Abstract
Background
The number of diagnostic and interventional cardiac catheterization procedures are increasing in the post-operative period of congenital heart diseases (CHD). The aim of this study was to evaluate data of patients who underwent cardiac catheterization in the early post-operative period after congenital heart surgery (CHS).
Methods
We retrospectively evaluated the data of patients who underwent cardiac catheterization within 30 days after CHS.
Results
Between 2010 and 2016 in our hospital, 2584 children had operations, and 2911 children underwent cardiac catheterization due to CHD. Cardiac catheterization was performed in 50 (1.9% of the surgeries) of these patients during the early post-operative period. Twenty-nine (58%) of the patients were males. The median age was 7.5 months (range: 15 days-12.5 years), and the median body weight was 6 kg (range: 3-35 kg). Twenty-eight (56%) of the patients had two-ventricle, and 22 (44%) had single ventricle physiology. The median RACHS-1 score was 3 (range: 1-6). Cardiac catheterization was performed under extracorporeal membrane oxygenation (ECMO) support in 16 of the patients. Twenty-four (48%) patients underwent diagnostic catheterization, while 26 (52%) had interventional procedures. Fifteen (30%) patients had a reoperation due to anatomic problems identified during catheterization. Major complications developed in 4 (8%) patients. There was no cases of procedural mortality due to catheterization.
Conclusions
Cardiac catheterization should be performed in post-operative cardiac patients without hesitation, even under ECMO, if significant hemodynamic or clinical problems cannot be identified clearly by other non- interventional diagnostic techniques.
Keywords: Cardiac catheterization, Cardiac surgery, Children, Congenital heart defect, Post-operative period
INTRODUCTION
Congenital heart diseases are important health problems in both developing and developed countries. In recent years, advances in surgical methods and improved intensive care units have led to a marked increase in survival rates after neonatal, infant, and childhood congenital heart defect operations.1
Congenital heart diseases are quite a heterogeneous and complex group of cardiac malformations. For this reason, the type of surgical treatment performed in each case and the post-operative intensive care unit period can be quite different. During the post-operative period after congenital heart surgery (CHS), hemodynamic or clinical problems may occur. Serious complications affecting morbidity and mortality such as low cardiac output, post-operative arrhythmia, and pulmonary hypertensive crisis can develop due to these problems. The residual defects and additional pathologies can be major factors in the development or worsening of the severity of these complications. Medical therapy or surgery can be the choice of treatment for these complications. Extracorporeal membrane oxygenation (ECMO) can be useful for the pre-operative hemodynamic stabilization of patients, and in certain patients with fatal arrhythmias resistant to medical treatment or low cardiac output syndrome after cardiac surgery.2-5
Non-interventional diagnostic methods such as echocardiography, computerized tomography (CT), and magnetic resonance imaging can be inadequate to identify the underlying pathology. Echocardiography, CT angiography and other imaging systems play important roles during the evaluation of a congenital heart disease, even if it occurs in the early post-operative period. Cardiac catheterization and angiographic evaluations would be a better choice if the residual lesion or the underlying condition has not been identified with echocardiography or CT angiography, or if a percutaneous intervention was planned.4-6
There are limited reports on early post-operative catheterization and interventional outcomes in infants with congenital heart disease.6,7 The aim of this study was to evaluate patients with congenital heart disease in whom cardiac catheterization and angiography were performed in the early post-operative period.
MATERIALS AND METHODS
Patients with congenital heart disease who had operations for the correction or palliation at our center between January 2010 and December 2016 were identified using the hospital’s database. The type of operation and degree of difficulty were determined for each case by using the following: the Risk Adjustment in Congenital Heart Surgery-1 (RACHS-1) score, Aristotle Basic Complexity (ABC) score, and Aristotle Comprehensive Complexity (ACC) score.8,9 The patients who underwent cardiac catheterization and angiography in the early postoperative period (≤ 30 days) constituted the study group.
Medical records were retrospectively reviewed for all patients. Variables collected included demographic data regarding age, weight, cardiac diagnosis, primary cardiac surgical procedure, indication for catheterization, timing of catheterization, and details of catheterization including the intervention, procedural complications, and clinical outcomes. The Institutional Review Board of our hospital approved this study.
