Abstract
Purpose
The objectives of this study were to: (i) evaluate endothelial function via fingertip reactive hyperemia peripheral arterial tonometry (RH-PAT) among heart failure (HF) patients receiving cardiac resynchronization therapy (CRT), (ii) assess the effects of CRT on RH-PAT score, and (iii) investigate whether RH-PAT score can identify CRT response.
Methods
A total of 63 patients (61.8 ± 10.3 years; 50 males; left ventricular (LV) ejection fraction 24.3 ± 3.9%) with HF who received CRT were enrolled. Endothelial function via RH-PAT was assessed 1 day before and 6 months after CRT. Minnesota Living with Heart Failure Questionnaire (MLWHFQ) was used to assess clinical improvements. CRT response was defined as a reduction in LV end-systolic volume ≥ 15% at 6 months.
Results
A RH-PAT score of < 1.7 signified a cut-off for endothelial dysfunction (ED). Baseline ED was observed among 43 (68.3%) patients and was more prevalent in responders (76.1% vs. 47.1%, p = 0.037). RH-PAT score improved 6 months after CRT (1.58 ± 0.35 vs. 1.71 ± 0.31, p = 0.012). A RH-PAT score of < 1.7 was a significant independent predictor of CRT response in multivariate logistic regression analysis (β = 1.275, OR = 3.512, 95% CI = 1.231-11.477, p = 0.032). The severity of ED was an independent predictor of LV reverse remodeling (β = -8.873, p = 0.015). Spearman’s correlation analysis revealed moderate positive correlations between an improvement in RH-PAT (ΔRH-PAT) and LV reverse remodeling (r = 0.461, p = 0.001) and MLWHFQ score (r = 0.440, p = 0.001).
Conclusions
ED detected via RH-PAT could predict the response to CRT. The RH-PAT score increased 6 months after CRT and was correlated with echocardiographic and clinical improvements.
Keywords: Cardiac resynchronization therapy, Endothelial dysfunction, Heart failure, Peripheral arterial tonometry, Response
INTRODUCTION
Endothelial dysfunction (ED) is a systemic pathological state of the endothelium which can be defined as an imbalance between vasodilator and vasoconstrictor substances produced by (or acting on) the endothelium.1 ED is known to be an important pathophysiological feature of several cardiovascular diseases.2-6 Heart failure (HF) is a life-threatening syndrome with substantial morbidity and mortality, and it is a burden to both patients and healthcare systems.7 In HF, ED of coronary and peripheral arteries has been shown regardless of an ischemic or non-ischemic etiology. The development of ED results in impaired coronary and systemic perfusion as well as a reduction of functional capacity.3-6
Cardiac resynchronization therapy (CRT) has been shown to improve functional capacity, left ventricular (LV) systolic and diastolic function, cardiac autonomic function, and survival in patients with HF.8-10 It has also been shown that endothelial function improves following successful treatment of HF with CRT.6 Furthermore, Akar et al.3 reported that ED assessed by flow-mediated dilation (FMD) could identify CRT responders.3
Most of the studies assessing ED have used FMD as the study outcome.3,6 However, the utility of FMD as a measure of ED is limited by operator dependence and it is known to be affected by biological, environmental, and methodological factors.11,12 Recently, another non-invasive method, fingertip reactive hyperemia peripheral arterial tonometry (RH-PAT), has been used to identify ED.11-14 RH-PAT probes measure pulse volume changes at the fingertips after occlusion of the brachial artery of the dominant arm. Its ease of use, standardized methodology and the availability of validated cut-off thresholds are advantages of RH-PAT for evaluating ED11-13 Endothelial function assessments via RH-PAT in patients with HF, particularly those receiving CRT, has not previously been investigated.
This study had three aims: (i) to evaluate endothelial function via RHPAT among HF patients receiving CRT, (ii) assess the effects of CRT on RH-PAT score, and (iii) investigate whether RH-PAT score can identify CRT response.
