Abstract
Background:
Ataluren was approved for the treatment of nmDMD, both the efficacy and safety have been previously reported only from clinical trials but no report exists about real-life experience.
Patient/methods:
we describe three Italian children with nmDMD treated with ataluren for 1 year. Measurements were made every 3 months and was evaluated the 6-Minute Walking Distance (6MWD).
Results:
Case1 involves a patient with a 6MWD at T0 of 360 m, who started ataluren therapy at age 10 years. Case2 is a child who began treatment with ataluren at age 8 years when he had severe ambulatory compromise (6MWD < 75 m at T0). A third patient (case3) had a 6MWD of 320 m when he started ataluren therapy at age 5 years. The best improvement in 6MWD was observed in case3, a patient in whom treatment with ataluren was started much earlier. In case1, ataluren was started relatively late and 6MWD was maintained at a stable level. Surprisingly, we observed a 50% improvement in 6MWD in case2, a patient who began therapy early, but with a severe loss of lower limb muscle function at the time.
Conclusions:
treatment responses depend on the patient’s age and disease severity when therapy was initiated. On the basis of our experience, the main factor that influences the effectiveness seems to be earlier instigation of therapy and positive results may still be achieved in patients with more severe muscle involvement. Interestingly, these three boys with phenotypically different nmDMD provide useful information regarding future therapeutic recommendations for the ataluren administration in real clinical practice.
Keywords: ataluren, Duchenne muscular dystrophy, neuromuscular disease, nonsense mutation
Introduction
Duchenne muscular dystrophy (DMD) is a severe and rare X-linked genetic condition characterized by progressive muscle weakness and atrophy due to the absence of functioning dystrophin, and premature death due to cardiac or respiratory failure.1–3 In approximately 10–15% of cases, the condition is caused by a nonsense mutation (nmDMD) in the gene encoding dystrophin, resulting in a premature stop codon in mRNA which leads to the decay of the transcript and the production of a truncated peptide which is degraded and nonfunctional.4
Management of DMD has been limited to corticosteroid therapy, which slows the progression of dystrophinopathy, but can cause significant side effects. Furthermore, it does not treat the underlying cause of nmDMD.5 For the specific form of the disease caused by a nonsense mutation, an innovative therapeutic approach is available. Ataluren is a first-in-class drug which was approved by the European Medicines Agency in July 2014 for the treatment of nmDMD in ambulatory patients aged 5 years and older. It targets the underlying cause of nonsense mutation DMD by promoting readthrough of the premature stop codon in the mRNA, thereby increasing the production of full-length functional dystrophin.5–7
While both the efficacy and safety of ataluren in patients with nmDMD have been previously reported from clinical trials,8,9 it is also important to describe the real-life experience of patients with rare diseases such as nmDMD. Clinical findings with ataluren in patients with nmDMD in everyday clinical practice will help extend our knowledge regarding its effectiveness and safety/tolerability, as well as aspects related to functionality, patient/family satisfaction and overall well-being/quality of life.
We describe here findings from three Italian children with nmDMD treated with ataluren 40 mg/kg/day for 1 year. All evaluations were performed by the same physician, measurements were made every 3 months (T0, T1, T2, T3, T4) and the following outcomes were evaluated: 6- Minute Walking Distance (6MWD),10 and the timed function tests (TFTs), stand from supine, four-stair ascend, four-stair descend and 10 m run/walk. All patients’ legally authorized representative (parent or guardian) provided written informed consent for inclusion in the case series and publication of medical data. Case series do not require approval from the Ethics Committee of University of Naples, Federico II.
Cases
Details of three cases of first-born boys with nmDMD treated at the Neuromuscular center of University Federico II of Naples are presented. All three cases were confirmed in a similar manner. Once DMD was suspected, initial assessment involved multiplex ligation-dependent probe amplification genetic testing to search for deletions/duplications in the dystrophin gene. As no mutations were found, each patient underwent muscle biopsy, which showed a dystrophic pattern with reduction of dystrophin levels (Figure 1). Sanger sequencing was subsequently performed, and a nonsense mutation in the dystrophin gene was identified in each case (Table 1). Following molecular confirmation of diagnosis in these three children, the genetic investigation was extended to their mothers and all three were confirmed to be carriers. No other family members with DMD were identified for any of the three boys. During the course of follow up, none of the children exhibited cardiac or respiratory involvement. Periodic echocardiograms, electrocardiograms and spirometry check ups were all normal and each patient received regular sessions of physical therapy and psychomotor testing.