In our pediatric heart center, transesophageal echocardiography (TEE) is the first option and is performed routinely to evaluate patients during CHS. Intraoperative epicardial echocardiography (IEE) was performed when the TEE system was in use in another operating room or in an interventional procedure. Intraoperative imaging is not routinely performed in BCPC (Glenn), Norwood, shunt or arterial switch operations, and arch surgeries. Post-operative transthoracic echocardiography (TTE) was performed routinely in all patients within 24 hours of the surgery, usually before extubation in the intensive care unit. Subsequent TTE studies were performed as needed.
Indications for cardiac catheterization and angiography included the following:
(a) Persistent/severe cyanosis;
(b) A low cardiac output state;
(c) Inability to wean from inotropic/ventilation support;
(d) Inability to wean from cardiopulmonary support;
(e) Echocardiographic diagnosis of severe residual defects;
(f) Severe effusions (pleural/ascites).
Catheterization data collected consisted of the procedure performed, procedural mortality, and procedural complications (minor/major). Major complications included death, persistent or complete atrioventricular (AV) block, cardiopulmonary arrest, and the need for unplanned emergent surgery or ECMO support because of complications. Other cases were considered to be minor complications.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) for Windows version 15 (SPSS, Chicago, IL, USA) was used for statistical analysis. Continuous variables are expressed as median (minimum-maximum), and categorical variables are expressed as percentages.
Patients
Between January 2010 and December 2016, 50 patients underwent cardiac catheterization and angiography in the early post-operative period. These patients constituted 1.9% (n = 2584) of all surgical cases, and 1.7% (n = 2911) of all cardiac catheterization cases in our pediatric cardiovascular surgery center. In 16 cases (14.5% of all ECMO cases), cardiac catheterization was performed under ECMO support.
RESULTS
The demographic and diagnostic characteristics of the patients are demonstrated in Table 1. The median RACHS-1 score was 3 (range: 1-6), the median Aristotle basic score was 9 (range: 6.5-14.5), and the median Aristotle comprehensive score was 10 (range: 6.5-27.5).
Table 1. The demographic data and the characteristics of the patients.
| Patient characteristics | n = 50 |
| Age | 7.5 months (15 day-12.5 year)* |
| Weight (kg) | 6 (3-35)* |
| Height (cm) | 65 (49-150)* |
| Sex (male/female) | 29/21 |
| Postoperative course | |
| Postoperative day of catheterization (days) | 12 (1-30)* |
| Mechanical circulatory support during catheterization n(%) | 16 (32%) |
| Duration of intensive care unit stay (days) | 20 (2-84) |
| Diagnosis | n |
| Pulmonary atresia/ventricular septal defect | 11 |
| Right atrial isomerism – complete AVSD (unbalanced) | 6 |
| Double outlet right ventricle | 5 |
| Transposition of the great arteries (ventricular septal defect/arcus hypoplasia/pulmonary stenosis) | 5 |
| Tetralogy of Fallot | 4 |
| Total anomalous pulmonary venous return | 3 |
| Shone’s complex | 3 |
| Coarctation of the aorta/arcus hypoplasia | 3 |
| Hypoplastic left heart syndrome | 3 |
| Others | 7 |
| Biventricular/univentricular | 28/22 |
* median (range). AVSD, atrioventricular septal defects.
The median procedure time was 30 min (range: 15-210 min), median fluoroscopic time was 10.8 min (range: 1.7-97 min), median radiation exposure in total air kerma was 87 mGy (range: 13.1-4435 mGy), and the dose area product was 5400 mGy/cm2 (range: 425-117710 mGy/cm2). The median contrast medium exposure was 4.2 cc/kg (range: 2.1-12.4 cc/kg).
The diagnoses were as follows: 11 cases had ventricular septal defect-pulmonary atresia; 6 cases had right atrial isomerism-complete atrioventricular septal defects-unbalanced ventricles; 5 cases had double outlet right ventricles; 5 cases had transposition of the great arteries; 4 cases had tetralogy of Fallot; 3 cases had hypoplastic left heart syndrome; 3 cases had shone complex; 3 cases had total anomalous pulmonary venous return; 3 cases had arcus hypoplasia; and 7 cases had other diagnoses. Twenty-eight patients (56%) had two-ventricle physiology, and 22 (44%) patients had single ventricle physiology (Table 1).
