg/kg) =
Xs x Vt x Df/Ws
Where;
- Ac =
peak area of the compound in the standard
- Mc =
mass of the compound injected in nanograms
- N =
number of calibration points in the external calibration curve
- AS =
peak area of the analyte in the sample
- CFav =
average calibration factor (for each analyte, the average of the different calibration points)
- Xs =
calculated mass of the analyte in the sample aliquot introduced into the instrument (in nanograms)
- Vt =
total volume of the concentrated extract (μL)
- Ws =
weight of soil sample extracted (g)
Soil pH and total organic carbon (TOC) were determined by standard methods using a Jenway 3310 pH meter in ratio 1:2 (wt/vol) and the Walkey-Black method, respectively.44 Approximately 0.5 g of each of the sieved samples were weighed, 10 mL of standard potassium dichromate solution added, and swirled to mix, 15 mL of concentrated sulphuric acid was added gently and mixed. The flasks were allowed to stand for 30 minutes. Five drops of ferroin indicator was added and the resulting mixtures were titrated against ferrous ammonium sulphate until color change from blue green to violet red was observed. Total organic carbon was determined using an appropriate mathematical expression and multiplied by a factor to obtain the total organic matter (TOM).22,24
Soil Contamination
The degree of contamination of the dumpsite and the control site soils was evaluated using four indices.
Geo-accumulation Index
Geo-accumulation Index (Igeo) shows the degree of anthropogenic pollution in soil samples by comparing soil metals concentrations to average shale values.41,45 It is expressed using Equation 1
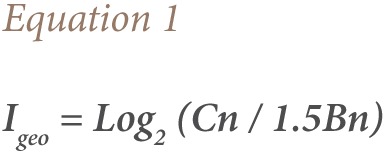 |
where,
Cn is the measured concentration of a particular metal in a particular soil sample; Bn is the geochemical background value in average shale of element n and 1.5 is a background matrix correction factor, accounting for lithogenic effects.46 We then classified each Igeo using Forstner et al. descriptive categories: <0, unpolluted; 0–1, unpolluted to moderately polluted; 1–2, moderately polluted; 2–3, moderately to highly polluted; 3–4, highly polluted; 4–5, highly to very highly polluted, and >5, very highly polluted.47
Contamination Factor
The contamination factor (Cfi) was used by Hakanson to assess soil contamination by comparing the contaminant concentration in the surface layer to a background value.39,48 We used a modified Cfi formula, using metals concentrations in the control samples instead of background values, which are currently lacking for Nigeria.49 It is expressed using Equation 2.
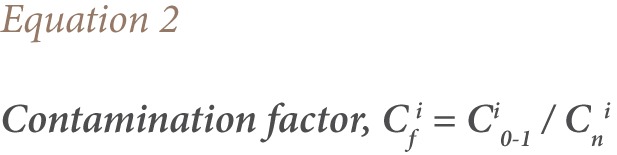 |
where,
Cfi = contamination factor; Ci0–1 = mean concentration of each metal in the soil; Cni = baseline or background value (concentration of each metal in the control sample was used); n = number of analyzed elements; i = ith element (or pollutants). We then classified the Cfi using descriptive categories: Cfi < 1, low contamination; 1 ≤ Cfi < 3, moderate contamination; 3 ≤ Cfi < 6, considerable contamination; and 6 ≤ Cfi, very high contamination.
Pollution Load Index
Pollution load index (PLI) was also used to assess the metal accumulation and multi-element contamination resulting in increased overall metal toxicity.50 Heavy metal contamination is associated with a mixture of contaminants rather than one metal contaminant.51 The higher the pollution load index, the more serious the heavy metal accumulation in the soil.50 We used the PLI to characterize the aggregate contamination of the six target metals using Equation 3.
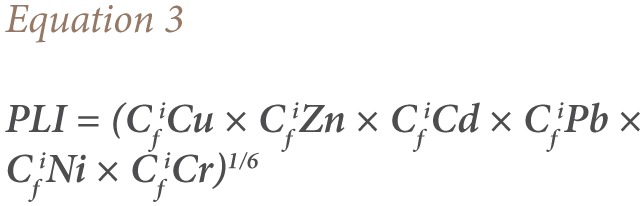 |
Where; Cfi is the contamination factor of each metal.40
Potential Ecological Risk Index
In this study, a simplified approach to risk assessment based on comparison of the measured level of contamination in the soil of the studied sites with the background value from the control sample was adopted.49 Although the ecological risk index (RI) is primarily intended by Hakanson to express the ecotoxic potential of increased concentrations of toxic metals such as arsenic, Cu, Ni, cobalt, Pb, Cd, and mercury in consumable fish, it can also be applied for the assessment of the potential risk from toxic substances to biota and non-human biota in other similar media such as contaminated soils.39,52 We used the RI introduced by Hakanson to characterize the metal contamination of each sample in terms of their potential ecotoxicity using the Equations 4 to 6.
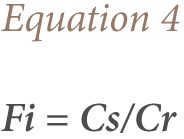 |
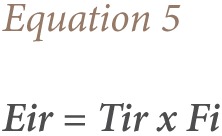 |
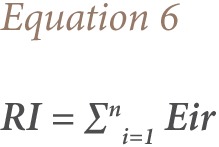 |
where;
Fi is the single metal pollution index; Cs is the concentration of metal in the samples; Cr is the reference value for the metal; Eir is the monomial potential ecological risk factor; Tir is the metal toxic response factor according to Hakanson, and Zn = 1< Cr = 2 < Cu = Ni = Pb = 5 < As = 10 < Cd 30.39,53 The ecological risk index is the potential ecological risk caused by the overall contamination categorized in the four classes as shown in Table 1. The potential ecological risk caused by Cu, Zn, Cd, Pb, Ni and Cr on the e-waste dumpsite soils in Lagos and Ibadan were calculated based on the potential ecological risk factor (Eir). The ecological RI value characterizes the sensitivity of the local ecosystem to the pollutants i.e., metals, and represents the ecological risks resulting from the overall contamination. The overall RI was calculated as the sum of all the four risk factors.
Table 1.
Ecological Risk Category of Metals
| Eir value | Level of Ecological Risk of Metal | RI Value | Ecological Risk Category |
|---|---|---|---|
| Eir < 40 | Low risk | RI < 110 | Low risk |
| 40 ≤ Eir < 80 | Moderate risk | 110 ≤ RI < 200 | Moderate risk |
| 80 ≤ Eir < 160 | Considerable risk | 200 ≤RI <400 | Considerable risk |
| 160 ≤ Eir < 320 | High risk | 400 ≤ RI | Very high risk |
| 320 ≤ Eir | Very high risk |
Abbreviations: RI, ecological risk index; Eir, ecological risk factor
Statistical Analysis
Obtained data (i.e., soil properties, metals concentrations and total concentrations of PAHs) were subjected to descriptive statistics and Pearson's correlation coefficient to determine whether there were significant relationships between total PAHs, metals concentrations and soil properties. The statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 16.0.
Results
Soil Characteristics and Total Metals Concentrations
The pH of topsoil (0–15 cm) ranged from 5.77–5.80 and 5.84 - 6.30, respectively, in samples collected in Lagos (LSS) and Ibadan (ISS), while total organic matter ranged from 8.32–8.85% and 3.27–8.65%, respectively (Table 2). In general, e-waste dumpsite soils were more acidic than the control soil. This might be attributed to the parent material and burning of wastes on the dumpsites. Among dumpsite soils, the Lagos samples were more acidic, with high TOM compared to Ibadan samples. Metals concentrations across dumpsites varied widely. Topsoil Pb ranged from 193–2,240 mg/kg in Lagos and 246–2,090 mg/kg in Ibadan, while Cu ranged from 50.5–5,390 mg/kg and 79.3–1,150 mg/kg, Zn ranged from 220–1930 mg/kg and 27.5–3420 mg/kg, Cd ranged from 0.43–5.85 mg/kg and not detectable-6.50 mg/kg, Ni ranged from 11.0–51.5 mg/kg and 27.7–128 mg/kg, and Cr ranged from 108–118 mg/kg and 94.0–325 mg/kg, in Lagos and Ibadan, respectively (Table 2). Metals concentrations in the control sample were generally lower than what was detected in e-waste dumpsite soils by over one hundred orders of magnitude in some metals, which may be at least partly explained by e-waste burning activity at the dumpsites. Most heavy metals determined in soils collected from Wenling, an emerging e-waste recycling city in Taizhou, China exceeded the respective Grade II value of soil quality standards from the State Environmental Protection Administration of China and also exceeded the Dutch optimum values.19 High levels of Cu (712 and 496 mg/kg) exceeding the new Dutch list action value (of 190 mg/kg) were reported in soil near a printer roller dumping area and a plastic burning site at an electronic waste recycling site at Guiyu, southeast China (Table 3).
