Abstract
To date, it is largely unknown which light settings define the optimum to steer alertness and cognitive control during regular daytime working hours. In the current article, we used a multimeasure approach combined with a relatively large sample size (N = 60) and a large range of intensity levels (20-2000 lux at eye level) to investigate the dose-dependent relationship between light and correlates of alertness and executive control during regular working hours in the morning and afternoon. Each participant was exposed to a single-intensity light level for 1 h after a 30-min baseline phase (100 lux at the eye) in the morning and afternoon (on separate days) during their daily routine. Results revealed no clear dose-dependent relationships between 1-h daytime light exposure and correlates of alertness or executive control. Subjective correlates showed only very modest linear relationships with the log-transformed illuminance, and we found no significant effects of light intensity on the behavioral and physiological indicators. Overall, these results suggest that daytime exposure to more intense light, at least for 1 h of exposure, may not systematically benefit alertness or executive functioning. However, future research is required to investigate effects of longer exposure durations and potential moderations by prior light exposure, personal characteristics, and spectrum.
Keywords: dose-response curve, light, alertness, executive control, daytime
Introduction
To date, multiple studies have investigated diurnal acute non–image forming (NIF) effects of light intensity on alertness (e.g., Badia et al., 1991; Daurat et al., 1993; Huiberts et al., 2015, 2016; Phipps-Nelson et al., 2003; Rüger et al., 2006; Vandewalle et al., 2006), complementing a large body of research on the nocturnal effects of light on alertness (e.g., Cajochen et al., 2000; Dijk et al., 1991; Chellappa et al., 2013; Correa et al., 2016; Figueiro et al., 2007; Lavoie et al., 2002; Lockley et al., 2006; Myers and Badia, 1993). While there are indications that light may induce acute alertness-enhancing effects during daytime, the current findings on subjective and objective indicators of alertness are inconclusive, as some studies reported beneficial effects while others reported no significant light-induced modulations in alertness (for reviews, see Lok et al., 2018a [this issue]; Souman et al., 2017). It should be noted that the study paradigms as well as the number of participants varied substantially between studies, which may—at least partly—explain the mixed results. In addition, most studies investigated a limited set of lighting conditions to test the acute NIF effect of exposure to more intense light on daytime alertness (Huiberts et al., 2015, 2016; Phipps-Nelson et al., 2003; Rüger et al., 2006; Smolders et al., 2012; Smolders and de Kort, 2014; Vandewalle et al., 2006), generally comparing 2 or 3 light intensity levels. Because of the substantial differences in intensity levels, measurement protocols, and experimental power between studies and a restricted number of lighting conditions employed within one study paradigm, it is difficult to determine to what extent and under which conditions diurnal exposure to more intense light induces acute alerting effects and which settings define the optimum. Investigating the daytime effects of a more varied range of intensities on both subjective and objective markers of alertness within one study paradigm among a relatively large sample is therefore crucial to gain additional insights into persons’ (potentially nonlinear) relative responsiveness to different intensity levels. This has been done for nighttime exposure: Cajochen and colleagues (2000) established dose-response relationships for subjective alertness, incidence of slow eye movements, and EEG power density (5- to 9-Hz range) as a function of illuminance for a 6.5-h light pulse at night (Cajochen et al., 2000). Moreover, a dose-response relationship for the alerting potential of light intensity has been established based on 8 previously published studies that investigated the effects of light intensity on subjective alertness at night or during the daytime using different study paradigms (Hommes and Giménez, 2015). Both methods revealed a good fit for a sigmoidal relationship between light intensity and alertness. However, it is unknown whether this dose-dependent relationship between subjective alertness and light intensity also holds for daytime, under natural conditions. Moreover, it is unknown whether a similar relationship for behavioral markers of alertness and executive control exists, as the occurrence, direction, and onset of light-induced modulations in daytime alertness and executive functioning may depend on the marker or type of indicator employed (Huiberts et al., 2015, 2016; Smolders et al., 2012; Smolders and de Kort, 2014; see also Lok et al., 2018a [this issue]). In addition, earlier research has provided indications for time-of-day–dependent modulations in responsiveness to light between morning and afternoon exposure (Huiberts et al., 2015, 2016, 2017; Smolders et al., 2012, 2013).
Given the fact that many persons nowadays spend most of their time indoors, knowledge on the impact of light on alertness and executive functioning—in addition to potential effects on sleep and health (see, e.g., Boyce, 2010; Chellappa et al., 2011; Figueiro et al., 2017)—is crucial to steer a healthy light regime to optimally support daytime functioning among, for instance, students and office workers. To this end, we aim to investigate the dose-response relationship for subjective, behavioral, and physiological correlates of alertness and executive functioning as a function of light intensity during daytime working hours in the morning and afternoon.
Materials and Methods
Design
A mixed design was employed, with illuminance manipulated between subjects and the timing of the lighting condition (morning vs. afternoon) manipulated within subjects. In total, there were 20 illuminances (divided equidistantly based on a logarithmic scale), ranging from 20 to 2000 lux (at the eye) at a corrected color temperature (CCT) of 4000 K. Participants were randomly assigned and exposed to one of the experimental levels for 1 h after an initial baseline exposure of 100 lux for 30 min. Both subjective (self-reports) and objective (performance and physiological) measures for alertness and executive control were employed once during the baseline and multiple times during the experimental lighting condition. Individual participants were exposed to the same illuminance in the morning (starting at 9:00 am or 11:00 am) and afternoon sessions (starting at 1:00 pm or 3:00 pm), which took place on 2 separate visits to the lab (with at least 2 days in between sessions).1 The experiment was initially performed in the spring and subsequently repeated during the winter months. The experimental sessions in the spring were conducted from May 17 to June 9, 2016, and in the winter, sessions were conducted from January 20 to February 20, 2017.
Participants
Thirty-eight Dutch-speaking subjects (14 men, 24 women; mean age = 21 years, SD = 3.75; range = 18-38 years) participated in the study in the spring. Twenty-two Dutch-speaking subjects (5 men, 17 women; mean age = 23 years, SD = 5.32; range = 18-43 years) participated in the study in the winter. We thus had 3 participants per illuminance on average. Healthy participants without hearing deficit, eye disease, and motoric impairments were recruited via the J.F. Schouten School for User-System Interaction Research database from the Eindhoven University of Technology. All participants gave their written informed consent and were compensated for their participation.