METHODS
Study population
Of the 68 consecutive patients who were initially enrolled in the study, three were excluded owing to inappropriate coronary sinus anatomy, and two survivors who did not come to the second visit. A total of 63 chronic HF patients (mean age, 61.8 ± 10.3 years, 50 males) with a QRS duration > 120 ms and LV ejection fraction (LVEF) ≤ 35% who remained in New York Heart Association (NYHA) functional class II, III and ambulatory IV despite adequate medical treatment were enrolled in the study. This study complies with the Declaration of Helsinki. Informed consent was obtained from all participants, and the study was approved by the Research Ethics Committee.
Patients with a mechanical tricuspid valve, acute coronary syndrome, atrial fibrillation, prior pacemaker implantation, and a life expectancy of less than 6 months were excluded. All patients were evaluated in terms of age, gender, coronary artery disease (CAD), diabetes mellitus (DM), hypercholesterolemia, hypertension (HT), and other concomitant diseases. Hypercholesterolemia was defined as a low-density lipoprotein cholesterol (LDL-C) level ≥ 100 mg/dL and/or currently on statin treatment. All patients underwent a complete physical examination, during which their systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (PP), height and weight were recorded. Transthoracic echocardiography, Minnesota Living with Heart Failure Questionnaire (MLWHFQ), and fingertip RH-PAT measurements were performed the day before and 6 months after CRT. The MLWHFQ contains 21 questions referring to the signs and symptoms of HF, social relationships, physical and sexual activity, work, and emotions. The answer for each question was chosen from a scale of 0 (none) to 5 (very much); the greater the score, the worse the quality of life.15,16
Transthoracic echocardiography
Transthoracic echocardiographic examinations were performed in the left lateral position using a GE Vingmed Vivid E9 scanner (GE Vingmed Ultrasound, Horten, Norway) with a 2.5 MHz M5S transducer. From the parasternal long axis, the LV end-diastolic diameter and LV end-systolic diameter were measured using M-mode (at the mitral chordae level perpendicular to the long axis of the ventricle). The endocardial boundaries were identified from the apical four-chamber view. LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV) and LVEF were calculated using the modified Simpson’s method. All measurements were based on the criteria proposed by the American Society of Echocardiography.17
Reactive hyperemia peripheral arterial tonometry
Assessments of endothelial function were performed with the participant in a fasting state, after having avoided stimulants for 12 hours, and at a pre-defined time (range 8-10 AM). Pulse amplitude tone (PAT) was measured with an RH-PAT device (EndoPAT2000; Itamar Medical, Caesarea, Israel) placed on the tip of each index finger. The RH-PAT device consisted of a pneumatic plethysmograph that applied uniform pressure to the surface of the distal finger, allowing measurements of pulse volume changes. During measurements, the RH-PAT finger probe was placed on the index finger of the right hand undergoing hyperemia, and a second probe was placed on the index finger of the left hand. After a 5-minute equilibration period, the cuff was inflated to a pressure of 50 mmHg above the systolic pressure or 200 mmHg for 5 minutes, and then deflated to induce reactive hyperemia. The RH-PAT hyperemia score was defined as the ratio of the average amplitude of PAT signal over a 60-second period starting 90 seconds after cuff deflation divided by the average PAT of a 210-second pre-occlusion baseline period.14 The fingertip RH-PAT measurement method, EndoPAT2000 device, finger alignment during the procedure, and two output examples are shown in Figure 1.
Figure 1.

Reactive hyperemia peripheral arterial tonometry measurement, (A) EndoPAT2000 device. (B) System and finger alignment were shown. On right side, an example of a HF patient with (C) endothelial dysfunction and (D) normal endothelial function were shown.