Indications for cardiac catheterization were as follows: persistent/severe cyanosis in 10 cases; low cardiac output state in 12 cases; inability to wean from inotropic/ventilation support in 8 cases; inability to wean from cardiopulmonary support in 8 cases [in 5 of the 8 patients who failed weaning from cardiopulmonary bypass, TEE or IEE was performed. In 3 patients (who received a Glenn operation (n = 1), arterial switch operation (n = 1) and Norwood operation (n = 1)), intraoperative imaging was not performed]; echocardiographic diagnosis of residual defects in 8 cases; and persistent/severe effusions (pleural/ascites) in 4 cases.
The median post-operative duration of catheterization was 12 days (range 1-30 days). The median duration from the initiation of ECMO support to the catheterization procedure was 3 days (range 1-11 days). Catheterization was performed during the first 3 days post-operatively in 20% of the cases, 7 days in 46% of the cases, 10 days of 54% of the cases, and 14 days in 66% of the cases.
During the postoperative period, TTE or TEE was performed in all of the patients (n = 50). A hemodynamic pathology was suspected in 32 of the patients. CT angiography was performed in 7 of 18 patients who had no important hemodynamic abnormalities in the echocardiographic evaluation, and 5 patients who had pathology on CT were catheterized. Thirteen patients without any determined hemodynamically important pathology on echocardiography or CT were catheterized for detailed evaluations.
Diagnostic catheterization was performed in 24 cases (48%) and interventional procedures were carried out in 26 cases. Diagnostic catheterization was performed in 7 cases under ECMO support, and invasive procedures were used in 9 cases under ECMO support. Pulmonary artery dilatation was performed in 5 cases, pulmonary artery stenting in 5 cases, and major aortopulmonary collateral artery (MAPCA) embolization in 3 cases. The interventional data are demonstrated in Table 2, and Figure 1 shows procedural images of a case.
Table 2. Transcatheter management of the patients.
| Intervention type | n = 26 (52%) |
| Balloon dilation of pulmonary artery | 5 |
| Pulmonary artery stenting | 5 |
| Stent implantation to increase pulmonary blood flow | 4 |
| BT shunt | 2 |
| MAPCA stenting | 1 |
| RVOT stenting | 1 |
| MAPCA embolization | 3 |
| Atrial septostomy | 3 |
| Balloon angioplasty for coarctation and aortic balloon valvuloplasty | 2 |
| Pulmonary vein stenting | 1 |
| Fistula embolization | 1 |
| Mitral balloon valvuloplasty | 1 |
| Transcatheter VSD closure | 1 |
BT, blalock taussig; MAPCA, major aortopulmonary collateral artery; RVOT, right ventricular outflow tract; VSD, ventricular septal defect.
Figure 1.
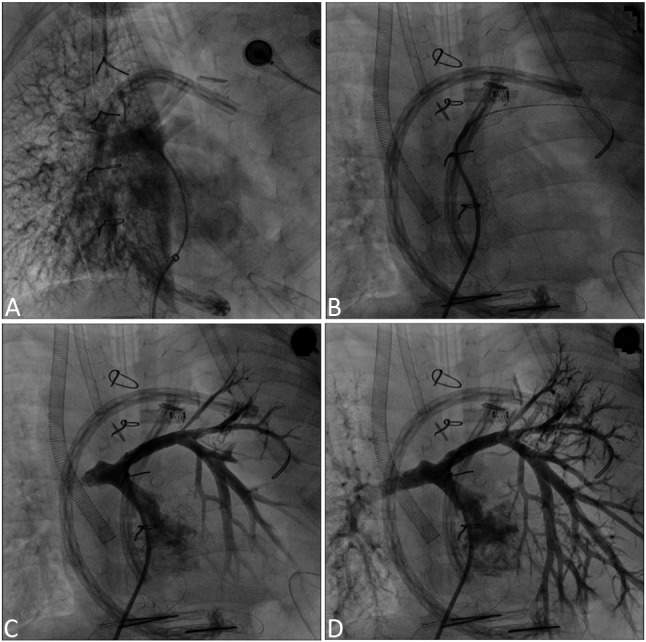
Angiogram of a five-year-old boy diagnosed with double outlet right ventricle, ventricular septal defect and severe pulmonary stenosis. After cardiac surgery, ECMO support initiated. During ECMO support cardiac catheterization performed. (A) No antegrade flow in the left pulmonary artery. (B & C) Balloon and stent angioplasty of left pulmonary artery. (D) Increased left pulmonary artery flow after stent implantation.
Fifteen cases (30%) required reoperations, and 12 of these cases had diagnostic catheterization, while the other 3 had interventional procedures. Based on the results of the cardiac catheterization, medical therapy was changed in 9 (18%) of the cases. Table 3 shows the indications for reoperation and medical therapy changes.
