FLOWERING LOCUS T3 (FT3) promotes spikelet formation but not floral development under long- and short-day conditions in barley.
Abstract
In many angiosperm plants, FLOWERING LOCUS T (FT)-like genes have duplicated and functionally diverged to control different reproductive traits or stages. Barley (Hordeum vulgare) carries several FT-like genes, the functions of which are not well understood. We characterized the role of HvFT3 in the vegetative and reproductive development of barley. Overexpression of HvFT3 accelerated the initiation of spikelet primordia and the early reproductive development of spring barley independently of the photoperiod. However, HvFT3 overexpression did not accelerate floral development, and inflorescences aborted under short days, suggesting that HvFT3 controls spikelet initiation but not floral development. Analysis of a nonfunctional HvFT3 allele supported the specific effects of this gene on spikelet initiation independent of the photoperiod. HvFT3 caused the up-regulation of the winter and spring alleles of the vernalization gene VERNALIZATION1 (VRN-H1) in nonvernalized plants and was therefore dominant over the repressive effects of the vernalization pathway. Global transcriptome analysis in developing main shoot apices of the transgenic lines showed that HvFT3 modified the expression of genes involved in hormone synthesis and response, of floral homeotic genes, and of barley row-type genes SIX-ROWED-SPIKE1 (VRS1), SIX-ROWED-SPIKE4 (VRS4), and INTERMEDIUM C. Understanding the specific functions of individual FT-like genes will allow modification of individual phases of preanthesis development and thereby adaptation to different environments and improved yield.
In most flowering plants, the transition from vegetative to reproductive development is triggered by a variety of environmental and endogenous cues (Boss et al., 2004; Michaels et al., 2005; Andrés and Coupland, 2012). The perception and integration of these cues ensure that floral transition is coordinated with favorable environmental conditions. In the model plant Arabidopsis (Arabidopsis thaliana), the photoperiod, vernalization, gibberellin, and the autonomous pathways have been defined as the main genetic pathways that regulate floral transition (Mouradov et al., 2002). The four pathways converge on a small set of genes known as the floral pathway integrator genes (Araki, 2001; Simpson and Dean, 2002). An important floral integrator gene is FLOWERING LOCUS T (FT), the product of which exhibits similarities to phosphatidylethanolamine-binding proteins and to Raf kinase inhibitor proteins (Kardailsky et al., 1999; Kobayashi et al., 1999). Under long day (LD) conditions, FT expression is induced in the leaves, and the FT protein moves to the shoot apical meristem (SAM) where it interacts with the bZIP transcription factor FLOWERING LOCUS D to activate floral meristem identity genes and the transition to flowering (Abe et al., 2005; Wigge et al., 2005; Corbesier et al., 2007).
The family of FT-like genes has expanded by gene duplications occurring independently in nearly all modern angiosperm lineages. The emergence of FT-like genes coincided with the evolution of flowering plants, and the potential of FT-like genes to promote floral transition seems conserved in angiosperms (Ballerini and Kramer, 2011). A few reports suggest that gene duplication was followed by diversification of FT functions within and between species. For example, in perennial poplar (Populus spp.), FT paralogs (FT1) and FLOWERING LOCUS T2 (FT2) have functionally diverged to coordinate the repeated cycles of vegetative and reproductive growth (Hsu et al., 2011). The basis for functional differentiation between FT-like genes appears to be controlled by expression pattern shifts, changes in proteins, and divergence in gene regulatory networks (Hsu et al., 2011). However, the role of different FT paralogs within most flowering species is as yet undescribed.
Genomes of cereal monocots are characterized by a large number of FT-like genes, i.e. wheat (Triticum aestivum) and barley (Hordeum vulgare) genomes each contain 12 FT paralogs (Halliwell et al., 2016), whereas 13 and 15 FT-like genes have been identified in rice (Oryza sativa) and maize (Zea mays), respectively (Chardon and Damerval, 2005; Danilevskaya et al., 2008). Functional diversification of FT-like genes has been demonstrated in rice, where HEADING DATE3a induces floral transition under inductive short day (SD) conditions, whereas its closest homolog, Rice FT1 (RFT1), acts as the floral activator under LD conditions (Kojima et al., 2002; Hayama et al., 2003; Komiya et al., 2008, 2009). In both wheat and barley, FT1 is the candidate gene for VERNALIZATION3 (VRN3), which promotes flowering under LDs (Yan et al., 2006). In contrast, HvFT3 was postulated as the candidate gene for the PHOTOPERIOD RESPONSE2 (Ppd-H2) locus, which promotes reproductive development under SDs (Laurie et al., 1995; Faure et al., 2007). In barley, two alleles of HvFT3 have been described; the dominant functional allele is prevalent in spring barley and winter barley from southern European cultivation areas, whereas the recessive nonfunctional allele with a large deletion in the transcribed coding region is typical for northern European winter barley (Kikuchi et al., 2009; Casao et al., 2011a, 2011b). Casao et al. (2011b) showed that the wild-type HvFT3 allele promotes flowering of winter cultivars under noninductive conditions, i.e. under SDs or in plants that have not satisfied their vernalization requirement.
In barley, vernalization requirement is determined by the interaction of two genes, VRN-H2, a strong inhibitor of HvFT1 and hence flowering under LD conditions, and VRN-H1. VRN-H1, an APETALA1 (AP1)/CAULIFLOWER/FRUITFULL (FUL)-like MADS box transcription factor, is upregulated during vernalization and represses VRN-H2 to release HvFT1 expression (Yan et al., 2003, 2004). A deletion of the VRN-H2 locus and deletions in the regulatory regions of the first intron of VRN-H1 induce the expression of HvFT1 independent of vernalization and cause a spring growth habit (Hemming et al., 2009; Rollins et al., 2013). In spring or vernalized winter barley, LDs strongly promote flowering, whereas SDs delay reproductive development. Flowering under LDs is controlled by the major photoperiod response gene Ppd-H1, which upregulates HvFT1 in the leaf under LDs (Turner et al., 2005). A natural mutation in the conserved CCT domain of Ppd-H1 prevalent in spring barley causes a delay in the induction of HvFT1 and later flowering under LDs (Turner et al., 2005; von Korff et al., 2006, 2010; Jones et al., 2008; Wang et al., 2010). In the leaf, HvFT1 expression is correlated with the up-regulation of the barley MADS box (BM) genes VRN-H1, HvBM3/FUL2, and HvBM8/FUL3 and successful inflorescence development and flowering (Hemming et al., 2008; Sasani et al., 2009; Digel et al., 2015; Ejaz and von Korff, 2017).
Reproductive development in temperate cereals can be divided into three major phases based on morphological changes of the shoot apex: leaf initiation (vegetative phase), spikelet initiation (early reproductive phase), and spike growth and floral development (late reproductive phase; Appleyard et al., 1982; Slafer and Rawson, 1994; González et al., 2002; Campoli and von Korff, 2014). Physiological studies of preanthesis development in barley and wheat show that vernalization controls flowering time, predominantly by reducing the duration of the vegetative phase (Griffiths et al., 1985; Roberts et al., 1988; González et al., 2002). Additionally, vernalization affects the late reproductive phase and floral development (González et al., 2002). In spring and vernalized winter barley, LDs shorten the vegetative phase but primarily accelerate the late reproductive phase of stem elongation and floral development (Roberts et al., 1988; Miralles and Richards, 2000; Digel et al., 2015, 2016). In spring barley, spikelet initiation occurs under both SDs and LDs whereas floral development depends on LDs and the expression of HvFT1 (Digel et al., 2015). The regulation of HvFT3 and its effects on spikelet initiation and floral development has not yet been examined.
By studying the effects of HvFT3 overexpression and the natural deletion of HvFT3 on reproductive development, we demonstrate that HvFT3 specifically promotes spikelet initiation and accelerates the early reproductive phase under SD and LD conditions but does not promote floral development in barley. Analysis of gene expression revealed that HvFT3 overexpression accelerates spikelet initiation through the down-regulation of putative floral repressors and the up-regulation of floral homeotic genes at the shoot apices of transgenic plants.
RESULTS
Overexpression of HvFT3 Accelerated Flowering Time of Spring Barley under LD But not under SD Conditions
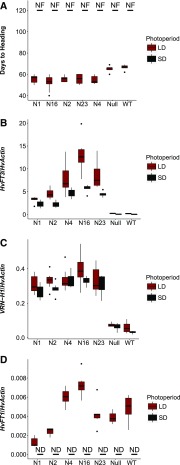
We investigated the effect of HvFT3 on flowering time by ectopically overexpressing HvFT3 in the spring variety Golden Promise (spring VRN-H1, spring vrn-H2, and reduced photoperiod sensitivity ppd-H1) and analyzing flowering time and development of the main shoot apex (MSA) under LDs and SDs. We scored flowering time in days from germination until heading where heading denotes the emergence of spike awns out of the sheath of the main shoot flag leaf (Zadoks stage 49; Zadoks et al., 1974). Under LDs, the constitutive overexpression of HvFT3 significantly accelerated flowering. The five independent transgenic Ubi::HvFT3 lines required 52 to 56 d to flower, whereas Golden Promise and the null segregants flowered 66 d after germination (DAG; Fig. 1A). In contrast, none of the plants flowered before 120 DAG, when the experiment was stopped, under SDs (Fig. 1A). Under both photoperiods, however, HvFT3 expression levels were significantly increased in the transgenic families compared to the control lines (Fig. 1B; Supplemental Fig. S1). Expression levels of VRN-H1 were also significantly higher in the presence of the transgene under both photoperiods (Fig. 1C). HvFT1 was only expressed under LDs but not SDs in the transgenic and wild-type lines. Overexpression of HvFT3 did not affect HvFT1 expression levels as these were not consistently altered in transgenic versus wild-type lines (Fig. 1D; Supplemental Fig. S1). We further examined whether variation at Ppd-H1, and thus HvFT1 expression levels, affected HvFT3 expression in wild-type plants. For this purpose, we evaluated HvFT3 expression levels in Golden Promise (ppd-H1) and a derived introgression line with a wild-type Ppd-H1 allele from the winter barley variety Igri over development under LDs and SDs. HvFT3 expression levels were reduced in the introgression line with a wild-type Ppd-H1 allele under LDs but not under SDs (Supplemental Fig. S2).
Figure 1.
Flowering time and gene expression levels in Ubi::HvFT3 transgenic lines under long-day and short-day conditions. Flowering time and expression levels of HvFT3, VRN-H1, and HvFT1 were estimated in Ubi::HvFT3 transgenic lines (N1, N2, N4, N16, and N23), a null segregant line (Null), and Golden Promise (wild type [WT]) under long day (LD; 16 h light, red) and short day (SD; 8 h light, black) conditions. Each column represents the average (A) flowering time, (B) HvFT3 expression levels, (C) VRN-H1 expression levels, and (D) HvFT1 expression levels. Flowering time was measured in days from germination until heading. For A, B, C, and D, four to 15 plants were used as biological replicates. Error bars, standard deviation. Expression analysis was performed on leaf samples collected 2 h before the end of the light period at day 5 after germination. Expression values were normalized to HvActin. NF, Did not flower; ND, not detected.