Device implantation
All patients received a CRT device in combination with a cardioverter defibrillator. The LV pacing lead was inserted transvenously via the subclavian route. A coronary sinus venogram was routinely obtained before the introduction of the LV lead. The LV electrode was placed in the posterolateral branch of the coronary sinus in 57 patients (90.5%), and in the antero-lateral branch of the coronary sinus in six patients (9.5%). The right ventricular lead was positioned at the apex, and the right atrial lead was positioned in the atrial appendage.
At 6 weeks post-CRT implantation, the patients were referred for routine echocardiography-guided CRT optimization. Optimization of atrioventricular (AV) delay was performed using Doppler echocardiography of trans-mitral flow to provide the maximum LV filling time without compromising CRT (iterative method). The AV delay was set at a value which provided maximum separation of the E- and A-waves, representing passive ventricular filling and atrial contraction, respectively. The VV delay (LV-RV) was set at a value which yielded maximum aortic TVI during VV optimization.18
Definition of the cut-off values
An echocardiographic response was defined as a response to CRT, and quantified according to LV reverse remodeling (percentage of decline in the LVESV) after CRT. A decrease of ≥ 15% in LVESV at the 6-month follow-up visit was defined as a CRT response.8,19,20 An RH-PAT score of < 1.7 signified ED. This RH-PAT score value is a widely accepted cut-off value as determined in the study of Bonetti et al.11
Statistical analysis
The distribution of data was assessed using a one-sample Kolmogorov-Smirnov test. Data are presented as mean ± standard deviation (SD) for normally distributed continuous variables, median (minimum-maximum) for continuous variables with a skewed distributed, and frequencies for categorical variables. An independent sample t-test and the Mann-Whitney U test were used for numerical variables, and Pearson’s χ2 test or Fisher’s exact test was used for categorical variables for inter-group comparisons (responders vs. non-responders).
Comparisons of the RH-PAT score, clinical variables and echocardiographic indices before and after CRT were performed used the paired sample t-test or Wilcoxon’s signed-rank test. Inter-observer and intra-observer agreements for LV volumes and LVEF were assessed with intra- and inter-class correlation coefficients and with the average difference between readings, corrected for their mean (variability). The percentage of change (Δ) in RH-PAT (ΔRH-PAT) was compared using the Mann-Whitney U test. Spearman’s correlation analysis was used to assess the relationship between LV reverse remodeling and ΔRH-PAT. Among the factors influencing the response to CRT; age, female gender, ischemic etiology, QRS duration, left bundle branch block (LBBB) type QRS morphology, NYHA functional class, and the presence of ED were examined by multivariate logistic regression analysis. Sensitivity, specificity, positive and negative predictive values of ED for predicting a CRT response were expressed as percentages. Confidence intervals (CIs) for sensitivity and specificity were "exact" Clopper-Pearson CIs, and CIs for the predictive values were standard logit CIs. Statistical analysis of the data was conducted using SPSS version 15 (SPSS Inc., Chicago, IL, USA), and a two-tailed p < 0.05 was considered to be statistically significant.
RESULTS
Baseline features
Among the 63 patients (mean age, 61.8 ± 10.3 years), 50 (79.4%) were males (mean age, 61.2 ± 10.3 years) and 13 (20.6%) were females (mean age, 64.2 ± 10.4 years). The etiology of HF was primarily ischemic (n = 44, 69.8%). Other non-ischemic etiologies (n = 19, 30.2%) included idiopathic in 10 cases, severe mitral regurgitation in three cases, severe aortic regurgitation in two cases, radiotherapy-induced cardiotoxicity in two cases, and chemotherapy-induced cardiotoxicity in two cases. DM was present in 21 (33.3%) patients, 14 patients were receiving insulin treatment, and seven patients were using metformin. None of the patients were taking sulfonylureas, meglitinides or sodium-glucose cotransporter 2 inhibitors (SGLT2i) that may have altered endothelium function. There were no complications with CRT device implantation, and none of the patients required LV lead repositioning during follow-up. The programmed AV delay was 126.2 ± 14.0 ms, and the VV delay (LV-RV) was 28.2 ms ± 5.8. The baseline clinical and demographic features of the study population, responders and non-responders are presented in Table 1.
