Abstract
Background
Hyperhomocysteinemia is a known risk factor for acute coronary syndrome (ACS) and is related with the severity of coronary artery disease (CAD). Previous studies have used less quantifiable scoring systems for assessing the severity of CAD. Therefore, we aimed to assess the relationship between homocysteine levels and SYNTAX score (SXscore), which is currently more widely used to grade the severity of CAD.
Methods
A total of 503 patients with adiagnosis of ACS were examined angiographically with SXscore. The patients were divided into three groups according to SXscore; Group 1 a low SXscore (≤ 22), Group 2 a moderate SXscore (23-32), and Group 3 a high SXscore (≥ 33).
Results
Plasma homocysteine levels were 16.3 ± 6.2 nmol/mL in Group 1, 18.1 ± 9.6 nmol/mL in Group 2, and 19.9 ± 9.5 nmol/mL in Group 3. Homocysteine levels were significantly higher in Group 2, and Group 3 compared to Group 1 (p = 0.023 and 0.007, respectively). In the correlation analysis, homocysteine levels were correlated with SXscore (r: 0.166, p < 0.01).
Conclusions
Serum homocysteine levels on admission were associated with an increased severity of CAD in the patients with ACS.
Keywords: Acute coronary syndrome, Coronary artery disease, Homocysteine, SYNTAX score
INTRODUCTION
Homocysteine is an amino acid compriseds of sulfide. It plays a major role in folate metabolism and is produced by the demethylation of methionine.1 Hyperhomocysteinemia can be caused by vitamin B12 and folic acid deficiency and by genetic defects in enzymes. Hyperhomocysteinemia can affect many atherogenic mechanisms. Elevated homocysteine levels can increases low density lipoprotein (LDL) intake into the vascular wall and stimulate vascular smooth muscle growth.2 Hyperhomocysteinemia has been recognized as a risk factor for the presence and acceleration of atherosclerosis, hypercoagulability and atherothrombosis leading to coronary artery disease (CAD).3,4 Hyperhomocysteinemia has been associated with a greater risk of adverse cardiovascular diseases such as acute coronary syndrome (ACS), stroke and cardiovascular mortality.5,6
CAD has become a major public health problem and is a main contributor to morbidity and mortality worldwide. The severity of CAD can be assessed with scoring systems such as Gensini and SYNTAX.7,8 The number of obstructed arteries can also be used to assess the severity of CAD. Well-known traditional risk factors for CAD include diabetes mellitus, hypertension, hyperlipidemia, smoking and advanced age. However, some patients with CAD (15-20%) do not have any known risk factors.9 Investigators have reported that hyperhomocysteinemia should be considered in patients with ACS and those without conventional risk factors. Therefore, this study aimed to determine the relationship between the severity of CAD and levels of homocysteine in patients with ACS.
METHODS
A total of 503 patients with a diagnosis of ACS who were admitted to our clinic between March 2013 and December 2015 were included in the present study. Coronary angiography was performed in all patients. The ACS spectrum was defined as unstable angina pectoris (USAP), non-ST elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI), and the diagnosis was established according to practice guidelines.10,11 The inclusion criteria were a diagnosis of ACS. The exclusion criterias were the presence of any of the followings: acute/chronic renal failure, chronic obstructive lung disease, any malignancy, active infectious disease, liver failure, previous history of heart failure, decompensated heart failure, previous history of revascularization (percutaneous or surgical), moderate or severe valvular heart disease, peripheral arterial disease, aortic dissection, pulmonary embolism, any thyroid disease, and any rheumatological disease.
Hemoglobin, platelets and white blood cell count, homocysteine, creatinine, glucose, high sensitivity C-reactive protein (hs-CRP), glycated hemoglobin (HbA1C) and cholesterol levels were assessed. The homocysteine level was determined using a commercially available kit (Chromsystems Instruments & Chemicals GmbH Am Haag 12, 82166 Gräfelfing, Germany) by high-pressure liquid chromatography and fluorometric methods in blood samples with ethylenediaminetetraacetic acid.