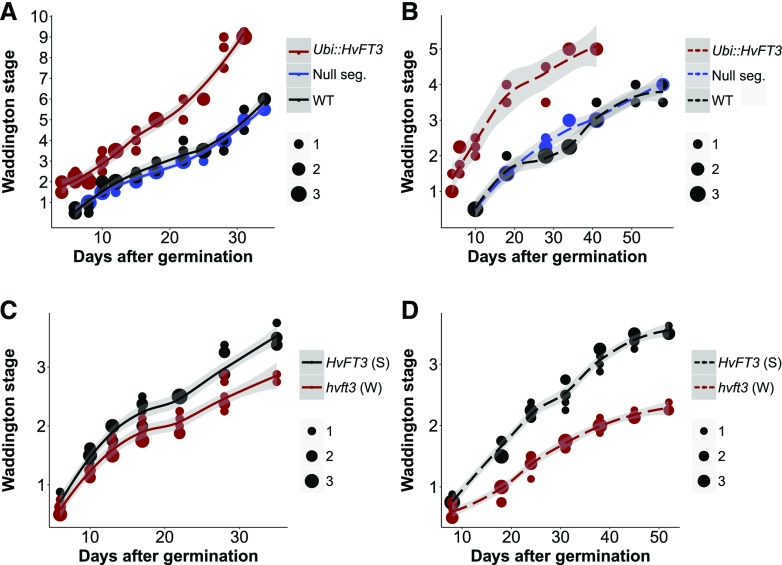
To further characterize the day-length-dependent effects of Ubi::HvFT3, we dissected the primary shoots of developing transgenic, null segregant and Golden Promise plants and determined differences in the timing of spikelet initiation and inflorescence development. We monitored phenotypic changes of the MSA and evaluated these according to the Waddington scale, a quantitative scale for barley and wheat development based on the morphogenesis of the shoot apex and carpels (Waddington et al., 1983; Fig. 2). The emergence of the first spikelet primordia on the shoot apex at the double-ridge stage (W1.5–W2.0) specifies a reproductive SAM. The first floral organ primordia differentiate and stem elongation initiates at the stamen primordium stage (W3.5). Anthesis and pollination of the most advanced floret occur at the last stage of the Waddington scale (W10.0).
Figure 2.
Effects of HvFT3 on MSA development in spring barley under LDs and SDs. A and B, Ubi::HvFT3 (red line), null segregant (dark blue), and wild-type (WT; black) plants were grown under (A) LD (16 h light) and (B) SD (8 h light), and meristem development was evaluated based on the Waddington scale. Two independent Ubi::HvFT3 lines, N16 and N23, were used under LD and SD, respectively. Each value represents the average of 2 to 4 replicates. C and D, Meristem development of spring introgression lines carrying either the functional HvFT3 (black) or the null hvft3 allele (red) under (C) LD (13 h light) and (D) SD (8 h light). Meristem development was evaluated based on the Waddington scale. Each value represents the average of 4 to 6 replicates. The size of the dots shows the number of MSA with the same Waddington stage at a given time point. The shaded area indicates the 95% confidence interval (Loess smooth line) calculated using a polynomial regression model.
Under LDs, early development of the MSA was strongly accelerated in Ubi::HvFT3 compared to the null segregant and the wild-type lines (Fig. 2A). Apical meristems of the transgenic plants had already initiated spikelet primordia 4 DAG, whereas the control plants required 8 more days to reach the same stage. The transgenic plants developed stamen primordia (W3.5) 12 d earlier than the null segregants and Golden Promise. During further floral development, no additional acceleration in development of the transgenic compared to the control plants was observed. Eventually, the transgenic line flowered 44 DAG, while the null segregant and wild-type lines flowered 54 and 55 DAG, respectively. Under SDs, spikelet initiation (W2.0) occurred six DAG in Ubi::HvFT3, 22 d earlier than in null segregant and wild-type plants (Fig. 2B). Early reproductive development was further accelerated in the transgenic line as it developed stamen primordia (W3.5), on average 33 d earlier than in the two control genotypes. The MSA of the control plants failed to develop further than the pistil primordium stage (W4.0), whereas the MSA of Ubi::HvFT3 plants reached the carpel primordium stage (W5.0). None of the plants produced fertile florets and seeds under SDs.
Under both photoperiods, null segregants, wild-type plants, and the majority of transgenic plants formed normal shoot meristems that developed fertile flowers and set seeds under LDs (Supplemental Fig. S3A), while the MSA of approximately 20% of transgenic Ubi::HvFT3 plants exhibited impaired development (Supplemental Fig. S3B). These meristems remained very small (Supplemental Fig. S1D), failed to develop floral primordia, and were prematurely aborted (Supplemental Fig. S3B). We also observed Ubi::HvFT3 plants that developed floret primordia, but the architecture of their inflorescences was severely impaired, i.e. the shoot apices were not symmetrical, very short and compacted, and some inflorescences were branched.
We further analyzed the effect of natural variation at HvFT3 on spikelet initiation and inflorescence development. We compared the MSA development of two sets of nontransgenic sister F4 lines derived from the cross Ubi::HvFT3 (Golden Promise) × Igri. These were isogenic for the vernalization genes (spring growth habit, VRN-H1/vrn-H2) and the mutated ppd-H1 allele but segregating for the functional spring HvFT3 allele from Golden Promise and the mutated nonfunctional allele hvft3 introgressed from winter barley (Casao et al., 2011a, 2011b). The primary shoots of the two genotypes were dissected under SDs of 8 h light and LDs of 13 h light, which correspond to the photoperiod during early reproductive development of spring-sown barley plants in northern European cultivation areas. Under 13-h LDs, the MSA of HvFT3 plants initiated spikelet primordia 4 d earlier and reached the stamen primordium stage 7 d earlier than hvft3 plants (Fig. 2C). Genotypes with the functional HvFT3 allele flowered on average in 84 DAG, significantly earlier than the hvft3 introgression lines, which flowered on average 19 d later (some plants failed to flower by 120 DAG).
Under SD conditions, early development of the MSA was strongly enhanced in the presence of the spring HvFT3 allele (Fig. 2D). Plants with the HvFT3 allele reached the double-ridge stage (W2.0) 21 DAG and the stamen primordium stage (W3.5) 45 DAG, while lines carrying the hvft3 allele initiated spikelet primordia 38 DAG but had not reached the glume primordia stage (W2.5) by 52 DAG. The inflorescences of HvFT3 plants did not develop beyond the stamen primordium stage (W3.5), while hvft3 plants stopped further development directly after spikelet initiation. Taken together, the natural variation in HvFT3 specifically affected spikelet initiation and early reproductive growth of spring barley under SDs, as we also observed in the transgenic lines. Under 13 h LDs, HvFT3 accelerated spikelet initiation and later inflorescence development.
We further explored the photoperiod- and stage-specific effects of HvFT3 by overexpressing HvFT3 in the Arabidopsis ft10 mutant (Yoo et al., 2005). Flowering time was scored in three homozygous 35S::HvFT3 T2 families, together with a mock family (ft10 transformed with an empty vector), 35S::AtFT, and the ft10 mutant plants under LDs and SDs (Supplemental Fig. S4). The 35S::HvFT3 plants flowered with 18 to 28 leaves and 22 to 28 leaves under LDs and SDs, respectively, which was significantly earlier than the mock plants and the ft10 mutant lines. These flowered with 33 to 35 leaves and >40 leaves under LDs and SDs, respectively. The 35S::AtFT plants flowered with only five leaves under both photoperiods. Consequently, the overexpression of HvFT3 accelerated flowering in Arabidopsis, but its effect was much smaller compared to that of AtFT.
Taken together, HvFT3 promoted the initiation of spikelet primordia and accelerated early inflorescence development under both SDs and LDs as demonstrated by HvFT3 overexpression and a natural HvFT3 knockout line. However, both transgenic and wild-type plants headed and flowered only under LDs, i.e. the inflorescences were aborted under SDs. Overexpression of HvFT3 was associated with a strong up-regulation of VRN-H1, but not HvFT1 in the leaf under both tested photoperiods. In Arabidopsis, the overexpression of HvFT3 accelerated flowering in the ft10 mutant plants under both photoperiods. The photoperiod-dependent effect of HvFT3 was consequently specific to barley, as overexpression of HvFT3 in Arabidopsis accelerated flowering time under both photoperiods.
Overexpression of HvFT3 Accelerated Flowering Time in Winter Barley
To analyze the genetic interaction of HvFT3 with the vernalization genes VRN-H1 and VRN-H2 and the photoperiod response gene Ppd-H1, we examined flowering time and gene expression in an F2 population developed by crossing Ubi::HvFT3 in the background of Golden Promise to the winter variety Igri. Golden Promise carries a functional HvFT3 gene, a natural mutation at Ppd-H1 that reduces the response to LD (Turner et al., 2005), a deletion in the first regulatory intron of VRN-H1 and a deletion of the VRN-H2 locus. As a consequence, this genotype does not require vernalization and shows a reduced photoperiod response. In contrast, Igri carries a nonfunctional allele of HvFT3, the wild-type allele of Ppd-H1, and the winter alleles of VRN-H1 and VRN-H2. Consequently, Igri requires vernalization to flower and shows a strong photoperiod response. The transgene Ubi::HvFT3, native HvFT3, Ppd-H1, VRN-H1, and VRN-H2 were the main flowering time genes segregating in the generated F2 population. To test whether overexpression of HvFT3 can overcome the vernalization requirement, we scored flowering time of F2 plants grown without vernalization under LDs.
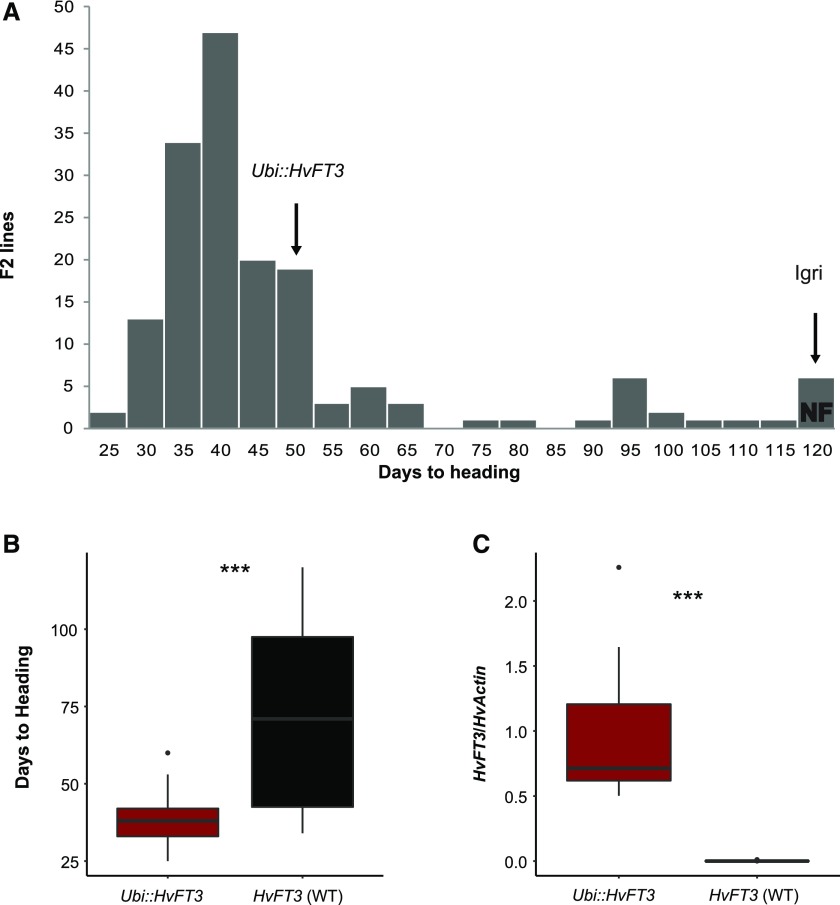
Flowering time of Ubi::HvFT3 × Igri F2 lines varied between 25 and 111 d (Fig. 3A). The population exhibited strong transgressive segregation, as 70% of the lines flowered earlier than the transgenic parent, which required on average 47 d to flower. A few F2 lines and the winter parent Igri did not flower before 120 DAG, when the experiment was stopped. The distribution of flowering time in the F2 population was strongly skewed with the majority of lines flowering as early or earlier than the transgenic parent. Of the 166 F2 lines from the Ubi::HvFT3 Golden Promise × Igri population, 127 F2 lines (76.51%) carried the transgene Ubi::HvFT3 and 39 (23.49%) did not, which showed that the transgene segregated as a single insert.