Table 1. Baseline clinical and echocardiographic parameters of the study population, responder and non-responder groups.
| Parameter | Total population (n = 63) | Responder group (n = 46) | Non-responder group (n = 17) | p value* |
| Age (years) | 61.8 ± 10.3 | 61.2 ± 10.1 | 63.5 ± 11.0 | 0.426 |
| Gender, male, n (%) | 50 (79.4) | 35 (76.1) | 15 (88.2) | 0.485 |
| Ischemic etiology, n (%) | 44 (69.8) | 30 (65.2) | 14 (82.4) | 0.230 |
| Hypertension, n (%) | 45 (71.4) | 34 (73.9) | 11 (64.7) | 0.473 |
| Diabetes mellitus, n (%) | 21 (33.3) | 17 (37) | 4 (23.5) | 0.316 |
| Hypercholesterolemia#, n (%) | 27 (42.9) | 20 (43.5) | 7 (41.2) | 0.870 |
| NYHA functional capacity | 2.82 ± 0.52 | 2.78 ± 0.51 | 2.94 ± 0.55 | 0.291 |
| MLWHFQ score | 29.2 ± 7.6 | 29.6 ± 6.6 | 27.9 ± 9.9 | 0.444 |
| Baseline ED†, % | 68.3 | 76.1 | 47.1 | 0.037 |
| Baseline RH-PAT score | 1.58 ± 0.34 | 1.52 ± 0.26 | 1.76 ± 0.46 | 0.01 |
| QRS morphology, LBBB/non-LBBB type | 19299 | 14763 | 43439 | 0.149 |
| QRS duration (ms) | 149.5 ± 22.9 | 148.4 ± 21.1 | 152.4 ± 27.8 | 0.550 |
| Left atrial diameter (mm) | 44.9 ± 5.2 | 44.5 ± 5.4 | 45.9 ± 4.1 | 0.312 |
| LV end-diastolic diameter (mm) | 66.8 ± 7.5 | 66.4 ± 7.4 | 67.0 ± 7.2 | 0.906 |
| LV end-systolic diameter (mm) | 56.1 ± 6.8 | 55.8 ± 6.5 | 56.2 ± 6.8 | 0.844 |
| LV end-diastolic volume (mL) | 172.1 ± 45.4 | 170.1 ± 46.1 | 173.1 ± 47.1 | 0.532 |
| LV end-systolic volume (mL) | 130.4 ± 39.1 | 130.2 ± 38.8 | 131.4 ± 39.5 | 0.412 |
| LV ejection fraction‡ (%) | 24.3 ± 3.9 | 25.2 ± 4.1 | 24.0 ± 4.2 | 0.386 |
| ACE-I or ARB use, n (%) | 63 (100). | 46 (100). | 17 (100) | NA |
| β-blocker use, n (%) | 58 (92.1) | 43 (93.5) | 15 (88.2) | 0.605 |
| Diuretic use, n (%) | 63 (100). | 46 (100). | 17 (100) | NA |
| Digoxin use, n (%) | 25 (39.7) | 16 (34.8) | 9 (52.9) | 0.249 |
| Spironolactone use, n (%) | 34 (54) | 27 (58.7) | 7 (41.2) | 0.262 |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CRT, cardiac resynchronization therapy; ED, endothelial dysfunction; LBBB, left bundle branch block; LV, left ventricular; MLWHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; RH-PAT, reactive hyperemia peripheralarterial tonometry.
Numerical variables were presented as the mean ± SD and categorical variables were presented as percentages. NA refers “not assessed” because parameter is constant in 2 groups.