Table 2.
Concentrations of Heavy Metals and Soil Characteristics of E-waste Dumpsites in Lagos and Ibadan
| Sampling Location | Depth (cm) | Cu | Zn | Cd | Pb | Ni | Cr | pH | TOM (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lagos | |||||||||
| LLS1 | 15 | 5,390 | 1,820 | 5.63 | 2,840 | 51.5 | 113 | 5.77 | 8.85 |
| 30 | 3,830 | 1,550 | 5.85 | 1,630 | 36.1 | 110 | 5.77 | 8.63 | |
| 45 | 2,780 | 1,480 | 2.63 | 260 | 25.1 | 108 | 5.76 | 6.73 | |
| LLS2 | 15 | 50.5 | 220 | 0.45 | 193 | 17.5 | 114 | 5.80 | 8.32 |
| 30 | 57.5 | 1,930 | 0.58 | 114 | 12.0 | 118 | 5.77 | 6.88 | |
| 45 | 50.0 | 1,750 | 0.43 | 142 | 11.0 | 111 | 5.77 | 3.48 | |
| Ibadan | |||||||||
| ISS1 | 15 | 1,150 | 3,420 | 4.53 | 2,090 | 48.7 | 114 | 5.93 | 8.65 |
| 30 | 440 | 161 | 2.28 | 768 | 27.7 | 107 | 5.88 | 6.79 | |
| 45 | 310 | 1,430 | 1.98 | 302 | 40.5 | 104 | 5.89 | 3.09 | |
| ISS2 | 15 | 101 | 1,840 | 6.30 | 1,030 | 46.4 | 103 | 5.84 | 7.95 |
| 30 | 356 | 571 | 2.88 | 365 | 27.7 | 111 | 5.88 | 6.54 | |
| 45 | 1,210 | 815 | 6.50 | 840 | 41.1 | 105 | 5.89 | 3.06 | |
| ISS3 | 15 | 79.3 | 105 | 0.03 | 246 | 128 | 325 | 6.30 | 3.27 |
| 30 | 51.8 | 77.8 | 0.03 | 253 | 104 | 117 | 6.17 | 0.88 | |
| 45 | 42.8 | 27.5 | ND | 178 | 47.5 | 94.0 | 6.10 | 0.36 | |
| CSS | 15 | 3.98 | 47.4 | 0.35 | 6.25 | 0.33 | 3.95 | 6.89 | 1.56 |
| 30 | 7.55 | 26.2 | 0.45 | 6.75 | 2.35 | 3.58 | 7.21 | 0.29 | |
| 45 | 4.75 | 19.8 | 0.50 | 13.8 | 2.23 | 5.08 | 7.07 | 0.24 | |
Values presented as mg/kg
Abbreviations: ND, not detected
Table 3.
Comparison of Heavy Metal Concentrations in Soil of E-waste Dumpsites Across Studies
| Location | Type of Soil or Sediment | Cr | Ni | Cu | Zn | Cd | Hg | Pb | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| mgkg−1 dry weight | ||||||||||
| Lagos, and Ibadan, Nigeria | E-waste dumpsite soils | This study | ||||||||
| Surface soil (0–15 cm) | Range | 103–325 | 17.5–128 | 50.0–5,390 | 105–3,420 | 0.03–6.30 | - | 246–2,840 | ||
| Subsoil (15–30 cm) | Range | 107–118 | 12.0–104 | 51.8–3,830 | 77.8–1,930 | 0.03–5.85 | - | 114–1,630 | ||
| Subsoil (30–45 cm) | Range | 47.5–111 | 11.0–47.5 | 42.8–2,780 | 27.5–1,750 | ND-6.50 | - | 142–840 | ||
| Taizhou, Zhejiang Province, China | Paddy soil (0–20 cm) in an e-waste recycling area (n = 6) | Range | 54.4–74.1 | 25.8–46.2 | 56.1–236.9 | - | 0.55–7.86 | 0.24–0.76 5 | 1.96–64.6 | 53 |
| Longtang Town, Northern Guangdong Province, China | Surface soil (0–15 cm) of a vegetable garden (n = 16) | Range | 12.3±5.1 | 8.83±2.9 | 324±172 | 122±55.7 | 0.9±0.8 | - | 95.6±19.5 | 54 |
| 9.66–19 | 7.04–10.3 | 210–450 | 92.4–142 | 0.26–1.17 | - | 73.3–134 | ||||
| Surface soil (0–15 cm) of a paddy field (n = 11) | Range | 17.3±8.1 | 34.5±26.6 | 155±94 | 166±76.7 | 1.0±0.4 | - | 61.8±24 | ||
| 10.5–24.1 | 10.8–66 | 40.1–260 | 62.1–252 | 0.04–1.43 | - | 48.1–97 | ||||
| Surface soil (0–15 cm) of an incineration site (n = 11) | Range | 68.9±53 | 60.1±59 | 11,140±9,000 | 3,690±2,680 | 17.1±12.5 | - | 4,500±3 370 | ||
| 23.6–122 | 12.2–132 | 1,500–21,400 | 682–8,970 | 3.05–46.8 | - | 629–7,720 | ||||
| Bangalore City, India | Soil of an e-waste recycling site in a slum area (n = 7) | Range | 46–160 | - | 61.7–4,790 | 126–2,530 | 0.385–38.9 | 0.09–59 | 90.4–2,850 | 55 |
| Soil of an e-waste recycling area (n = 3) | Range | 50–62 | - | 154–2,190 | 119–499 | 0.301–0.906 | - | 79.1–262 | ||
Metals Contamination Indices
The Igeo analysis showed that the soil of LLS1 was very highly polluted with Pb and Cu, moderately to highly polluted with Zn and Cd, and unpolluted with Cr and Ni (Table 4). The second dumpsite in Lagos, LLS2, was highly polluted with Zn and Pb, unpolluted with Cu, Ni, and Cr, and unpolluted to moderately polluted with Cd. The same trend was observed in Ibadan dumpsites samples, while the control sample was not found to be polluted with any of the target metals. The Dugbe dumpsite (ISS3) in Ibadan, where e-waste burning was not typically observed, was generally unpolluted with the targeted metals except for Pb (Igeo range 2-3) and Ni (Igeo range 0–1), which may be attributed to other possible sources of contamination such as vehicular emissions and atmospheric deposition.
Table 4.