Judkins technique was used for coronary angiography using a digital angiographic system (Siemens Axiom Artis zee 2011; Siemens Healthcare, Erlangen, Germany). All coronary arteries were visualized in at least two different projections. The severity and complexity of CAD was evaluated by SYNTAX score (SXscore). At least 50% stenosis was accepted as significant CAD. All lesions with a diameter of at least 1.5 mm in a coronary artery that were causing significant stenosis were included in the calculation of SXscore. The website (http://www.syntaxscore.com) was used to calculate SXscore. The SXscore was calculated separately by two different interventional cardiologists who were blinded to the patients and study protocol. In cases of disagreement between the two calculations, a senior interventional cardiologist was consulted and a common consensus was obtained from the three operators. After calculating the SXscore, the patients were divided into three groups. The first group (low SXscore ) was composed of patients with an SXscore of ≤ 22, the second group (intermediate SXscore) was composed of patients with an SXscore of 23-32, and the third group (high SXscore) was composed of patients with an SXscore of ≥ 33.
Statistical analysis was performed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL). The distribution of variables was analyzed using the Kolmogorov-Smirnov test. The chi-square test was used to compare categorical variables, and the Student’s t-test and Mann-Whitney U tests were used to compare parametric and non-parametric variables. Statistical correlations were assessed using Pearson’s and Spearman’s correlation analyses according to the distribution type. Analysis of variance (ANOVA) with post-hoc analysis was used to compare means among the three groups to assess each between two-group differences. Multiple linear regression analysis was then performed with standard and forward stepwise selection to identify independent factors associated with the severity of atherosclerosis. Data are expressed as mean ± SD for parametric variables and median (minimum-maximum) for non-parametric variables. A p < 0.05 was accepted as being statistically significant. The study was approved by the local ethics committee, and informed consent was obtained from all study participants.
RESULTS
In total, 503 patients with ACS were included in the study, of whom 291 (57%) had STEMI, 154 (30%) had NSTEMI, and 58 (11%) had USAP. According to ANOVA with post-hoc analysis, homocysteine levels were not different in the subgroups of ACS (17.4 ± 8.0 in patients with STEMI, 16.7 ± 6.2 in patients with NSTEMI, 15.7 ± 7.6 in patients with USAP, p > 0.05 for between each groups). The demographic and clinical characteristics of the patients are shown in Table 1. The first group was compared with the second and the third groups separately. The statistical difference between the first group and second groups was termed p1, and the difference between the first and third groups was termed p2. When the first and second groups were compared, age and prevalence of diabetes were significantly lower (p1 = 0.003, and p1 = 0.044, respectively), haemoglobin levels were significantly higher in the first group (p1 = 0.003). When the first and third groups were compared, age, prevalence of diabetes, creatinine, glucose and HbA1c levels were significantly lower (p2 < 0.001, p2 = 0.004, p2 = 0.002, p2 = 0.002, and p2 = 0.003, respectively), haemoglobin levels, smoking and family history of CAD were significantly higher (p2 = 0.012, p2 = 0.013, and p2 = 0.023, respectively) in the first group (Table 2).
Table 1. Baseline clinical features.
| Baseline clinical features | Group I | Group II | Group III | p1 | p2 |
| Age (mean ± SD) | 59.69 ± 12.2 | 63.62 ± 12.4 | 69.89 ± 13 | 0.003 | < 0.001 |
| Male sex (%) | 249 (70.7) | 74 (63.8) | 26 (74.3) | 0.161 | 0.659 |
| Arterial hypertension (%) | 162 (46.7) | 62 (54.4) | 18 (51.4) | 0.154 | 0.592 |
| Hypercholesterolemia (%) | 122 (35.2) | 40 (35.1) | 8 (22.9) | 0.989 | 0.143 |
| Diabetes (%) | 105 (30.2) | 46 (40.4) | 19 (54.3) | 0.044 | 0.004 |
| Family history of CAD (%) | 62 (17.9) | 18 (15.8) | 1 (2.9) | 0.611 | 0.023 |
| Smokers (%) | 165 (47.6) | 43 (37.7) | 9 (25.7) | 0.067 | 0.013 |
| Previous CVA (%) | 7 (2) | 2 (1.8) | 2 (5.7) | 0.860 | 0.169 |
CAD, coronary artery disease; CVA, cerebrovascular accident.
