Abstract
Background:
Serous cystadenomas of pancreas are rare benign epithelial neoplasms, which predominantly occur in the pancreatic body and tail of elderly females. Majority of these tumors have microcystic appearance. Macrocystic and solid variants have also been described. A number of more aggressive cystic pancreatic lesions are included in the differential diagnosis. Distinction from such lesions is important for optimal management.
Objective:
Our aim was to study the clinical and histological features of serous cystadenomas which would be helpful in making their correct diagnosis and understanding their behavior.
Methods:
We reviewed 23 cases of serous cystadenomas diagnosed in our institution between January 2001 and June 2018.
Results:
Mean age at presentation was 53.43 years. Female to male ratio was 4.75:1. Over half (56.5%) of the cases were diagnosed incidentally. Abdominal pain was the most common symptom. Body and tail (either alone or in combination) were the most common locations. Tumor size ranged from 2 to 16 cm. Central scar was seen in 43.4% cases. Two cases were unilocular (macrocystic). Microscopically, all cases showed simple cuboidal to flattened epithelium with round, uniform nuclei, and glycogen-rich clear cytoplasm. Focal micropapillae formation was seen in eight cases (34.7%). Surgical resection was performed in 82.6% cases. Recurrence occurred in only one single case.
Conclusion:
Pancreatic serous cystadenomas are benign neoplasms with excellent prognosis. The tumors showed typical morphological features in all cases. Surgical resection was performed in the majority of cases in our study owing to lack of optimal and complete radiological workup pre-operatively and the concern for not missing and adequately treating pancreatic mucinous cystic neoplasms.
Keywords: Serous cystadenoma, microcystic, macrocystic, pancreas, central scar
Introduction
Serous cystadenomas (SCs) are rare but distinct benign neoplasms of pancreas. According to various published articles, they comprise 1%–2% of all pancreatic neoplasms and 10%–29% of pancreatic cystic neoplasms.1–3 SCs are more common in the elderly and show a female predominance.4 Abdominal pain and palpable abdominal mass are the most common presenting complaints, but a significant proportion is asymptomatic and is diagnosed as an incidental finding.5,6 SCs are associated with von Hippel–Lindau (VHL) disease and with many sporadic tumors.4,7,8 Majority of these lesions involve the body and/or tail of pancreas.3,9 Radiologically, typical cases have a polycystic (honeycomb) appearance with a central stellate scar seen in few cases.10
Grossly, majority of SCs are well circumscribed, and the cut surface exhibits numerous small cystic spaces filled with clear serous fluid (microcystic SCs). These cystic spaces are separated by thin fibrous septae, which coalesce to form the fibrous stellate scar. Of the other variants, the macrocystic/oligocystic variant is poorly demarcated and has fewer but larger cystic spaces. The solid variant appears as a well-circumscribed solid nodule lacking any grossly visible cystic spaces.1,4,11–13 All these macroscopic variants are histologically composed of characteristic flattened to cuboidal, glycogen-containing epithelial cells with round uniform nuclei. Intervening stroma shows a network of small vascular channels.1,3,4
SCs run a benign, indolent clinical course and have an excellent prognosis. They are slow growing, malignant progression is rare, and disease mortality is almost nil. Surgical excision is curative but is required only in minority of cases when tumors are large or causing significant (or related) symptoms or when preoperative diagnosis was uncertain in spite of extensive workup including computed tomography (CT) scan, magnetic resonance imaging (MRI), and endoscopic ultrasonography. It is also required in the exceptional cases where concern of malignancy exists. Large majority of these tumors may require conservative management only.14–16 Recurrence is seen rarely in locally aggressive tumors.5
The aim of our study was to describe the clinical and histopathological features of SCs of pancreas which would be helpful in making their correct diagnosis and understanding their behavior.