Geo-accumulation Indices of Metals in Dumpsite and Control Soil Samples
| Sampling Location | Depth (cm) | Cu | Zn | Cd | Pb | Ni | Cr |
|---|---|---|---|---|---|---|---|
| Lagos | |||||||
| LLS1 | 15 | 6.32 | 3.67 | 3.65 | 6.56 | 0.99 | −0.26 |
| 30 | 5.83 | 3.44 | 3.70 | 5.76 | −1.50 | −0.30 | |
| 45 | 5.36 | 3.38 | 2.55 | 3.11 | −2.02 | −0.32 | |
| LLS2 | 15 | −0.42 | 0.63 | 3.2 × 10−16 | 2.69 | −2.54 | −0.24 |
| 30 | −0.23 | 3.76 | 0.37 | 1.93 | −3.09 | −0.19 | |
| 45 | −0.43 | 3.62 | −0.07 | 2.24 | −3.21 | −0.28 | |
| Ibadan | |||||||
| ISS1 | 15 | 3.09 | 4.58 | 3.33 | 6.12 | −1.07 | −1.47 |
| 30 | 1.70 | 0.18 | 2.34 | 4.68 | −1.88 | −2.29 | |
| 45 | 1.20 | 3.33 | 2.14 | 3.33 | −1.33 | −1.74 | |
| ISS2 | 15 | −0.42 | 3.69 | 3.81 | 5.10 | −1.14 | −1.54 |
| 30 | 1.40 | 2.00 | 2.68 | 3.60 | −1.88 | −2.29 | |
| 45 | 3.16 | 2.52 | 3.85 | 4.81 | −1.31 | −1.72 | |
| ISS3 | 15 | −0.77 | −0.44 | −4.17 | 3.04 | 0.38 | −0.08 |
| 30 | −1.38 | −0.87 | −4.17 | 3.08 | 0.03 | −0.38 | |
| 45 | −1.66 | −2.37 | 0 | 2.57 | −1.10 | −1.51 | |
| CSS | 15 | −4.08 | −1.59 | −0.36 | −2.26 | −8.27 | −5.09 |
| 30 | −3.16 | −2.44 | 3.2 × 10−16 | −2.15 | −5.44 | −5.24 | |
| 45 | −3.83 | −2.85 | 0.15 | −1.12 | −5.52 | −4.73 | |
The Cfi analyses using Hakanson's classification showed that the dumpsites soils were highly contaminated with all of the metals except for Cd, which showed moderate contamination at the sites LLS2 and ISS3 (Table 5).39 Contamination of site LLS1 was the highest, possibly attributable to the large volume of e-waste handled at Alaba, an international electronics market for both Nigeria and West Africa. The PLI calculations showed serious metals accumulation at all the e-waste dumpsites with the Alaba market (LLS1) having the highest pollution load index of 109 (Table 5). In the Eir analyses, Zn showed low levels of ecological risk at all the dumpsites except for ISS1 in Ibadan, which had moderate risk (Table 6). In most cases, Cu, Cd, Pb, and Ni showed moderate to very high ecological risk, while Cr showed moderate to high ecological risk. The RI values were between 584 and 10,402 at all of the dumpsites, signifying very high ecological risk.
Table 5.
Metal Contamination Factors and Pollution Load Indices in Dumpsites
| Sites | Cu | Zn | Cd | Pb | Ni | Cr | Total Cfi | PLI |
|---|---|---|---|---|---|---|---|---|
| Lagos | ||||||||
| LLS1 | 1354 | 38.4 | 16.1 | 454 | 156 | 28.6 | 2,047 | 109 |
| LLS2 | 12.7 | 4.64 | 1.29 | 30.9 | 53.0 | 28.9 | 131 | 12.4 |
| Ibadan | ||||||||
| ISS1 | 289 | 72.2 | 12.9 | 334 | 148 | 28.9 | 885 | 85.1 |
| ISS2 | 25.4 | 38.8 | 18.0 | 165 | 141 | 26.1 | 414 | 46.9 |
| ISS3 | 19.9 | 2.22 | 0.07 | 39.4 | 388 | 82.3 | 532 | 12.6 |
Abbreviations: Cf
i, contamination factor; PLI, pollution load index
Table 6.
Metals Ecological Risk Factors and Risk Indices in Dumpsites
| E-waste Dumpsites | Eir | RI | |||||
|---|---|---|---|---|---|---|---|
| Cu | Zn | Cd | Pb | Ni | Cr | ||
| LLS1 | 6,771 | 38.4 | 483 | 2,270 | 780 | 57.2 | 10,402 |
| LLS2 | 63.4 | 4.64 | 38.6 | 154 | 265 | 57.7 | 584 |
| ISS1 | 1,445 | 72.2 | 388 | 1,672 | 738 | 57.7 | 4,373 |
| ISS2 | 127 | 38.8 | 540 | 824 | 703 | 52.2 | 2,285 |
| ISS3 | 99.6 | 2.22 | 2.14 | 197 | 1,939 | 165 | 2,405 |
Abbreviations: Eir, ecological risk factor; RI, risk index
PAH Concentrations
The USEPA identified 16 priority PAHs, which can be classified as being of low or high molecular weight. Low molecular weight (LMW) PAHs (i.e., acenaphthylene, naphthalene, acenaphthene, fluorene, phenanthrene and anthracene), also referred to as petrogenic (formed during the emission of non-combustion-derived matter, including inadvertent oil spills), have molecular weights ranging from 128.2 to 178.2 g/mol. High molecular weight (HMW), pyrolytic PAHs, are fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoranthene, benzo(a) fluoranthene, benzo(k)pyrene, dibenzo(a, h)anthracene, benzo(g, h, i) perylene and indeno(1,2,3-cd) pyrene and have molecular weights ranging from 202.3 to 278.4 g/mol.56,57 Pyrogenic PAHs are formed during the incomplete combustion of coal, oil, gas wood and garbage. The % HMW PAHs was higher than % LMW PAHs at the dumpsites and the control site. The ranges of % LMW and HMW at 0–15 cm were 27.6–34.9 and 48.5–52.9, respectively, and 24.1–32.8 and 47.4–54.8, respectively, at the 15–30 cm depth. The percentage at both depths in the control soil was 18% and 65.5%, respectively (Tables 7 and 8). This indicates that high molecular weight PAHs were the predominant PAHs throughout the dumpsites. The total concentrations of the 16 target PAHs, including 5 carcinogenic PAHs, in the dumpsites and control soils at the 0–15 cm and 15–30 cm depths are presented in Tables 7 and 8, respectively. Total PAHs ranged from 1,756–2,224 μg/kg and 1,664–2,152 μg/kg at depths of 0–15 cm and 15–30 cm, respectively. Total PAHs obtained from the control soil was 278 μg/kg at both depths. Total PAHs were generally an order of magnitude greater in the dumpsites soils compared to the control sample, possible evidence of contamination by burning activities at the dumpsites. Anthracene and 2-methyl naphthalene were not detected in any of the samples, while phenanthrene, pyrene and fluoranthene were found in the highest concentrations for the PAHs determined in this study. Five and six-membered ring PAHs were the most prevalent at all of the sites, which may be due to their very high level of resistance to environmental degradation.54 Among the 7 carcinogenic PAHs (i.e., banz(a) anthracene, chrysene, benzo(a) fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd) pyrene and benzo(g,h,i)perylene), benzo(g,h,i)perylene was not detected in any of the soil samples except for soil collected at the ISS2 dumpsite at a 15–30 cm depth. Benzo(a) fluoranthene and benzo(a)pyrene were not determined in any of the samples. The % carcinogenic PAHs in the soils of e-waste dumpsites in Lagos and Ibadan ranged from 29.5–39.6 and 31.2–47.5 at the 0–15 cm and 15–30 cm level, respectively.
Table 7.
Polycyclic Aromatic Hydrocarbon Concentrations in Dumpsite and Control Soil Samples, 0–15 cm Depth
| Target Analytes | LLS1 | LLS2 | ISS1 | ISS2 | CSS |
|---|---|---|---|---|---|
| Naphthalene (Nap) | 10.0 | ND | ND | ND | ND |
| 2-methyl Naphthalene (mNap) | ND | ND | ND | ND | ND |
| Acenaphthylene (Acy) | 37.0 | 38.0 | 38.0 | 38.0 | ND |
| Acenaphthene (Ace) | 9.00 | 9.00 | 9.00 | 9.00 | ND |
| Fluorene (Flu) | 140 | 137 | 141 | 143 | ND |
| Phenanthrene (Phe) | 323 | 593 | 345 | 392 | 50.0 |
| Anthracene (Ant) | ND | ND | ND | ND | ND |
| Fluoranthene (Fla) | 251 | 293 | 240 | 260 | 41.0 |
| Pyrene (Pyr) | 255 | 384 | 245 | 271 | 37.0 |
| Benz(a)anthracene (BaA) | 227 | 247 | 235 | 227 | 45.0 |
| Chrysene (Chr) | 213 | 199 | 190 | 267 | 33.0 |
| Benzo(k)fluoranthene (BkF) | 215 | 211 | 204 | 224 | 40.0 |
| Benzo(b)fluoranthene (BbF) | 109 | 113 | 109 | 111 | ND |
| Perylene (Per) | ND | ND | ND | 51.0 | 6.00 |
| Benzo(g,h,i)perylene (BghiP) | ND | ND | ND | ND | ND |
| Dibenz(a,h)anthracene (DahA) | ND | ND | ND | ND | 26.0 |
| Indeno(1,2,3-cd)pyrene (IcdP) | ND | ND | ND | 119 | ND |
| Total PAH | 1,789 | 2,224 | 1,756 | 2,112 | 278 |
| %LMW PAH | 29.0 | 34.9 | 30.4 | 27.6 | 18.0 |
| %HMW PAH | 52.9 | 50.5 | 51.8 | 48.5 | 65.5 |
| % C PAH | 36.6 | 29.5 | 35.8 | 39.6 | 51.8 |
Values presented as μg/kg.