# Hypercholesterolemia is defined as low-density lipoprotein cholesterol (LDL-C) ≥ 100 mg/dL and/or currently on statin treatment. † Baseline ED (RH-PAT values less than 1.7). ‡ Measured by modified Simpson’s method.
* p values for difference between responder and non-responder groups.
Clinical parameters and echocardiographic indices after CRT
NYHA functional capacity, MLWHFQ score, QRS duration, LVEDV, LVESV, and LVEF improved 6 months after CRT. Among the study population, 46 (73.0%) were responders (i.e. decline in LVESV ≥ 15%). The responder and non-responder groups were similar with respect to age (61.2 ± 10.1 vs. 63.5 ± 11.0 years, p = 0.426), gender distribution [(male/female) 35/11 vs. 15/2, p = 0.485], CAD (65.2 vs. 82.4%, p = 0.230), HT (73.9 vs. 64.7%, p = 0.473), DM (37.0 vs. 23.5%, p = 0.316), MLWHFQ score (27.9 ± 9.9 vs. 29.6 ± 6.6, p = 0.444), programmed AV delay (125.2 ± 12.9 vs. 128.8 ± 16.5, p = 0.367) and VV delay (28.0 ± 5.8 vs. 28.8 ± 6.0, p = 0.641). Comparisons of clinical and echocardiographic features of the patients 6 months after CRT are presented in Table 2.
Table 2. Clinical parameters, echocardiographic indices 6 months after CRT.
| Parameter | Before CRT | After CRT | p-value |
| NYHA functional capacity | 2.82 ± 0.52 | 1.74 ± 0.67 | 0.001 |
| Basal HR (bpm) | 73.5 ± 4.9 | 72.1 ± 5.3 | 0.088 |
| QRS (ms) | 149.5 ± 22.9 | 132.8 ± 22.7 | 0.001 |
| LVEDV (ml) | 172.1 ± 45.4 | 157.7 ± 42.2 | 0.001 |
| LVESV (ml) | 130.4 ± 39.1 | 105.1 ± 33.4 | 0.001 |
| LVEF (Simpson’s method) (%) | 24.3 ± 3.9 | 30.2 ± 7.3 | 0.001 |
| MLWHFQ score | 29.2 ± 7.6 | 19.4 ± 8.6 | 0.029 |
| RH-PAT score | 1.58 ± 0.35 | 1.71 ± 0.31 | 0.012 |
| SBP (mmHg) | 116.2 ± 8.7 | 117.7 ± 7.1 | 0.101 |
| DBP (mmHg) | 60.9 ± 10.1 | 60.2 ± 8.9 | 0.516 |
| PP (mmHg) | 55.2 ± 14.8 | 57.6 ± 12.6 | 0.098 |
CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; HR, heart rate; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; MLWHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; PP, pulse pressure (PP = SBP-DBP); RH-PAT, reactive hyperemia peripheral arterial tonometry; SBP, systolic blood pressure.
Numerical variables were presented as the mean ± SD. Numerical variables with a skewed distribution were presented as the median (minimum-maximum) values.
RH-PAT score and CRT
An RH-PAT score of < 1.7 was accepted as a cut-off value for ED. Baseline ED was observed among 43 (68.3%) patients and was more prevalent in the responders (76.1% vs. 47.1%, p = 0.037). The baseline RH-PAT score was lower in the responders (1.52 ± 0.26 vs. 1.76 ± 0.46, p = 0.01) than in the non-responders. Among the study population, the RH-PAT score significantly improved 6 months after CRT (1.58 ± 0.35 vs. 1.71 ± 0.31, p = 0.012). The responders had a marked improvement in RH-PAT score (1.52 ± 0.26 vs. 1.67 ± 0.29, p = 0.001) after CRT, whereas there was no improvement in RH-PAT score in the non-responders (1.76 ± 0.46 vs. 1.82 ± 0.34, p = 0.640) after CRT (Figure 2). There was no significant difference in the prevalence of ED between the responders and non-responders (47.8% vs. 41.2%, p = 0.778) 6 months after CRT.
