Abstract
Background
The outcome of pulmonary hypertension (PH) mainly depends on the development of right ventricular (RV) dysfunction, and survival among patients with different etiologies of PH varies. Chronic hypoxia is a major cause of secondary PH, however the mechanisms of its associated RV dysfunction are largely unknown. Herein, we studied the role of microRNA-21 (miR-21) in hypoxia-induced RV dysfunction.
Methods
In this longitudinal, prospective study, we enrolled 41 patients with hypoxia-induced PH. Echocardiography was conducted and circulating miR-21 was measured. The expression of miR-21 was also evaluated in hypoxia-treated human pulmonary microvascular endothelial cells (HPECs) and conditioned media. Through the over-expression of miR-21 in H9C2 cells, we further identified crosstalk between the pulmonary circulation and RV.
Results
Among the studied patients, 10 developed RV dysfunction. Notably, the expression of circulating miR-21 was correlated with the severity of RV dysfunction. Likewise, miR-21 was up-regulated in the hypoxia-treated HPECs and its conditioned media in a time-dependent manner. I addition, hypertrophic changes were observed in the hypoxia-treated HPECs. The up-regulation of heart failure-associated markers in H9C2 cells over-expressing miR-21 implied the influence of pulmonary circulatory miR-21 on RV function.
Conclusions
The expression of systemic and pulmonary miR-21 is associated with the severity of RV dysfunction in patients with hypoxia-induced PH.
Keywords: Hypoxia-induced pulmonary hypertension, miR-21, Right ventricular dysfunction
INTRODUCTION
Pulmonary hypertension (PH) is a complex vascular disease defined clinically as a maladaptive process in the pulmonary circulation.1-3 The survival rate in patients with PH is mainly correlated to right ventricular (RV) dysfunction.4 Among different etiologies of PH, chronic hypoxia is a major cause of secondary PH, however the mechanisms of its associated RV dysfunction are largely unknown.5,6 From another aspect, microRNAs (miRs) are small endogenous non-coding RNAs which regulate the expression of complementary target messenger RNAs.7,8 Dysregulation of miRs has been shown to be involved in the development of various cardiac diseases including PH.8,9 miR-21 has been associated with cardiac hypertrophy, heart failure and pulmonary artery remodeling,8-10 however the impact of miR-21 on RV dysfunction in hypoxia-induced PH remains undetermined. In this study, we investigated the role of miR-21 in hypoxia-induced PH and its involvement in the severity of RV dysfunction. Furthermore, by measuring the effect of hypoxia on the expression of miR-21 in human pulmonary microvascular endothelial cells (HPECs) and conditioned media, we evaluated the potential influence of pulmonary circulating factors on RV. It is our hope that these results will provide new insights into the miR-21-mediated pathophysiology of hypoxia-induced PH.
METHODS
Objectives
In this longitudinal, prospective study, we enrolled patients with hypoxia-induced PH at a pulmonary pressure above 25 mmHg detected by either right heart catheterization (RHC; 43% of the studied population) or echocardiography. After excluding patients with a left ventricular ejection fraction (LVEF) less than 50%, poor echocardiographic window, and those diagnosed with other etiologies of PH, a total of 41 patients were enrolled, including 19 with chronic obstructive pulmonary diseases, 9 with bronchiectasis, 7 with pulmonary tuberculosis, and 6 with idiopathic pulmonary fibrosis. Informed consent was obtained from each patient, and the study was conducted according to the recommendations of the 1975 Declaration of Helsinki on Biomedical Research involving human subjects. In addition, the study was approved by the local Ethics Committee (IRB: B-ER-106-056). All participants underwent echocardiography and blood tests.
Human blood samples
Peripheral blood samples of the participants were collected and centrifuged at 3,000 rpm for 15 minutes at 4 °C. Portions of the samples were used for RNA extraction while the rest were frozen and sent to a central laboratory for N-terminal prohormone of brain natriuretic peptide (NT-pro BNP) analysis using a Triage BNP kit (BIOSITE, San-Diego, CA, USA) according to the manufacturer’s instructions.