Abbreviations: LMW, low molecular weight; HMW, high molecular weight; C, carcinogenic PAH; ND, not detected
Table 8.
Polycyclic Aromatic Hydrocarbon Concentrations in Dumpsite and Control Soil Samples, 15–30 cm Depth
| Target Analytes | LLS1 | LLS2 | ISS1 | ISS2 | CSS |
|---|---|---|---|---|---|
| Naphthalene (Nap) | ND | ND | ND | ND | ND |
| 2-methylNaphthalene (mNap) | ND | ND | ND | ND | ND |
| Acenaphthylene (Acy) | 37.0 | 38.0 | 38.0 | 37.0 | ND |
| Acenaphthene (Ace) | 9.00 | 9.00 | 9.00 | 8.00 | ND |
| Fluorene (Flu) | 141 | 136 | ND | 140 | ND |
| Phenanthrene (Phe) | 294 | 523 | 392 | 324 | 50.0 |
| Anthracene (Ant) | ND | ND | ND | ND | ND |
| Fluoranthene (Fla) | 229 | 277 | 241 | 259 | 41.0 |
| Pyrene (Pyr) | 226 | 349 | 244 | 274 | 37.0 |
| Benz(a)anthracene (BaA) | 229 | 245 | 227 | 242 | 45.0 |
| Chrysene (Chr) | 188 | 214 | 204 | 227 | 33.0 |
| Benzo(k)fluoranthene (BkF) | 202 | 213 | 206 | 225 | 40.0 |
| Benzo(b)fluoranthene (BbF) | 109 | 113 | 110 | 110 | ND |
| Perylene (Per) | ND | 35.0 | ND | 59.0 | 6.00 |
| Benzo(g,h,i)perylene (BghiP) | ND | ND | ND | 195 | ND |
| Dibenz(a,h)anthracene (DahA) | ND | ND | ND | ND | 26.0 |
| Indeno(1,2,3-cd)pyrene (IcdP) | ND | ND | ND | 116 | ND |
| Total PAH | 1,664 | 2,152 | 1,671 | 2,116 | 278 |
| % LMW PAH | 28.9 | 32.8 | 26.3 | 24.1 | 18.0 |
| % HMW PAH | 52.4 | 50.4 | 54.8 | 47.4 | 65.5 |
| % C PAH | 37.2 | 31.2 | 38.1 | 47.5 | 51.8 |
Values presented as μg/kg
Abbreviations: LMW, low molecular weight; HMW, high molecular weight; C, carcinogenic PAH
According to the Dutch Ministry of Housing, Spatial Planning and the Environment (VROM), the total concentrations of ten VROM PAHs (napthalene, anthracene, phenanthrene, fluoranthene, benzo(a) anthracene, chrysene, benzo(a) pyrene, benzo(g,h,i)perylene, benzo(k) fluoranthene and indeno(1,2,3-cd) pyrene) in soil should not exceed the maximum value of 1000 μg/kg.58 The concentrations of nine VROM PAHs determined in the soil samples at depths of 0–15 cm and 15–30 cm exceeded this value. The concentrations of nine VROM PAHs (benzo(a)pyrene was not determined) ranged from 1,231- 1,543 μg/kg and 1,142–1,588 μg/kg at the depths of 0–15 cm and 15–30 cm, respectively. The Institute of Soil Science and Plant Cultivation (Pulawy, Poland) classification showed that soils with total PAH < 1,000 μg/kg dry weight (dw) can be considered to be unpolluted.59 The total PAHs concentrations of all of the samples in the dumpsite soils and the control exceeded the typical concentration of arable topsoil (around 200 μg/kg) in Sweden.60 The target established by the Dutch government for PAHs in uncontaminated soil is 20–50 μg/kg (dw).61 The total PAHs concentrations at depths of 0–15 cm and 15–30 cm in all the e-waste dumpsites in Lagos and Ibadan exceeded the 50 μg/kg limit. Thus, all of the study sites were considered to be highly polluted by PAHs.
The ratio of PAH profiles maybe used to track their origin as petrogenic, biogenic and pyrogenic sources.20,62,63 Petrogenic sources are characterized with the predominance of LMW PAHs (naphthalene, 2-methyl naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene and anthracene) over the HMW PAHs (fluoranthene, pyrene, benz(a) anthracene, chrysene, benzo(a) pyrene, dibenz(a,h)anthracene). A ratio of LMW to HMW greater than 1 indicates a petrogenic source.64 In the soils of e-waste dumpsites in Lagos and Ibadan, we obtained a LMW/HMW range of 0.55–0.69 and 0.48–0.65 at the 0–15 cm and 15–30 cm level, respectively, which indicated pollution of pyrolytic origin (Table 9). The ratios of fluoranthene to fluoranthene plus pyrene, benzo(a) anthracene to benzo(a)anthracene plus chrysene and indeno(1,2,3-cd) pyrene to indeno(1,2,3-cd)pyrene plus benzo(g,h,i)perylene were also used for source identification.19,30,65,66 The ranges obtained were 0.43–0.50, 0.44–0.50; 0.46–0.55, 0.52–0.55; 0–1.0, 0–0.37, respectively, at the 0–15 cm and 15–30 cm levels, respectively, for these PAHs. These values indicated that PAHs had both pyrolytic and petrolytic origins. The results obtained in this study were compared with those in the literature and are presented in Table 10. It was reported that total PAHs in soil collected from Wenling, an emerging e-waste recycling area in Taizhou, China ranged from 371.8 to 1231.2 μg/kg, and relatively higher PAHs concentrations were found in soils taken from simple household workshops.19
Table 9.
Diagnostic Ratio of Polycyclic Aromatic Hydrocarbons
| Soil depth | LMW/HMW | Fla/(Fla + Pyr) | BaA/(BaA + Chr) | IcdP/(IcdP + BghiP) |
|---|---|---|---|---|
| 0–15 cm | ||||
| LLS1 | 0.55 | 0.50 | 0.52 | 0 |
| LLS2 | 0.69 | 0.43 | 0.55 | 0 |
| ISS1 | 0.59 | 0.49 | 0.55 | 0 |
| ISS2 | 0.57 | 0.49 | 0.46 | 1.0 |
| 15–30 cm | ||||
| LLS1 | 0.55 | 0.50 | 0.55 | 0 |
| LLS2 | 0.65 | 0.44 | 0.53 | 0 |
| ISS1 | 0.48 | 0.50 | 0.53 | 0 |
| ISS2 | 0.51 | 0.49 | 0.52 | 0.37 |
| Control | 0.27 | 0.53 | 0.58 | 0 |
Abbreviations: Fla, fluoranthene; Pyr, pyrene; BaA, benz(a)anthracene; Chr, chrysene; IcdP, indeno(1,2,3-cd)pyrene; BghiP, benzo(g,h,i)perylen
Table 10.