Materials and methods
We retrieved 23 cases of SC of pancreas from the surgical pathology database of the Section of Histopathology, Aga Khan University Hospital reported between January 2001 and June 2018 through “Integrated Laboratory Management System (ILMS)” software. Clinical information regarding age, sex, exact location in pancreas, presenting complaints, tumor size, and gross appearance was obtained from the surgical pathology reports. Hematoxylin and Eosin (H&E)-stained microscopic glass slides were reviewed by two authors (M.U.T. and N.U.D.) to assess histological features such as micropapillary architecture, epithelial cell morphology, and calcification and extension into peripancreatic adipose tissue. Follow-up information for nine patients treated at our institution was available from the patients’ records which were reviewed. These records contained findings of CT scans performed on each follow-up visit. Follow-up information for patients treated at other institutions was obtained from the patients verbally via telephonic conversation.
Results
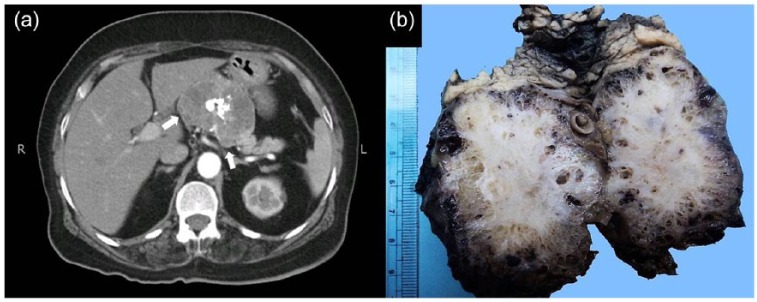
Ages of patients at the time of presentation ranged from 23 to 79 years with mean and standard deviation (SD) of 53.43 years ± 14.88 and 50 years. Of the 23 patients, 19 (82.6%) were females and 4 (17.4%) were males. The F:M ratio was median of 4.7:1. Symptoms related to pancreatic lesions were seen in 10 cases (43.5%) with abdominal pain being the most common (5 cases or 21.7%), followed by jaundice (3 or 13%). None of the 19 female patients in the study had any history of alcohol consumption and/or smoking. Clinical information regarding body mass index (BMI) was not available in any of the cases. Preoperative serum carcinoembryonic antigen (CEA) levels were available in two cases and were within normal limits. Serum CA 19-9 levels were available in three patients and were found to be elevated in two cases. In five cases (21.7%), the tumors were incidentally identified during routine radiological examination or radiological examination done for detection of non-pancreatic lesions (Table 1). Approximately, two-thirds were located in body and tail of pancreas. Surgical resection was performed in 19 of 23 cases (82.6%). Distal pancreatectomy with splenectomy was the commonest type of surgical resection performed (Table 1). Tumor size ranged from 2 to 16 cm in maximum dimension with median and mean size of 5.5 and 6.4 cm ± 4.6 SD, respectively. According to the preoperative radiological films, majority of these lesions presented as well-defined, hypodense, cystic masses, mainly involving the body and tail of the pancreas. In two cases, the lesions showed predominantly solid appearance. Cystic locules of variable sizes, internal septations, calcifications, and peripancreatic fat stranding were also observed in few cases (Figure 1(a)). However, convincing radiological evidence of infiltration into retroperitoneal fat was seen in only a single case. Grossly, the lesions in our study were well circumscribed with smooth or bosselated outer surfaces and cystic spaces of variable sizes filled with clear or straw colored serous fluid on cut surface. In few cases, cut surfaces showed solid areas without grossly visible lumina. A gray white, fibrotic, variably calcified central scar was seen in 10 cases (43.4%). The cystic spaces ranged from 1 mm to 3 cm in diameter, but the majority was smaller than 1 cm (Figure 1(b)). Of 23 cases, 2 (8.6%) were unilocular (Figure 2). Macroscopic extension into retroperitoneal fat was seen in a single case which had radiological evidence of retroperitoneal fat involvement.
Table 1.