Echocardiography
Standard echocardiography was performed (iE33, Philips) with a 3.5-MHz multiphase-array probe in accordance with the recommendations of the American Society of Echocardiography.11 LVEF was measured using the biplane Simpson’s method. In addition, left ventricular (LV) diastolic function-associated parameters including transmitral and tricuspid early filling velocity (E) to atrial velocity (A) ratio and tissue Doppler imaging were obtained from the apical four-chamber view. Peak systolic annular velocity (S′) and early (e′) and late (a′) annular diastolic velocities were measured at both ventricles. RV dimensions including mid-cavity diameters and wall thickness were measured at the mid-cavity in diastole as described previously. The fractional area change (FAC) was measured as (RV end diastolic area – RV end systolic area)/RV end diastolic area × 100%. RV pressure was calculated according to the trans-tricuspid flow velocity using the Bernoulli equation. Tricuspid annular plane systolic excursion (TAPSE) represents the distance of systolic excursion of the RV annular plane towards the apex. Patients with a TAPSE < 1.6 cm and tissue Doppler RV S′ < 10 cm/s were defined as having RV dysfunction.11 All the analyzed images were acquired in three consecutive cardiac cycles and stored digitally with a frame rate of 50-90 frames per second.
Cell culture and in vitro hypoxic stimulation
HPECs were a gift from Professor Liu’s laboratory in National Cheng Kung University Hospital. The cells were placed in either a normoxic or a hypoxic chamber (0.2% O2, 5% CO2, Biospherix, Lacuna, NY) for 24 or 48 hours. The cells and their conditioned media were harvested at the corresponding time points.
Quantitative real time-polymerase chain reaction
Total RNA was isolated using Trizol (Invitrogen, CA) or Trizol LS (Invitrogen, CA). cDNA was generated using a miRCURY LNATM Universal cDNA Synthesis Kit (Exiqon, DK). The primer sequences used for miR-21-5p, brain natriuretic peptide (BNP) and β-actin reverse real time polymerase chain reaction (PCR) were: 5′UAGCUUAUCAGACUGAUGUUGA3′, 5′GTCAGTCGCTTGGGCTGT3′, 3′CCAGAGCTGGGGAAAGAAG5′ and 5′CTAAGGCCAACCGTGAAAAG3′, 3′GCCTGGATGGCTACGTACA5′ respectively. miRs were detected with ExiLENT SYBR Green Master Mix (Exiqon, DK), and mRNAs were evaluated using LightCycler TaqMan Master (Roche, DE) with 100 ng RNA. To evaluate circulating miRs, spike-in UniSp6 (Exiqon, DK) was used as an internal control. Otherwise β-actin was used for normalization of RT-PCR. Quantitative real-time PCR was done on a LightCycler 2.0 System (Roche, DE).
Transfection
H9C2 myoblast cells were transfected with miR-21-5p miRCURY LNA miR Mimic (Exiqon, DK), 0, 10 and 20 nM using a TrsnsIT-X2 Dynamic Delivery System (Mirus Bio, US). Further experiments were conducted after 48 hours.
Immunostaining
H9C2 cells over-expressing miR-21 were fixed with 4% PFA. F-actin was stained with rhodamine phalloidin (Thermo Fisher, USA) and the nuclei were visualized using 4′,6-diamidino-2-phenylindole (Thermo Fisher, USA).
Statistics
All values are expressed as mean ± SD. Statistical analyses were performed using the Student’s unpaired t test with GraphPad Prism software (Version 5.03). A p value < 0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
Compared with the 31 patients without RV dysfunction, those with RV dysfunction presented with no specific differences in age, gender, body weight, height and baseline serum chemistry except for a slight increase in NT-pro BNP. There were no significant differences in the etiologies of PH or prescribed medications between the two groups (Table 1). Notably, the expression of circulating miR-21 was significantly increased in the patients with RV dysfunction (6.9 ± 4.4 vs. 87.4 ± 55.3, p = 0.001). Regarding echocardiographic parameters, the patients with RV dysfunction showed a significant decline in S′ (13.3 ± 2.5 cm/s vs. 6.5 ± 1.8 cm/s, p = 0.001) and TAPSE (1.9 ± 0.3 cm vs. 1.0 ± 0.2 cm, p = 0.001). Likewise, FAC was relatively reduced compared with those with preserved RV function (45.3.5 ± 6.5% vs. 21.3 ± 8.2%, p = 0.09) (Table 2). There were no specific differences in LV, RV dimensions, severity of tricuspid regurgitation, RV pressures or diastolic function.