Setting and Apparatus
The experiment was conducted in a laboratory at the Eindhoven University of Technology. In the laboratory, 2 cabinets were created with a wall-mounted light panel (Philips Strato luminaire, TPH710; 1.2 m × 1.2 m) positioned in the gazing direction of the participants. The dimensions of the cabinets were 1.2 m × 2.5 m. A small desk (55 cm × 40 cm) with a chair, laptop (in spring: Dell Latitude D630, and in winter: Dell Latitude E6500), mouse, headphones, and physiological measurement device was placed in each cabinet at a 55-cm distance from the wall-mounted luminaire. Each luminaire contained 6 fluorescent tubes of 28W, of which 3 tubes were 2700K (TL5-28W/827) and 3 tubes were 6500K (TL5-28W/865) and had a translucent cover. With the described setup, the light intensity could be set between 100 and 2000 lux at 4000K. To achieve light intensities below 100 lux, a neutral-density filter was used. The filter used was a Rosco E-Colour+#211:.9 Neutral Density (transmission = 13%). A spectral power density diagram of the light at 100 lux and 4000K (Suppl. Fig. S1.1) is in the supplementary materials as are the photometric values, including alpha-opic lux levels (Lucas et al., 2014), at the eye level of the 20 lighting conditions (Suppl. Table S1.1). It should be noted that all these measurements were performed with the display of the laptop on.
Procedure
All participants completed the Munich Chronotype Questionnaire (MCTQ; Roenneberg et al., 2003) before the start of the first session. At least 3 days before the start of the first experimental session, participants picked up an Actiwatch, a printed sleep diary, and a wearable light sensor. Participants were instructed to wear the Actiwatch and adhere to their regular sleep-wake pattern for the 3 nights prior to the start of the experimental session. Moreover, they were instructed to wear the wearable light measurement device on the day of the experimental session from sleep offset until the start of the experimental procedure. After this 3-day protocol, participants came to the laboratory for the first 90-min session (which took place either in the morning or afternoon).
At the start of the session, participants received information about the procedure of the experiment and attached the sensors for the physiological measurements according to written instructions. They put on headphones, read instructions on the laptop explaining the 3 performance tasks, and started with the trial version for each of the tasks. Subsequently, the actual experiment started. Participants engaged in auditory performance tasks and completed short questionnaires in 5 repeated measurement blocks. Each measurement block consisted of a 5-min Psychomotor Vigilance Task (PVT), followed by a 5-min Go-NoGo task, a 4-min 2-Back task, and a short questionnaire probing alertness, vitality, tension, and mood. Moreover, autonomic nervous activity (heart rate [HR] and skin conductance level [SCL]) was monitored continuously during these blocks. The first block (block 0) was administered during the baseline phase (100 lux at eye level), and the other 4 blocks (blocks 1-4) were administered during the lighting manipulation phase. At the end of the experimental session, participants completed some additional questionnaires concerning their behavior prior to the session (e.g., food consumption, travel time outdoors) and lighting appraisals. See Table 1 for a schematic overview of the experimental procedure. After the first session was completed, the second session was confirmed with the subjects. The procedure of the second experimental session was similar to the first session, except that a few additional questionnaires were included at the end of the second session. After completing those questionnaires, subjects were thanked for their participation, debriefed, and compensated for their participation. The experiment was approved by the HTI Ethical Review Board at the Eindhoven University of Technology, the Netherlands.
Table 1.
Overview of experimental procedure of one experimental session.
| Instructions, Practice, and Baseline Phase |
Experimental Light Exposure Phase |
|||||
|---|---|---|---|---|---|---|
| 100 lx at Eye | Exposure to Single Illuminance in Range of 20-2000 lx at Eye | |||||
| Instructions, and task practice | Block 0: measurements of (correlates of) alertness and executive functioning | Block 1: measurements of (correlates of) alertness and executive functioning | Block 2: measurements of (correlates of) alertness and executive functioning | Block 3: measurements of (correlates of) alertness and executive functioning | Block 4: measurements of (correlates of) alertness and executive functioning | Quest. |
| 10 min | 15 min | 15 min | 15 min | 15 min | 15 min | 5 min |
Measures
As dependent variables, we included both subjective and objective indicators related to alertness (Karolinska Sleepiness Scale [KSS], PVT, HR) and to executive control (vitality, Go-NoGo, 2-Back, SCL). In addition, we logged sleep-wake timing and light exposure prior to the session and probed mood and participants’ lighting appraisals during the experimental sessions.
Actigraphy and Wearable Light Sensor
Sleep-wake timing was measured by means of actigraphy and self-reports. The Actiwatch Spectrum Pro was worn at the wrist and measured participants’ physical activity, sleep timing, and light exposure at wrist. The paper-and-pencil sleep diary consisted of 6 questions probing self-reported sleep onset, sleep offset, sleep duration, sleep latency, sleep inertia, and number of awakenings. A wearable light sensor (Lightlog) was worn on participants’ clothes, close to their chin, and used to quantify the light history of the participant in the vertical plane close to their eyes during the waking episode prior to the start of the experimental session.
Performance Indicators
Three performance tasks were administered for collecting objective indicators of alertness (PVT) and executive functioning (Go-NoGo task and 2-Back task). A 5-min auditory PVT was employed as a neurobehavioral measure of alertness and sustained attention (Dinges and Powell, 1985). During this task, participants had to press the spacebar as fast as possible when they heard a beep (400 Hz). Each beep was generated with a random interstimulus interval between 1 and 9 s. Participants’ reaction speed (1/mean reaction time in seconds) was used as marker for performance on this task.
A 5-min auditory Go-NoGo task was administered to measure inhibitory capacity. During this task, 2 different beeps were presented to the user: target beeps (400 Hz; same beeps as used in PVT) and nontarget beeps (600 Hz). Participants had to press the spacebar when they heard the target beep but inhibit their response when they heard the nontarget beep. The different beeps were generated randomly with a random interstimulus interval between 1 and 9 s, and targets were presented in 50% of the trials. Reaction speed to targets (1/mean response time in seconds) and percentage correct were used as performance indicators for this executive functioning task.
A 4-min auditory 2-Back task (Mackworth, 1959) was used to measure participants’ working memory capacity. Participants were presented with a sequence of 1-syllable consonants spoken in Dutch and had to press the spacebar as fast as possible when the letter they heard at that moment was the same as the letter they heard 2 positions back. The letters were presented at a frequency of 1 Hz. Inverted mean response time to targets (1/s) and percentage correct were used as markers for speed and accuracy, respectively, on this task.
The mean reaction times for these 3 performance tasks were computed based on the raw reaction times excluding outliers for each block per participant. In this computation, values more than 3 standard deviations from the participant’s mean for a specific block were considered as outliers. The average percentage of outliers was estimated marginal means (EMM) = 1.9% (SE = 0.1) for the PVT, EMM = 1.5% (SE = 0.3) for the Go-NoGo task, and EMM = 2.1% (SE = 0.2) for the 2-Back task.
Physiological Indicators
Electrocardiography (ECG) and electrodermal activity were measured continuously during the baseline and experimental lighting phase using TMSi software to investigate participants’ level of autonomic nervous activity. For the ECG measurements, 3 Kendall H124SG ECG electrodes were applied using the lead II placement. The average HR (in beats per minute [bpm]) during the PVT was used as marker for HR and computed based on the RR intervals in the raw ECG data with MATLAB R2015b. An auxiliary sensor was used to measure SCL. The sensor consists of 2 electrodes that were attached to the participants’ soft part of the first phalanx of the middle finger and the ring finger. The average SCL (in µSiemens) during the PVT in each block was computed by means of MATLAB R2015b.