Table 2. Comparision of baseline blood features in all groups.
| Baseline blood features | Group I | Group II | Group III | p1 | p2 |
| Glucose (mg/dL, mean ± SD) | 144 ± 77 | 160 ± 86 | 188 ± 99 | 0.06 | 0.002 |
| HbA1c (mg/dL, mean ± SD) | 6.7 ± 1.7 | 7.0 ± 1.8 | 7.4 ± 2.0 | 0.211 | 0.03 |
| Creatinine (mg/dL, mean ± SD) | 1.06 ± 0.3 | 1.11 ± 0.29 | 1.23 ± 0.28 | 0.196 | 0.002 |
| Total cholesterol (mg/dL, mean ± SD) | 194 ± 54 | 191 ± 48 | 186 ± 57 | 0.614 | 0.399 |
| HDL-C (mg/dL, mean ± SD) | 40 ± 10 | 40 ± 8 | 43 ± 9 | 0.761 | 0.062 |
| LDL-C (mg/dL, mean ± SD) | 123 ± 41 | 121 ± 42 | 112 ± 44 | 0.659 | 0.161 |
| Triglycerides (mg/dL, mean ± SD) | 165 ± 126 | 169 ± 121 | 160 ± 90 | 0.731 | 0.836 |
| Hs-CRP (mg/dL, mean ± SD) | 6.4 ± 3.9 | 7.0 ± 3.8 | 7.5 ± 3.8 | 0.157 | 0.108 |
| Hemoglobin (g/dL, mean ± SD) | 14.2 ± 1.8 | 13.6 ± 1.9 | 13.3 ± 1.5 | 0.003 | 0.012 |
| Platelets (103/mm3, mean ± SD) | 237 ± 73 | 240 ± 65 | 226 ± 94 | 0.68 | 0.43 |
| White blood cells (106/μL,mean ± SD) | 10.3 ± 3.2 | 10.7 ± 3.8 | 10.2 ± 3.9 | 0.176 | 0.888 |
| Homocysteine (nmol/mL, mean ± SD) | 16.3 ± 6.2 | 18.1 ± 9.6 | 19.9 ± 9.5 | 0.023 | 0.007 |
HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein-cholesterol; Hs-CRP, high sensitive C-reactive protein; LDL, low-density lipoprotein; LDL-C, low-density lipoprotein-cholesterol; SD, standard deviation.
Plasma homocysteine levels were 16.3 ± 6.2 nmol/mL in the first group, 18.1 ± 9.6 nmol/mL in the second group and 19.9 ± 9.5 nmol/mL in the third group. Homocysteine levels were significantly higher in the second group and third groups compared to the first group (p1 = 0.023 and, p2 = 0.007). According to the correlation analysis, homocysteine levels were correlated with SXscore (r = 0.166, p < 0.01, Figure 1).
Figure 1.
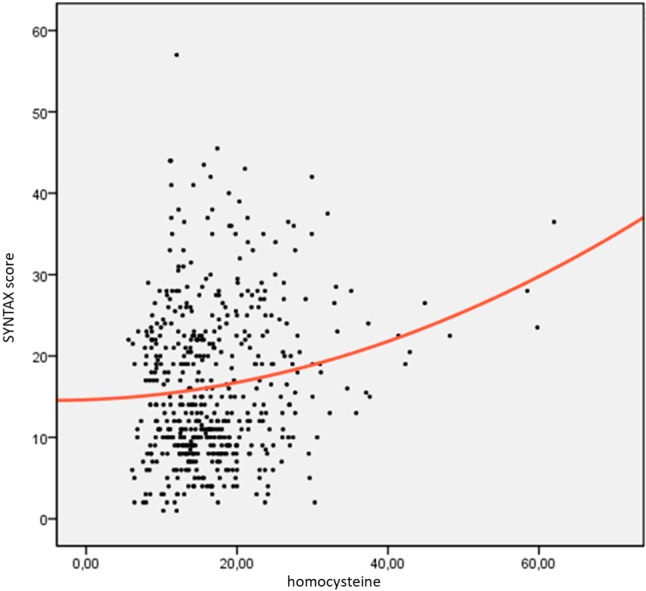
Correlation analysis of serum homocysteine levels and SYNTAX score (r: 0.166, p < 0.01).
According to the multiple linear regression analysis, age (B = 0.170, p < 0.001), diabetes (B = 2.6, p = 0.005), creatinine level (B = 3.1, p = 0.032) and homocysteine level (B = 0.144, p = 0.017) were independently associated with SXscore (Table 3).