Figure 2.
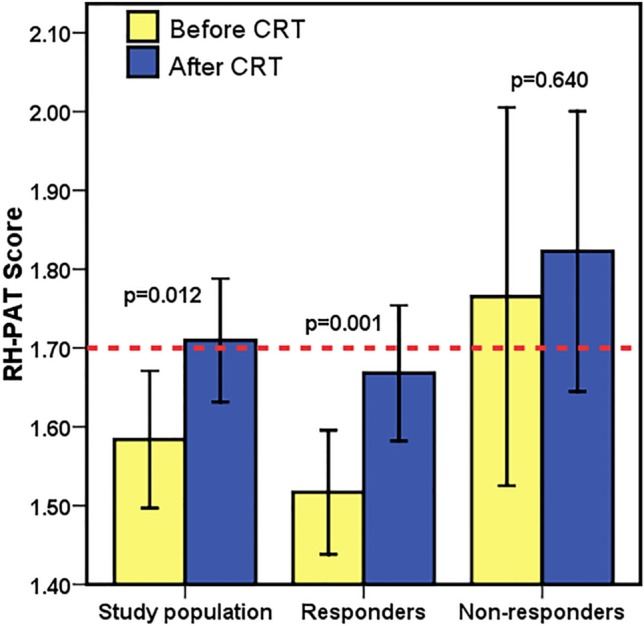
Changes in RH-PAT scores were shown. Error bars represents 95% confidence interval.
Among the factors influencing the response to CRT, age, female gender, ischemic etiology, QRS duration, LBBB type QRS morphology, NYHA functional class and the presence of ED were examined in multivariate logistic regression analysis. The results showed that the presence of ED had an independent influence on the response to CRT (Table 3). The presence of ED (RH-PAT score of < 1.7) was a significant independent predictor of CRT response [β = 1.275, Odds ratio (OR) = 3.512, 95% CI = 1.231-11.477, p = 0.032]. The severity of ED was an independent predictor of LV reverse remodeling (β = -8.873, p = 0.015).
Table 3. Results of multivariate logistic regression analysis for the predictors of CRT response.
| Variable | β | p value | OR | CI (95%) | |
| Lower | Upper | ||||
| Age | -0.011 | 0.744 | 0.989 | 0.925 | 1.057 |
| Female gender | 0.589 | 0.566 | 1.803 | 0.241 | 13.462 |
| Ischemic etiology | -0.343 | 0.690 | 0.710 | 0.131 | 3.834 |
| QRS duration | -0.023 | 0.118 | 0.977 | 0.949 | 1.006 |
| LBBB type QRS morphology | 1.316 | 0.242 | 2.506 | 0.538 | 11.671 |
| NYHA functional capacity | -0.289 | 0.656 | 0.749 | 0.210 | 2.666 |
| Presence of ED* | 1.275 | 0.032 | 3.512 | 1.231 | 11.477 |
CI, confidence interval; ED, endothelial dysfunction; LBBB, left bundle branch block; LV, left ventricular; NYHA, New York Heart Association; OR, Odds ratio.
* Presence of ED (RH-PAT values less than 1.7).
Spearman’s correlation analysis revealed moderate positive correlations of ΔRH-PAT with the degree of reduction in LVESV (r = 0.461, p = 0.001) and MLWHFQ score (r = 0.440, p = 0.001) (Figure 3). When an RH-PAT score of < 1.7 was used as a cut-off value for ED, the sensitivity of ED for predicting CRT response was 76.1% (61.2% to 87.4%, 95% CI), and the specificity was 52.9% (27.8% to 77.1%, 95% CI). The positive predictive value of ED was 81.4% (72.1% to 88.1%, 95% CI), and the negative predictive value was 45.0% (29.2% to 61.8%, 95% CI).
Figure 3.