Figure 3.
Flowering time and expression of HvFT3 in the F2 population Ubi::HvFT3 × Igri grown under LD conditions. A, Distribution of flowering time in the Ubi::HvFT3 × Igri F2 lines. One hundred sixty-six F2 plants derived from the cross Ubi::HvFT3 × Igri were grown under LD conditions (16 h light). Plants were not subjected to vernalization, and flowering time was measured in days from germination until heading. The average flowering time of the two parental genotypes is indicated by arrows. NF, Did not flower after 120 d. B, Average flowering time and (C) expression of HvFT3 normalized to HvActin of F2 lines classified according to the presence/absence of the transgene Ubi::HvFT3. Expression analysis was performed on leaf samples from 69 selected F2 lines collected 2 h before the end of the light period in LD (16 h light) at day 5 after germination. Error bars, standard deviation; WT, wild type. Significant differences were calculated based on a Student’s t test. ***refers to a significant difference at P < 0.001.
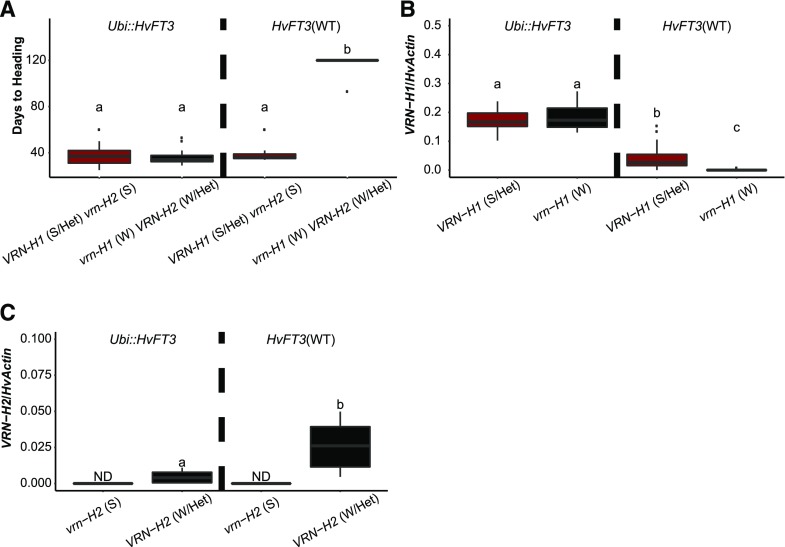
Association of flowering time with the presence of Ubi::HvFT3 and genetic variation at Ppd-H1, VRN-H1, and VRN-H2 in the F2 population showed that the transgene Ubi::HvFT3 had the strongest effect on flowering time in the population (Supplemental Table S1). Transgenic F2 lines flowered on average after 38 d, 37 d earlier than those without the transgene (Fig. 3B). Ppd-H1 affected flowering time only in the transgenic subpopulation (Supplemental Fig. S5). In the presence of Ubi::HvFT3, lines with a photoperiod-responsive Ppd-H1 allele flowered on average 9 d earlier than their siblings with the mutated ppd-H1 allele. The allelic status of Ppd-H1 had no significant effect on flowering in the nontransgenic F2 lines, suggesting that in these lines the vernalization pathway was dominant over the photoperiod pathway. Allelic variation at VRN-H2 and VRN-H1 contributed to the overall observed variation in flowering time but did not affect the flowering time of the transgenic F2 lines (Fig. 4A). Consequently, transgenic lines with the winter alleles vrn-H1 and VRN-H2 flowered even without vernalization. Overexpression of HvFT3 was thus dominant over the vernalization pathway. By contrast, flowering of the nontransgenic F2 lines was significantly delayed by the combination of the winter alleles of VRN-H1 and VRN-H2. We could not evaluate the effects of the native HvFT3 on flowering time in this population as only a limited number of genotypes (39) were not transgenic, and these carried additional background variation at Ppd-H1, VRN-H1, and VRN-H2, which masked the effect of the wild-type HvFT3 locus on flowering time and gene expression.
Figure 4.
Effects of the transgene Ubi::HvFT3 on flowering time, its interaction with allelic variation at VRN-H1 and VRN-H2, and expression of VRN-H1 and VRN-H2 in the F2 population Ubi::HvFT3 × Igri under LD conditions. A, Average flowering time. B, Expression levels of VRN-H1. C, Expression levels of VRN-H2. F2 lines were classified according to the presence of Ubi::HvFT3 and allelic variation of VRN-H1 and VRN-H2: S, Spring allele; Het, heterozygote; W, winter allele; WT, wild type. One hundred sixty-six F2 lines derived from the cross Ubi::HvFT3 × Igri were grown under LD conditions (16 h light). Plants were not subjected to vernalization, and flowering time was measured in days from germination until heading. Expression analysis was performed on leaf samples from 69 selected F2 lines collected 2 h before the end of the light period in LD (16 h light) at day 5 after germination. Expression values were normalized to HvActin. Error bars, standard deviation. Significant differences were calculated based on a one-way ANOVA with a post-hoc Tukey HSD (honestly significant difference) test. Different letters above the columns indicate significant differences at P < 0.05. ND, Expression not detected.
To further characterize the molecular control of flowering time in the F2 population under LDs, we analyzed the effects of Ubi::HvFT3 and genetic variation at Ppd-H1, VRN-H1, and VRN-H2 on expression levels of selected flowering time regulators. Flowering time exhibited the highest positive and negative correlations with expression levels of VRN-H2 (0.80) and VRN-H1 (−0.69; Supplemental Table S2), respectively. Flowering time also negatively correlated with expression of HvFT3 (−0.48), HvFT1 (−0.46), and Ppd-H1 (−0.39). F2 lines carrying the transgene had on average 650 times higher expression levels of HvFT3 compared to their siblings with the wild-type gene only (Fig. 3C). The presence of the transgene was associated with a strong up-regulation of VRN-H1 independently of allelic variation at VRN-H1 (Fig. 4B). In contrast, the transgene caused a down-regulation of VRN-H2 (Fig. 4C). Expression levels of HvFT1 correlated negatively and positively with those of VRN-H2 (−0.38) and Ppd-H1 (0.39), but not with HvFT3 or VRN-H1 (Supplemental Table S2). Furthermore, HvFT1 expression was mainly controlled by the presence/absence of VRN-H2 (41%) and allelic variation at Ppd-H1 (18%) and their interaction (16%; Supplemental Table S3). Consequently, overexpression of HvFT3 controlled the expression of VRN-H1 and VRN-H2 and was therefore dominant over the repressive effects of the vernalization genes VRN-H1 and VRN-H2 on flowering.
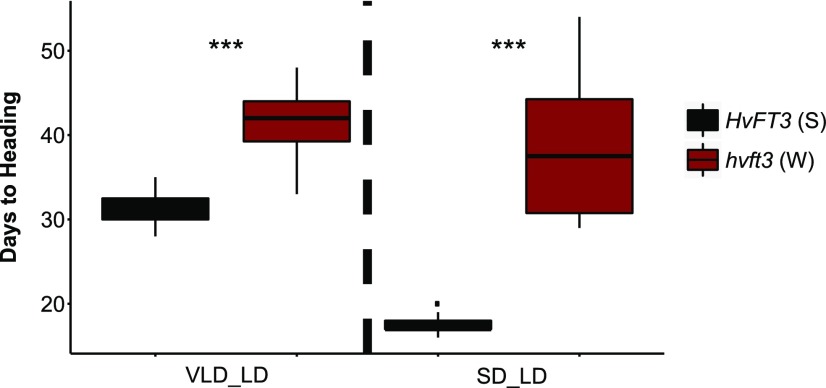
We further investigated the effects of HvFT3 on flowering time of winter barley in response to vernalization or SD treatment. For this purpose, we selected two groups of nontransgenic sister F4 families from the cross Ubi::HvFT3 × Igri to carry the photoperiod-responsive allele Ppd-H1, the winter alleles vrn-H1 and VRN-H2, and either the winter nonfunctional allele hvft3 or the spring functional allele HvFT3. The two groups of genotypes were tested under two different treatments: vernalization for 8 weeks under LDs followed by LDs (VLD_LD) and 8 weeks under SDs followed by LDs (SD_LD; Fig. 5). In the VLD_LD treatment, HvFT3 plants flowered at 31 DAG, which was significantly earlier than hvft3 genotypes (42 DAG; Fig. 5). HvFT3 plants in the SD_LD treatment required only 18 d to flower, while hvft3 plants flowered 39 d after transfer to LDs. Consequently, natural variation at HvFT3 affected flowering time under both conditions. The differences in flowering time between both groups of genotypes were, however, much more pronounced following the SD treatment compared to vernalization. We therefore concluded that HvFT3 does not only counteract the repressive effect of the vernalization pathway but also induces early reproductive development of winter barley under SD conditions.
Figure 5.
Effect of allelic variation at HvFT3 on flowering time of winter barley grown under different experimental regimes. Flowering time of two winter F4 families (Ppd-H1 vrn-H1 VRN-H2) carrying the spring (S) HvFT3 (black) or the winter (nonfunctional, W) hvft3 allele (red) was scored as days to heading after 8 weeks of vernalization in LD followed by LD (VLD_LD) and after 8 weeks of SD treatment followed by LD (SD_LD). Flowering time was measured in days from germination until heading. Each box represents the average flowering time of 10 plants. Error bars, standard deviation. Significant differences were calculated based on a Student’s t test. ***refers to a significant difference in flowering time at P < 0.001.
Transcriptional Changes at the SAM during the Vegetative and Early Reproductive Phases under LD and SD Conditions
Ubi::HvFT3 plants exhibited a faster initiation of spikelet primordia and an accelerated early reproductive development under LDs and SDs. We therefore aimed to detect candidate HvFT3 targets that affected spikelet initiation and early reproductive development independently of photoperiod. For this purpose, we conducted a global transcriptional profiling of MSA samples of Ubi::HvFT3 and the null segregants collected at the spikelet initiation stage (W2.0) and at the stamen primordium stage (W3.0–3.5) under LD and SD conditions. We focused on transcripts that were differentially regulated between genotypes and stages under both photoperiods. Since a large number of transcripts have strong diurnal expression profiles that may vary between LDs and SDs, we did not compare transcript expression between photoperiods.