Table 3. Relationship between Syntax Score and risk factors in multiple linear regression analysis.
| Variables | Unstandardized coefficients | Standardized coefficients | t | p | |
| B | Std. error | Beta | |||
| Age | 0.170 | 0.035 | 0.228 | 4.810 | < 0.001 |
| Diabetes | 2.639 | 0.927 | 0.130 | 2.847 | 0.005 |
| Creatinine | 3.154 | 1.464 | 0.102 | 2.154 | 0.032 |
| Homocysteine | 0.144 | 0.060 | 0.112 | 2.390 | 0.017 |
DISCUSSION
In the present study, homocysteine levels were higher in the high SXscore group than in the low SXscore group of ACS patients. Although a few studies have reported a positive correlation between homocysteine levels and severity of CAD,12-15 no previous study has reported an association between homocysteine levels and severity of CAD using SXscore. Several methods can be used to assess the severity of the CAD; such as the number of diseased vessels, Gensini and SXscore. We used SXscore in this study because it is the most widely used and specific of these methods.
Previous studies have shown that serum homocysteine activates vascular smooth muscle proliferation and decreases nitric oxide levels through inhibiting nitric oxide synthetase enzyme.16-18 More recent epidemiological studies have shown an association between elevated homocysteine levels and increased thrombogenicity, oxidative stress and endothelial dysfunction, thereby playing a role in atherosclerotic plaque progression.19,20 These studies have shown that homocysteine is an independent risk factor for CAD. Similar studies have shown an increased risk of myocardial infarction in patients with hyperhomocysteinemia.21,22 Fu and colleagues also showed that hyperhomocysteinemia in elderly ACS patients was an important predictor for both all-cause mortality and major adverse cardiovascular events.23 Another study reported that increased plasma total homocysteine levels in STEMI patients increased the 30-day cardiovascular event rate.24
An increased mortality rate has been reported in parallel with increased severity of CAD.23 Relationships between hyperhomocysteinemia and all-cause/cardiovascular mortality were shown in these studies, however the underlying mechanisms are not clear. An increased severity of atherosclerosis in patients with hyperhomocysteinemia (as in the current study), and a relationship between the severity of atherosclerosis and increased mortality in patients with CAD may be the underlying mechanism.
Few studies have investigated the relationship between the severity of CAD and hyperhomocysteinemia, and the results of these studies have been inconsistent. Homocysteine concentrations were more elevated in three-vessel disease than in single vessel disease in the study of Joubran et al.14 Wu et al. found that homocysteine levels were strongly correlated with the severity of CAD.13 Shenoy et al. used Gensini score to assess the severity of CAD, and found that homocysteine levels were elevated more in the CAD group, and that homocysteine levels were also correlated with the extent of the disease.12 In our study, we found that homocysteine levels were higher in the high SXscore group of ACS patients. This study differs from previous studies in that we used SXscore, which is a more specific method to assess the severity of CAD.
Although the physiopathogenesis of the association between elevated homocysteine and extent of CAD is not completely understood, multiple mechanisms may be involved. First, inflammatory processes are known to play an important role in atherosclerosis. Elevated homocysteine levels, an important inflammatory marker, may play a role in the progression of atherosclerosis and increased severity of CAD. Second, associations among elevated homocysteine levels and increased thrombogenicity, oxidative stress and endothelial dysfunction have been reported. This mechanism may explain the relationship between high homocysteine levels and CAD extent. Finally, serum homocysteine levels have been positively associated with cardiac risk factors, which contribute to atherosclerotic processes such as hypertension, diabetes, and metabolic syndrome.26 These diseases which are also associated with high homocysteine levels may play a role in the pathogenesis of the increased severity of CAD in hyperhomocysteinemic patients.
There are several limitations to this study. This was a single-center study and did not have a control group. In addition, there were fewer patients in group III. Homocysteine is not widely used as a marker, and we did not have any data on long-term cardiovascular events. Further prospective randomized studies on a larger scale are needed to confirm these findings.
CONCLUSIONS
In conclusion, serum homocysteine levels on admission were associated with an increased severity of CAD in patients with ACS. It should be kept in mind that CAD may be present in patients with high homocysteine levels. If there are accompanying risk factors, pre-intervention preparations should be made appropriately.