Table 1. Clinical characteristics of patients with hypoxia induced pulmonary hypertension (PH) and preserved or impaired right ventricular (RV) function.
| PH without RV dysfunction (n = 31) | PH with RV dysfunction (n = 10) | p-value | |
| Age (years) | 56.2 ± 8.5 | 51.6 ± 19.4 | 0.37 |
| Male (%) | 21 (67.7) | 6 (60) | 0.11 |
| Body height (cm) | 156.7 ± 7.4 | 157.5 ± 10.6 | 0.61 |
| Body weight (kg) | 53.4 ± 7.4 | 58.2 ± 1.1 | 0.68 |
| Diabetes mellitus (%) | 9 (29) | 2 (20) | 0.69 |
| Hypertension (%) | 6 (19.3) | 3 (30) | 0.82 |
| Malignancy (%) | 3 (9.7) | 1 (10) | 0.74 |
| Etiologies | |||
| COPD | 15 (48.4) | 4 (40) | 0.41 |
| Bronchiectasis | 6 (19.3) | 3 (30) | 0.32 |
| Pulmonary tuberculosis | 6 (19.3) | 1 (10) | 0.72 |
| IPF | 4 (12.9) | 2 (20) | 0.63 |
| Medication | |||
| Theophylline | 18 (58) | 6 (60) | 0.74 |
| Β-agonist | 27 (87) | 8 (80) | 0.63 |
| Digoxin | 8 (25.8) | 3 (30) | 0.82 |
| Diuretics | 17 (54.8) | 7 (70) | 0.41 |
| CCB | 15 (48.3) | 5 (50) | 0.87 |
| Systemic steroid | 11 (35.4) | 3 (30) | 0.62 |
| SpO2 | 89 ± 4.3 | 91 ± 2.8 | 0.61 |
| RHC | 11 (35.4) | 7 (70) | 0.28 |
| Creatinine clearance rate (ml/min) | 102.7 ± 42.8 | 97.5 ± 24.8 | 0.8 |
| NT-proBNP (pg/ml) | 847.1 ± 643.2 | 1814.8 ± 1377.8 | 0.26 |
| Hemoglobin (g/dl) | 13.1 ± 2.1 | 14.3 ± 2.3 | 0.17 |
| miR-21 expression | 6.9 ± 4.4 | 87.4 ± 55.3 | 0.001 |
Data are expressed as mean ± standard deviation.
CCB, calcium channel blockers; COPD, chronic obstructive pulmonary diseases; IPF, idiopathic pulmonary fibrosis; miR, microRNA; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PH, pulmonary hypertension; RHC, right heart catheterization; RV, right ventricle.
Table 2. Echocardiographic parameters of patients with hypoxia induced pulmonary hypertension (PH) and preserved or impaired right ventricular (RV) function.
| PH without RV dysfunction (n = 31) | PH with RV dysfunction (n = 10) | p-value | |
| Right heart parameters | |||
| RV base diameter, mm | 3.0 ± 0.5 | 3.2 ± 0.9 | 0.82 |
| RV midventricular diameter, mm | 2.2 ± 0.8 | 2.1 ± 0.4 | 0.51 |
| RV length, mm | 5.3 ± 1.1 | 5.5 ± 0.9 | 0.72 |
| Tricuspid regurgitation grade > II (%) | 24 (77.4) | 7 (70) | 0.48 |
| RV pressure (mmHg) | 44.2 ± 16.1 | 44.3 ± 32.6 | 0.91 |
| RVFAC (%) | 45.3.5 ± 6.5 | 21.3 ± 8.2 | 0.09 |
| RV S′ (cm/s) | 13.3 ± 2.5 | 6.5 ± 1.8 | 0.001 |
| TAPSE (cm) | 1.9 ± 0.3 | 1.0 ± 0.2 | 0.001 |
| Left heart parameters | |||
| LVMI (g/m2) | 64.0 ± 22.2 | 56.0 ± 8.5 | 0.25 |
| LVEDVi (ml) | 49.3 ± 21.3 | 43.5 ± 13.8 | 0.49 |
| LVEF (%) | 69.1 ± 9.0 | 67.0 ± 3.8 | 0.21 |
| E (ms) | 75.6 ± 27.5 | 52.8 ± 23.8 | 0.15 |
| DT (ms) | 209.3 ± 62.5 | 196.3 ± 51.1 | 0.48 |
| e′ (ms) | 9.9 ± 3.7 | 9.9 ± 2.3 | 0.93 |
| E/e′ (mean) | 7.5 ± 2.1 | 6.1 ± 2.3 | 0.61 |
Data are expressed as mean ± standard deviation.
DT, deceleration time; E, transmitral valve early filling velocity; e′, early diastolic mitral annular velocity; LVEDVi, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RV, right ventricular; RVFAC, right ventricular fractional area change; S′, tissue Doppler systolic velocity; TAPSE, tricuspid annular plane systolic excursion.