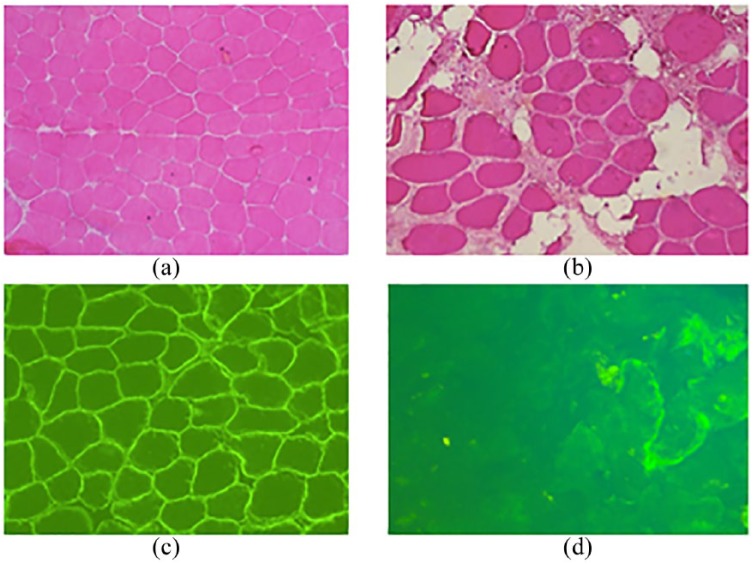
Figure 1.
Muscle biopsy findings in a normal subject compared with case 1.
(a), (b) HE of a normal subject (a) and case 1 (b) with dystrophic changes; (c) and (d) dystrophin immunohistochemistry normally expressed in (c) and reduced with few reverted fibers in muscle of case 1 (d).
HE, haemotoxylin–eosin staining.
Table 1.
Results for the 6-minute walk test and timed function tests in three patients with nonsense mutation Duchenne muscular dystrophy treated with ataluren 40 mg/kg/day over a period of 1 year.
| T0 | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|
|
Case 1 age at diagnosis:
5 years CPK ~14,000 IU/l; no steroids due to side effects; mutation: c.2077 T > C, exon 17; ataluren treatment started at 10 years of age | |||||
| 6-minute walk distance (m) | 360 | 303 | 375 | 400 | 370 |
| Timed 10 m run/walk (s) | 10 | 6.5 | 7.5 | 6.7 | 7.5 |
| Timed four-stair ascend (s) | 7.0 | 7.2 | 7.5 | 8.3 | 7.6 |
| Timed four-stair descend (s) | 4.5 | 6.1 | 5.2 | 5.5 | 5.5 |
| Timed stand from supine (s) | 35 | 26 | 25 | 16 | 44 |
|
Case 2 age at diagnosis:
5 years CPK > 10,000 IU/l; on steroids (deflazacort; 20 mg/d) since 5 years of age; mutation: c.3242C >A, exon 24; ataluren treatment started at 7 years of age | |||||
| 6-minute walk distance (m) | 64 | 100 | 101 | 119 | 118 |
| Timed 10 m run/walk (s) | 30 | 19 | 22 | 16 | 17 |
| Timed four-stair ascend (s) | NA* | NA* | NA* | NA* | NA* |
| Timed four-stair descend (s) | NA* | NA* | NA* | NA* | NA* |
| Timed stand from supine (s) | NA* | NA* | NA* | NA* | NA* |
|
Case 3 age at diagnosis:
4 years CPK >10,000 IU/l, no steroids (mother refused to start the treatment) mutation: c.7471C > T, exon 51; ataluren treatment started at 5 years of age | |||||
| 6-minute walk distance (m) | 320 | 330 | 355 | 409 | 400 |
| Timed 10 m run/walk (s) | 10 | 5.6 | 5.9 | 6.8 | 8.6 |
| Timed 4-stair ascend (s) | 14 | 10 | 11 | 15 | 14 |
| Timed 4-stair descend (s) | 9 | 9 | 10 | 9.7 | 10 |
| Timed stand from supine (s) | 4.5 | 5.6 | 4.2 | 5 | 7 |
Not able to perform test.