Subjective Indicators
The KSS (Åkerstedt and Gillberg, 1990) was used to measure subjective sleepiness. State vitality was assessed with 4 items (“energetic,” “depleted” [reversed], “alert,” and “sleepy” [reversed]) on a 5-point rating scale ranging from 1 = not at all to 5 = very much. In addition to these self-reported correlates of alertness and executive control, participants reported on their affective state in terms of tension (2 items; “tense” and “calm”), positive affect (1 item; “happy”), and negative affect (1 item; “sad”) on similar rating scales.
Participants were also asked to evaluate the lighting in the room by means of six 5-point bipolar rating scales (unpleasant/pleasant, uncomfortable/comfortable, warm/cold, not disturbing/disturbing, dim/bright, and calming/activating) adopted from Smolders et al. (2012).
After each session, participants completed a questionnaire probing caffeine intake, food intake, and time spent outside. Furthermore, participants completed a questionnaire on trait vitality (Trait Vitality Scale; Ryan and Frederick, 1997), self-reported light sensitivity (Smolders et al., 2012), and general beliefs on the potential effects of light after the second session. Questions about participants’ general beliefs consisted of 6 self-formulated items probing how much they thought light influenced their cognitive and affective state: level of alertness, vitality, performance, concentration problems, mood, and motivation.
Statistical Analyses
First, preparatory analyses were performed to test whether participants adhered to their regular sleep-wake pattern (as assessed with the MCTQ) and to investigate whether there were significant differences in sleep timing, sleep duration, sleep latency, and sleep inertia prior to the spring versus winter sessions and prior to the morning versus afternoon laboratory sessions. Moreover, we explored potential differences in confounding variables related to participants’ behavior prior to the experimental sessions (i.e., caffeine consumption, food intake, and traveling time outdoors) as well as potential baseline differences in the dependent measures between the 2 seasons and between the morning and afternoon laboratory sessions.
To determine the dose-response relationship between light intensity and markers of alertness and executive functioning, a curve-fitting procedure was performed for each dependent variable. In addition, similar analyses were performed for the various indicators of mood and appraisals. For each variable, 2 functions were investigated: a linear relationship and a 4-parameter logistic model. In these analyses, the log-transformed illuminance was used as an independent variable. Detailed information on preparatory and subsequent analyses is reported in the supplementary materials (statistical analyses).
Results
Results of the preparatory analyses are reported in the supplementary materials
Effect of Light Intensity, Time of Day, Season, and Measurement Block
Linear mixed model (LMM) analyses were performed to fit a linear relationship between light intensity (log transformed) and markers of alertness and executive functioning (difference from baseline scores) and to test the effects of the timing of the light exposure (morning vs. afternoon), season (spring vs. winter), and block on the various markers. The EMMs for all indicators of alertness and executive functioning—in the morning and afternoon sessions and for the spring and winter sample— are displayed in Table 2. As can be seen, speed on the PVT and Go-NoGo task significantly decreased compared with baseline, across all light intensity levels, regardless of the timing of the experimental sessions and the season in which the data collection took place. In contrast, speed on the 2-Back task was only slower compared with baseline in the morning sessions in spring. Changes in accuracy on the Go-NoGo task and 2-Back task compared with baseline did not significantly differ from zero, except for an increase in accuracy compared with baseline on the Go-NoGo task in the afternoon sessions in the winter. The self-report measures revealed a significant increase in sleepiness and decrease in vitality compared with baseline in the spring but no significant changes compared with baseline in the winter sample (again, averaged over all light intensity levels). HR significantly decreased compared with baseline, except for the HR measured in the morning sessions in spring. SCL was significantly higher during the experimental phase than in the baseline, except for the morning sessions in the winter.
Table 2.
Difference scores (estimated marginal means) of behavioral, subjective, and physiological indicators for morning and afternoon experimental sessions in spring and winter.
| Spring |
Winter |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Morning |
Afternoon |
Morning |
Afternoon |
||||||
| Variable | EMM | SE | EMM | SE | EMM | SE | EMM | SE | |
| Behavioral indicators | PVT | −0.45 | 0.04 | −0.46 | 0.04 | −0.43 | 0.06 | −0.40 | 0.06 |
| Go-NoGo task, speed | −0.44 | 0.13 | −0.61 | 0.14 | −0.77 | 0.17 | −0.79 | 0.17 | |
| Go-NoGo task, accuracy | 0.01 | 0.01 | −0.01 | 0.01 | −0.01 | 0.01 | 0.03 | 0.01 | |
| 2-Back task, speed | −0.03 | 0.01 | −0.02 | 0.01 | −0.03 | 0.01 | −0.02 | 0.01 | |
| 2-Back task, accuracy | −0.01 | 0.01 | <0.01 | 0.02 | −0.02 | 0.02 | −0.02 | 0.02 | |
| Subjective indicators | Sleepiness | 0.89 | 0.25 | 0.78 | 0.26 | 0.29 | 0.33 | 0.19 | 0.33 |
| Vitality | −0.32 | 0.10 | −0.43 | 0.11 | −0.22 | 0.14 | −0.14 | 0.14 | |
| Physiological indicators | HR | −0.95 | 0.64 | −1.72 | 0.67 | −2.92 | 0.86 | −2.84 | 0.84 |
| SCL | 1.42 | 0.37 | 1.37 | 0.39 | 0.14 | 0.54 | 1.63 | 0.48 | |
EMM = estimated marginal means; SE = standard error; PVT = Psychomotor Vigilance Task; HR = heart rate; SCL = skin conductance level. Difference scores significantly different from zero are displayed in bold.
Results of the LMM analyses revealed no significant linear relationship between light intensity (in log lux) and speed or accuracy on any of the cognitive performance tasks (all p > 0.05). The PVT and Go-NoGo tasks showed a significant effect of block on speed, suggesting a decrease in speed with time in session (PVT: B = −0.07; F1,341 = 57.35, p < 0.01, R2 = 0.02; Go-NoGo task: B = −0.13; F1,343 = 9.22, p < 0.01, R2 = 0.01). Time of day had a significant main effect on Go-NoGo accuracy (F1,357 = 4.21, p = 0.04, R2 = 0.002). This effect was moderated by season (F1,357 = 9.99, p < 0.01, R2 = 0.02), suggesting that —as indicated above— accuracy significantly differed from baseline only in the afternoon sessions in the winter, with an increase in the percentage correct (p < 0.01; see Table 2).