Table 3. Surgical or medical management of the patients.
| Reoperation | n = 15 (30%) |
| Pulmonary artery reconstruction | 4 |
| RV-PA conduit placement/repair | 3 |
| Aortic arch reconstruction | 2 |
| Pulmonary artery banding | 2 |
| Glenn operation | 1 |
| Pulmonary vein repair | 1 |
| Glenn takedown | 1 |
| Right atrial maze +fenestration + pacemaker implantation | 1 |
| Change in medical treatment | n = 9 (18%) |
| Anti pulmonary hypertension treatment | 4 |
| Inhaled nitric oxide | 2 |
| Others (Sildenafil, Iloprost, Bosentan) | 2 |
| Antithrombotic treatment (heparin or LMWH) | 3 |
| Additional inotropic support (Levosimendan) | 2 |
LMWH, low-molecular-weight heparin; PA, pulmonary artery; RV, right ventricle.
There were no cases of procedural mortality due to catheterization. Six of our patients had minor adverse events during the cardiac catheterization procedure. Four of these cases had hemorrhage at the femoral puncture site, and a short duration of hypotension was observed in the other 2. Major complications developed in 4 cases, including cardiac arrest requiring cardiopulmonary resuscitation (CPR) in 2, 1 who had a complete AV block that recovered within 12 hours after the procedure, and 1 who had an ECMO cannula emerge.
Overall patient outcomes
Nine patients who were not under ECMO support (9 of 34 patients) died during the post-operative period. In 6 of these cases, diagnostic cardiac catheterization was conducted, while interventional procedures were used in the remaining 3 cases. Reoperation was performed in 5 of these cases. Of the 9 patients who died, 5 were due to multi-organ failure, 2 were attributed to neurological damage, and 2 had gram-negative Klebsiella Pneumonia septicemia.
Twelve of the 16 patients (75%) under ECMO who underwent pre-operative cardiac catheterization were successfully weaned. Five of these cases were re-operated on soon after catheterization, including conduit replacement in 3 cases, repair of the stenosis in the branch pulmonary arteries in 1 case, and repair of the supravalvular aortic stenosis with coronary ostium stenosis in the remaining case. Two of the patients died despite successful ECMO weaning during intensive care follow-up. One of the cases, who was lost despite successful ECMO weaning, had neurological damage, and the other died of multi-organ failure. Both had undergone diagnostic catheterization.
Four cases (25%) died under ECMO support. Two of these cases had severe neurological damage, 1 case had septicemia, and the other case had multi-organ failure. Two of them received a diagnostic catheter, and the other 2 cases had pulmonary artery stenting and a ballooning procedure. The total survival rate after cardiac catheterization in the post-operative patients was 70% (non-ECMO patient survival 73.5%, ECMO patient survival 62.5%).
DISCUSSION
Cardiac catheterization has been used for many years as a standard diagnostic method in the pre-operative period. Due to technological advances and developments, it is now used as the main treatment method for many congenital heart diseases.4,5 In the current study, we analyzed catheter indications, interventional procedures, outcomes and survival of the patients who had cardiac catheterization in the early post-operative period after CHS. Few prior studies have investigated this issue. The results showed that cardiac catheterization was performed successfully, with acceptable complication rates, in such cases for diagnostic or therapeutic purposes. Congenital heart diseases have various types and numbers of pathologies. Although there were a limited number of patients in this series, most of the patients who were catheterized during the early post-operative period had right ventricular outflow tract (RVOT) or pulmonary artery surgery.
A benefit of cardiac catheterization in the early post-operative period is the correct delineation of the anatomy and physiology, which allows for effective therapy; thus, a substantial number of patients undergo reoperation based on the findings during cardiac catheterization. This may be avoided in the early post-operative period due to associated risks, such as difficulties transporting these critically ill patients, concerns regarding worsening clinical status because of the procedure, and fear of disrupting recently placed suture lines.6,7,9
In one previous study, early post-operative cardiac catheterization was followed by a return to the operating room to address important residual lesions in 43% of the patients. In the interventional early post-operative cardiac catheterization group, 32 patients (49%) returned to the operating room within 10 days of the catheterization, and findings during catheterization prompted reoperation in 17 patients. In the early post-operative diagnostic cardiac catheterization group, 51 patients (40%) were brought back to the operating room within 10 days of catheterization, of whom 33 reoperations were attributed to findings during catheterization. The most common reoperations were branch pulmonary arterioplasty (n = 11), systemic-to-pulmonary artery shunt revision (n = 9), and Fontan or Glenn shunt revision (n = 9).4 Recently, successful applications such as dilation of the pulmonary artery stenosis, conduit stenosis or residual aortic coarctation, and occlusion of MAPCA or anterograde flow in patients with bidirectional Glenn anastomosis have been reported in patients under ECMO or mechanical ventilation support in the early post-operative period, such as in cases with Glenn shunt revision.11-13 Similarly, the most frequent indication for reoperation in the present study was pulmonary artery reconstruction.