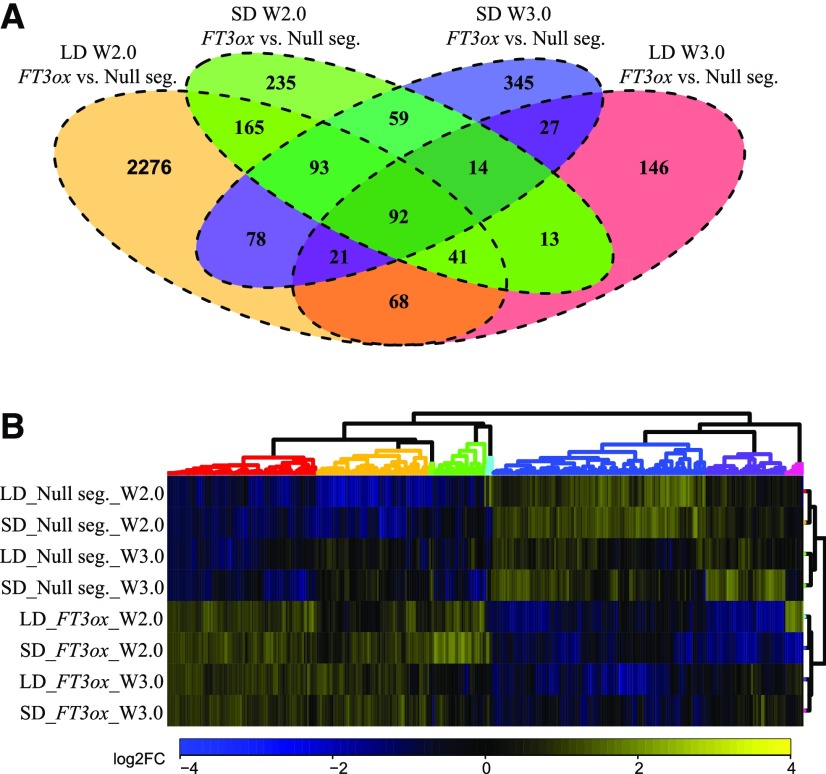
We detected a total of 20,282 transcripts with expression levels greater than five reads in at least three libraries under each condition (LD, SD). Differentially expressed transcripts (DETs) between genotypes and developmental stages were identified under both photoperiods. Principal component analysis demonstrated that the majority of transcripts were differentially regulated between stages, whereas DETs differentiated genotypes most strongly at the spikelet initiation stage (Supplemental Fig. S6). A total of 3,034 and 1,183 DETs were regulated between Ubi::HvFT3 and the null segregant in at least one of the two stages under LDs and SDs, respectively. Between the stages, 4,824 transcripts were differentially regulated under LDs compared to 3,474 DETs under SDs in at least one of the genotypes (Fig. 6A). The detailed description of the expression of transcripts in the reference set in different genotypes, photoperiods, and developmental stages, and their functional annotations can be found in Supplemental Table S4.
Figure 6.
Global transcriptome analysis in developing main shoot apices in the Ubi::HvFT3 and null segregant lines. A, Venn diagram of transcripts differentially regulated between Ubi::HvFT3 (FT3ox) and the null segregant (Null seg.) at two different stages under LD and SD. B, Coexpression clustering of 391 DETs regulated between the Ubi::HvFT3 and null segregant genotypes at the spikelet initiation stage. Colors represent log2 fold changes (log2-FC) in expression levels relative to the mean transcript abundance across the tested conditions. LD, Long day; SD, short day; W2.0, Waddington stage W2.0; W3.0, Waddington stage W3.0.
At the spikelet initiation stage (W2.0), a total of 391 transcripts were differentially regulated between the transgenic plants and their null segregants under both photoperiods. Of these, 195 DETs were upregulated, 180 downregulated, and 16 differentially regulated under LDs and SDs in Ubi::HvFT3 compared to the wild type (Fig. 6B). At the lemma primordium phase (W3.0–3.5), 154 transcripts were differentially regulated between genotypes under both photoperiods, with 84 DETs being upregulated and 69 downregulated and one transcript differentially regulated under LDs and SDs in Ubi::HvFT3 compared to the null segregant (Supplemental Fig. S7). A subset of 92 transcripts were significantly affected by Ubi::HvFT3 under both developmental stages and photoperiods. Consequently, overexpression of HvFT3 affected more transcripts at the spikelet initiation than the lemma primordium stage under both photoperiods. In addition, the majority of transcripts regulated at the lemma primordium stage were also differentially expressed between genotypes at the spikelet initiation stage.
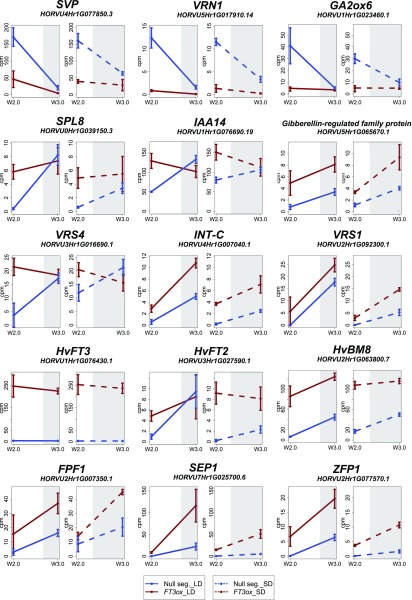
Genes that were differentially regulated at the spikelet initiation stage had functions in meristem and floral development and hormone biosynthesis, signaling, and response. Among the transcripts strongly downregulated at the spikelet initiation stage in apices of the transgenic lines compared to the null segregants were transcription factors involved in the suppression of floral determinacy in Arabidopsis (Hartmann et al., 2000; Yant et al., 2010; Licausi et al., 2013; Huang et al., 2017). These included two homologs of MADS box transcription factors AGAMOUS-LIKE1 (HORVU6Hr1G002330) and SHORT VEGETATIVE PHASE (SVP; HORVU4Hr1G077850), two AP2-like ethylene responsive-element-binding proteins (AP2/EREB; HORVU7Hr1G116220, HORVU5Hr1G112440) and AP2/B3 domain-containing proteins (VRN1; HORVU5Hr1G017910, HORVU5Hr1G017890; Fig. 7). In addition, in the transgenic line, we observed a reduced expression of an F-box-domain-containing protein ABERRANT PANICLE ORGANIZATION1 (HORVU7Hr1G108970). In rice, an ABERRANT PANICLE ORGANIZATION1 homolog suppresses the precocious conversion of inflorescence meristems to spikelet meristems (Ikeda et al., 2007).
Figure 7.
Expression profile of selected DETs. Ubi::HvFT3 (red) and wild-type (blue) expression either at W2.0, W3.0, or both stages under long day (LD, left) and short day (SD, right) conditions. White, light period; gray, dark period. W, Waddington stage; cpm, counts per million.
Among the transcripts upregulated in the overexpression line were homologs of the SQUAMOSA PROMOTER-BINDING-LIKE8 (SPL8; HORVU0Hr1G039150) and SPL9 (HORVU5Hr1G073440) and a homolog of LEAFY (LFY; HORVU2Hr1G102590). We also observed higher expression levels for three cytochrome p450 mono-oxygenase proteins CYP78A9 (HORVU7Hr1G057100), CYP89A6 (HORVU7Hr1G081610), and CYP71B34 (HORVU7Hr1G096560) in the transgenic compared to null segregants. Cytochrome P450 oxygenases and SPL proteins have been implicated in the control of leaf initiation, axillary meristem outgrowth, and floral development in model and crop plants (Miyoshi et al., 2004; Schwarz et al., 2008; Mascher et al., 2014; Gou et al., 2017). We also detected a strong up-regulation of homeobox-Leucine zipper protein family genes in the Ubi::HvFT3 lines. These included SIX-ROWED SPIKE1 (VRS1/HvHOX1; HORVU2Hr1G092290, HORVU2Hr1G092300), its close paralog, the homeobox gene HOX2 (HORVU2Hr1G036680) and an additional homologous homeodomain zipper protein (HORVU4Hr1G070610). VRS1 has evolved specific functions to suppress the development of lateral spikelets and thus controls the dimorphism between two- and six-rowed barley spikes (Komatsuda et al., 2007). Interestingly, VRS4 (LATERAL ORGAN BOUNDARIES; LOB; HORVU3Hr1G016690), the upstream positive regulator of VRS1 (Koppolu et al., 2013) and another modifier of lateral spikelet development INTERMEDIUM-C (INT-C; HORVU4Hr1G007040; Ramsay et al., 2011) were also upregulated by HvFT3 overexpression.
Transcripts related to hormone synthesis, signaling, and response were differentially regulated between genotypes. The transcript levels of a homolog of GIBBERELLIN2-OXIDASE6 (HORVU1Hr1G023460) and a brassinosteroid responsive B-box zinc finger family protein BZS1 (HORVU7Hr1G091180) were reduced in the transgenic compared to wild-type lines. Several auxin-related genes, such as INDOLEACETIC ACID-INDUCED PROTEIN14 (IAA14, HORVU1Hr1G076690), IAA15 (HORVU1Hr1G025670), and IAA17 (HORVU3Hr1G031460), a putative INDOLE-3-ACETIC ACID-AMIDO SYNTHASE GH3.9 (HORVU3Hr1G077360.1), and an AUXIN TRANSPORTER-LIKE1 (HORVU3Hr1G084750) were upregulated in the transgenic compared to the null segregant genotypes. These transcript changes suggested an increase in or redistribution of auxin and gibberellin concentration, which matched the earlier differentiation of spikelets and floral primordia in the transgenic line.
At the lemma primordium phase (W3.0–3.5), 154 transcripts were differentially regulated. Among those were genes implicated in meristem and floral development, disease resistance, and abiotic stress tolerance. Among the genes upregulated at both stages, W2.0 and W3.0, were the transgene HvFT3 (HORVU1Hr1G076430) and its homolog HvFT2 (HORVU3Hr1G027590), suggesting that HvFT3 affects HvFT2 expression in the MSA. We also detected the up-regulation of many floral homeotic genes that have conserved functions in controlling floral development across plant species. These included the AP1-like genes HvBM3 (HORVU0Hr1G003020), HvBM8 (HORVU2Hr1G063800), a Homeobox-Leu zipper protein homologous to Arabidopsis thaliana HOMEOBOX (ATHB7; HORVU5Hr1G081090), SEPALLATA1 (SEP1; HORVU7Hr1G025700), and a PISTILLATA like gene (PI, HORVU3Hr1G068900). In addition, two SPL-like proteins, SPL17 (HORVU0Hr1G020810) and SPL16 (HORVU5Hr1G076380), were expressed at higher levels in the transgenic than null segregant MSAs. SPLs are plant-specific transcription factors that play important roles in plant phase transition, the juvenile-to-adult vegetative transition, and the vegetative-to-reproductive transition and floral development (Hyun et al., 2017).