Self-reported sleepiness showed a significant linear relationship with log-transformed illuminance, suggesting a larger decrease in sleepiness compared with baseline with increasing light intensity (B = −0.63, F1,60 = 5.02, p = 0.03, R2 = 0.04). In line with these results, self-reported vitality significantly increased with increasing intensity (in log lux; B = 0.32, F1,59 = 7.80, p < 0.01, R2 = 0.05). Both variables also showed a significant main effect of block with decreasing sleepiness (B = −0.09, F1,355 = 4.05, p = 0.04, R2 = 0.001) and increasing vitality (B = 0.06, F1,357 = 4.07, p = 0.04, R2 = 0.003) with time in session. The effect of light intensity on sleepiness and vitality remained when adding the 2-way interactions of light intensity with season, time of day, and block, while the main effect of block disappeared. The 2-way interactions of light intensity with season, time of day, and block were not significant (all p > 0.05).
HR and SCL did not show a significant linear relationship with illuminance (in log lux; both F < 1, ns). There was only a significant main effect of block on HR, revealing decreased HR with time in session (B = −0.41, F1,332 = 41.92, p < 0.01, R2 = 0.004). For SCL, there was only a significant block × time of day interaction, suggesting a stronger increase in SCL with time in session in the afternoon sessions (B = 0.30, F1,288 = 8.14, p < 0.01, R2 = 0.003).
Inspection of the difference scores for mood revealed that positive affect significantly decreased compared with baseline in the morning and afternoon in spring (EMMMorning = −0.30, SE = 0.10; EMMAfternoon = −0.34, SE = 0.10) as well as in the morning in the winter (EMMMorning = −0.37, SE = 0.13) but not in the afternoon sessions in the winter (EMMAfternoon = −0.05, SE = 0.13). Difference scores for self-reported tension and negative affect were not significantly different from zero (all p > 0.05). Self-reported tension, positive affect, and negative affect revealed no significant linear relationship with illuminance (in log lux) nor significant main or interaction effects of time of day, season, or block (all p > 0.05). Results on the lighting appraisals revealed a significant linear relationship between the log-transformed illuminance and the brightness as well as the degree to which the lighting was experienced as activating, with higher ratings when participants were exposed to more intense light (B = 1.20, F1,60 = 31.63, p < 0.01, R2 = 0.42; B = 1.02, F1,60 = 25.41, p < 0.01, R2 = 0.30, respectively). In addition, the color of the lighting was experienced as colder when exposed to more intense light (B = 0.78, F1,59 = 16.63, p < 0.01, R2 = 0.20). Effects of light intensity on brightness and the degree to which the lighting was experienced as activating were not significantly moderated by time of day or season (both p > 0.05). In contrast, the effect of light intensity on the experienced color was moderated by time of day (B = 0.49, F1,59 = 4.57, p = 0.04, R2 = 0.02), suggesting a higher correlational strength in the afternoon than in the morning sessions. Season and time of day had no significant effects on the appraisals (p > 0.05), except for a significant time of day effect on perceived color (B = −1.33, F1,59 = 6.06, p = 0.02, R2 = 0.03), which appeared to be moderated by light intensity.
Four-Parameter Logistic Model Fitting
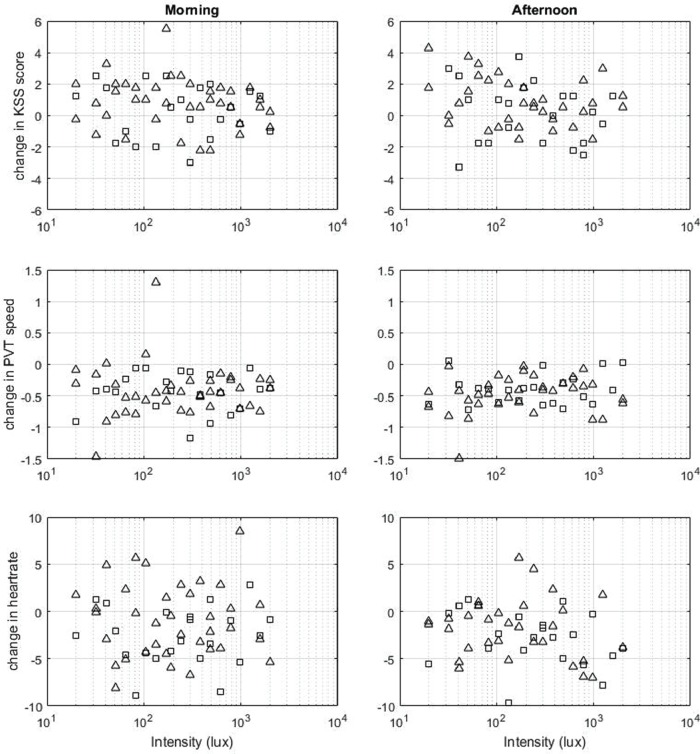
We fitted a 4-parameter logistic model, similar to the model constructed by Cajochen et al. (2000) and used by Hommes and Giménez (2015): f(x) = d + (a − d)/(1 + (x/b)^c) (see the supplementary materials for additional details on the statistical analyses). Inspection of the 95% confidence intervals for the parameters of the sigmoidal function for the various performance, self-report, and physiological correlates of alertness and executive functioning showed that, for none of the models, the parameters for the Hill’s slope of the curve (parameter c) nor the inflection point (parameter b) seemed to differ from zero (see Tables 3 and 4). All confidence intervals for these parameters include zero and show generally large uncertainty of the parameter estimates. This was confirmed by the low goodness-of-fit values of the models, with R2 ranging from <0.01 to 0.13 in the morning and from <0.01 to 0.28 in the afternoon. Moreover, confidence intervals for parameters a and d always overlapped, except for subjective vitality and SCL in the afternoon. Figure 1 displays the raw data for the various markers of alertness in the morning and afternoon for the spring and winter sample separately, which confirms that there is no clear sigmoidal relationship for the various correlates as function of light intensity.
Table 3.
Parameter estimates with confidence intervals for the 4-parameter logistic models of behavioral, self-report, and physiological correlates of alertness and executive functioning and goodness of fit based on data of the morning sessions.