Concentrations of the 16 USEPA Identified Polycyclic Aromatic Hydrocarbons in Soil and Sediment Samples from E-waste Processing Areas in China Compared with this Study
| Location | Type of Soil or Sediment | Range | Mean | Reference |
|---|---|---|---|---|
| μg/kg dry weight | ||||
| Lagos and Ibadan, Nigeria | Topsoil (0–15cm) of e-waste dumpsite (n = 20) | 1,756–2,224 | This study | |
| Subsoil (15–30 cm) of e-waste dumpsite (n = 20) | 1,664–2,152 | |||
| Control (0–15 cm; 15–30 cm) (n = 5) | 278 | |||
| Guiyu, Guangdong Province, | Surface soil (0–10 cm) of a burnt plastic dump site (n = 3) | 428 | 34 | |
| Surface soil (0–10 cm) near an open burning site (n = 8) | 851 | 30 | ||
| Surface soil (0–10 cm) of an open burning site (n = 5) | 2,065 | |||
| Surface soil (0–10 cm) of an open burning site (n = 5) | 1,066 | 67 | ||
| Surface soil (0–10 cm) of an open burning site (n = 5) | 899.9 | |||
| Surface soil (0–10 cm) of an open burning site (n = 5) | 2,777 | |||
| Taizhou, Zhejiang Province, China | Surface soil (0–20 cm) of large recycling plants (n = 5) | 128.8–6,687.2 | 68 | |
| Surface soils (0–20 cm) of small recycling workshops (n = 3) | 135.3–228.8 | |||
| Surface soils (0–20 cm) of control sites (n = 3) | 5.2–29.4 | |||
| Local agricultural surface soil (0–20 cm) from an e-waste recycling facility (n = 10) | 330–20,000 | 69 | ||
| Topsoil (0–30 cm) of large-scale e-waste recycling plants in Wenling (n = 14) | 488.0–764.0 | 19 | ||
| Topsoil (0–30 cm) of large-scale gold recycling plants in Wenling (n = 5) | 371.8–850.7 | |||
| Topsoil (0–30 cm) of household e-waste recycling workshops in Wenling (n = 18) | 730.5–1,231.2 | |||
| Reference site (n = 1) | 0.4 | |||
| Near household e-waste recycling workshops | 809–7,880 | 70 | ||
| Near industrial parks | 2,820–3,020 | |||
| Qingyuan, Guangdong Province, China | Road soils mixed with deposited dust near dismantling workshops (n = 29) | 190.8–9,156.0 | 2,689.1 | 71 |
Statistical Analysis
Statistical analyses of the results obtained in the e-waste dumpsites in Lagos and Ibadan using Pearson's correlation coefficient (Tables 11 and 12) showed very strong and negatively significant correlations between total PAHs versus Cd (r = −0.955, p< 0.05), Ni (r = −0.973, p< 0.05) and TOC (r = −0.899, p< 0.05) in Ibadan, suggesting that these contaminants might have originated from similar sources, such as burning of e-waste at dumpsites. There was no significant correlation between total PAHs and TOC (r = −0.395, p< 0.05), and no significant correlation with most of the metals except for Zn (r = 0.648, p< 0.05) in soils of e-waste dumpsites in Lagos, suggesting different emission sources.
Discussion
Migration of Cd from topsoil to the subsurface soil was observed in both the Lagos and Ibadan dumpsites. In most cases, Cu, Zn and Pb concentrations were highest in topsoil, which was evidence of recent/anthropogenic contamination, but with limited evidence of migration to the subsoil.72 This indicates that there is little risk of groundwater contamination at these sites. All of the e-waste dumpsites in Lagos and Ibadan exhibited multi-element contamination from anthropogenic inputs, most likely from e-waste burning activity. The indices of potential ecological risk were found in the following order at the different sites:
LLS1: Cu > Pb > Ni > Cd > Cr > Zn;
LLS2: Ni > Pb > Cu > Cr > Cd > Zn;
ISS1: Pb > Cu > Ni > Cd > Zn > Cr;
ISS2: Pb > Ni > Cd > Cu > Cr > Zn;
ISS3: Ni > Pb > Cr > Cu > Zn > Cd.
The total PAH concentrations of all of the samples in the dumpsite soils and the control exceeded the typical concentration of arable topsoil (around 200 μg/kg) in Sweden and the target established by the Dutch government for PAHs in uncontaminated soil of 20–50 μg/kg (dw).60,61 This is a public health concern, since several of the measured PAHs are considered probable human carcinogens (benz(a)anthracene, benzo(a)pyrene, benzo(k)fluoranthene, chrysene, dibenz(a,h)anthracene and indeno(1,2,3-c,d)pyrene) or possibly (benzo(a)fluoranthene, benzo(k) fluoranthene and indeno(1,2,3-c,d) pyrene).73 Indeno(1,2,3-c,d)pyrene and dibenz(a,h)anthracene were not detected in any of the samples except at a site in Ibadan, ISS2, and the control soil, respectively, at depths of 0–15 cm and 15–30 cm. Phenantherene (LMW) PAH is a thermodynamically stable compound mainly derived from petrogenic sources (from the release of uncombusted petroleum products such as gasoline, diesel fuel and fuel oil from vehicle traffic).30 Its predominance in the soil of e-waste dumpsites in Lagos and Ibadan and the control indicates a petrogenic source. Comparison of pollution in the four dumpsites considered in Lagos and Ibadan showed the following trend:
0–15 cm: LLS2 > ISS2 > LLS1 > ISS1
15–30 cm: LLS2 > ISS2 > ISS1 > LLS1
The PAHs profile pattern in soils of four e-waste dumpsites in Lagos and Ibadan were similar. In most cases, the concentrations of individual PAHs were higher in soil at the 0–15 cm level compared to soil at the 15–30 cm level. Phenanthrene and pyrene were the most abundant pollutants at all of the sites at the 0–15 cm and 15–30 cm levels, except at LLS1, where phenanthrene and fluoranthene were the most abundant, while 2-methylnaphthalene and anthracene were not detected in any of the e-waste dumpsites or the control site. However, pyrene, fluoranthene, benz(a)anthracene, and chrysene (HMW) typically have a pyrogenic source (from combustion of fossil fuels). Hence, the PAH profile in the soil of e-waste dumpsites in Lagos and Ibadan suggests both petrogenic and pyrogenic sources.
Conclusions
The degree of contamination and ecological risk posed by metals in e-waste dumpsite soils in Lagos and Ibadan, Nigeria were evaluated in the present study. The results provide evidence that open burning, stockpiling, and other improper e-waste management practices may have resulted in toxic metal accumulation in soils of e-waste dumpsites in Lagos and Ibadan, corroborating previous results at e-waste dumpsites in other countries. Various metals contamination indices showed moderate to very high levels of contamination in the dumpsite soils, indicating potential threats to human and ecological health. We found PAHs at levels exceeding 1,000 μg/kg in dumpsite soils, suggesting anthropogenic contamination from both petrogenic and pyrogenic sources. It was previously shown that leachates from municipal solid waste dumpsites in Nigeria contain high concentrations of metals, PAHs and PCBs.56
Our work shows that improper e-waste handling at these sites may contribute additional metals and PAH contamination and highlights the need for regular soil monitoring at major dumpsites in Nigeria.