HvFT3 overexpression also caused an up-regulation of genes encoding proteins involved in light signaling such as FAR-RED IMPAIRED RESPONSE1 homologs (HORVU2Hr1G087170, HORVU3Hr1G011190, HORVU6Hr1G019030) a homolog of ELONGATED MESOCOTYL (HORVU5Hr1G059310) essential for phytochrome synthesis, signaling, and light control of development in Arabidopsis (Sawers et al., 2004; Wang and Wang, 2015) and a basic helix-loop-helix protein (HORVU0Hr1G017110) involved in blue/far-red light signaling (Hyun and Lee, 2006; Castelain et al., 2012)
Furthermore, numerous genes regulated between genotypes were associated with the transport of carbon, nutrients, and ions including a POLYOL/MONOSACCHARIDE TRANSPORTER1 (HORVU2Hr1G036570), metal ion transporters (HORVU7Hr1G106570, HORVU1Hr1G071930), amino acid transporters (AMINO ACID PERMEASE, HORVU2Hr1G084780; BIDIRECTIONAL AMINO ACID TRANSPORTER1, HORVU3Hr1G053090), and a sulfotransferase (HORVU2Hr1G117700). Furthermore, genes with functions in disease resistance were upregulated in the transgenic line, i.e. barley homologs of nucleotide-binding adaptor shared by APAF-1, R proteins, and CED-4 (NB-ARC) domain-containing disease resistance proteins (HORVU3Hr1G002130, HORVU7Hr1G002260, HORVU7Hr1G002290, HORVU7Hr1G111700).
Taken together, the overexpression of HvFT3 altered the expression levels of genes involved in floral development, hormone homeostasis, and transport. Strong and precocious expression of genes with roles in floral development, spike architecture, and hormone homeostasis was linked to the earlier spikelet initiation observed in the Ubi::HvFT3 plants.
DISCUSSION
HvFT3 Affects Spikelet Initiation But not Floral Development
HvFT3 (Ppd-H2 locus) was originally described as a floral promoter under SD conditions in barley (Laurie et al., 1995; Laurie, 1997; Faure et al., 2007; Kikuchi et al., 2009). In this study, however, overexpression of HvFT3 significantly accelerated flowering of spring barley under LDs but did not lead to successful flowering under SDs. In contrast to HvFT3, elevated expression levels of HvFT1 were associated with day-neutral early flowering. For example, barley genotypes with a hypermorphic mutation in PHYTOCHROME C, a loss-of-function mutation in the circadian clock gene EARLY FLOWERING3 and a hypomorphic mutation in the clock gene LUX1/ARRHYTHMO are characterized by photoperiod independent up-regulation of HvFT1 and early flowering under long and short days (Faure et al., 2012; Campoli et al., 2013; Pankin et al., 2014). Consequently, HvFT1 and HvFT3 have different photoperiod-dependent effects on flowering; the induction of HvFT1 but not HvFT3 causes day length independent flowering in barley. Interestingly, overexpression of HvFT3 in an Arabidopsis ft mutant resulted in a photoperiod-independent acceleration of flowering time. Both barley and Arabidopsis are considered long-day plants; however, both species differed in their response to photoperiod, and this difference was independent of HvFT3 overexpression. It was previously shown that continuous exposure to long days is crucial for floral development and flowering in barley (Digel et al., 2015), whereas in Arabidopsis, a transient shift for a few days to LD growth conditions is sufficient to irreversibly commit the plants to flower (Corbesier et al., 2007).
Monitoring MSA development revealed that HvFT3 overexpression specifically accelerated vegetative and early reproductive growth independently of photoperiod. Likewise, natural variation at HvFT3 affected spikelet initiation and early reproductive growth under SDs and to a lesser extent under LDs. However, spring genotypes with the functional and nonfunctional alleles of HvFT3 failed to develop beyond the lemma primordium stage under SDs and inflorescences aborted. In contrast, variation in HvFT1 expression levels as controlled by Ppd-H1 were associated with the acceleration of all phases of MSA development under LDs, particularly the late reproductive phase of floral development (Hemming et al., 2008; Digel et al., 2015). Therefore, we propose that HvFT3 specifically controls spikelet initiation but fails to promote floral development. Successful floral development likely depends on the LD induction of HvFT1 expression.
In both Arabidopsis and temperate cereals, the vernalization and the photoperiod pathways converge on FT (Hemming et al., 2008; Amasino and Michaels, 2010). In barley, VRN-H2 counteracts the LD activation of flowering by repressing FT expression before vernalization (Yan et al., 2006; Hemming et al., 2008; Mulki and von Korff, 2016). We showed that overexpression of HvFT3 caused an up-regulation of the winter and spring alleles of VRN-H1, and this was associated with the down-regulation of VRN-H2. Consequently, transgenic winter lines flowered early without vernalization. Similarly, the functional HvFT3 variant accelerated flowering time in winter genotypes grown under nonvernalizing conditions. Casao et al. (2011b) has already reported that HvFT3 can significantly accelerate flowering of winter genotypes that were not or were only partially vernalized. In Arabidopsis, FT and TWIN SISTER OF FT suppress the late-flowering phenotype of winter accessions, even when FLOWERING LOCUS C was overexpressed (Michaels et al., 2005). Similarly, introducing the Hope-dominant allele of the wheat homolg TaFT1, which shows high transcript levels of FT1, into winter wheat led to an early-flowering phenotype (Yan et al., 2006). Consequently, HvFT3 counteracts the repression of the vernalization pathway as demonstrated for the FT1 homolog in wheat.
Interestingly, for plants with a functional HvFT3 allele, treatment with SDs was significantly more effective than vernalization in promoting flowering. This indicated that HvFT3 plays a major role in accelerating flowering of winter barley in response to SD treatment. Since VRN-H2 is not expressed under SDs, HvFT3 accelerates spikelet initiation of winter genotypes under SDs as already demonstrated for spring barley. Evans (1987) also found that development of the inflorescences in unvernalized winter wheat was more rapid under SDs than LDs. By showing that SDs actively induced development, our results thus supported the earlier proposed dual SD-LD induction of flowering in cereals (McKinney and Sando, 1935; Evans, 1987; Dubcovsky et al., 2006). HvFT3 seems to play a dual role in the induction of flowering by promoting spikelet initiation under SDs and by reducing the requirement for vernalization under LDs as HvFT3 caused a down-regulation of VRN-H2 in the absence of vernalization.
High levels of VRN-H1 caused an up-regulation of HvFT1 (Hemming et al., 2008), and a positive feedback loop between VRN1 and FT has been suggested in wheat (Shimada et al., 2009; Distelfeld and Dubcovsky, 2010). Furthermore, Deng et al. (2015) have suggested that VRN-H1 upregulates HvFT1 by directly binding to its promoter. In this study, however, the strong and early up-regulation of VRN-H1 in Ubi::HvFT3 plants under LDs and SDs was not associated with a consistent up-regulation of HvFT1. This is in contrast to results from Casao et al. (2011a), who demonstrated that the up-regulation of the native HvFT3 transcript correlated with increased levels of VRN-H1 and HvFT1 expression. However, in the transgenic lines, relatively high expression levels of VRN-H1 were not correlated with an induction of HvFT1 expression levels as normally observed in wild-type plants. This altered ratio of high-VRN-H1 and low-HvFT1 expression in the transgenic lines may explain the observed early abortion of the main inflorescence of some transgenic plants (Supplemental Fig. S3). While overexpression of HvFT3 did not clearly affect HvFT1 expression levels, we demonstrated that native HvFT3 expression levels were influenced by natural variation at Ppd-H1 and thus differences in HvFT1 expression levels. This is in contrast to results in a transgenic wheat line carrying a highly expressed FT1 allele, where FT3 expression levels were increased under both LDs and SDs, while in wheat and Brachypodium (Brachypodium distachyon L.) FT1RNAi lines, FT3 expression levels were not altered (Lv et al., 2014). Similarly, in rice, cosilencing of the FT1 homologs HEADING DATE3a and RFT1 also showed no effect on the expression of the rice homologs of FT3 (Komiya et al., 2008). However, expression profiling of FT-like genes in the cultivar Morex demonstrated that HvFT3 expression preceded the expression of HvFT1, but HvFT3 expression levels decreased when HvFT1 expression increased (Kikuchi et al., 2009). These findings support our results that in barley HvFT1 expression is associated with a down-regulation of HvFT3 transcript levels.
Overexpression of HvFT3 Induces the Expression of Floral Regulators and Row-Type Genes
We used whole-transcriptome sequencing in the dissected MSAs at the spikelet initiation and lemma primordium stages to identify HvFT3-dependent molecular changes observed under both photoperiods. Early spikelet initiation in the transgenic lines was associated with a down-regulation of putative floral repressors in the MSA. These included an SVP-like gene, which delays floral transition by repressing the flowering pathway integrators FT, FLOWERING LOCUS D, the MADS domain gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) and gibberellin biosynthesis in Arabidopsis (Lee et al., 2007; Li et al., 2008; Andrés et al., 2014). We also observed an HvFT3-dependent down-regulation of floral homeotic genes with an AP2 domain, i.e. VRN1 (AP2/B3) and AP2/EREB. AP2 transcription factors play essential roles in growth, development, and stress response and have been implicated in various signal transduction pathways mediated by hormones such as abscisic acid, ethylene, cytokinin, and jasmonate (Yant et al., 2010). The transcription of floral activators like SOC1 and FT is directly repressed by proteins with an AP2 domain and a B3-type DNA binding domain (Castillejo and Pelaz, 2008; Yant et al., 2010) and overexpression of AP2 domain-containing proteins causes late flowering in Arabidopsis (Aukerman and Sakai, 2003; Schmid et al., 2003; Chen, 2004; Jung et al., 2007). The down-regulation of AP2/EREB (AP2) and VRN1 (AP2/B3) has previously been associated with spikelet initiation in barley (Digel et al., 2015). Thus, their down-regulation by HvFT3 overexpression might have accelerated the transition to reproductive growth in the transgenic lines.
The strong down-regulation of a GIBBERELLIN2-OXIDASE6 that initiates the major catabolic pathway for gibberellin (GA) suggested that spikelet initiation co-occurred with an increase in GA in the shoot apex. Pearce et al. (2013) have shown that the induction of FT1 expression causes the up-regulation of GA biosynthetic genes in the shoot apex. The authors proposed that FT1 is expressed in the leaves and the protein is then transported to the SAM, where it induces GA biosynthetic genes necessary for the up-regulation of the early floral meristem genes and development of the wheat spike. Similarly in rice, GA2ox1 expression is strongly decreased in the shoot apex at the transition from vegetative to reproductive growth, and ectopic expression of OsGA2OX1 in transgenic rice inhibits stem elongation and the development of reproductive organs (Sakamoto et al., 2001). Our expression analysis suggested that the up-regulation of FT3 also causes GA levels to rise in the MSA, as has been shown for FT1 in wheat. The up-regulation of several auxin-related genes, such as indole-3-acetic acid-like and auxin transporter genes, suggested that HvFT3 overexpression also induced changes in auxin homeostasis at the MSA. An increase in auxin may be important for floral organ initiation in barley, as demonstrated in Arabidopsis, where auxin distribution within the periphery of the inflorescence meristems specifies the site of floral meristem initiation and mediates organ growth and patterning (Krizek, 2011).
Down-regulation of putative floral repressors was correlated with an up-regulation of SPL8 and SPL9 specifically at spikelet initiation. The up-regulation of SPL genes in the transgenic plants was associated with an up-regulation of genes putatively involved in floral development such as LFY, AP1, and FUL-like genes at spikelet initiation and the lemma primordium stage. In Arabidopsis, SPL genes are repressed by SVP (Torti et al., 2012), and SPL proteins control floral transition by binding directly to and activating the transcription of SOC1, AP1, FUL, and LFY (Wang et al., 2009; Yamaguchi et al., 2009). These results suggested that HvFT3 regulates floral integrator genes as shown for HvFT1 (Digel et al., 2015) and that gene regulatory networks controlling floral transition are at least in part conserved between Arabidopsis and barley.