| Variable | a | b | c | d | R 2 | |
|---|---|---|---|---|---|---|
| Behavioral indicators | PVT | −0.51 (−0.72, −0.31) | 1.91 (−495, −498) | 270 (−9.83 × 107, 9.83 × 107) | −0.40 (−0.52, −0.28) | 0.02 |
| Go-NoGo task, speed | 0.06 (−0.76, 0.88) | 1.52 (−18.81, 21.85) | 158 (−2.22 × 105, 2.23 × 105) | −0.61 (−0.81, −0.41) | 0.07 | |
| Go-NoGo task, accuracy | 0.14 (−1.14 × 105, 1.14 × 105) | 0.57 (−1.23 × 106, 1.23 × 106) | 0.05 (−3.82 × 104, 3.82 × 104) | −0.12 (−1.01 × 105, 1.01 × 105) | <0.01 | |
| 2-Back task, speed | 0.03 (−0.24, 0.29) | 1.96 (−0.20, 4.12) | 5.36 (−29.55, 40.28) | −0.06 (−0.21, 0.09) | 0.05 | |
| 2-Back task, accuracy | −0.25 (−3316, 3316) | 1.24 (−1.73 × 104, 1.73 × 104) | 0.21 (−2676, 2677) | 0.21 (−2671, 2671) | <0.01 | |
| Subjective indicators | Sleepiness | 0.96 (0.36, 1.56) | 2.38 (−1955, 1960) | 409 (−1.56 × 109, 1.56 × 109) | 0.24 (−0.39, 0.88) | 0.05 |
| Vitality | −0.43 (−0.67, −0.18) | 2.36 (−1.57, 6.29) | 174 (−3.38 × 104, 3.41 × 104) | −0.13 (−0.37, 0.10) | 0.05 | |
| Physiological indicators | HR | 0.53 (−2.81, 3.86) | 1.65 (−1.35, 4.65) | 171 (−1.34 × 104, 1.38 × 104) | −1.82 (−2.99, −0.65) | 0.04 |
| SCL | 0.94 (−0.12, 2.00) | 3.11 (−1040, 1046) | 475 (−5.14 × 107, 5.14 × 107) | 5.31 (1.06, 9.56) | 0.13 |
PVT = Psychomotor Vigilance Task; HR = heart rate; SCL = skin conductance level. All values correspond to difference scores compared with baseline. Parameters b and c are printed in bold if significantly different from 0; parameters a and d are printed in bold if their 95% confidence intervals do not overlap.
Table 4.
Parameter estimates with confidence intervals for the 4-parameter logistic models of behavioral, self-report, and physiological correlates of alertness and executive functioning and goodness of fit based on data of the afternoon sessions.
| Variable | a | b | c | d | R 2 | |
|---|---|---|---|---|---|---|
| Behavioral indicators | PVT | −0.58 (−0.74, −0.41) | 1.82 (−55.91, 59.53) | 233 (−8.44 × 106, 8.44 × 106) | −0.40 (−0.49, −0.31) | 0.07 |
| Go-NoGo task, speed | −0.88 (−1.27, −0.48) | 2.17 (−109, 113) | 375 (−8.00 × 105, 8.00 × 105) | −0.53 (−0.86, −0.19) | 0.04 | |
| Go-NoGo task, accuracy | −0.25 (−7574, 7573) | 0.66 (−4.18 × 104, 4.18 × 104) | 0.12 (−3298, 3298) | 0.23 (−5748, 5749) | <0.01 | |
| 2-Back task, speed | −0.59 (−1984, 1983) | 0.70 (−4596, 4597) | 0.22 (−661, 661) | 0.45 (−1219, 1220) | 0.03 | |
| 2-Back task, accuracy | −0.83 (−533, 531) | 0.18 (−274, 275) | 0.58 (−152, 153) | 0.18 (−32.11, 32.48) | 0.07 | |
| Subjective indicators | Sleepiness | 7.51 (−252, 267) | 0.97 (−16.30, 18.23) | 3.07 (−28.59, 34.73) | −0.10 (−4.35, 4.15) | 0.10 |
| Vitality | −0.60 (−0.85, −0.35) | 2.40 (−86.38, 91.18) | 316 (−1.64 × 106, 1.64 × 106) | 0.02 (−0.27, 0.32) | 0.18 | |
| Physiological indicators | HR | −1.27 (−2.67, 0.13) | 2.74 (−618, 623) | 619 (−7.52 × 106, 7.52 × 106) | −4.27 (−6.79, −1.75) | 0.09 |
| SCL | 7.04 (4.33, 9.74) | 1.49 (−25.43, 28.41) | 136 (−2.72 × 105, 2.72 × 105) | 0.85 (0.17, 1.54) | 0.28 |
PVT = Psychomotor Vigilance Task; HR = heart rate; SCL = skin conductance level. All values correspond to difference scores compared with baseline. Parameters b and c are printed in bold if significantly different from 0; parameters a and d are printed in bold if their 95% confidence intervals do not overlap.
Figure 1.
Scatter plots spring (triangles) and winter (squares) data displayed in morning and afternoon sessions. Scores represent changes compared with the corresponding baseline score.
The 95% confidence intervals of the 4-parameter logistic models for the indicators of mood and light appraisals similarly revealed that parameters a and d for none of the estimates differed from each other, except that the lighting was experienced as colder in terms of color and more activating with increasing intensity (see Suppl. Tables S2.3 and S2.4). For none of the models was the Hill’s slope different from zero. The point of inflection did differ from zero for experienced pleasantness and in the afternoon for perceived color and brightness (see Suppl. Tables S2.3 and S2.4), but this is quite irrelevant in view of the estimates of a, c, and d for these indicators.
Discussion
The current laboratory study aimed to determine the dose-response relationship between illuminance and correlates of alertness and executive functioning during regular daytime working hours. To this end, we investigated, for the first time, diurnal NIF effects of light intensity on alertness and executive functioning using a large range of illuminances. Moreover, we employed a multimeasure approach among a relatively large sample to establish to what extent various markers for alertness and executive functioning varied in a dose-dependent manner.
No Clear Dose-Dependent Relationships for Alertness and Executive Control
Results of this study revealed no clear dose-response relationships between illuminance and markers of alertness. In contrast to earlier established dose-response curves for nighttime (Cajochen et al., 2000) or for daytime and nighttime studies combined (Hommes and Giménez, 2015), self-reported alertness showed no clear sigmoidal relationship as a function of light intensity in the morning or afternoon. In line with this finding, the behavioral and physiological markers for alertness (PVT and HR, respectively) also showed no clear dose-response curve. In addition to a sigmoidal function, we also explored linear relationships. Results of these explorative analyses revealed only a significant linear relationship for self-reported sleepiness as a function of the log-transformed illuminance. Based on the goodness of fit of this relationship, however, the relational strength appeared extremely modest. Although these findings deviate substantially from earlier dose-response curves for alertness that were wholly, or at least partly, based on nighttime exposure (Cajochen et al., 2000; Hommes and Giménez, 2015), the results are largely in line with the study by Lok et al. (2018b [this issue]), which was performed in parallel to the current study and revealed no significant dose-dependent effect of illuminance on diverse correlates of alertness.
Two main differences between the current studies (ours and the study by Lok et al., 2018b [this issue]) and the study by Cajochen and colleagues (2000) are the timing and the duration of the light exposure. Whereas we investigated the effect of 1 h of exposure during daytime working hours, the participants in the study by Cajochen et al. (2000) were exposed to the light for 6.5 h in the early biological night. Circadian and homeostatic sleep pressure are generally high at night, whereas circulating melatonin levels are negligible and sleep pressure is generally relatively low during the biological day. Earlier research has shown that the NIF effect of light may depend on the circadian and homeostatic phase (Vandewalle et al., 2011) and revealed differences in responsiveness to bright light between daytime and nighttime exposure but mainly for physiological measures of autonomic nervous activity (Rüger et al., 2006). A very recent systematic literature review (Souman et al., 2017) including day- and nighttime studies revealed no clear indications for a moderation in the alerting potential of light (assessed with the KSS and PVT) by the timing of the light exposure. In fact, the number of studies reporting an effect of light on subjective reports was not higher at night than during daytime, and very few indications were found for a robust effect on vigilance. Nevertheless, as our study investigated only effects of diurnal exposure, we must limit our reflections to daytime and be open to the idea that exposure to more intense light during the day may not always benefit alertness.