Summary of clinical and histological features of serous cystadenoma of pancreas patients (n = 23).
| Tumor location | Frequency, n (%) |
|---|---|
| Body and tail of pancreas | 6 (26.1) |
| Body of pancreas only | 2 (8.6) |
| Tail of pancreas only | 5 (21.7) |
| Head of pancreas | 6 (26.1) |
| Uncinate process | 1 (4.3) |
| Site not mentioned | 3 (13.0) |
| Surgical procedure and type of biopsy | Frequency |
| Surgical resection | 18 (81.8) |
| Distal pancreatectomy | 13 (59) |
| Whipple’s procedure | 4 (17.4) |
| Enucleation | 1 (4.3) |
| Wedge/incisional biopsy | 4 (17.4) |
| Block received for second opinion | 1 (4.3) |
| Splenectomy | 11 (50) |
| Histological features | Frequency |
| Micropapillae formation | 8 (34.7) |
| Calcification | 7 (30.4) |
| Hemorrhage | 4 (17.4) |
| Lymphocytic infiltration | 4 (17.4) |
| Peripancreatic fat extension | 4 (17.4) |
| Entrapment of native pancreatic tissue | 8 (34.7) |
| List of other non-pancreatic lesions (5 cases) | |
| Ovarian thecoma | |
| Gastric gastrointestinal stromal tumor (GIST) | |
| Renal oncocytoma and uterine fibroid | |
| Hepatocellular carcinoma/liver cirrhosis | |
| Tuberculous lymphadenitis | |
Figure 1.
(a) CT scan abdomen showing a well-defined, solid, hypodense mass lesion in pancreatic body with central popcorn-like amorphous calcification. (b) Gross appearance of serous cystadenoma. Central fibrotic scar is evident along with multiple small-sized cystic spaces. Normal pancreatic tissue is present at the periphery.
Figure 2.
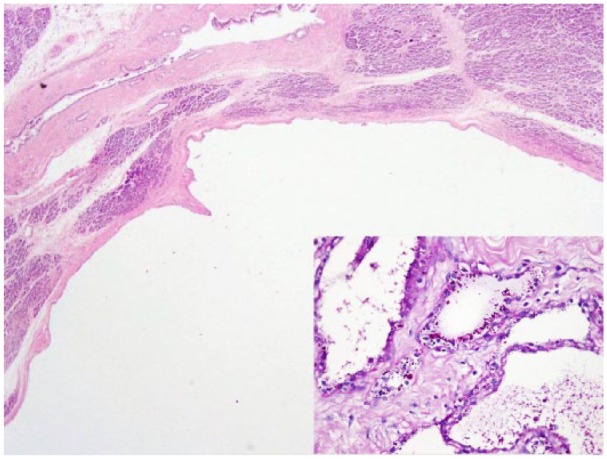
Low-power view of unilocular variant exhibiting a single large cystic locule lined by single layer of cells. Inset: PAS special stain highlighting intracytoplasmic glycogen granules.
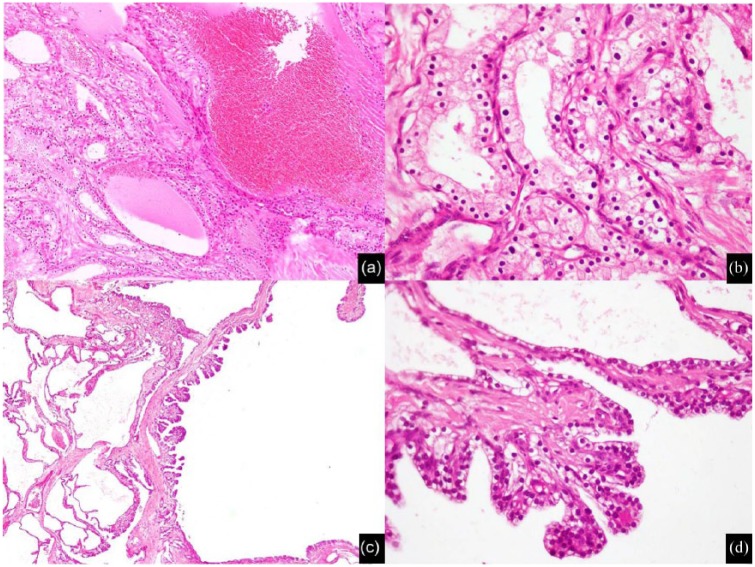
Microscopically, cystic spaces in all cases were lined by a single layer of flattened to cuboidal epithelial cells with moderate amount of clear to faintly eosinophilic cytoplasm. One case showed ciliated cuboidal epithelium while another showed decapitation secretions. Epithelial cell nuclei were round, uniform, and centrally placed (Figure 3(a) and (b)). Significant nuclear atypia or mitotic activity was not seen in any of the cases. Cytoplasmic glycogen granules were highlighted on periodic acid Schiff (PAS) stain and were washed out with diastase reaction (Figure 3, inset). In few cases, micropapillae formation was seen focally (Figures 3(c) and (d)). Intraluminal eosinophilic secretions were seen in some cases (Figure 4(a)). Since H&E staining and PAS with diastase stains were sufficient for establishing the diagnosis, immunohistochemical (IHC) stains were not performed. Central stellate scar, when present, was composed histologically of acellular fibrous tissue with variable degree of hyalinization and edematous change. Scattered foci of dystrophic calcification, hemorrhage, and collections of hemosiderin macrophages were also identified (Figure 4(b) and (c)). In addition to the case with radiological and macroscopic evidence of retroperitoneal fat involvement, focal extension into peripancreatic fat and entrapment of native pancreatic tissue was seen microscopically in four cases (17.4%) (Figure 4(d) and Table 1).