At the lemma primordium stage, overexpression of HvFT3 induced the expression of barley homologs of the Arabidopsis floral homeotic genes AP1/FUL, SEP1, SEP3, and PI, which are involved in floral organ formation (Mandel et al., 1992; Goto and Meyerowitz, 1994; Pelaz et al., 2000). These transcripts were upregulated under LD and SD conditions, even though successful floral development only proceeded under LDs. Interestingly, HvFT3 overexpression also induced the expression of the row-type genes VRS1, VRS4, and INT-C. VRS4 encodes a LATERAL ORGAN BOUNDARY transcription factor that is homologous to maize RAMOSA2 and upregulates the expression of the a HD-ZIP transcription factor VRS1 (HvHox1; Koppolu et al., 2013). VRS1 is a key inhibitor of lateral spikelet development, and loss of function or a complete down-regulation leads to the development of six-rowed spikes. A partial down-regulation of VRS1 as observed in intermedium (int) mutants results in an intermedium spike phenotype with varying two- or six-rowed patterns or enlarged lateral florets, which may or may not develop into kernels depending on the position on the spike and the environment. The most frequent int mutant is int-c, which has been identified as a barley homolog of TEOSINTE-BRANCHED1, a TCP transcription factor and major domestication-related gene affecting shoot branching in maize. Boden et al. (2015) have shown that a modified spikelet arrangement in wheat is controlled by FT1, where higher levels of FT1 expression as controlled by PPD1 inhibited paired spikelet formation. We show that modulated expression of an FT-like gene in barley acts upstream of important row-type genes and thus provides a direct link between a flowering gene and spike architecture.
CONCLUSION
Overexpression of HvFT3 accelerated the spikelet initiation and the early reproductive development of spring barley independently of the photoperiod but did not accelerate floral development. Transgenic lines did not flower under SD conditions, suggesting that overexpression of HvFT3 could not compensate for an LD-dependent signal necessary for successful floral development. This strongly suggests that HvFT3 is functionally different from HvFT1, which has been associated with both spikelet initiation and floral development. HvFT3 was dominant over the repressive effect of the vernalization pathway and induced spikelet initiation of winter and spring barley under SD conditions. HvFT3 overexpression modified the expression of a number of floral regulators, floral homeotic genes, and genes involved in GA and auxin synthesis, signaling, and response pathways in the shoot apex. It also upregulated the expression of barley row-type genes VRS4, VRS1, and INT-C, which suggested that FT-like genes may control spike architecture in addition to modulating developmental timing.
MATERIALS AND METHODS
Generation and Growth Conditions of Transgenic Ubi::HvFT3 Lines
Barley (Hordeum vulgare) plants of the spring variety Golden Promise were transformed with an overexpression construct generated with the genomic DNA clones of HvFT3 from Golden Promise driven by the maize (Zea mays) ubiquitin promoter (Ubi-I; Christensen et al., 1992). The overexpression cassette was inserted into the pWBVEC8 binary vector (Wang et al., 1998) and introduced into Agrobacterium tumefaciens. A. tumefaciens-mediated transformation was then performed on excised barley embryos (Tingay et al., 1997; Matthews et al., 2001).
Independent barley transformants were regenerated, and T1 and T2 plants were screened for the presence of the transgene using two pairs of primers that bind to the hygromycin selectable marker gene and the HvFT3 genomic DNA (gDNA) sequence (Supplemental Table S5).
Five independent transgenic T2 families designated as Ubi::HvFT3 lines N1, N2, N4, N16, and N23, in addition to two control lines, a null segregant line that does not carry the transgene, and Golden Promise, were all sown in soil and grown under SDs (8 h light/16 h dark) and LDs (16 h light/8 h dark) in the greenhouse (temperature 20°C/16°C days/nights). Four to 15 plants of each of the lines were used to score flowering time, which was measured in days from germination until heading (days after germination [DAG]). Heading was scored as the emergence of spike awns out of the sheath of the main shoot flag leaf (Zadoks stage 49; Zadoks et al., 1974), and the experiment was stopped 120 DAG under SDs. For each tested line, leaf material from every plant (each plant represents a biological replicate) was collected 5 DAG, 2 h before the end of the light (Zeitgeber, T14 in LD and T6 in SD) to perform RNA extraction and gene expression analysis. In addition, expression of HvFT3 and HvFT1 was surveyed in transgenic, null, and wild-type genotypes during development and 6, 13, 20, and 28 d after sowing under LD conditions. Leaf samples were harvested 2 h before the end of the day, and three biological replicates were analyzed per line and time point.
We further examined whether variation at Ppd-H1 affected HvFT3 expression in wild-type plants under LDs and SDs. For this purpose, we evaluated HvFT3 expression levels in Golden Promise (ppd-H1) and a derived introgression line with a wild-type Ppd-H1 allele from the winter barley Igri over development under LDs and SDs. Three replicate leaves per genotype and time point were harvested 2 h before the end of the day for RNA extraction and expression analysis.
Generation of Arabidopsis Transgenic 35S::HvFT3 Lines and Their Growth Conditions
The genomic DNA of HvFT3 from Golden Promise was ligated into a 35S promoter-driven pLeela binary vector following the manufacturer’s instructions (Invitrogen). The resulting construct was introduced into the Arabidopsis (Arabidopsis thaliana) ft-10 mutant (Transfer DNA, T-DNA insertion alleles in the Columbia background; Yoo et al., 2005) through A. tumefaciens-mediated transformation by the floral-dip method (Clough and Bent, 1998). An empty pLeela vector was also introduced into ft-10 to serve as a control line (mock). 35S::FT seeds were kindly provided by Dr. Fernando Andres. Plants from three independent ft-10 35S::HvFT3 lines in addition to the mock, 35S::FT, and ft-10 lines were grown in the greenhouse under LD (16 h light/8 h dark) and SD (8 h light/16 h dark) at a temperature of 21°C/19°C light/dark. Flowering-time was measured as the total number of leaves when the first flower opened. Seven to 12 plants per line were scored.
Inflorescence Development of Transgenic Ubi::HvFT3 Lines
Transgenic Ubi::HvFT3 plants in addition to null segregant and wild-type Golden Promise plants were grown under SDs (8 h light/16 h dark) or LDs (16 h light/8 h dark) in the greenhouse (temperature 20°C/16°C days/nights). The transgenic T4 plants grown under SD and LD were generated from lines N23 and N16, respectively. Primary shoots of Ubi::HvFT3, null segregant, and wild-type plants were dissected each 2 to 3 d under LD and 6 to 8 d under SD. Two to four replicates were dissected at each time point. Phenotypic changes of the SAM were monitored and evaluated according to the Waddington scale for inflorescence development (Waddington et al., 1983).
Generation and Growth Conditions of Ubi::HvFT3 × Igri F2 Population
The transgenic line Ubi::HvFT3 N23 was crossed with the winter barley variety Igri. Golden Promise (wild type), the genetic background of the transgenic line, is a spring variety that carries a functional allele of the HvFT3 gene (Laurie et al., 1995; Faure et al., 2007). Golden Promise also carries a mutation in the CONSTANS, CONSTANS-like and TOC1 (CCT) domain of Ppd-H1, which causes reduced photoperiod sensitivity and delayed flowering under LDs (Turner et al., 2005). In addition, Golden Promise is characterized by a spring allele at VRN-H1 and a deletion of the VRN-H2 locus and consequently does not require vernalization for the induction of flowering. In contrast, Igri carries the winter null allele of HvFT3, a dominant Ppd-H1 allele with a strong photoperiod response, and winter alleles at VRN-H1 and VRN-H2 and thus needs vernalization to flower.
One hundred and sixty-six F2 plants derived from the cross Ubi::HvFT3 × Igri were sown in soil and grown in the greenhouse (temperature 20°C/16°C days/nights) under LD (16 h/8 h light/dark) conditions. Seedlings were not subjected to vernalization, and flowering time was scored as number of days until heading (Zadoks stage 49). Leaf material was harvested from parental lines and 69 F2 lines 5 DAG at T14 under LD and subsequently used for RNA extraction and gene expression analysis. The selection of F2 lines for gene expression analysis under LD was based on the genotypic information to ensure balanced allele combinations at the analyzed flowering time genes (the transgene, HvFT3 [wild type], Ppd-H1, VRN-H1, and VRN-H2).
Generation and Growth Conditions of HvFT3 F4 Spring Families
For testing the effect of natural variation at HvFT3 on MSA development, three F2:F4 families were selected from the cross Ubi::HvFT3 × Igri that varied at HvFT3 but carried spring alleles at Ppd-H1, VRN-H1, and VRN-H2. The development of the MSA of these selected families was scored under SD (8 h light/16 h dark, temperature 20°C/16°C) and LD (13 h light/11 h dark, temperature 20°C/16°C). Primary shoots of six plants from each allelic combination (two plants/family) were dissected each 3 to 7 and 6 to 10 d, under LDs and SDs, respectively. None of the plants reached heading under SDs, whereas only some plants did not flower by 120 DAG and were given a score of 120 DAG.
Generation, Growth Conditions, and the Vernalization/SD Response Experiment of HvFT3 F4 Winter Families
Four nontransgenic F2:F4 families were developed from selected F2 lines of the cross Ubi::HvFT3 × Igri. Plants of the developed families carried the winter alleles vrn-H1 and VRN-H2, the photoperiod-responsive allele Ppd-H1, and either the winter null allele hvft3 or the spring functional allele HvFT3. Plants from both families were grown under two different treatments: VLD_LD, 8 weeks of vernalization under LDs (16 h light/8 h dark, temperature 4°C) followed by LDs (16 h light/8 h dark, temperature 20°C/16°C); and SD_LD, 8 weeks of SDs (8 h light/16 h dark, temperature 20°C/16°C) followed by LDs (16 h light/8 h dark, temperature 20°C/16°C). Flowering time was scored for 10 plants per treatment and genotype.
DNA Extraction and Genotyping of the Segregating Populations
Genomic DNA of individual F2 genotypes was extracted from leaf samples following the Biosprint DNA extraction protocol (Qiagen). F2 genotypes of all analyzed populations were genotyped for the presence of the transgene and allelic diversity of the major flowering genes Ppd-H1 (Turner et al., 2005), VRN-H1 (Hemming et al., 2009), VRN-H2 (Dubcovsky et al., 2005), and HvFT3 (Faure et al., 2007; Kikuchi et al., 2009). Polymerase chain reactions (PCRs) were performed as described in the original references (list of primers in Supplemental Table S5).
RNA Extraction, cDNA Synthesis, and Reverse Transcription Quantitative PCR
Total RNA extraction, first-strand cDNA synthesis, and reverse transcription quantitative PCR (RT-qPCR) for transgenic and wild-type plants as well as F2 plants were performed as described in Campoli et al. (2012). RT-qPCR was performed using gene-specific primers (Supplemental Table S5). Two technical replicates were used for each cDNA sample, and starting amounts for each data point were calculated based on the titration curve for each target gene and the reference (HvActin) gene using the LightCycler 480 Software (Roche; version 1.5).