In addition to the differences in timing of the onset of the light manipulation, the duration of light exposure also differed substantially between the studies (1 h in the current study and the study by Lok et al., 2018b [this issue]), versus 6.5 h in the study by Cajochen et al., 2000). This leaves open the possibility that a longer exposure period would induce stronger alerting effects. It is, however, worth mentioning that other diurnal studies have found acute alerting effects of light within an hour of exposure (Huiberts et al., 2015, 2016, 2017; Kaida et al., 2006; Phipps-Nelson et al., 2003; Smolders et al., 2012; Smolders and de Kort, 2014; see also Souman et al., 2017). Moreover, in the analysis by Hommes and Giménez (2015), studies investigating the effects of light on subjective alertness employing exposure durations shorter than 6.5 h were also included. More precisely, they included studies assessing self-reported alertness (with the KSS) with exposure durations ranging from 1 h to 8.25 h of exposure. In addition, the recent literature review by Souman et al. (2017) suggested no clear moderations by exposure duration for those daytime nor nighttime studies that did reveal significant effects of light intensity on the KSS and/or PVT. In the current study, we have chosen to test potential alertness-enhancing effects of 1 h of exposure to the light manipulation to be able to determine time-of-day–dependent moderations in the dose-response relationship. As stated above, this duration has been shown to be sufficient to determine acute effects on subjective and objective measures and correlates of alertness. In contrast to Cajochen et al. (2000), we focused only on the acute effects of light on alertness and did not assess potential phase-shifting effects of the light manipulation. In fact, the experimental paradigm by Cajochen et al. (2000) was designed such that dose-response relationships for both acute and circadian effects could be determined. Results on the dose-response relationship between light intensity and the magnitude of the phase-shift for nighttime exposure, assessed in the same experiment as Cajochen et al. (2000), are reported in Zeitzer et al. (2000).
Another factor that differed between the current study paradigm and the experimental procedure employed in the study by Cajochen et al. (2000) is the prior light exposure (very dim compared with regular light levels in the current study). In the current study, the participants came to the laboratory during their daily routine, meaning they were not light deprived beforehand. In contrast, participants in the nighttime study were light deprived before the actual experiment as they were exposed to (~3 lux) prior to the light manipulation on the experimental day preceded by <150 lux during 3 baseline days and a subsequent ~50 h constant routine in dim light (<10 lux at eye level) in the laboratory, which possibly increased the alerting potential of light (e.g., Chang et al., 2011, 2013; Hébert et al., 2002; Jasser et al., 2006). To date, most studies have investigated the effect of prior light history on responsiveness to nocturnal light exposure, mainly in terms of melatonin suppression. Whether prior light exposure moderates the alerting potential of light during daytime is still largely unknown.
It is also worth mentioning that we used 100 lux at eye level during the 30-min baseline phase prior to the light manipulation, which corresponds to the inflection point in the study by Cajochen et al. (2000) and the analysis by Hommes and Giménez (2015). This may have already resulted in higher alertness and vitality prior to the start of the lighting manipulation and decreased the sensitivity to the light manipulation. Nevertheless, inspection of the baseline scores suggests that a ceiling effect is unlikely because the scores on, for instance, the KSS at the end of the baseline phase were relatively high for daytime assessment, indicating that participants felt rather sleepy prior to the light manipulation. In addition, the participants in the study by Lok et al. (2018b [this issue]) were exposed to levels below 10 lux at eye level prior to and in between the lighting manipulations provided at fixed times during the day, and their study revealed no significant dose-dependent effects of light during daytime. The current results, combined with the findings by Lok et al. (2018b [this issue]), suggest no clear dose-response relationship between light intensity and correlates of alertness during daytime, at least not for 1 h of exposure, regardless of pre-exposure lighting conditions.
In contrast to the nighttime study by Cajochen et al. (2000) and the analysis by Hommes and Giménez (2015), we also investigated the potential dose-dependent relationship between light intensity and correlates of executive control. In line with the current results on alertness, the correlates of executive control showed very minimal effects: no clear sigmoidal dose-response relationship between light intensity and self-reported (subjective vitality), behavioral (performance on inhibitory control and working memory tasks), or physiological (SCL) indicators for morning nor afternoon exposure. Subjective vitality showed only a significant linear relationship with the log-transformed illuminance, suggesting increased vitality under exposure to more intense light. The R2 value was again rather low, suggesting that only a modest portion of the variance in self-reported vitality could be explained by the intensity of the lighting. Nevertheless, the effect of light was stronger than moderations in subjective vitality as a function of time in session or timing of the light exposure.
As discussed in Lok et al. (2018a [this issue]), earlier findings on behavioral and physiological markers for executive functioning have revealed quite mixed results with positive, null, as well as negative effects of daytime exposure to bright versus dim light on executive control. Tuning of the light intensity may therefore be crucial to optimally support executive functioning, but it is still largely unclear to what extent and under which conditions light can benefit executive control. In the current study, we aimed to gain more knowledge on the optimal intensity level to steer executive control in terms of inhibitory capacity and working memory during individuals’ regular daily routine in the morning and afternoon. The current study was, at least to our knowledge, the first attempt to investigate the dose-response relationship for self-reported, behavioral, and physiological correlates of executive control, employing more than 3 light intensities. In line with the results on alertness, illuminance did not reveal clear dose-dependent relationships for the various measures, except for a linear trend for self-reported vitality. This result on subjective vitality, although modest, is in line with earlier correlational findings in the field, also suggesting increased vitality when exposed to more intense light during one’s daily routine (Smolders et al., 2013). The results on objective markers are in line with earlier studies reporting null effects on executive control but contrast with studies reporting beneficial or performance-undermining effects of exposure to bright light on executive control (for an overview of earlier findings, see Lok et al., 2018a [this issue]). It should be noted that in the current study, we tested only a linear and sigmoidal relationship for the various markers as a function of light intensity, while earlier studies have also proposed that the effects of light on cognitive tasks probing executive control may, in line with the Yerkes-Dodson Law, follow an inverted U-shape function. Yet the current evidence for such a parabolic relationship is still inconclusive (e.g., Huiberts et al., 2015, 2016; Veitch, 2001). Visual inspection of the data also revealed no indications for a parabolic function. We acknowledge that we used only a limited set of performance tasks probing executive control and did not manipulate the difficulty level within tasks. The results can therefore not be applied to other tasks probing different cognitive abilities and/or difficulty levels. In line with the results on alertness, the current findings on executive control also apply for only 1 h of light exposure during daytime, in the absence of sleep deprivation, and/or preexposure to very dim light.