Spearman’s correlation analysis between percentage of improvement in RH-PAT score with (A) echocardiographic response, (B) clinical improvement after CRT. r, correlation coefficient.
Reproducibility
Two measurements were obtained and averaged for all echocardiographic variables. For intra-observer reliability analysis, a sample of 15 patients was re-analyzed in a period ranging from 5 to 7 days between the first and second analysis. The intra-observer correlation coefficients and variability were 0.980 and 1.1% for LVESV and 0.913 and 1.7% for LVEDV, respectively. For the inter-observer reliability analysis, there was good correlation between the observers in LV volumes. The inter-observer correlation coefficients and variability were 0.921 and 1.6% for LVESV and 0.876 and 2.5% for LVEDV, respectively.
DISCUSSION
To the best of our knowledge, this is the first study to assess ED via RH-PAT among HF patients receiving CRT. The main findings of the present study are as follows: (i) ED is commonly observed among patients with HF, (ii) the presence of baseline ED as assessed by RH-PAT can independently identify a CRT response, and (iii) improvements in ED are correlated with LV reverse remodeling and clinical improvements as assessed by MLWHFQ.
ED is a powerful predictor of adverse events in patients with a variety of cardiovascular diseases.2-4,6,21 It usually manifests as impaired FMD of the brachial artery. ED is prevalent in HF and has emerged as a predictor of poor outcomes.3,5,6 In our study, we found that ED as assessed via RH-PAT was present in nearly 70% of the patients with HF. Patients with advanced HF have increased sympathetic tone, altered hemodynamics, peripheral shear stress and an activated renin-angiotensin system, all of which are thought to contribute to ED.3,6 In our study, we found that during follow-up, ED as assessed by RH-PAT was ameliorated in the responders and did not change in the non-responders. In addition, this amelioration of ED was correlated with LV reverse remodeling and clinical improvements as assessed by the MLWHFQ. In accordance with our results, improvements in FMD have previously been demonstrated after CRT implantation.3,6 Because CRT does not have a direct peripheral effect on the vasculature, any improvement in ED derived from CRT is likely to be a consequence of enhanced production of endothelium-derived nitric oxide due to improved hemodynamics, peripheral shear stress, cardiac loading conditions, and neurohormonal activation.3,6
Baseline ED was more common in the CRT responders compared to the non-responders, implying that although the two groups appeared to be similar clinically, there were differences as reflected in the degree of ED. This raises the possibility that among patients who are currently eligible for CRT, those with ED and lower RHPAT score are more likely to respond. There is mounting evidence that a response to CRT is not all or nothing but rather a spectrum.3 As such, it is likely that patients with advanced HF will derive the greatest benefit from the incremental effects of CRT. Furthermore, consistent with our results, the presence of baseline ED was found to be an independent predictor of CRT response in the study by Akar et al.3 Importantly, the current study is the first to demonstrate that the severity of ED as evaluated by RHPAT score was an independent predictor of LV reverse remodeling.
An important strength of our study is that we used an operator-independent method to assess ED. Although FMD is a common research method for peripheral, noninvasive assessments of ED. It differs from RH-PAT in several ways. While FMD assesses a single conduit vessel, RH-PAT measures several vascular beds composed of small vessels and microcirculation. Furthermore, RH-PAT corrects for systemic changes by using simultaneous measurements from the (un-occluded) contra-lateral arm. With minimal training, RH-PAT is practically operator-independent, while FMD requires a trained ultrasound technician and is highly user-dependent in both data acquisition and analysis.12,22
The major limitations of the present study are the relatively small number of patients, and the results are based on a single center experience. In addition, invasive hemodynamic measurements of endothelial function were not obtained in the patients. RH-PAT was found to be correlated with coronary endothelial function using the gold standard method of assessment which is injection of acetylcholine during coronary catheterization.11
CONCLUSIONS
In conclusion, ED is commonly observed in patients with HF. CRT decreases mortality and morbidity in patients with HF, however around one third fail to demonstrate an improvement following CRT. Therefore, potential predictors of CRT response are crucial. RH-PAT, a non-invasive evaluation method of ED via pulse volume changes at the fingertips, can be used to predict the response to CRT at 6 months. Improvements in ED (ΔRH-PAT) were moderately correlated with LV reverse remodeling and clinical improvements. ED may be considered when selecting patients for CRT. However before making a general recommendation, larger studies are needed to confirm its predictive power and determine why patients with severe baseline ED are more likely to respond to CRT.