References
- 1. Oketola AA, Adebisi AA, Morakinyo O.. Distribution and Bioavailability of Metals in Gasoline Contaminated Sites in Lagos, Nigeria. Journal of Solid Waste Technology and Management 2013; 39 3: 161– 172. [Google Scholar]
- 2. Jibiri NN, Isinkaye MO, Momoh HA.. Assessment of radiation exposure levels at Alaba e-waste dumpsite in comparison with municipal waste dumpsites in southwest Nigeria. J Radiat Res Appl Sci [Internet]. Elsevier Ltd; 2014; 7 4: 536– 41. Available from: http://www.sciencedirect.com/science/article/pii/S1687850714000909 [Google Scholar]
- 3. Bakare AA, Alabi OA, Gbadebo AM, Ogunsuyi OI, Alimba CG.. In vivo cytogenotoxicity and oxidative stress induced by electronic waste leachate and contaminated well water. Chall [Internet] 2013. [cited 2017 Aug 10]; 4 2: 169– 87. Available from: http://www.mdpi.com/2078-1547/4/2/169/htm [Google Scholar]
- 4. Babatunde B, Anabuike F.. In Vivo Cytogenotoxicity of Electronic Waste Leachate from Iloabuchi Electronic Market, Diobu, Rivers State, Nigeria on Allium Cepa. Challenges [Internet]. 2015; 6 1: 173– 87. Available from: http://www.mdpi.com/2078-1547/6/1/173/ [Google Scholar]
- 5. Ogungbuyi O, Nnorom CI, Osinbanjo O, Schluep M.. e-Waste Country Assessment Nigeria. Basel Conv [Internet]. 2012;( May). Available from: http://www.ewasteguide.info/files/Ogungbuyi_2012_BCCC-Empa.pdf
- 6. Ogu OG, Ogw PA.. Assessment of heavy metals concentrations in soils of acid battery waste dumpsites in aba southeastern Nigeria. Journal of Environmental Sciences and Resources Management. 2014; 6 1: 12– 22. [Google Scholar]
- 7. Puckett J, Westervelt S, Gutierrez R, Takamiya Y.. The digital dump: exporting re-use and abuse to Africa [Internet]. Basel Action Network: Seattle, Washington; 2005. October 24 [cited 2017 Aug 10] 85 p. Available online: http://archive.ban.org/library/TheDigitalDump.pdf [Google Scholar]
- 8. Nnorom IC, Osibanjo O.. Overview of electronic waste (e-waste) management practices and legislations, and their poor applications in the developing countries. Resour Conserv Recycl [Internet]. 2008. April [cited 2017 Aug 10]; 52 6: 843– 58. Available from: http://www.sciencedirect.com/science/article/pii/S0921344908000165 Subscription required to view. [Google Scholar]
- 9. Adaramodu AA, Osuntogun AO, Ehi-Eromosele CO.. Heavy metal concentration of surface dust present in e-waste components: the Westminister Electronic Market, Lagos case study. Resour Environ [Internet]. 2012. [cited 2017 Aug 10]; 2 2: 9– 13. Available from: https://www.researchgate.net/publication/255729971_Heavy_Metal_Concentration_of_Surface_Dust_Present_in_EWaste_Components_The_Westminister_Electronic_Market_Lagos_Case_Study [Google Scholar]
- 10. Bakare AA, Adeyemi AO, Adeyemi A, Alabi OA, Osibanjo O.. Cytogenotoxic effects of electronic waste leachate in Allium cepa. Caryologia [Internet]. 2012. [cited 2017 Aug 10]; 65 2: 94– 100. Available from: http://www.tandfonline.com/doi/abs/10.1080/00087114.2012.709786 [Google Scholar]
- 11. Dave S, Dave SR, Shah MB, Tipre DR.. E-waste : Metal Pollution Threat or Metal Resource ? J Adv Res Biotech 2016;( March) 1 2: 14. [Google Scholar]
- 12. Zhang J-H, Fan W-W.. Metal partitioning and relationships to soil microbial properties of submerged paddy soil contaminated by electronic waste recycling. Chem Ecol [Internet]. 2014; 31 2: 147– 59. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84922237421&partnerID=tZOtx3y1 [Google Scholar]
- 13. Ademola AK, Olaoye MA, Abodunrin PO.. Radiological safety assessment and determination of heavy metals in soil samples from some waste dumpsites in Lagos and Ogun state, south-western, Nigeria. J Radiat Res Appl Sci [Internet]. Elsevier Ltd; 2015; 8 1: 148– 53. Available from: http://www.sciencedirect.com/science/article/pii/S1687850714001320 [Google Scholar]
- 14. Needhidasan S, Samuel M, Chidambaram R.. Electronic waste - an emerging threat to the environment of urban India. J Environ Heal Sci Eng [Internet]. Journal of Environmental Health Science and Engineering; 2014; 12 1: 36 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3908467&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Jinhui, Duan Huabo, Shi Pixing. . Heavy metal contamination of surface soil in electronic waste dismantling area: site investigation and sourceapportionment analysis. Waste Manag Res [Internet]. 2011; 29 7: 727– 38. Available from: http://wmr.sagepub.com/cgi/doi/10.1177/0734242X10397580%5Cnhttp://www.scopus.com/inward/record.url?eid=2-s2.0-79959903960&partnerID=tZOtx3y1 [DOI] [PubMed] [Google Scholar]
- 16. Amfo-Otu R, Bentum JK, Omari S.. Assessment of Soil Contamination through E-Waste Recycling Activities in Tema Community One. Environ Pollut [Internet]. 2013; 2 2: 66– 70. Available from: http://www.ccsenet.org/journal/index.php/ep/article/view/25792 [Google Scholar]
- 17. Zhang D, An T, Qiao M, Loganathan BG, Zeng X, Sheng G, Fu J.. Source identification and health risk of polycyclic aromatic hydrocarbons associated with electronic dismantling in Guiyu town, South China. J Hazard Mater [Internet]. 2011. August 15 [cited 2017 Aug 10]; 192 1: 1– 7. Available from: http://www.sciencedirect.com/science/article/pii/S0304389411004250 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 18. Olafisoye OB, Adefioye T, Osibote OA.. Heavy metals contamination of water, soil, and plants around an electronic waste dumpsite. Pol J Environ Stud [Internet]. 2013. [cited 2017 Aug 10]; 22 5: 1431– 9. Available from: http://www.pjoes.com/abstracts/2013/Vol22/No05/18.html [Google Scholar]
- 19. Tang X, Shen C, Shi D, Cheema SA, Khan MI, Zhang C, Chen Y.. Heavy metal and persistent organic compound contamination in soil from Wenling: an emerging e-waste recycling city in Taizhou area, China. J Hazard Mater [Internet]. 2010. January 15 [cited 2017 Aug 10]; 173 1–3: 653– 60. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304-3894(09)01433-2 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Tian Z, Zhu H, Cheng Z, Kang M, Luo C, . et al. Polycyclic aromatic hydrocarbons (PAHs) in soils and vegetation near an e-waste recycling site in South China: Concentration, distribution, source, and risk assessment. Sci Total Environ [Internet]. Elsevier B.V.; 2012; 439: 187– 93. Available from: 10.1016/j.scitotenv.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 21. Gupta S, Modi G, Saini R, Agarwala V.. A review on various electronic waste recycling techniques and hazards due to its improper handling. Int Ref J Eng Sci [Internet]. 2014. May [cited 2071 Aug 10]; 3 5: 5– 17. Available from: http://www.irjes.com/Papers/vol3-issue5/B350517.pdf [Google Scholar]
- 22. Adeyi AA, Babalola B.. Lead and cadmium levels in residential soils of Lagos and Ibadan, Nigeria. J Health Pollut [Internet]. 2017. March [cited 2017 Aug 10]; 7 13: 42– 55. Available from: www.journalhealthpollution.org/doi/full/10.5696/2156-9614-7-13.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toxicological profile for lead [Internet]. Atlanta, Georgia: Agency for Toxic Substances and Disease Registry: 2007. August [cited 2017 Aug 10] 582 p. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf [PubMed] [Google Scholar]
- 24. Adeyi AA, Omidiran OM, Osibanjo O.. Assessment of soil contamination of a cattle market around River Ogun Basin, Isheri, Nigeria [Internet]. : Hernandez-Soriano MC, . editor. Environmental Risk Assessment of Soil Contamination Rijeka, Croatia: InTech; 2014. [cited 2017 Aug 10]. Chapter 7. p 225– 55. Available from: https://www.intechopen.com/books/environmental-riskassessment-of-soil-contamination/soil-contaminationrisk-assessment-and-remediation [Google Scholar]