For both the correlates of alertness and executive control, we investigated the potential moderating role of the timing of the onset of the light manipulation (morning vs. afternoon) and explored potential differences in responsiveness between seasons. The results showed no clear time-of-day– nor season-dependent differences in the responsiveness based on the parameter estimates and goodness of fit for the 4-parameter logistic models nor for the linear relationships of self-reported sleepiness and vitality. This is in contrast to some earlier studies reporting more pronounced effects of exposure to more intense light on self-reported sleepiness and vitality in the morning compared with the afternoon (Huiberts et al., 2015; Smolders et al., 2013) and in the winter compared with spring (Huiberts et al., 2017), but it is in line with the overall results in the current literature (Souman et al., 2017). While time of day and block were manipulated within subjects, different subjects participated in the spring and winter data collection phase. It is important to note that the number of participants (and therefore also the number of sessions and statistical power) in the winter was lower than in spring. Comparisons between seasons should therefore be considered with some caution.
Overall, the current results revealed mainly null effects of light intensity on the various correlates of alertness and executive control in the morning and afternoon. In fact, none of the objective indicators revealed a clear dose-dependent relationship as a function of the light intensity, and exposure to more intense light for 1 h during regular daytime working hours resulted in only subtle decreases in feelings of sleepiness and increases in feelings of vitality. As also indicated by Lok et al. (2018a [this issue]) and Souman et al. (2017), the inconsistent findings in the current literature with studies reporting significant as well as nonsignificant effects of exposure to bright versus dim light could (at least partly) be explained by rather low sample sizes and corresponding power in multiple studies investigating acute NIF effect of light on correlates of alertness and executive control. In the current study, we used, however, a relatively large sample (total N = 60) and repeated measurements within subjects for morning and afternoon exposure. It is therefore unlikely that we were not able to detect small to medium effect sizes. In fact, we were able to demonstrate rather subtle effects, as also reflected in the low R2 values for significant effects. Inspection of the regression coefficients showed that subjective sleepiness decreased, on average, about 1.3 scale points on a 9-point scale, and self-reported vitality increased, on average, about 0.6 scale points on a 5-point scale over a range of 20 lux to 2000 lux at eye level. Based on the current findings and the null results by Lok et al. (2018b [this issue]), it is questionable whether increasing the illuminance from 200 lux at the eye (which roughly corresponds with 500 lux at the desk [EN12464-1] and is in line with commonly experienced intensity levels during the day; Smolders et al., 2013) to, for instance, 2000 lux at the eye for 1 h during daytime hours would result in meaningful changes in alertness and executive control.
Exploration of Dose-Dependent Relationships for Mood and Appraisals
Exploration of participants’ mood and appraisals of the lighting revealed that mood was not significantly influenced by light in a dose-dependent manner. Participants rated the lighting, as expected, as brighter with increasing intensity levels. Moreover, they experienced the lighting as more activating when exposed to more intense light. Both appraisals showed an increase according to a linear function of the log-transformed illuminance. This linear increase in the extent to which the lighting was perceived as activating is in line with the results on alertness and vitality but showed a more pronounced relationship as reflected in higher R2 values. The increase in experienced level of activation of the lighting was, however, not reflected in an increased performance or autonomic nervous activity. The experienced color of the lighting was also significantly influenced by the intensity level, despite the fact that the CCT level was kept constant across the lighting conditions. While earlier studies reported that exposure to bright light may be experienced as less pleasant (Huiberts et al., 2015, 2016; Smolders et al., 2012; Smolders and de Kort, 2014), the current results revealed no significant dose-dependent modulation in perceived pleasantness of the lighting nor a clear transition point at which the lighting becomes less pleasant.
Potential Limitations
Similar to the study by Cajochen et al. (2000) and Lok et al. (2018b [this issue]), the lighting manipulation in the current study occurred between subjects. In contrast, many earlier studies comparing 2 or 3 light conditions on correlates of alertness and executive control used a within-subjects design (see Lok et al., 2018a [this issue]). The use of a between-subjects design may have increased variance in responsiveness to the lighting manipulation. In fact, visual inspection of the current data revealed substantial interindividual variation in the difference scores within lighting conditions. However, because of the low number of participants per lighting condition, we were not able to test whether there were structural patterns in responsiveness to the lighting manipulation between persons as a function of, for instance, their chronotype, age, or gender. Therefore, additional research would be needed employing a within-subjects design, combined with a relatively large number of lighting conditions, to determine whether effects are more pronounced when controlling for interindividual differences in responsiveness and to explore to what extent lighting requires personal tuning of the light intensity.
Another potential limitation of the current study is that we, similar to Cajochen et al. (2000) and Lok et al. (2018b [this issue]), tested the effects of illuminance for only 1 CCT level. While there are clear indications for the maximal sensitivity to light in terms of melatonin suppression in the blue part of the region (e.g., Brainard et al., 2001; Thapan et al., 2001), to date no spectral sensitivity curve has been established for other markers of alertness or for correlates of executive control. The analysis by Hommes and Giménez (2015) showed that the use of melanopsin activation instead of photopic light levels resulted in better fit to describe the dose-dependent relationship between light and subjective alertness. However, the current findings concerning the effect of CCT and monochromatic or narrowband light on self-report and behavioral indicators of alertness and executive control are still inconclusive (see, e.g., Smolders and de Kort, 2017; Souman et al., 2017). Whether spectral tuning aimed at providing stronger modulations in melanopsin activation increases the alerting potential of light as well as the relative contribution of the various photoreceptors in the effects of light on subjective, behavioral, and physiological NIF functioning, therefore, remains to be investigated.
Conclusion
The current study, combined with the findings of the study by Lok et al. (2018b [this issue]), indicate that the results for a sigmoidal dose-response relationship between light intensity and alertness as established for nighttime exposure by Cajochen et al. (2000) may not directly be applied to everyday daytime situations. Employing a large range of light levels and relatively large sample size, we were not able to establish clear dose-dependent relationships between 1-h daytime light exposure and correlates of alertness or executive control. In fact, results revealed only very modest linear relationships between the log-transformed illuminance and the subjective correlates and no significant effects of light intensity on the behavioral and physiological indicators. Overall, these results suggest that daytime exposure to more intense light at a fixed local time, at least for 1 h of exposure, may not systematically benefit alertness or executive functioning. Yet future research is required to investigate the effects of longer exposure durations and potential moderations by prior light exposure. Moreover, additional research is needed to quantify and model potential interindividual variations in responsiveness to light intensity and to test the alerting potential of light for different spectral compositions.
Supplemental Material
Supplemental material, supplementary_materials_-_dose-response_relationships_for_effects_of_white_light_exposure for Investigation of Dose-Response Relationships for Effects of White Light Exposure on Correlates of Alertness and Executive Control during Regular Daytime Working Hours by Karin C. H. J. Smolders, Samantha T. Peeters, Ingrid M. L. C. Vogels and Yvonne A. W. de Kort in Journal of Biological Rhythms
Acknowledgments
We would like to thank Stichting onderzoek licht en gezondheid (SOLG) for their financial support.