DISCLOSURES
None.
REFERENCES
- 1.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 3.Akar JG, Al-Chekakie MO, Fugate T, et al. Endothelial dysfunction in heart failure identifies responders to cardiac resynchronization therapy. Heart Rhythm. 2008;5:1229–1235. doi: 10.1016/j.hrthm.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagoner DR. Is homocysteine a mediator of atrial dysfunction or just another marker of endothelial dysfunction? Europace. 2008;10:899–900. doi: 10.1093/europace/eun182. [DOI] [PubMed] [Google Scholar]
- 5.Terzi S, Emre A, Yesilcimen K, et al. The endothelial nitric oxide synthase (NOS3-786T>C) genetic polymorphism in chronic heart failure: effects of mutant -786C allele on long-term mortality. Acta Cardiol Sin. 2017;33:420–428. doi: 10.6515/ACS20161215B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enomoto K, Yamabe H, Toyama K, et al. Improvement effect on endothelial function in patients with congestive heart failure treated with cardiac resynchronization therapy. J Cardiol. 2011;58:69–73. doi: 10.1016/j.jjcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32:400–411. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okutucu S, Aytemir K, Evranos B, et al. Cardiac resynchronization therapy improves exercise heart rate recovery in patients with heart failure. Europace. 2011;13:526–532. doi: 10.1093/europace/euq410. [DOI] [PubMed] [Google Scholar]
- 9.Aksoy H, Okutucu S, Kaya EB, et al. Clinical and echocardiographic correlates of improvement in left ventricular diastolic function after cardiac resynchronization therapy. Europace. 2010;12:1256–1261. doi: 10.1093/europace/euq150. [DOI] [PubMed] [Google Scholar]
- 10.Aksoy H, Okutucu S, Aytemir K, et al. Improvement in right ventricular systolic function after cardiac resynchronization therapy correlates with left ventricular reverse remodeling. Pacing Clin Electrophysiol. 2011;34:200–207. doi: 10.1111/j.1540-8159.2010.02919.x. [DOI] [PubMed] [Google Scholar]
- 11.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 12.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 13.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp. 2010;(44):2167. doi: 10.3791/2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulsmann M, Berger R, Sturm B, et al. Prediction of outcome by neurohumoral activation, the six-minute walk test and the Minnesota Living with Heart Failure Questionnaire in an outpatient cohort with congestive heart failure. Eur Heart J. 2002;23:886–891. doi: 10.1053/euhj.2001.3115. [DOI] [PubMed] [Google Scholar]
- 16.White JA, Yee R, Yuan X, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 18.Hasan A. How should echocardiography be used in CRT optimization? J Am Soc Echocardiogr. 2010;23:867–871. doi: 10.1016/j.echo.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Okutucu S, Aytemir K, Oto A. Cardiac resynchronization therapy and arterial blood pressure: a bonus for hemodynamic improvement. Expert Rev Cardiovasc Ther. 2011;9:571–574. doi: 10.1586/erc.11.45. [DOI] [PubMed] [Google Scholar]
- 20.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 21.Katz SD, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 22.Woo JS, Jang WS, Kim HS, et al. Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron Artery Dis. 2014;25:421–426. doi: 10.1097/MCA.0000000000000094. [DOI] [PubMed] [Google Scholar]