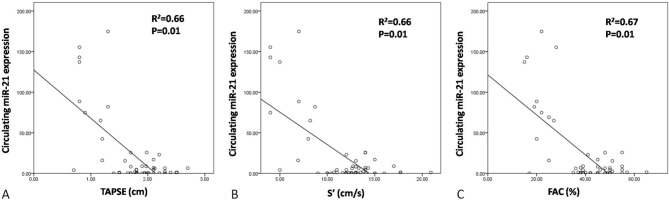
Negative correlation between circulating miR-21 expression and RV function
In linear regression analysis, the circulating miR-21 expression was negatively correlated with TAPSE (R2 = 0.66, p = 0.01), tissue Doppler S′ (R2 = 0.66, p = 0.01) and FAC (R2 = 0.67, p = 0.01) in the patients with hypoxia-induced PH (Figure 1). This indicated that the expression of miR-21 was associated with the severity of RV dysfunction.
Figure 1.
The negative correlations between circulating miR-21 expression with (A) Tricuspid annular plane systolic excursion (TAPSE), (B) Tissue Doppler S′ and (C) Fractional area change (FAC) in patients with hypoxia induced pulmonary hypertension.
Hypoxia-induced up-regulation of miR-21 in HPECs and conditioned media
Compared with HPECs grown in a normoxic environment, those treated with hypoxia for 48 hours showed a significant increase in miR-21 expression (Figure 2A). Correspondingly, under hypoxia treatment, the conditioned media collected at 48 hours showed an elevation of miR-21 expression compared with the other groups (Figure 2B). Notably, there were also increased expressions of miR-21 in the conditioned media post 48 hours compared with those treated with 24 hours in both normoxia and hypoxia groups. This may have been due to the increased number of cells and a corresponding miR-21 expression post-cell cycle. In addition, the cells developed hypertrophy 48 hours after hypoxia (Figure 2C). To evaluate the influence of up-regulated miR-21 in the pulmonary circulation on RV, we over-expressed miR-21 in cardiac myoblast H9C2 cells and observed a significantly hypertrophic change with an increase in cell surface area of H9C2 post miR-21 treatment (Figure 3A, B). In addition, there was also a significant elevation in the expression of BNP in miR-21 over-expressed H9C2 cells (Figure 3C).
Figure 2.
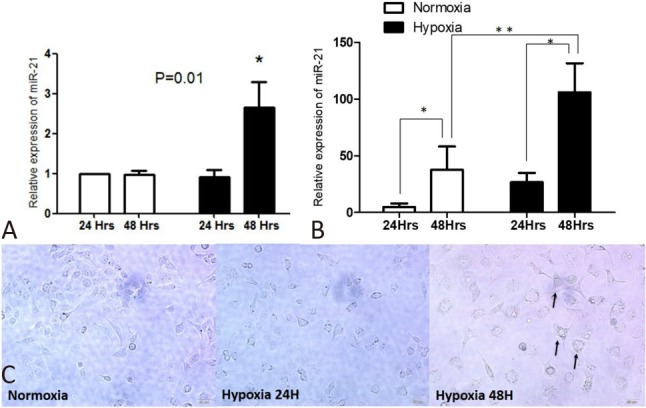
Hypoxia induced up-regulation of miR21 expression in (A) Pulmonary microvascular endothelial cells (HPEC) and (B) Its conditioned media (N = 6; * p < 0.05; ** p < 0.001). (C) The hypertrohic changes in hypoxia treated HPEC (arrow; N = 3).
Figure 3.
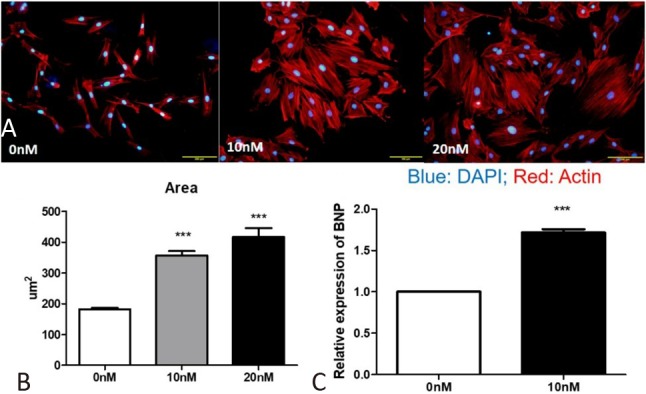
(B) The increased cell surface area in miR-21 over-expressed H9C2 cells. (C) The increased expression of brain natriuretic peptide (BNP) in miR-21 over-expressed H9C2 cells (N = 2).