- 25. Jarup L. Hazards of heavy metal contamination. Br Med Bull [Internet]. 2003. [cited 2017 Aug 10]; 68: 167– 82. Available from: https://academic.oup.com/bmb/articlelookup/doi/10.1093/bmb/ldg032 [DOI] [PubMed] [Google Scholar]
- 26. Martins S, Griswold W.. Human health effects of heavy metals. Environ Sci Technol Briefs Citiz [Internet]. 2009. March [cited 2017 Aug 10]; 15: 1– 6. Available from: https://www.engg.ksu.edu/chsr/files/chsr/outreach-resources/15HumanHealthEffectsofHeavyMetals.pdf [Google Scholar]
- 27. Buljac M, Bogner D, Bralic M, Peris N, Buzuk M, Brinic S, Vladislavic N.. Cadmium and lead distribution in marine soil sediments, terrestrial soil, terrestrial rock, and atmospheric particulate matter around Split, Croatia. Anal Lett [Internet]. 2014. [cited 2017 Aug 10]; 47 11: 1952– 64. Available from: http://www.tandfonline.com/doi/abs/10.1080/00032719.2014.888725 Subscription required to view. [Google Scholar]
- 28. Karim Z, Qureshi BA.. Health Risk Assessment of Heavy Metals in Urban Soil of Karachi, Pakistan. Hum Ecol Risk Assess An Int J [Internet]. 2014; 20 3: 658– 67. Available from: http://www.tandfonline.com/doi/abs/10.1080/10807039.2013.791535 [Google Scholar]
- 29. Wei L, Liu Y.. Present Status of e-waste Disposal and Recycling in China. Procedia Environ Sci [Internet]. 2012; 16: 506– 14. Available from: http://www.sciencedirect.com/science/article/pii/S1878029612006081 [Google Scholar]
- 30. Yu XZ, Gao Y, Wu SC, Zhang HB, Cheung KC, Wong MH.. Distribution of polycyclic aromatic hydrocarbons in soils at Guiyu area of China, affected by recycling of electronic waste using primitive technologies. Chemosphere [Internet]. 2006. November [cited 2017 Aug 10]; 65 9: 1500– 9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045-6535(06)00432-2 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 31. Oliveira RF, Varandas L, Arbilla G.. Characterization of polycyclic aromatic hydrocarbon levels in the vicinity of a petrochemical complex located in a densely populated area of the Rio de Janeiro, Brazil. Atmos Pollut Res. 2014. January [cited 2017 Aug 10]; 5 1: 87– 95. Available from: http://www.sciencedirect.com/science/article/pii/S1309104215303457 [Google Scholar]
- 32. The determination of polycyclic aromatic hydrocarbons in soil by dichloromethane extraction using gas chromatography with mass spectrometric detection: methods for the examination of waters and associated materials. Rotherham, UK: Environment Agency; 2003. 26 p. [Google Scholar]
- 33. Khan S, Cao Q.. Human health risk due to consumption of vegetables contaminated with carcinogenic polycyclic aromatic hydrocarbons. J Soils Sediment [Internet]. 2012. February [cited 2017 Aug 11]; 12 2: 178– 84. Available from: https://link.springer.com/article/10.1007/s11368-011-0427-3 Subscription required to view. [Google Scholar]
- 34. Leung A, Cai ZW, Wong MH.. Environmental contamination from electronic waste recycling at Guiyu, southeast China. J Mater Cycles Waste Manag [Internet]. 2006. March [cited 2017 Aug 11]; 8 1: 21– 33. Available from: https://link.springer.com/article/10.1007/s10163-005-0141-6 Subscription required to view. [Google Scholar]
- 35. Annex: polycyclic aromatic hydrocarbons - occurrence in foods, dietary exposure and health effects [Internet]. Brussels, Belgium: European Commission; 2002. December 4 [cited 2017 Aug 11]. Report No.: SCF/CS/CNTM/PAH/29 ADD1 Final. p A1– 194. Available from: https://ec.europa.eu/food/sites/food/files/safety/docs/scicom_scf_out154_en.pdf [Google Scholar]
- 36. Polycyclic aromatic hydrocarbons (PAHs) [Internet]. Atlanta, Georgia: Agency for Toxic Substances and Disease Registry: 1996. September [cited 2017 Aug 11] 2 p. Available from: https://www.atsdr.cdc.gov/toxfaqs/tfacts69.pdf [PubMed] [Google Scholar]
- 37. Palm LM, Carboo D, Yeboah PP, Quasie WJ, Gorleku MA, Darko A.. Characterization of polycyclic aromatic hydrocarbons (PAHs) present in smoked fish from Ghana. Adv J Food Sci Technol [Internet]. 2011. [cited 2017 Aug 11]; 3 5: 332– 8. Available from: http://maxwellsci.com/print/ajfst/v3-332-338.pdf [Google Scholar]
- 38. Tsibart AS, Gennadiev AN.. Polycyclic aromatic hydrocarbons in soils: sources, behavior, and indication significance (a review). Eurasian Soil Sci [Internet]. 2013. July [cited 2017 Aug 11]; 46 7: 728– 41. Available from: https://link.springer.com/article/10.1134%2FS1064229313070090 Subscription required to view. [Google Scholar]
- 39. Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res [Internet]. 1980. [cited 2017 Feb 25]; 14 8: 975– 1001. Available from: http://www.sciencedirect.com/science/article/pii/0043135480901438 Subscription required to view. [Google Scholar]
- 40. Tomlinson LD, Wilson JG, Harris CR, Jeffery DW.. Problems in the assessments of heavy-metal levels in estuaries and formation of a pollution index. Helgoländer Meeresuntersuchungen [Internet]. 1980. March [cited 2017 Feb 25]; 33 1: 566– 75. Available from: https://link.springer.com/article/10.1007/BF0241478042. [Google Scholar]
- 41. Muller G. Index of geoaccumulation in sediments of the Rhine River. Geol J. 1969; 2 3: 108– 18. [Google Scholar]
- 42. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2. Washington, DC: United States Environmental Protection Agency, 1996. [Cited 2017 Aug 18] Available from: https://www.epa.gov/sites/production/files/2015-06/documents/epa-3050b.pdf [Google Scholar]
- 43. Method 827°C Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). Washington, DC: United States Environmental Protection Agency, 1996. [Cited 2017 Aug 18] Available from: https://www.epa.gov/sites/production/files/2015-07/documents/epa-8270d.pdf [Google Scholar]
- 44. Walkley A, Black IA.. Methods of soil analysis. Soil Sci. 1934; 37: 29– 38. [Google Scholar]
- 45. Chai M, Shi F, Li R, Shen X.. Heavy metal contamination and ecological risk in Spartina alterniflora marsh in intertidal sediments of Bohai Bay, China. Mar Pollut Bull [Internet]. 2014. July 15 [cited 2017 Aug 11]; 84 1–2: 115– 24. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0025-326X(14)00306-3 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 46. Ghrefat H, Yusuf N.. Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere [Internet]. 2006. December [cited 2017 Aug 11]; 65 11: 2114– 21. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045-6535(06)00801-0 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 47. Forstner U, Ahlf W, Calmano W.. Sediment quality objectives and criteria development in Germany. Water Sci Technol [Internet]. 1993. [cited 2017 Aug 11]; 28 8–9: 307– 16. Available from: https://tubdok.tub.tuhh.de/bitstream/11420/453/1/S0001208.pdf [Google Scholar]
- 48. Liu WH, Zhao JZ, Ouyang ZY, Söderlund L, Liu GH.. Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environ Int [Internet]. 2005. August [cited 2017 Aug 11]; 31 6: 805– 12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0160-4120(05)00113-3 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 49. Adeyi AA, Torto N.. Profiling heavy metal distribution and contamination in soil of old power generation station in Lagos, Nigeria. Am J Sci Technol [Internet]. 2014. [cited 2017 Aug 11]; 1 1: 1– 10. Available from: http://www.fulviofrisone.com/attachments/article/532/9020729.pdf [Google Scholar]
- 50. Al Obaidy AH, Al Mashhadi AA.. Heavy metal contaminations in urban soil within Baghdad City, Iraq. J Environ Prot [Internet]. 2013. January [cited 2017 Aug 11]; 4 1: 72– 82. Available from: https://file.scirp.org/Html/8-6701677_27185.htm [Google Scholar]
- 51. Jung MC. Heavy metal contamination of soils and waters in and around the Imcheon Au–Ag mine, Korea. Appl Geochem [Internet]. 2001. [cited 2017 Aug 11]; 16 2001: 1369– 75. Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.553.6821&rep=rep1&type=pdf [Google Scholar]