Nicholson et al.6 evaluated cardiac catheterization and angiography cases in the early post-operative period. They performed 219 catheterizations in 193 cases, in patients aged between 1 day and 18 years of age. Twenty-two of the cases were under ECMO support, 132 of the cases were on mechanical ventilatory support, and 129 of the cases were receiving inotropic support. Ninety-one (42%) of these procedures were interventional, and 128 (58%) were diagnostic. Indications for cardiac catheterization were residual defects in 62 cases, cyanosis in 54 cases, and low cardiac output syndrome in 35 cases. There were 7 major complications including death in 1 case, the need for ECMO support in 2 cases, device/stent migration in 2 cases, and vascular rupture in 2 cases. In contrast to the study of Nicholson et al., the most frequent indication was low cardiac output in the present study, and residual defects and cyanosis were less common.
In a study by Zahn et al., 62 patients underwent 66 catheterization procedures approximately 9 days after surgery (median = 9 days; range = 0 to 42 days).10 In 35 cases, 50 interventional procedures were carried out. Nine patients required ECMO support. Success rates respectively by procedure were angioplasty (100%), stent implantation (87%), vascular/septal occlusion (100%), and palliative pulmonary valvotomy (75%). Complications included stent migration (1 patient), cerebral vascular injury (1 patient), and left pulmonary artery stenosis (1 patient).
In the present study, interventional catheterizations were more frequent than diagnostic catheterizations, which is consistent with the findings of Zahn et al. Additionally, the interventional success and complication rates were also similar.
In the literature, the incidence of cardiac catheterization under ECMO has been reported to be 3.5-7.1 patients/year, a rate of roughly 30% of patients under ECMO.13-15 In our center, the incidence was 3.2 patients/year (14.5% of patients under ECMO). This low incidence may be related to the learning period of our clinic.
Various catheter indications under ECMO have been reported in different studies.13-15 Callahan et al.16 performed 40 cardiac catheterizations in a series of 36 cases, in which the indications for catheterization included hemodynamic/anatomic assessments of post-operative (n = 16) and non-operative (n = 7) patients, planned catheter interventions (n = 12), and cardiomyopathy assessment (n = 5). Unexpected diagnostic information was found in 21 patients (52%), and catheter interventions were performed in 18 patients (45%). The unexpected diagnostic information included the following: stenting of vessels/surgical shunts (n = 9); balloon atrial septostomy (n = 4); device closure of septal defects/vessels (n = 3); thrombectomy of thrombosed vessels (n = 2); endomyocardial biopsy (n = 2); and temporary cardiac pacing lead placement (n = 1). In our study, half of the patients underwent interventional procedures, and the remaining half had only diagnostic catheterizations under ECMO support.
There are limited data on the safety and utility of cardiac catheterization in the immediate post-operative period after surgical repair of CHS. There is a commonly accepted notion that one should wait 6 weeks after surgery before performing an intervention.16 Yet, there are no published data indicating unusual suture fragility in the early post-operative period. Zahn et al.10 evaluated patients who underwent cardiac catheterization within 6 weeks after cardiac surgery, and reported that although 42% of the cohort (26 patients) underwent interventions across suture lines, no vascular disruptions were noted. Nicholson et al.6 reported patients who underwent catheterization within 4 weeks after surgery, of whom two-thirds underwent catheterization within 12 days of surgery at a median age of 3.8 months. They observed a total of 18 (8.3%) complications (7 major and 11 minor). There was no significant difference in the incidence of major or minor complications between the interventional and diagnostic catheterization cohorts. Various complications can be observed during cardiac catheterization and angiography. The complication rate was 16% (range 5% to 18%) in a multicenter study reported by Bergersen et al.17
In the present study, the minor complication rate was 12% (6 of 50), and the major complication rate was 8% (4 of 50), resulting in an overall complication rate of 20% (n = 10).