CPK, creatine phosphokinase; NA, not applicable.
Case 1: mutation: c.2077 T > C, p.Q693X exon 17 mutation
This patient is an 11-year-old male with nmDMD (c.2077T > C, exon 17). Past medical history was uneventful with normal milestones until the age of 5 years. The patient had his first neurological examination at the age of 5 years after elevated transaminase levels were detected in the absence of any liver function impairment. Subsequently, markedly raised creatine phosphokinase levels (>10,000 U/I) were recorded and nmDMD was confirmed as described above. The patient was immediately started on corticosteroids (deflazacort 15 mg/day). However, this treatment had to be stopped when the patient was 7-years old as a result of poor tolerability (agitation and sleep alterations). When the child reached the age of 10 years, the 6MWD ranged between 300 and 400 m, and treatment with ataluren was started at 40 mg/kg/day (Table 1). Surprisingly, considering the patient’s age and absence of steroid treatment, he was still able to walk 360 m at timepoint T0. His good performance on the 6-minute walk test (6MWT) may be explained by the presence of a faint reaction of dystrophin or revertant fibers in his muscle biopsy (Figure 1D).11 During the course of 12 months’ treatment, the patient’s disease remained stable with no marked worsening of motor performance; 6MWD remained between 300 and 400 m, and at the 12-month timepoint it was 370 m (Table 1). Other measures of functional performance such as the TFTs (10 m walk/run, timed four-stair ascent and descent, and the time to stand from the supine position) also confirmed stabilization of the disease with respect to motor performance during treatment with ataluren (Table 1).
Case 2: mutation: c.3242C > A, p.S1081X exon 24
This patient is an 8-year-old male with nmDMD (c.3242C > A, exon 24). At 5 years of age he presented with poor language ability and mild cognitive impairment; nmDMD was subsequently phenotypically and genotypically confirmed. Treatment with deflazacort (20 mg/day) was initiated almost immediately postdiagnosis. Ataluren therapy (40 mg/kg/day) was started when the boy was 7-years old and this motor impairment was already very severe (6MWD < 75 m). In fact, he presents a more severe clinical impairment when compared with natural history studies.12 The premature worsening of his motor performance was due to inadequate surgical correction of a contracted Achilles tendon. Unfortunately, in contrast with our opinion and guidelines’ recommendations, the patient was treated by an orthopedic surgeon at age 6 who lacked specific neuromuscular training. 5
Over the course of the 12-month ataluren treatment period, the 6MWD almost doubled and was ⩾100 m at all timepoints. The patient could also perform the 10 m walk/run much faster (30 s at baseline and 17.3 s after 12 months) (Table 1).
Case 3: mutation: c.7471 C > T, p.Glu2491X exon 51
This patient is currently a 6-year-old boy with nmDMD (c.7471C > T, exon 51). At 4 years of age, during the first neurological examination, he showed attention difficulties and waddling gait (mainly on the tips of the toes) with hyperlordosis. He also had a positive Gower’s sign and pseudohypertrophy of the quadriceps and gastrocnemius muscles with associated moderate proximal weakness. A few months later, the diagnosis of DMD was confirmed by genetic testing. At the age of 5 years, treatment with ataluren was initiated and mobility measures (6MWD, TFTs) remained the same or improved during the 12-month treatment period. The baseline 6MWD in this patient was 320 m and increased to 400 m after 12 months’ treatment with ataluren. The results for the TFTs are shown in Table 1.
For each of the three cases described above, the respective caregivers reported less fatigue and a reduction in the number of falls per month. Additionally, all three children achieved better school performance ratings, were more participative and spent more time involved in social and entertainment-based activities. Ataluren was generally well tolerated and no adverse events (AEs) reported.
Discussion
Ataluren is a first-in-class drug indicated for the treatment of nmDMD; therefore, it is important to collect information on its effectiveness and safety/tolerability in routine clinical practice. Unlike clinical trials where the population is divided in homogeneous groups for the correct interpretation of results, in clinical practice, the patients are heterogeneous and the observed results are different from those expected. Here, we report the clinical follow up of three Italian boys with nmDMD who were treated with ataluren for 1 year. The findings provide several points to reflect upon, given the wide phenotypic spectrum of the three cases. In particular, they started treatment at different ages with different degrees of muscle involvement, and they achieved individual responses to therapy with some interesting aspects to analyze.