- 52. Haciyakupoglu S, Esen AN, Erenturk S, Okka M, Genceli M, Mercimek M, . et al. Determining distribution of heavy metal pollution in terms of ecological risk levels in soil of industrially intensive areas around Istanbul. Toxicol Environ Chem [Internet]. 2015; 97 1: 62– 75. Available from: 10.1080/02772248.2014.993640 [DOI] [Google Scholar]
- 53. Fu J, Zhou Q, Liu J, Liu W, Wang T, Zhang Q, Jiang G.. High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere [Internet]. 2008. April [cited 2017 Aug 11]; 71 7: 1269– 75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045-6535(07)01531-7 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 54. Luo C, Liu C, Wang Y, Liu X, Li F, Zhang G, Li X.. Heavy metal contamination in soils and vegetables near an e-waste processing site, South China. J Hazard Mater [Internet]. 2011. February 15 [cited 2017 Aug 11]; 186 1: 481– 90. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304-3894(10)01448-2 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 55. Ha NN, Agusa T, Ramu K, Tu NP, Murata S, Bulbule KA, Parthasaraty P, Takahashi S, Subramanian A, Tanabe S.. Contamination by trace elements at e-waste recycling sites in Bangalore, India. Chemosphere [Internet]. 2009. June [cited 2017 Aug 11]; 76 1: 9– 15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045-6535(09)00258-6 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 56. Oketola AA, Akpotu SO.. Assessment of solid waste and dumpsite leachate and topsoil. Chem Ecol [Internet]. 2015; 31 2: 134– 46. Available from: http://www.tandfonline.com/doi/abs/10.1080/02757540.2014.907280 [Google Scholar]
- 57. Duke O. Source determination of polynuclear aromatic hydrocarbons in water and sediment of a creek in the Niger Delta region. Afr J Biotechnol [Internet]. 2008. February 5 [cited 2017 Aug 11]; 7 3: 282– 5. Available from: https://www.researchgate.net/publication/27798115_Source_determination_of_polynuclear_aromatic_hydrocarbons_in_water_and_sediment_of_a_creek_in_the_Niger_Delta_region_Okoro_Duke [Google Scholar]
- 58. Intervention values and target values: soil quality standards. The Hague, Netherlands: Netherlands Ministry of Housing, Spatial Planning and Environment, Department of Soil Protection; 1994. [Google Scholar]
- 59. Sieciechowicz A, Sadecka Z, Myszograj S, Wlodarczyk-Makula M, Wisniowska E, Turek A.. Occurrence of heavy metals and PAHs in soil and plants after application of sewage sludge to soil. Desalination Water Treat [Internet]. 2014. [cited 2017 Aug 11]; 52 19–21: 4014– 26. Available from: http://www.tandfonline.com/doi/abs/10.1080/19443994.2014.922292 Subscription required to view. [Google Scholar]
- 60. Berset JD, Holzer R.. Organic micropollutants in Swiss agriculture: distribution of polynuclear aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB) in soil, liquid manure, sewage sludge, and compost samples: a comparative study. Int J Environ Anal Chem [Internet]. 1995. [cited 2017 Aug 11]; 59 2–4: 145– 65. Available from: http://www.tandfonline.com/doi/abs/10.1080/03067319508041324 Subscription required to view. [Google Scholar]
- 61. Van Brummelen TC, Verweij RA, Wedzinga SA, Van Gestel CA.. Enrichment of polycyclic aromatic hydrocarbons in forest soils near a blast furnace plant. Chemosphere [Internet]. 1996. January [cited 2017 Aug 11]; 32 2: 293– 314. Available from: http://www.sciencedirect.com/science/article/pii/0045653595003398 Subscription required to view. [Google Scholar]
- 62. Adedosu TA, Adeniyi OK, Adedosu HO.. Distribution, sources and toxicity potentials of polycyclic aromatic hydrocarbons in soil around the vicinity of Balogun-Birro Dumpsite of Oshogbo, Nigeria. Anal Sci [Internet]. 2015. [cited 2017 Aug 11]; 19 3: 636– 48. Available from: http://www.ukm.my/mjas/v19_n3/pdf/Adedosu_19_3_20.pdf [Google Scholar]
- 63. Zhang WH, Wu YX, Simonnot MO.. Soil Contamination due to e-waste disposal and recycling activities: a review with special focus on China. Pedosphere [Internet]. 2012. August [cited 2017 Aug 11]; 22 4: 434– 55. Available from: http://www.sciencedirect.com/science/article/pii/S1002016012600307 Subscription required to view. [Google Scholar]
- 64. Magi E, Bianco R, Ianni C, Di Carro M.. Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Environ Pollut [Internet]. 2002. [cited 2017 Aug 11]; 119 1: 91– 8. Available from: http://www.sciencedirect.com/science/article/pii/S0269749101003219 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 65. Khalili NR, Scheff PA, Holsen TM.. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ [Internet]. 1995. March [cited 2017 Aug 11]; 29 4: 533– 42. Available from: http://www.sciencedirect.com/science/article/pii/135223109400275P Subscription required to view. [Google Scholar]
- 66. Nganje TN, Edet AE, Ekwere SJ.. Distribution of PAHs in surface soils from petroleum handling facilities in Calabar. Environ Monit Assess [Internet]. 2007. July [cited 2017 Aug 11]; 130 1–3: 27– 34. Available from: 10.1007/s10661-006-9453-9 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 67. Hang W, Wang H, Zhang R, Yu XZ, Qian PY, Wong MH.. Bacterial communities in PAH contaminated soils at an electronic-waste processing center in China. Ecotoxicology [Internet]. 2010. January [cited 2017 Aug 11]; 19 1: 96– 104. Available from: 10.1007/s10646-009-0393-3 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 68. Shen C, Huang S, Wang Z, Qiao M, Tang X, Yu C, Shi D, Zhu Y, Shi J, Chen X, Setty K, Chen Y.. Identification of ah receptor agonists in soil of E-waste recycling sites from Taizhou area in China. Environ Sci Technol [Internet]. 2008. January 1 [cited 2017 Aug 11]; 42 1: 49– 55. Available from: http://pubs.acs.org/doi/pdf/10.1021/es071162z Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 69. Shen C, Chen Y, Huang S, Wang Z, Yu C, Qiao M, Xu Y, Setty K, Zhang J, Zhu Y, Lin Q.. Dioxinlike compounds in agricultural soils near e-waste recycling sites from Taizhou area, China: chemical and bioanalytical characterization. Environ Int [Internet]. 2009. January [cited 2017 Aug 11]; 35 1: 50– 5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0160-4120(08)00122-0 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 70. Chen L, Yu C, Shen C, Zhang C, Liu L, Shen K, Tang X, Chen Y.. Study on adverse impact of e-waste disassembly on surface sediment in East China by chemical analysis and bioassays. J Soil Sediment [Internet]. 2010. April [cited 2017 Aug 11]; 10 3: 359– 67. Available from: https://link.springer.com/article/10.1007/s11368-009-0176-8 Subscription required to view. [Google Scholar]
- 71. Luo Y, Luo XJ, Lin Z, Chen SJ, Liu J, Mai BX, Yang ZY.. Polybrominated diphenyl ethers in road and farmland soils from an e-waste recycling region in Southern China: concentrations, source profiles, and potential dispersion and deposition. Sci Total Environ [Internet]. 2009. January 15 [cited 2017 Aug 11]; 407 3: 1105– 13. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0048-9697(08)01070-X Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 72. Gowd SS, Reddy MR, Govila PK.. Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J Hazard Mater [Internet]. 2010. February 15 [cited 2017 Aug 11]; 174 1–3: 113– 21. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304-3894(09)01470-8 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 73. Toxicological profile for polycyclic aromatic hydrocarbons [Internet]. Atlanta, Georgia: Agency for Toxic Substances and Disease Registry; 1995. August [cited 2017 Aug 11] 487 p. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp69.pdf [PubMed] [Google Scholar]