Statistical Analysis
The statistical significance of differences in flowering time and gene expression levels between each of the Ubi::HvFT3 genotypes and the wild-type and the null controls (wild type + Null combined) grown under LDs and SDs was determined using Student’s t test. A fixed-model ANOVA for unbalanced designs was used to calculate significant effects and two-way interaction effects of the transgene and allelic variation at Ppd-H1, VRN-H1, VRN-H2, and HvFT3 on flowering time and gene expression in all tested F2 populations. Pearson correlation coefficients were calculated between flowering time and gene expression values in the tested population.
RNA Sequencing Experiment and Analysis
RNA was isolated from tissue of the MSA harvested from Ubi::HvFT3 and null segregant plants grown under LD and SD conditions. MSA tissue was harvested at the developmental stages W2.0 and W3.0 to 3.5 2 h before the end of the light period. Leaves surrounding the MSA were removed manually, and the apex was cut using a microsurgical stab knife (5-mm blade at 15° [SSC#72-1551]). For each of three biological replicates, at least 10 MSA were pooled. The MSA harvested for RNA extraction were frozen immediately in liquid nitrogen and stored at −80°C. The RNA was isolated as described in van Esse et al. (2017). The Illumina cDNA libraries were prepared according to the TruSeq RNA sample preparation (version 2; Illumina). A cBot (Illumina) was used for clonal sequence amplification and generation of sequence clusters. Single-end sequencing was performed using a HiSeq 2500 (Illumina) platform by multiplexing 8 libraries resulting in ∼18 million reads per library. The sequencing data quality was verified using FastQC software (version 0.10.1, http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/) before further processing using the CLC Genomics workbench (version 6.0.4, CLCbio). PCR duplicates were removed from the raw sequencing data using the Duplicate Read Removal plugin of CLC. The reads were trimmed with an error probability limit calculated from the Phred scores of 0.05, allowing for a maximum of two ambiguously called nucleotides per read. Reads shorter than 60 bp, subsequent to the quality-based trimming, were removed from the dataset.
The obtained RNA sequencing reads were mapped to a barley high confidence transcripts reference (Beier et al., 2017; Mascher et al., 2017) using Salmon in quasi-mapping-based mode as described in van Esse et al. (2017). When building the quasi-mapping-based index, an auxiliary k-mer hash over k-mers of length 31 was used. U (unstranded single end read) was chosen as library type to quantify the reads of each library. The expected number of reads (NumReads) that have originated from each transcript given the structure of the uniquely mapping and multimapping reads and the relative abundance estimates for each transcript and transcripts per million values were extracted using Salmon (Patro et al., 2017). The NumReads was used in downstream analysis. Transcripts with expression levels greater than five NumReads in at least three libraries under each condition (LD, SD) were retained. The expected number of reads and normalized counts per million values are provided in Supplemental Table S4. Differentially regulated reads were called using the R bioconductor package Limma-vroom (Ritchie et al., 2015) using a Benjamin and Hochberg adjustment for multiple testing for calculation of the adjusted P values (false discovery rate; FDR values). For expression analysis, an FDR value of 0.05 was used as cut-off value for the selection of differentially-expressed transcripts (DETs). DETs were extracted per developmental stage between the genotypes and per genotype between the developmental stages. Hierachical cluster analysis was done in R using Pearson correlation coefficients. The overrepresentation analysis of particular GO terms was performed using the R-package TopGo (Alexa and Rahnenfuhrer, 2016). The Venn diagram was drawn using the R package VennDiagram (Chen and Boutros, 2011). The correspondence of MLOC to HORVU gene identifiers was estimated using reciprocal blastn analysis (identity score > 95%). The gene names were extracted based on the MLOC identifiers as annotated in Digel et al. (2015).
Illumina data is available in the European Short Read Archive, EBI ArrayExpress E-MTAB-7158. Accession numbers of major flowering-time genes are listed in Supplemental Table S5.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression of HvFT3 and HvFT1 in Ubi::HvFT3 transgenic lines, null segregant and Golden Promise plants 6, 13, 20, and 28 d after germination under LD conditions.
Supplemental Figure S2. Expression of HvFT3 in Golden Promise (GP) and a derived introgression line with a wild-type Ppd-H1 allele under LDs and SDs at 6, 13, 20, and 28 d after germination.
Supplemental Figure S3. Developing shoot apices in wild-type and transgenic plants.
Supplemental Figure S4. Complementation experiment in Arabidopsis.
Supplemental Figure S5. Effects of the transgene Ubi::HvFT3 and its interaction with allelic variation at Ppd-H1 on flowering time of the F2 population Ubi::HvFT3 × Igri under LD conditions.
Supplemental Figure S6. Principal component analysis of normalized expression from all expressed genes under LDs and SDs.
Supplemental Figure S7. Coexpression clustering of 154 DETs regulated between the Ubi::HvFT3 and null segregant genotypes at the lemma primordium stage.
Supplemental Table S1. ANOVA of flowering time of the F2 population Ubi::HvFT3 × Igri grown under LD conditions.
Supplemental Table S2. Pearson correlation coefficients of flowering time and expression levels of tested flowering genes in the F2 population Ubi::HvCFT3 × Igri grown under LD conditions.
Supplemental Table S3. ANOVA of expression of all tested flowering genes in the F2 population Ubi::HvFT3 × Igri grown under LD conditions.
Supplemental Table S4. Annotation of differentially expressed transcripts, log-fold change, FDR values, number of reads, and normalized counts per million values per genotype, photoperiod, stage, and replicate.
Supplemental Table S5. List of primers used in this study.
Acknowledgments
The authors cordially thank Kerstin Luxa, Caren Dawidson, and Andrea Lossow for excellent technical assistance and Chiara Campoli for preparing the Ubi::HvFT3 constructs.
Footnotes
This research was funded by the German Cluster of Excellence on Plant Sciences (CEPLAS) EXC1028, the Priority Programme (SPP1530 Flowering time control: from natural variation to crop improvement), and the Max Planck Society. M.A.M. received a fellowship from the DAAD (German Academic Exchange Service). X.B. received a fellowship from the CSC (Chinese Scholarship Council).
Articles can be viewed without a subscription.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Alexa A, Rahnenfuhrer J (2016) topGO: Enrichment Analysis for Gene Ontology. R package version 2.30.0. [Google Scholar]
- Amasino RM, Michaels SD (2010) The timing of flowering. Plant Physiol 154: 516–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13: 627–639 [DOI] [PubMed] [Google Scholar]
- Andrés F, Porri A, Torti S, Mateos J, Romera-Branchat M, García-Martínez JL, Fornara F, Gregis V, Kater MM, Coupland G (2014) SHORT VEGETATIVE PHASE reduces gibberellin biosynthesis at the Arabidopsis shoot apex to regulate the floral transition. Proc Natl Acad Sci USA 111: E2760–E2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard M, Kirby EJM, Fellowes G (1982) Relationships between duration of phases in the pre-anthesis life cycle of spring barley. Aust J Agric Res 33: 917–925 [Google Scholar]
- Araki T. (2001) Transition from vegetative to reproductive phase. Curr Opin Plant Biol 4: 63–68 [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini ES, Kramer EM (2011) In the light of evolution: a reevaluation of conservation in the CO-FT regulon and its role in photoperiodic regulation of flowering time. Front Plant Sci 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier S, Himmelbach A, Colmsee C, Zhang XQ, Barrero RA, Zhang Q, Li L, Bayer M, Bolser D, Taudien S, et al. (2017) Construction of a map-based reference genome sequence for barley, Hordeum vulgare L. Sci Data 4: 170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden SA, Cavanagh C, Cullis BR, Ramm K, Greenwood J, Jean Finnegan E, Trevaskis B, Swain SM (2015) Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nat Plants 1: 14016. [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell 16 (Suppl): S18–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli C, von Korff M (2014) Genetic Control of Reproductive Development in Temperate Cereals. In Jacquot J-P, Gadal P, Fornara F, eds, Advances in Botanical Research: Vol. 72. The Molecular Genetics of Floral Transition and Flower Development. Academic Press, San Diego, CA, pp 131–158. [Google Scholar]
- Campoli C, Drosse B, Searle I, Coupland G, von Korff M (2012) Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J 69: 868–880 [DOI] [PubMed] [Google Scholar]
- Campoli C, Pankin A, Drosse B, Casao CM, Davis SJ, von Korff M (2013) HvLUX1 is a candidate gene underlying the early maturity 10 locus in barley: phylogeny, diversity, and interactions with the circadian clock and photoperiodic pathways. New Phytol 199: 1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casao MC, Igartua E, Karsai I, Lasa JM, Gracia MP, Casas AM (2011a) Expression analysis of vernalization and day-length response genes in barley (Hordeum vulgare L.) indicates that VRNH2 is a repressor of PPDH2 (HvFT3) under long days. J Exp Bot 62: 1939–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casao MC, Karsai I, Igartua E, Gracia MP, Veisz O, Casas AM (2011b) Adaptation of barley to mild winters: a role for PPDH2. BMC Plant Biol 11: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelain M, Le Hir R, Bellini C (2012) The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiol Plant 145: 450–460 [DOI] [PubMed] [Google Scholar]
- Castillejo C, Pelaz S (2008) The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr Biol 18: 1338–1343 [DOI] [PubMed] [Google Scholar]
- Chardon F, Damerval C (2005) Phylogenomic analysis of the PEBP gene family in cereals. J Mol Evol 61: 579–590 [DOI] [PubMed] [Google Scholar]
- Chen X. (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Boutros PC (2011) VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18: 675–689 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Danilevskaya ON, Meng X, Hou Z, Ananiev EV, Simmons CR (2008) A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol 146: 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Casao MC, Wang P, Sato K, Hayes PM, Finnegan EJ, Trevaskis B (2015) Direct links between the vernalization response and other key traits of cereal crops. Nat Commun 6: 5882. [DOI] [PubMed] [Google Scholar]
- Digel B, Pankin A, von Korff M (2015) Global transcriptome profiling of developing leaf and shoot apices reveals distinct genetic and environmental control of floral transition and inflorescence development in barley. Plant Cell 27: 2318–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digel B, Tavakol E, Verderio G, Tondelli A, Xu X, Cattivelli L, Rossini L, von Korff M (2016) Photoperiod-H1 (Ppd-H1) controls leaf size. Plant Physiol 172: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Dubcovsky J (2010) Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol Genet Genomics 283: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Chen C, Yan L (2005) Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Mol Breed 15: 395–407 [Google Scholar]
- Dubcovsky J, Loukoianov A, Fu D, Valarik M, Sanchez A, Yan L (2006) Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol Biol 60: 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz M, von Korff M (2017) The genetic control of reproductive development under high ambient temperature. Plant Physiol 173: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. (1987) Short day induction of inflorescence initiation in some winter wheat varieties. Aust J Plant Physiol 14: 277–286 [Google Scholar]
- Faure S, Higgins J, Turner A, Laurie DA (2007) The FLOWERING LOCUS T-like gene family in barley (Hordeum vulgare). Genetics 176: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA (2012) Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc Natl Acad Sci USA 109: 8328–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González FG, Slafer GA, Miralles DJ (2002) Vernalization and photoperiod responses in wheat pre-flowering reproductive phases. Field Crops Res 74: 183–195 [Google Scholar]