DISCUSSION
In this study, we found that (1) in patient with hypoxia-induced PH, the expression of circulating miR-21 was negatively associated with the severity of RV dysfunction; (2) hypoxic stimulation up-regulated miR-21 in HPECs and the conditioned media; and (3) the overexpression of miR-21 in H9C2 cells enhanced heart failure-associated markers. Together, our findings imply that an increased expression of miR-21 in the pulmonary circulation may be responsible for RV dysfunction in hypoxia-induced PH.
Chronic hypoxia causes pulmonary vascular remodeling and leads to RV dysfunction.3,5,12 Despite a high prevalence of hypoxia-induced PH, it is mainly under-diagnosed while hypoxia-associated clinical presentations are usually diagnosed as pulmonary diseases instead of PH.6 Data on the response of RV to current therapies are particularly limited.6 Since symptoms of hypoxia-induced PH are non-specific, a disease-specific biomarker is of clinical value.13 Dysregulation of miRs has been described in various cardiac diseases including PH.8 Among different signature expression patterns of miRNAs, miR-21 is a key regulator suppressing apoptosis and promoting proliferation, mainly associated with cardiac hypertrophy and heart failure.8,10,14 In the context of PH, miR-21 has been shown to be involved in pulmonary vasculature remodeling,8,9,14 and previous studies have reported that progressive hypoxia significantly affects levels of circulating miR-21, and that this is significantly correlated with increased pulmonary artery pressure.10,15 Aberrantly expressed miR-21 has also been described to contribute to altered bone morphogenetic protein receptor type II (BMPR2) signaling and PH development.14 In addition, hypoxia-induced miR-21 has been shown to regulate proliferative responses in the pulmonary circulation.16 Recently, Deng and colleagues reported that the activation of miRs regulate smooth muscle and endothelial cell crosstalk in PH.17 Nevertheless, prior to this study, the effect of miR-21 on RV remodeling remains largely undetermined. According to our findings, miR-21 may serve as a surrogate for the detection of RV failure, and also as a communicator between the pulmonary circulation and RV under hypoxic stimulation. Several expression profiling studies have indicated that miR-21 is one of the most abundantly dysregulated miRNAs in cardiovascular disorders.8,10 However, the function and mechanism of miR-21 are controversial.10 One previous study showed that miR-21 induces cardiac hypertrophy in a paracrine manner by crosstalk between cardiac fibroblasts and cardiomyocytes.18 Likewise, inhibition of miR-21 in a mouse model of angiotensin II-induced heart failure blunted cardiac hypertrophy.19 On the other hand, using a TAC-induced cardiac pressure overload mouse model, Yan et al. identified the intrinsic anti-hypertrophic function of miR-21 in cardiomyocytes.20 In addition, through inhibiting excessive autophagy via the Akt/mTOR pathway, miR-21 has been reported to protect against cardiac hypoxia in H9c2 cells.21 In contrast, our study focused on the interplay between pulmonary endothelial cells and H9C2 cells. We found a significant elevation in miR-21 expression in both HPEC cells and the conditioned media post hypoxia treatment. This over-expression of miR-21 in H9C2 cells resulted in significant cell hypertrophy and up-regulation of BNP expression. This finding indicated that circulating miR-21 may play an important role in the development of RV dysfunction and may help to distinguish whether or not PH patients will develop RV dysfunction.
During the development of PH, endothelial cell migration and proliferation are thought to be most responsible for the regeneration of injured endothelium.22 Nevertheless, similar to smooth muscle cells, hypoxia also causes pulmonary vasoconstriction and results in vascular remodeling with pulmonary artery cell proliferation and hypertrophy,23 which may explain the hypertrophic changes in HPECs post hypoxia in our study.
There are several limitations to this study. First, among the studied population only 10 patients developed RV dysfunction which may have attenuated the statistical power. In addition, only 43% of them received RHC, and echocardiography cannot replace RHC when measuring PA. Although patients with impaired LV systolic function were excluded initially, and in general there was no significant diastolic dysfunction among the studied population, the potential influence of LV cannot be ignored. Third, even though previous studies have reported that hypoxic culturing systems can successfully mimic hypoxia-induced PH,24,25 the development of PH in humans remains quite different from in vitro models. Further investigations of the interplay among the pulmonary and cardiac systems involved in PH development and progression may contribute to novel interventional targets.