The first important point to consider is that, in contrast with guidelines’ recommendations and our advice, only one of the patients continued corticosteroid therapy.5,13 For cases 1 and 3 the caregivers refused to continue glucocorticoids, as they had concerns regarding side effects. While this lack of compliance represents a therapeutic failure, it also demonstrates that there are still some problems regarding compliance and the urgent requirement to develop glucocorticoids with a more selective mechanism of action for a better balance of efficacy versus side effects.14
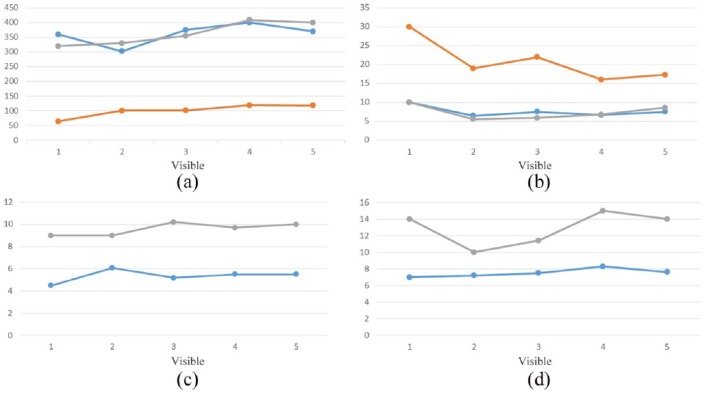
It must be emphasized that in 1 year’s treatment with ataluren, the disease did not significantly progress in these three patients who achieved an improved (cases 2 and 3) or stable (case 1) response from baseline evaluation at clinic based upon 6MWT results (Figure 2). Natural history studies have shown that in patients with DMD and a 6MWD of 370 m, a moderate to severe decline of 50–117 m, respectively, can be expected.12 The lack of disease progression in such a devastating, unrelentingly progressive disease as nmDMD, which was observed in the three cases described herein, should be considered an important therapeutic outcome. These findings are in line with the results of a recent study9 in which the group treated with ataluren, compared with placebo, had less of a reduction in 6MWT distances
Figure 2.
Timed function tests in three patients with nonsense mutation Duchenne muscular dystrophy treated with ataluren 40 mg/kg/day over a period of 1 year.
 Case 1
Case 1
 Case 2
Case 2
 Case 3
Case 3
(a) 6 MWD (m); (b) timed 10 m walk/run; (c) timed four-step descent; (d) timed four-step ascent.
6MWD, 6-minute walking distance.
We noted an improvement in walking distance in two of the three patients (cases 2 and 3). The hypothesis that improvement may have been due to the early initiation of ataluren treatment which helped to prevent muscle degeneration is supported by a muscle biopsy study in DMD patients which found that fibrotic tissue rapidly peaks (to about 30%) in children aged 6 to 7 years, and then slowly increases until the age of 10 years.15 This is a crucial time for patients with DMD, since the muscle tissue loses its ability to self-regenerate and declines toward fibrotic degeneration. This suggests that this age period may be critical for making treatment decisions regarding when to begin therapy for children with DMD. It may also suggest beginning treatment well before this age period, which may thus explain why we observed a better result in case 3, who started therapy early, (5 years) compared with case 1, who started therapy late (10 years). This is despite the fact that at the T0 visit, the 6MWD for case 1 was longer than it was for case 3. It is important to underscore that on the basis of natural history studies, the 5-year-old patient in case 3 could have a spontaneous improvement of disease at the age of 5 years;16 therefore, it is mandatory to continue a strict and longer follow up.
Case 2 presents some interesting information relating to a patient with severe mobility loss. At the start of ataluren treatment, the boy’s muscle impairment was already very severe (6MWD < 75 m) and we expected him to lose ambulation in a very short time. It was therefore a surprise that this patient achieved an approximate 50% improvement in motor performance. This observation highlights the fact that while the best results may be obtained when ataluren is administered at a younger age, positive results may still be achieved in patients with more severe muscle involvement. Our data therefore confirm the importance of an early diagnosis with gene sequencing, since starting treatment early with ataluren can help prevent muscle degeneration and thereby obtain a better therapeutic result in children with nmDMD. Of course, this observation was noted in a single case, and further study is needed in a larger group of patients with the similar characteristics.