Figure 3.
Microscopic features of serous cystadenoma: (a) low-power view showing small cystic space lined by single layer of cuboidal cells, (b) high-power view showing cuboidal cells with moderate amount of lightly eosinophilc granular cytoplasm and centrally placed, rounded, uniform nuclei, (c) low-power view of micropapillae, and (d) high-power view of micropapillae.
Figure 4.
Microscopic features of serous cystadenoma: (a) eosinophilic secretions in cyst lumina, (b) central scar exhibiting hyalinization and calcification, (c) hemorrhage and hemosiderin-laden macrophages, and (d) normal pancreatic tissue entrapped within cyst walls/septae.
Follow-up was available in all 23 cases. Follow-up duration ranged from 4 to 183 months with mean follow-up of 77.8 months ± 62.2 SD and median of 58 months. None of the patients received adjuvant chemotherapy or radiotherapy. Only one patient (with tumor extension into retroperitoneal fat) developed local recurrence 4 years after initial surgery. None of the patients developed metastasis or died of disease. One patient died of liver disease (unrelated to pancreatic tumor) 1 year after the diagnosis of SC. Compared to 23 cases of SC, 11 cases of pancreatic mucinous cystic neoplasms (MCNs) and 2 cases of intraductal papillary mucinous neoplasm (IPMN) were diagnosed during the study period (January 2001–June 2018). During the same period, 115 cases of invasive pancreatic adenocarcinoma and 177 cases of ampullary adenocarcinoma were diagnosed and managed.
Discussion
A variety of cystic lesions can occur in the pancreas and range from benign entities such as pseudocysts and SCs to tumors with potential for aggressive behavior such as MCNs and IPMNs. Moreover, solid pancreatic tumors such as solid pseudopapillary tumor and invasive ductal adenocarcinoma can acquire a cystic appearance due to degenerative or necrotic changes.17 In 1978, SCs of pancreas were separated from pancreatic mucinous cystic neoplasms.18,19 Based on the IHC and ultrastructural findings, SCs are believed to be derived from intercalated duct cell or centroacinar cell lineage.20,21
SCs occur over a wide age range. Various published studies, have reported age ranges from 24 to 93 years with mean age at presentation in sixth and seventh decades.1,4 We also observed similar age distribution in our series, as majority (65.2%) of our patients were in their fifth to seventh decades when diagnosed. Females are three times more commonly affected than males.5 In our study also, marked female preponderance was noted. These tumors can present with abdominal pain, palpable mass, vomiting, nausea, weight loss, and so on. Jaundice is uncommon but can be seen in tumors involving the head of pancreas secondary to occlusion of common bile duct.1,5,20 However, almost half are detected incidentally during radiological examination performed for detection of possible non-pancreatic lesions.14 It is possible nowadays to accurately diagnose SCs by performing MRI or endoscopic ultrasound, and so on, without need of biopsy other invasive techniques.16 In our study, over 55% patients were asymptomatic and were diagnosed incidentally. Abdominal pain, jaundice, abdominal mass, nausea, and vomiting were the main symptoms noticed in our patients. Only two patients in our series (with tumors in pancreatic head) presented with jaundice. Tumor sizes in published studies have ranged from 1 to 25 cm with mean size of 6 cm.1 In our series also, wide range was seen in tumor size from as small as 2 cm to as large as 16 cm. Grossly, the usual microcystic forms appear as well-circumscribed lesions with smooth rounded or bosselated outer surfaces. Cut surfaces show numerous microcysts with irregular contours and diameters ranging from less than a millimeter to more than a centimeter.1 A fibrous stellate scar with or without calcification is