REFERENCES
- 1.Fowler B. Homocystein an independent risk factor for cardiovascular and thrombotic diseases. Ther Umsch. 2005;62:641–646. doi: 10.1024/0040-5930.62.9.641. [DOI] [PubMed] [Google Scholar]
- 2.Antoniades C, Antonopoulos AS, Tousoulis D, et al. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 3.Malinow M. Hyperhomocyst(e)inemia. A common and easily reversible risk factor for occlusive atherosclerosis. Circulation. 1990;81:2004–2006. doi: 10.1161/01.cir.81.6.2004. [DOI] [PubMed] [Google Scholar]
- 4.Abraham R, John MJ, Calton R, et al. Raised serum homocysteine levels in patients of coronary artery disease and the effect of vitamin B12 and folate on its concentration. Indian J Clin Biochem. 2006;21:95–100. doi: 10.1007/BF02913073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. [PubMed] [Google Scholar]
- 6.Tanne D, Haim M, Goldbourt U, et al. Prospective study of serum homocysteine and risk of ischemic stroke among patients with preexisting coronary heart disease. Stroke. 2003;34:632–636. doi: 10.1161/01.STR.0000060203.58958.35. [DOI] [PubMed] [Google Scholar]
- 7.Çağdaş M, Karakoyun S, Yesin M, et al. The association between monocyte HDL-C ratio and SYNTAX score and SYNTAX score II in STEMI patients treated with primary PCI. Acta Cardiol Sin. 2018;34:23–30. doi: 10.6515/ACS.201801_34(1).20170823A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Hsu CY, Huang PH, et al. Association of serum bilirubin with SYNTAX score and future cardiovascular events in patients undergoing coronary intervention. Acta Cardiol Sin. 2016;32:412–419. doi: 10.6515/ACS20150708C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SC. Current and future directions of cardiovascular risk prediction. Am J Cardiol. 2006;97:28–32. doi: 10.1016/j.amjcard.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 10.ACCF/AHA guideline for the management of ST-elevation myocardial infarction. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 11.AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndrome. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 12.Shenoy V, Mehendale V, Prabhu K, et al. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem. 2014;29:339–344. doi: 10.1007/s12291-013-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Yang L, Zhong L. Decreased serum levels of thioredoxin in patients with coronary artery disease plus hyperhomocysteinemia is strongly associated with the disease severity. Atherosclerosis. 2010;212:351–355. doi: 10.1016/j.atherosclerosis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Joubran R, Asmi M, Busjahn A, et al. Homocysteine levels and coronary heart disease in Syria. J Cardiovasc Risk. 1998;5:257–261. [PubMed] [Google Scholar]
- 15.Tokgözoğlu S, Alikaşifoğlu M, Atalar E, et al. Homosistein ve MTHFR genotipinin koroner arter hastalığı risk ve yaygınlığının belirlenmesindeki önemi. Türk Kardiyol Dern Arş. 1999;27:598–603. [Google Scholar]
- 16.Bilsborough W, Green DJ, Mamotte CD, et al. Endothelial nitric oxide synthase gene polymorphism, homocysteine, cholesterol and vascular endothelial function. Atherosclerosis. 2003;169:131–138. doi: 10.1016/s0021-9150(03)00147-3. [DOI] [PubMed] [Google Scholar]
- 17.Kanani PM, Sinkey CA, Browning RL, et al. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocysteinemia in humans. Circulation. 1999;100:1161–1168. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- 18.Cavalca V, Cighetti G, Bamonti F, et al. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. 2001;47:887–892. [PubMed] [Google Scholar]
- 19.Weiss N, Heydrick SJ, Postea O, et al. Influence of hyperhomocysteinemia on the cellular redox state–impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41:1455–1461. doi: 10.1515/CCLM.2003.223. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi N, Sedoris KC, Steed M, et al. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–H2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 21.Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–282. doi: 10.1007/BF03195624. [DOI] [PubMed] [Google Scholar]
- 22.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Z, Qian G, Xue H, et al. Hyperhomocysteinemia is an independent predictor of long-term clinical outcomes in Chinese octogenarians with acute coronary syndrome. Clin Interv Aging. 2015;10:1467. doi: 10.2147/CIA.S91652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Li L, Geng XB, et al. Correlation between hyperhomocysteinemia and outcomes of patients with acute myocardial infarction. Am J Ther. 2016;23:e1464–e1468. doi: 10.1097/MJT.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 25.Burggraf GW, Parker JO. Prognosis in coronary artery disease. Angiographic, hemodynamic, and clinical factors. Circulation. 1975;51:146–156. doi: 10.1161/01.cir.51.1.146. [DOI] [PubMed] [Google Scholar]
- 26.Faeh D, Chiolero A, Paccaud F. Homocysteine as a risk factor for cardiovascular disease: should we (still) worry about it? Swiss Med Wkly. 2006;136:745–756. doi: 10.4414/smw.2006.11283. [DOI] [PubMed] [Google Scholar]