Limitation
This was a retrospective, single center study with a small sample size.
CONCLUSIONS
Cardiac catheterization should be performed in patients with operated CHS, even under ECMO if significant hemodynamic or clinical problems are observed in the early post-operative period while the patient is in an intensive care unit. The decision of whether to choose interventional or surgical treatment should be made together with the cardiologist and surgeon on a case by case basis according to the center preferences. Medical and interventional treatments performed on account of the catheterization results may have a positive effect on mortality and morbidity.
REFERENCES
- 1.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Pan JY, Lin CC, Chang JP. Fontan operation in a patient with severe hypoplastic right pulmonary artery, single ventricle, and heterotaxy syndrome. Acta Cardiol Sin. 2016;32:612–615. doi: 10.6515/ACS20160105A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Küçük M, Özdemir R, Karaçelik M, et al. Risk factors for thrombosis, overshunting and death in infants after modified blalock-taussig shunt. Acta Cardiol Sin. 2016;32:337–342. doi: 10.6515/ACS20150731A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth KL, Roth SJ, Perry SB, et al. Cardiac catheterization of patients supported by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2002;40:1681–1686. doi: 10.1016/s0735-1097(02)02343-4. [DOI] [PubMed] [Google Scholar]
- 5.Güzeltaş A, Kasar T, Tanıdır İC, et al. Cardiac catheterization procedures in pediatric patients undergoing extracorporeal membrane oxygenation cardiac catheterization, ECMO. Anatol J Cardiol. 2017;18:425–430. doi: 10.14744/AnatolJCardiol.2017.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson GT, Kim DW, Vincent RN, et al. Cardiac catheterization in the early post-operative period after congenital cardiac surgery. JACC Cardiovasc Interv. 2014;7:1437–1443. doi: 10.1016/j.jcin.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Siehr SL, Martin MH, Axelrod D, et al. Outcomes following cardiac catheterization after congenital heart surgery. Catheter Cardiovasc Interv. 2014;84:622–628. doi: 10.1002/ccd.25490. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 9.Lacour-Gayet F, Clarke D, Jacobs J, et al. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25:911–924. doi: 10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Zahn EM, Dobrolet NC, Nykanen DG, et al. Interventional catheterization performed in the early postoperative period after congenital heart surgery in children. J Am Coll Cardiol. 2004;43:1264–1269. doi: 10.1016/j.jacc.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Bhole V, Wright JG, De Giovanni JV, et al. Transcatheter interventions in the early postoperative period after the Fontan procedure. Catheter Cardiovasc Interv. 2011;77:92–98. doi: 10.1002/ccd.22667. [DOI] [PubMed] [Google Scholar]
- 12.Asoh K, Hickey E, Dorostkar PC, et al. Outcomes of emergent cardiac catheterization following pediatric cardiac surgery. Catheter Cardiovasc Interv. 2009;73:933–940. doi: 10.1002/ccd.21919. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal HS, Hardison DC, Saville BR, et al. Residual lesions in postoperative pediatric cardiac surgery patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg. 2014;147:434–441. doi: 10.1016/j.jtcvs.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Abraham BP, Gilliam E, Kim DW, et al. Early catheterization after initiation of extracorporeal membrane oxygenation support in children is associated with improved survival. Catheter Cardiovasc Interv. 2016;88:592–599. doi: 10.1002/ccd.26526. [DOI] [PubMed] [Google Scholar]
- 15.Panda BR, Alphonso N, Govindasamy M, et al. Cardiac catheter procedures during extracorporeal life support: a risk-benefit analysis. World J Pediatr Congenit Heart Surg. 2014;5:31–37. doi: 10.1177/2150135113505297. [DOI] [PubMed] [Google Scholar]
- 16.Callahan R, Trucco SM, Wearden PD, et al. Outcomes of pediatric patients undergoing cardiac catheterization while on extracorporeal membrane oxygenation. Pediatr Cardiol. 2015;36:625–632. doi: 10.1007/s00246-014-1057-5. [DOI] [PubMed] [Google Scholar]
- 17.Bergersen L, Marshal A, Gauvreau K, et al. Adverse events rates in congenital cardiac catheterization—a multi-center experience. Catheter Cardiovasc Interv. 2010;75:389–400. doi: 10.1002/ccd.22266. [DOI] [PubMed] [Google Scholar]