seen in up to 30% cases and provides a useful diagnostic clue.3 In our study, central scar was seen in 43.4% tumors. One of the recent studies divided the microcystic form of SC into “microcystic, classical type” which have very few larger cystic locules (measuring in centimeters) and “microcystic with prominent macrocystic component” which have a prominent component of large cystic locules, although the usual microcystic pattern still predominates.4 The less-common macrocystic variant was described in older studies as “serous oligocystic ill-demarcated adenoma of pancreas.” Macrocystic variants are poorly demarcated and contain larger cystic locules, which measure more than a centimeter in diameter each and are typically less than 10 in number. In some cases, the lesion comprises of a single locule only.1,4,11,12 Such unilocular lesions occur more frequently in pancreatic head of elderly males.1,2 However, a recent study on a larger cohort of cases reported that this variant occurs in a younger age group as compared to microcystic form (50 vs 61 years) but shows similar female predominance and predilection for body and tail region.4 In our cohort, two patients had unilocular tumors. One of these patients was a 23-year-old female with tumor in pancreatic tail. The other patient was a 68-year-old male with tumor in the head of the pancreas. Solid variant of SC is rare, and so far, only 20 sporadic cases have been reported. It comprised only 2% of cases in a large study on SCs. It has no gender or site (head vs body and tail) predilection and usually occurs in the sixth and seventh decades of life. In most cases, tumor size is between 2 and 4 cm. Grossly, these are well-circumscribed, grayish brown nodules with shiny cut surfaces, and do not have a central scar. Microscopically, the solid variant of SC is composed of closely packed, small, uniform tubules, and acini with little or no lumina formation.4,22 Turcotte et al.23 reported 10 (18.2%) cases of solid microcystic SC in a cohort of 55 VHL patients with SC. A few cases in our study grossly showed solid areas, but this appearance was not predominant on the cut surface of the lesion and these tumors did not meet the criteria for “solid variant” of SC. Fine needle aspiration of these lesions does not provide a good yield of diagnostic material due to paucicellular nature of these lesions and cohesiveness of the epithelial cells.4,24
Pancreatic lesions are seen in 35%–70% cases of VHL disease.25 Majority of these lesions are asymptomatic simple cysts, which usually do not require excision. According to World Health Organization (WHO) classification of pancreatic serous neoplasms, “VHL-associated serous cystic neoplasms” are a group of lesions, which can appear in microcystic, macrocystic, and solid forms. In VHL disease patients, they can diffusely involve pancreas or occur as multiple lesions. Microscopically, they have simple cuboidal epithelial lining of glycogen-rich, clear cells.1,23 Apart from their association with pancreatic neuroendocrine tumors (PanNET) in VHL disease, they are also seen in patients with various other neoplastic and non-neoplastic pancreatic and non-pancreatic lesions.1,3,4 Most frequent molecular abnormalities observed in sporadic SC cases include allelic losses on 10q (50%) and 3p (40%). In addition, somatic inactivating mutations of VHL gene are observed in 22% of sporadic SCs.26 We encountered a number of non-pancreatic lesions in our group of patients (Table 1). Molecular testing for VHL disease was not performed in any of our cases, since it was not available in any laboratory in the country. Association of these neoplastic lesions with SCs has not been explained until now. However, in our opinion, frequent simultaneous occurrence of these lesions raises a possibility of their common association with some other syndrome.