From our experience in these three cases, we found that ataluren was relatively straightforward to administer with no compliance issues, and it was generally very well tolerated, with no AEs reported. Last but not least, all caregivers anecdotally reported an improvement in endurance and school performance in each of the three children and they were able to spend more time playing. There was an improvement in the quality of life of the three children and also their caregivers. Given that studies on quality of life of caregivers and ataluren-treated children are scarce, we feel that this is an important area for future research.
In conclusion, this is the first report of ataluren administration in real clinical practice, and on the basis of our limited experience, the main factor influencing the effectiveness of treatment seems to be the earlier instigation of therapy. This aspect has yet to be emphasized in clinical trials.
Acknowledgments
The authors thank Dr Steve Clissold (Content Ed Net) and Leonardi Efthimia for editorial assistance that was funded by PTC Therapeutics (Switzerland).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest statement: Editorial assistance for this manuscript was funded by PTC Therapeutics (Switzerland).
Contributor Information
Lucia Ruggiero, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Via Sergio Pansini, 5, 80131 Naples, Italy.
Rosa Iodice, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
Marcello Esposito, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
Raffaele Dubbioso, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
Stefano Tozza, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
Floriana Vitale, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
Lucio Santoro, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
Fiore Manganelli, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University Federico II of Naples, Naples, Italy.
References
- 1. Bushby K, Finkel R, Birnkrant DJ, et al. ; on behalf of the DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010; 9: 77–93. [DOI] [PubMed] [Google Scholar]
- 2. Pichavant C, Aartsma-Rus A, Clemens PR, et al. Current status of pharmaceutical and genetic therapeutic approaches to treat DMD. Mol Ther 2011; 19: 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. J Paediatr Child Health 2015; 51: 759–764. [DOI] [PubMed] [Google Scholar]
- 4. Dent KM, Dunn DM, von Niederhausern AC, et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet A 2005; 134: 295–298. [DOI] [PubMed] [Google Scholar]
- 5. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018; 17: 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007; 447: 87–91. [DOI] [PubMed] [Google Scholar]
- 7. Haas M, Vlcek V, Balabanov P, et al. European Medicines Agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul Disord 2015; 25: 5–13. [DOI] [PubMed] [Google Scholar]
- 8. Bushby K, Finkel R, Wong B, et al. ; for the PTC124-GD-007-DMD study group. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 2014; 50: 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald C, Campbell C, Torriccelli ER, et al. Ataluren in patients with nonsense Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 1489–1498. [DOI] [PubMed] [Google Scholar]
- 10. McDonald C, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve 2013; 48: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fanin M, Danieli GA, Cadaldini M, et al. Dystrophin-positive fibers in Duchenne dystrophy: origin and correlation to clinical course. Muscle Nerve 1995; 18:1115–1120. [DOI] [PubMed] [Google Scholar]
- 12. Mercuri E, Signorovotch JE, Swallow E, et al. ; DMD Italian Group and Trajectory Analysis Project. Categorizing natural histories of ambulatory function measured by the 6-minute walk distance in patients with Duchenne muscular dystrophy. Neuromuscular Disord 2016; 26: 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle Nerve 2007; 36: 424–435. [DOI] [PubMed] [Google Scholar]
- 14. Hoffman EP, Reeves E, Damsker J, et al. Novel approaches to corticosteroid treatment in Duchenne muscular dystrophy. Phys Med Rehabil Clin N Am 2012; 23: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peverelli L, Testolin S, Villa L, et al. Histologic muscular history in steroid-treated and untreated patients with Duchenne dystrophy. Neurology 2015; 85: 1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Angelini C, Pegoraro E, Perini F, et al. A trial with a new steroid in Duchenne muscular dystrophy. In: Angelini C, Danieli GA, Fontanari D. (eds) Muscular dystrophy research: from molecular diagnosis toward therapy. Amsterdam: Excerpta Medica, 1991, pp.173–179. [Google Scholar]