Supplemental material is available for this article online.
It should be noted that 2 subjects participated twice in the morning (once at 9:00 am and once at 11:00 am) in spring on 2 separate days because of some issues with the registration.
Footnotes
Conflict of Interest Statement: The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Åkerstedt T, Gillberg M. (1990) Subjective and objective sleepiness in the active individual. Int J Neurosci 52:29-37. [DOI] [PubMed] [Google Scholar]
- Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. (1991) Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav 50(3):583-588. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. (2001) Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21:6405-6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce P. (2010) Review: the impact of light in buildings on human health. Indoor Built Environ 19:8-20. [Google Scholar]
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. (2000) Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res 115:75-83. [DOI] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA. (2011) The human circadian system adapts to prior photic history. J Physiol (Lond) 589:1095-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA, Aeschbach D. (2013) Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep 36:1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Gordijn MC, Cajochen (2011) Can light make us bright? Effects of light on cognition and sleep. Prog Brain Res 190:119-133. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Oelhafen P, Lang D, Götz T, Krebs J, Cajochen C. (2013) Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res 22(5):573-580. [DOI] [PubMed] [Google Scholar]
- Correa A, Barba A, Padilla F. (2016) Light effects on behavioural performance depend on the individual state of vigilance. PLoS ONE 11(11):e0164945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daurat A, Aguirre A, Foret J, Gonnet P, Keromes A, Benoit O. (1993) Bright light affects alertness and performance rhythms during a 24-h constant routine. Physiol Behav 53(5):929-936. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Cajochen C, Borbély AA. (1991) Effect of a single 3-hour exposure to bright light on core body temperature and sleep in humans. Neurosci Lett 121:59-62. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Powell JW. (1985). Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods 17:652-655. [Google Scholar]
- Figueiro MG, Bullough JD, Bierman A, Fay CR, Rea MS. (2007). On light as an alerting stimulus at night. Acta Neurobiol Exp 67(2):171-178. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Nagare R, Price LLA. (2017) Non-visual effects of light: how to use light to promote circadian entrainment and elicit alertness. Lighting Res Technol 50:38-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. (2002) The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res 33:198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes V, Giménez MC. (2015) A revision of existing Karolinska Sleepiness Scale responses to light: a melanopic perspective. Chronobiol Int 32:750-756. [DOI] [PubMed] [Google Scholar]
- Huiberts LM, Smolders KCHJ, de Kort YAW. (2015) Shining light on memory: effects of bright light on working memory performance. Behav Brain Res 294:234-245. [DOI] [PubMed] [Google Scholar]
- Huiberts LM, Smolders KCHJ, de Kort YAW. (2016) Non-image forming effects of illuminance level: exploring parallel effects on physiological arousal and task performance. Physiol Behav 164:129-139. [DOI] [PubMed] [Google Scholar]
- Huiberts LM, Smolders KCHJ, de Kort YAW. (2017) Seasonal and time-of-day variations in acute non-image forming effects of illuminance level on performance, physiology, and subjective well-being. Chronobiol Int 34:827-847. [DOI] [PubMed] [Google Scholar]
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. (2006) Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms 21:394-404. [DOI] [PubMed] [Google Scholar]
- Kaida K, Takahashi M, Haratani T, Otsuka Y, Fukasawa K, Nakata A. (2006). Indoor exposure to natural bright light prevents afternoon sleepiness. Sleep 29(4):462-469. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Paquet J, Selmaoui B, Rufiange M, Dumont M. (2003) Vigilance levels during and after bright light exposure in the first half of the night. Chronobiol Int 20(6):1019-1038. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. (2006) Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 29:161-168. [PubMed] [Google Scholar]
- Lok R, Smolders KCHJ, Beersma DGM, de Kort YAW. (2018. a) Light, alertness, and alerting effects of white light: a literature overview. J Biol Rhythms 33(6):589-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok R, Woelders T, Gordijn MCM, Hut RA, Beersma DGM. (2018. b) White light during daytime does not improve alertness in well-rested individuals. J Biol Rhythms 33(6):637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, et al. (2014) Measuring and using light in the melanopsin age. Trends Neurosci 37:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackworth JF. (1959) Paced memorizing in a continuous task. J Exp Psychol 58:206. [DOI] [PubMed] [Google Scholar]
- Myers BL, Badia P. (1993). Immediate effects of different light intensities on body temperature and alertness. Physiol Behav 54(1):199-202. [DOI] [PubMed] [Google Scholar]
- Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. (2003) Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep 26:695-700. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18(1):80-90. [DOI] [PubMed] [Google Scholar]
- Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. (2006) Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol 290:R1413-R1420. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Frederick C. (1997) On energy, personality, and health: subjective vitality as a dynamic reflection of well-being. J Pers 65:529-565. [DOI] [PubMed] [Google Scholar]
- Smolders KCHJ, de Kort YAW. (2014) Bright light and mental fatigue: effects on alertness, vitality, performance and physiological arousal. J Environ Psychol 39:77-91. [Google Scholar]
- Smolders KCHJ, de Kort YAW. (2017) Investigating daytime effects of correlated colour temperature on experiences, performance, and arousal. J Environ Psychol 50:80-93. [Google Scholar]
- Smolders KCHJ, de Kort YAW, Cluitmans PJM. (2012) A higher illuminance induces alertness even during office hours: findings on subjective measures, task performance and heart rate measures. Physiol Behav 107:7-16. [DOI] [PubMed] [Google Scholar]
- Smolders KCHJ, de Kort YAW, van den Berg SM. (2013) Daytime light exposure and feelings of vitality: results of a field study during regular weekdays. J Environ Psychol 36:270-279. [Google Scholar]
- Souman JL, Tinga AM, te Pas SF, van Ee R, Vlaskamp BN. (2017) Acute alerting effects of light: a systematic literature review. Behav Brain Res 337:228-239. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. (2001) An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol (Lond) 535:261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle G, Archer SN, Wuillaume C, Balteau E, Degueldre C, Luxen A, Dijk DJ, Maquet P. (2011) Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J Biol Rhythms 26:249-259. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, Albouy G, Darsaud A, Desseilles M, Dang-Vu TT, et al. (2006) Daytime light exposure dynamically enhances brain responses. Curr Biol 16:1616-1621. [DOI] [PubMed] [Google Scholar]
- Veitch JA. (2001) Lighting quality contributions from biopsychological processes. J Illum Eng Soc 30:3-16. [Google Scholar]
- Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. (2000) Sensitivity of the human circadian peacemaker to nocturnal light, melatonin phase resetting and suppression. J Physiol 526(3):695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary_materials_-_dose-response_relationships_for_effects_of_white_light_exposure for Investigation of Dose-Response Relationships for Effects of White Light Exposure on Correlates of Alertness and Executive Control during Regular Daytime Working Hours by Karin C. H. J. Smolders, Samantha T. Peeters, Ingrid M. L. C. Vogels and Yvonne A. W. de Kort in Journal of Biological Rhythms