In the majority of cases, the diagnosis is straightforward due to peculiar cytomorphology. However, these tumors may show heterogeneity in their histological features. We observed heterogeneous features including micropapillae formation, calcification, hemorrhage, lymphocytic infiltration, peripancreatic fat extension, and entrapment of native fat in a number of our cases. In cases with denuded epithelial lining, benign vascular lesions such as lymphangioma and hemangioma need to be considered in the differential diagnosis. The presence of smaller tubules with intact epithelium at the periphery provides a diagnostic clue. The presence of cytoplasmic glycogen granules and lack of cytoplasmic mucin rules out other pancreatic mucinous cystic neoplasms. Metastatic renal cell carcinoma (RCC) is another important differential diagnosis in cases where the tubules are smaller and closely packed. Clinical history and PAX-8 expression in RCC help in distinction between the two entities. PanNETs and clear-cell sugar tumors are other close differentials, especially in solid variant of SC. PanNETs occurring in patients with VHL disease exhibit clear-cell morphology, but expression for neuroendocrine markers helps in differentiation from pancreatic SCs.1 SCs demonstrate positive expression for epithelial membrane antigen (EMA), cytokeratins, α-inhibin, MUC6 and MUC1 stains, and negative expression for neuroendocrine markers.20 All cases in our study showed typical cuboidal to flattened epithelial cells with round, uniform nuclei, and clear, glycogen-rich cytoplasm. The epithelial lining can exhibit focal pseudopapillae formation. The central scar may show hyalinization, edema, hemorrhage, chronic inflammation, and calcification in variable proportions. We have reported the frequency and percentages of these features in our cases in detail (Table 1). Few studies have described these morphological features in such detail. Despite gross demarcation and circumscription, 4 (17.4%) tumors in our study showed focal extension into the peripancreatic fat on microscopic examination. However, none of these tumors had recurred at the time of last follow-up. The only case in our study which showed recurrence had radiological and macroscopic evidence of retroperitoneal fat extension. We feel that surgical excision with clear margins is sufficient to prevent recurrence.
Complete surgical resection is curative in all cases of pancreatic SC but is recommended only in a small minority of cases in which preoperative diagnosis remained uncertain in spite of extensive radiological workup or when tumors were large in size or were causing significant tumor-related symptoms. It is also recommended in the exceptional cases in which a concern of malignancy exists.16 However, in poor developing countries such as Pakistan and Afghanistan, radiological facilities for accurate non-invasive diagnosis of pancreatic neoplasms are suboptimal in most areas, and the importance of not missing and adequately treating mucinous pancreatic neoplasms, which have aggressive malignant potential, means that at least for the near future, surgical resection may continue to be performed for the majority of pancreatic cystic neoplasms regardless of whether they are serous or mucinous. In view of the postoperative morbidity and mortality associated with pancreatic surgeries, asymptomatic, and uncomplicated small tumors are managed conservatively. Surgical excision may be recommended for symptomatic and larger (>4 cm) tumors.14,16 Of the 23 patients in our study, 16 had tumors larger than 4 cm in diameter. In the remaining three cases in which resection was performed, the diameter was less than 4 cm. Since surgery in these three cases was performed outside our institution, exact indication for surgery is not known. Although majority of pancreatic SCs are benign, few are locally aggressive and sometimes extend into the peripancreatic tissues. These locally aggressive SCs have a greater potential for recurrence and therefore, warrant close follow-up.5 Most of our patients underwent surgical resection, and the exact type of surgical procedure was chosen on the basis of exact location of these tumors in the pancreas. Four patients only had incisional biopsy and the disease remained stable (until the time of last follow-up). One patient in our series with a pancreatic head tumor and radiological and gross extension into the retroperitoneal fat developed recurrence 4 years after initial resection of tumor.
Serous cystic neoplasms are slow-growing tumors with excellent prognosis. The incidence of malignancy was reported as 3% in a large study.27 According to the WHO classification, distant metastasis is the only criterion for malignancy.1 A recent large study by Reid et al.4 showed that cases of SC of pancreas with hepatic involvement actually represented synchronous and metachronous multifocal primary hepatic and pancreatic SCs rather than hepatic extension/metastasis from the pancreatic tumor. All patients in our study had a very good clinical course with not a single instance of metastasis or tumor-related death even several years after surgical removal.