CONCLUSIONS
In patients with hypoxia-induced PH, miR-21 may be a sentinel of RV remodeling. In addition, the up-regulation of miR-21 in the pulmonary circulation under hypoxic stimulation may result in the development of RV dysfunction.
Acknowledgments
We thank Professor Ping Yen Liu for technical support with the cell lines.
CONFLICT OF INTEREST
None.
FUNDING
This work was supported by the National Health Research Institute, Taiwan (NHRI-EX106-10618SC), Taiwan Society of Cardiology, and Chi-Mei Medical Center.
REFERENCES
- 1.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. doi: 10.1161/01.CIR.0000156326.48823.55. [DOI] [PubMed] [Google Scholar]
- 2.Wang KY. The changing landscape of pulmonary arterial hypertension in 21st century. Acta Cardiol Sin. 2017;33:510–513. doi: 10.6515/ACS20170810A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LY, Lee KT, Lin CP, et al. Long-term survival of patients with pulmonary arterial hypertension at a single center in Taiwan. Acta Cardiol Sin. 2017;33:498–509. doi: 10.6515/ACS20170612A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini P, Rossi A, Pasotti M, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 5.Ghofrani HA, Voswinckel R, Reichenberger F, et al. Hypoxia- and non-hypoxia-related pulmonary hypertension - established and new therapies. Cardiovasc Res. 2006;72:30–40. doi: 10.1016/j.cardiores.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Vender RL. Chronic hypoxic pulmonary hypertension. Cell biology to pathophysiology. Chest. 1994;106:236–243. doi: 10.1378/chest.106.1.236. [DOI] [PubMed] [Google Scholar]
- 7.Han M, Toli J, Abdellatif M. Micrornas in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Zhang C. Microrna-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–255. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh VN, Jin RC, Rabello S, et al. Microrna-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duygu B, Da Costa Martins PA. MiR-21: a star player in cardiac hypertrophy. Cardiovasc Res. 2015;105:235–237. doi: 10.1093/cvr/cvv026. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; 786-688. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Caruso P, MacLean MR, Khanin R, et al. Dynamic changes in lung microrna profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 13.Swaminathan AC, Dusek AC, McMahon TJ. Treatment-related biomarkers in pulmonary hypertension. Am J Respir Cell Mol Biol. 2015;52:663–673. doi: 10.1165/rcmb.2014-0438TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed MI, Mardaryev AN, Lewis CJ, et al. Microrna-21 is an important downstream component of bmp signalling in epidermal keratinocytes. J Cell Sci. 2011;124:3399–3404. doi: 10.1242/jcs.086710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blissenbach B, Nakas CT, Kronke M, et al. Hypoxia-induced changes in plasma micrornas correlate with pulmonary artery pressure at high altitude. Am J Physiol Lung Cell Mol Physiol. 2018;314:L157–L164. doi: 10.1152/ajplung.00146.2017. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Banerjee S, Freitas A, et al. MiR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302:L521–L529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MiR-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2017;120:e6. doi: 10.1161/RES.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 18.Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast-derived microrna passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thum T, Gross C, Fiedler J, et al. Microrna-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 20.Yan M, Chen C, Gong W, et al. MiR-21-3p regulates cardiac hypertrophic response by targeting histone deacetylase-8. Cardiovasc Res. 2015;105:340–352. doi: 10.1093/cvr/cvu254. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Wu S, Kong F, et al. MicroRNA-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J Cell Mol Med. 2017;21:467–474. doi: 10.1111/jcmm.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranchoux B, Harvey LD, Ayon RJ, et al. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape. Pulm Circ. 2018;8:2045893217752912. doi: 10.1177/2045893217752912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther. 2001;92:1–20. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 24.Porter KM, Kang BY, Adesina SE, et al. Chronic hypoxia promotes pulmonary artery endothelial cell proliferation through H2O2-induced5-lipoxygenase. PLoS One. 2014;9:e98532. doi: 10.1371/journal.pone.0098532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu L, Hales CA. Hypoxia does neither stimulate pulmonary artery endothelial cell proliferation in mice and rats with pulmonary hypertension and vascular remodeling nor in human pulmonary artery endothelial cells. J Vasc Res. 2011;48:465–475. doi: 10.1159/000327005. [DOI] [PMC free article] [PubMed] [Google Scholar]