- Goto K, Meyerowitz EM (1994) Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev 8: 1548–1560 [DOI] [PubMed] [Google Scholar]
- Gou J, Fu C, Liu S, Tang C, Debnath S, Flanagan A, Ge Y, Tang Y, Jiang Q, Larson PR, et al. (2017) The miR156-SPL4 module predominantly regulates aerial axillary bud formation and controls shoot architecture. New Phytol 216: 829–840 [DOI] [PubMed] [Google Scholar]
- Griffiths FEW, Lyndon RF, Bennett MD (1985) The effects of vernalization on the growth of the wheat shoot apex. Ann Bot 56: 501–511 [Google Scholar]
- Halliwell J, Borrill P, Gordon A, Kowalczyk R, Pagano ML, Saccomanno B, Bentley AR, Uauy C, Cockram J (2016) Systematic investigation of FLOWERING LOCUS T-like Poaceae gene families identifies the short-day expressed flowering pathway gene, TaFT3 in wheat (Triticum aestivum L.). Front Plant Sci 7: 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P (2000) Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J 21: 351–360 [DOI] [PubMed] [Google Scholar]
- Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719–722 [DOI] [PubMed] [Google Scholar]
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B (2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 147: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming MN, Fieg S, Peacock WJ, Dennis ES, Trevaskis B (2009) Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Mol Genet Genomics 282: 107–117 [DOI] [PubMed] [Google Scholar]
- Hsu CY, Adams JP, Kim H, No K, Ma C, Strauss SH, Drnevich J, Vandervelde L, Ellis JD, Rice BM, et al. (2011) FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA 108: 10756–10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shi T, Zheng B, Yumul RE, Liu X, You C, Gao Z, Xiao L, Chen X (2017) APETALA2 antagonizes the transcriptional activity of AGAMOUS in regulating floral stem cells in Arabidopsis thaliana. New Phytol 215: 1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun Y, Lee I (2006) KIDARI, encoding a non-DNA Binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mol Biol 61: 283–296 [DOI] [PubMed] [Google Scholar]
- Hyun Y, Richter R, Coupland G (2017) Competence to flower: age-controlled sensitivity to environmental cues. Plant Physiol 173: 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y (2007) Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant J 51: 1030–1040 [DOI] [PubMed] [Google Scholar]
- Jones H, Leigh FJ, Mackay I, Bower MA, Smith LMJ, Charles MP, Jones G, Jones MK, Brown TA, Powell W (2008) Population-based resequencing reveals that the flowering time adaptation of cultivated barley originated east of the Fertile Crescent. Mol Biol Evol 25: 2211–2219 [DOI] [PubMed] [Google Scholar]
- Jung JH, Seo YH, Seo PJ, Reyes JL, Yun J, Chua NH, Park CM (2007) The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19: 2736–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kawahigashi H, Ando T, Tonooka T, Handa H (2009) Molecular and functional characterization of PEBP genes in barley reveal the diversification of their roles in flowering. Plant Physiol 149: 1341–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Komatsuda T, Pourkheirandish M, He C, Azhaguvel P, Kanamori H, Perovic D, Stein N, Graner A, Wicker T, Tagiri A, et al. (2007) Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc Natl Acad Sci USA 104: 1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135: 767–774 [DOI] [PubMed] [Google Scholar]
- Komiya R, Yokoi S, Shimamoto K (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136: 3443–3450 [DOI] [PubMed] [Google Scholar]
- Koppolu R, Anwar N, Sakuma S, Tagiri A, Lundqvist U, Pourkheirandish M, Rutten T, Seiler C, Himmelbach A, Ariyadasa R, et al. (2013) Six-rowed spike4 (Vrs4) controls spikelet determinacy and row-type in barley. Proc Natl Acad Sci USA 110: 13198–13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA. (2011) Auxin regulation of Arabidopsis flower development involves members of the AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) family. J Exp Bot 62: 3311–3319 [DOI] [PubMed] [Google Scholar]
- Laurie DA. (1997) Comparative genetics of flowering time. In Sasaki T and Moore G, eds, Oryza: From Molecule to Plant. Springer, Dordrecht, Germany, pp 167–177 [PubMed] [Google Scholar]
- Laurie DA, Pratchett N, Snape JW, Bezant JH (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter x spring barley (Hordeum vulgare L.) cross. Genome 38: 575–585 [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH (2007) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev 21: 397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H (2008) A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell 15: 110–120 [DOI] [PubMed] [Google Scholar]
- Licausi F, Ohme-Takagi M, Perata P (2013) APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol 199: 639–649 [DOI] [PubMed] [Google Scholar]
- Lv B, Nitcher R, Han X, Wang S, Ni F, Li K, Pearce S, Wu J, Dubcovsky J, Fu D (2014) Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS One 9: e94171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277 [DOI] [PubMed] [Google Scholar]
- Mascher M, Jost M, Kuon JE, Himmelbach A, Aßfalg A, Beier S, Scholz U, Graner A, Stein N (2014) Mapping-by-sequencing accelerates forward genetics in barley. Genome Biol 15: R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, Wicker T, Radchuk V, Dockter C, Hedley PE, Russell J, et al. (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544: 427–433 [DOI] [PubMed] [Google Scholar]
- Matthews PR, Wang MB, Waterhouse PM, Thornton S, Fieg SJ, Gubler F, Jacobsen JV (2001) Marker gene elimination from transgenic barley, using co-transformation with adjacent twin T-DNAs’ on a standard Agrobacterium transformation vector. Mol Breed 7: 195–202 [Google Scholar]
- McKinney HH, Sando WJ (1935) Earliness of sexual reproduction in wheat as influenced by temperature and light in relation to growth phases. J Agric Res 51: 621–641 [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM (2005) Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol 137: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles DJ, Richards RA (2000) Response of leaf and tiller emergence and primordium initiation in wheat and barley to interchanged photoperiod. Ann Bot 85: 655–663 [Google Scholar]
- Miyoshi K, Ahn B-O, Kawakatsu T, Ito Y, Itoh J-I, Nagato Y, Kurata N (2004) PLASTOCHRON1, a timekeeper of leaf initiation in rice, encodes cytochrome P450. Proc Natl Acad Sci USA 101: 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 14 (Suppl): S111–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulki MA, von Korff M (2016) CONSTANS controls floral repression by up-regulating VERNALIZATION2 (VRN-H2) in Barley. Plant Physiol 170: 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankin A, Campoli C, Dong X, Kilian B, Sharma R, Himmelbach A, Saini R, Davis SJ, Stein N, Schneeberger K, et al. (2014) Mapping-by-sequencing identifies HvPHYTOCHROME C as a candidate gene for the early maturity 5 locus modulating the circadian clock and photoperiodic flowering in barley. Genetics 198: 383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C (2017) Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14: 417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Vanzetti LS, Dubcovsky J (2013) Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION1. Plant Physiol 163: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405: 200–203 [DOI] [PubMed] [Google Scholar]
- Ramsay L, Comadran J, Druka A, Marshall DF, Thomas wild-type, Macaulay M, MacKenzie K, Simpson C, Fuller J, Bonar N, et al. (2011) INTERMEDIUM-C, a modifier of lateral spikelet fertility in barley, is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1. Nat Genet 43: 169–172 [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EH, Summerfield RJ, Cooper JP, Ellis RH (1988) Environmental control of flowering in barley (Hordeum vulgare L.). I. Photoperiod limits to long-day responses, photoperiod-insensitive phase and effects of low-temperature and short-day vernalization. Ann Bot 62: 127–144 [Google Scholar]
- Rollins JA, Drosse B, Mulki MA, Grando S, Baum M, Singh M, Ceccarelli S, von Korff M (2013) Variation at the vernalisation genes Vrn-H1 and Vrn-H2 determines growth and yield stability in barley (Hordeum vulgare) grown under dryland conditions in Syria. Theor Appl Genet 126: 2803–2824 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kobayashi M, Itoh H, Tagiri A, Kayano T, Tanaka H, Iwahori S, Matsuoka M (2001) Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol 125: 1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasani S, Hemming MN, Oliver SN, Greenup A, Tavakkol-Afshari R, Mahfoozi S, Poustini K, Sharifi HR, Dennis ES, Peacock WJ, et al. (2009) The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J Exp Bot 60: 2169–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RJ, Linley PJ, Gutierrez-Marcos JF, Delli-Bovi T, Farmer PR, Kohchi T, Terry MJ, Brutnell TP (2004) The Elm1 (ZmHy2) gene of maize encodes a phytochromobilin synthase. Plant Physiol 136: 2771–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Uhlenhaut NH, Godard F, Demar M, Bressan R, Weigel D, Lohmann JU (2003) Dissection of floral induction pathways using global expression analysis. Development 130: 6001–6012 [DOI] [PubMed] [Google Scholar]
- Schwarz S, Grande AV, Bujdoso N, Saedler H, Huijser P (2008) The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol Biol 67: 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Ogawa T, Kitagawa S, Suzuki T, Ikari C, Shitsukawa N, Abe T, Kawahigashi H, Kikuchi R, Handa H, et al. (2009) A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J 58: 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C (2002) Arabidopsis, the Rosetta stone of flowering time? Science 296: 285–289 [DOI] [PubMed] [Google Scholar]
- Slafer GA, Rawson HM (1994) Sensitivity of wheat phasic development to major environmental factors: a re-examination of some assumptions made by physiologists and modellers. Funct Plant Biol 21: 393–426 [Google Scholar]
- Tingay S, McElroy E, Kalla R, Fieg S, Wang M, Thornton S, Brettell R (1997) Agrobacterium tumefaciens-mediated barley transformation. Plant J 11: 1369–1376 [Google Scholar]
- Torti S, Fornara F, Vincent C, Andrés F, Nordström K, Göbel U, Knoll D, Schoof H, Coupland G (2012) Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell 24: 444–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310: 1031–1034 [DOI] [PubMed] [Google Scholar]
- van Esse GW, Walla A, Finke A, Koornneef M, Pecinka A, von Korff M (2017) Six-rowed Spike3 (VRS3) is a histone demethylase that controls lateral spikelet development in barley. Plant Physiol 174: 2397–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Korff M, Wang H, Léon J, Pillen K (2006) AB-QTL analysis in spring barley: II. Detection of favourable exotic alleles for agronomic traits introgressed from wild barley (H. vulgare ssp. spontaneum). Theor Appl Genet 112: 1221–1231 [DOI] [PubMed] [Google Scholar]
- von Korff M, Léon J, Pillen K (2010) Detection of epistatic interactions between exotic alleles introgressed from wild barley (H. vulgare ssp. spontaneum). Theor Appl Genet 121: 1455–1464 [DOI] [PubMed] [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC (1983) A quantitative scale of spike initial and pistil development in barley and wheat. Ann Bot 51: 119–130 [Google Scholar]
- Wang H, Wang H (2015) Multifaceted roles of FHY3 and FAR1 in light signaling and beyond. Trends Plant Sci 20: 453–461 [DOI] [PubMed] [Google Scholar]
- Wang G, Schmalenbach I, von Korff M, Léon J, Kilian B, Rode J, Pillen K (2010) Association of barley photoperiod and vernalization genes with QTLs for flowering time and agronomic traits in a BC2DH population and a set of wild barley introgression lines. Theor Appl Genet 120: 1559–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Czech B, Weigel D (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138: 738–749 [DOI] [PubMed] [Google Scholar]
- Wang MB, Matthews PR, Upadhyaya NM, Waterhouse PM (1998) Improved vectors for Agrobacterium tumefaciens-mediated transformation of monocot plants. Acta Hortic 461: 401–407 [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Wu MF, Yang L, Wu G, Poethig RS, Wagner D (2009) The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell 17: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol 139: 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14: 415–421 [Google Scholar]