It is absolutely essential to make an accurate diagnosis. Confusion with pancreatic ductal adenocarcinomas may be catastrophic for the patient. Ductal adenocarcinomas of the pancreas are highly aggressive tumors and the vast majority have already spread beyond the pancreas at the time of diagnosis. They are fatal in almost all cases with mean survival times ranging from 3 to 5 months in untreated patients and 10–20 months after surgical resection. The tumors are surgically resectable at the time of diagnosis in only 10%–20% cases. Current newly developed neoadjuvant treatments are employed in unresectable cases. However, even adjuvant chemotherapy with gemcitabine or 5-fluorouracil prolongs survival times very slightly. On the other hand, SCs have excellent prognosis and can often be managed conservatively and as discussed above requires surgical resection in only selected cases. Neoadjuvant treatments are definitely not required, and in case of incorrect diagnosis, patients may be subjected to unnecessary, even harmful surgery (potentially significant postoperative morbidity and mortality especially in elder patients) and totally unnecessary getting harmful neoadjuvant chemotherapy. The importance of correct diagnosis and the need to distinguish SC from pancreatic adenocarcinomas cannot be overemphasized.1,28–33
Conclusion
SCs of pancreas are benign pancreatic neoplasms with distinct clinical, radiological, and histological features in the majority of cases. Distinction from more aggressive pancreatic cystic lesions is important and can be done by radiological and, in some cases, by histologic evaluation of adequate biopsy material. Clinical course is excellent. Recurrence is uncommon and tumor-related deaths are almost nil. Large majority of cases require only conservative initial management, and surgical resection is required only in a minority of cases. However, in poor developing countries such as ours, surgical resection may still be performed in the majority of cases owing to lack of optimum radiological services hindering non-invasive diagnosis of these tumors and the importance of ensuring that mucinous pancreatic neoplasms owing to their aggressive potential are not missed and are treated adequately.
Acknowledgments
M.U.T., N.U.D., and Z.A. performed the histological and immunohistochemical evaluation. M.U.T. did literature review and drafted the manuscript; J.A.-G. provided intellectual input for designing the study and analysis. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Since this was a retrospective observational study and did not involve actual patients, patients’ images, or videos, it was granted exemption from requiring ethics approval from Ethics Review Committee (ERC) of Aga Khan University Hospital (4518-Pat-ERC-16).
ORCID iD: Muhammad Usman Tariq  https://orcid.org/0000-0003-2963-9871
https://orcid.org/0000-0003-2963-9871
References
- 1. Terris B, Fukushima N, Hruban RH. Serous neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al. (eds) WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press, 2010, pp. 296–299. [Google Scholar]
- 2. Hruban R, Pitman M, Klimstra D. Serous cystic neoplasms. In: Silverberg S, Sobin L. (eds) AFIP atlas of tumor pathology: tumors of the pancreas (4th series). Washington, DC: ARP Press, 2007, pp. 33–50. [Google Scholar]
- 3. Colonna J, Plaza JA, Frankel WL, et al. Serous cystadenoma of the pancreas: clinical and pathological features in 33 patients. Pancreatology 2008; 8(2): 135–141. [DOI] [PubMed] [Google Scholar]
- 4. Reid MD, Choi HJ, Memis B, et al. Serous neoplasms of the pancreas: a clinicopathologic analysis of 193 cases and review with new insights on macrocystic and solid variants and critical reappraisal of so-called “serous cystadenocarcinoma.” Am J Surg Pathol 2015; 39(12): 1597–1610. [DOI] [PubMed] [Google Scholar]
- 5. Galanis C, Zamani A, Cameron JL, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg 2007; 11(7): 820–826. [DOI] [PubMed] [Google Scholar]
- 6. Yoon WJ, Lee JK, Lee KH, et al. Cystic neoplasms of the exocrine pancreas: an update of a nationwide survey in Korea. Pancreas 2008; 37(3): 254–258. [DOI] [PubMed] [Google Scholar]
- 7. Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg 2012; 16(7): 1422–1428. [DOI] [PubMed] [Google Scholar]
- 8. Blandamura S, Parenti A, Famengo B, et al. Three cases of pancreatic serous cystadenoma and endocrine tumour. J Clin Pathol 2007; 60(3): 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 2004; 445(2): 168–178. [DOI] [PubMed] [Google Scholar]
- 10. Choi JY, Kim MJ, Lee JY, et al. Typical and atypical presentations of serous cystadenoma of pancreas: imaging findings with pathologic correlation. Am J Roentgenol 2009; 193(1): 136–142. [DOI] [PubMed] [Google Scholar]
- 11. Egawa N, Maillet B, Schroder S, et al. Serous oligocystic and ill demarcated adenoma of the pancreas: a variant of serous cystic adenoma. Virchows Arch 1994; 424(1): 13–17. [DOI] [PubMed] [Google Scholar]
- 12. Chatelain D, Hammel P, O’Toole D, et al. Macrocystic form of serous pancreatic cystadenoma. Am J Gastroenterol 2002; 97(10): 2566–2571. [DOI] [PubMed] [Google Scholar]
- 13. Perez-Ordonez B, Naseem A, Lieberman PH, et al. Solid serous adenoma of the pancreas. The solid variant of serous cystadenoma? Am J Surg Pathol 1996; 20(11): 1401–1405. [DOI] [PubMed] [Google Scholar]
- 14. Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg 2005; 242(3): 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol 2015; 6(4): 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016; 65(2): 305–312. [DOI] [PubMed] [Google Scholar]
- 17. Adsay NV. Cystic lesions of the pancreas. Mod Pathol 2007; 20: S71–S93. [DOI] [PubMed] [Google Scholar]
- 18. Hodgkinson DJ, ReMine WH, Weiland LH. Pancreatic cystadenoma. A clinicopathologic study of 45 cases. Arch Surg 1978; 113(4): 512–519. [DOI] [PubMed] [Google Scholar]
- 19. Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol 1978; 69(3): 289–298. [DOI] [PubMed] [Google Scholar]
- 20. Kosmahl M, Wagner J, Peters K, et al. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol 2004; 28(3): 339–346. [DOI] [PubMed] [Google Scholar]
- 21. Alpert LC, Truong LD, Bossart MI, et al. Microcystic adenoma (serous cystadenoma) of the pancreas. A study of 14 cases with immunohistochemical and electron-microscopic correlation. Am J Surg Pathol 1988; 12(4): 251–263. [DOI] [PubMed] [Google Scholar]
- 22. Katsourakis A, Dimitriou I, Noussios G, et al. Solid serous adenoma of the pancreas: a case report and review of the literature. Case Rep Surg 2016; 2016: 3730249 DOI: 10.1155/2016/3730249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turcotte S, Turkbey B, Barak S, et al. von Hippel-Lindau disease-associated solid microcystic serous adenomas masquerading as pancreatic neuroendocrine neoplasms. Surgery 2012; 152(6): 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belsley NA, Pitman MB, Lauwers GY, et al. Serous cystadenoma of the pancreas: limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2008; 114(2): 102–110. [DOI] [PubMed] [Google Scholar]
- 25. Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet 2003; 361(9374): 2059–2067. [DOI] [PubMed] [Google Scholar]
- 26. Moore PS, Zamboni G, Brighenti A, et al. Molecular characterization of pancreatic serous microcystic adenomas: evidence for a tumor suppressor gene on chromosome 10q. Am J Pathol 2001; 158(1): 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strobel O, Z’graggen K, Schmitz-Winnenthal FH, et al. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion 2003; 68(1): 24–33. [DOI] [PubMed] [Google Scholar]
- 28. Jemal A, Siegel R, Ward E, et al. Cancer statistics 2009. CA Cancer J Clin 2009; 59(4): 225–249. [DOI] [PubMed] [Google Scholar]
- 29. Conlon KC, Klimstra DS, Brennan MF. Long term survival after curative resection for pancreatic ductal adenocarcinoma. clinicopathologic analysis of 5-year survivors. Ann Surg 1996; 223(3): 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer 2004; 40(4): 549–558. [DOI] [PubMed] [Google Scholar]
- 31. Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 1999; 189(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 32. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350(12): 1200–1210. [DOI] [PubMed] [Google Scholar]
- 33. Octtle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007; 297(3): 267–277. [DOI] [PubMed] [Google Scholar]