Endogenous free para-aminobenzoic acid levels modulate the auxin-ethylene crosstalk necessary for root gravitropism
Abstract
Plants respond to gravitational force through directional growth along the gravity vector. Although auxin is the central component of the root graviresponse, it works in concert with other plant hormones. Here, we show that the folate precursor para-aminobenzoic acid (PABA) is a key modulator of the auxin-ethylene interplay during root gravitropism in Arabidopsis (Arabidopsis thaliana). In gravistimulated roots, PABA promotes an asymmetric auxin response, which causes the asymmetric growth responsible for root curvature. This activity requires the auxin response transcription factors AUXIN RESPONSE FACTOR7 (ARF7) and ARF19 as well as ethylene biosynthesis and signaling, indicating that PABA activity requires both auxin and ethylene pathways. Similar to ethylene, exogenous PABA reverses the agravitropic root growth of the auxin transport mutant pin-formed2 (pin2) and the auxin biosynthetic double mutant with loss of function of weak ethylene insensitive (wei) genes, wei8wei2, but not the pin2wei8wei2 triple mutant. This finding suggests that PABA regulates the ethylene-dependent reciprocal compensation between auxin transport and biosynthesis. Furthermore, manipulation of endogenous free PABA levels by modulating the expression of the gene encoding its glucosylation enzyme, UDP-GLYCOSYL TRANSFERASE75B1, impacts the root graviresponse, suggesting that endogenous free PABA levels may play a crucial role in modulating the auxin-ethylene cross talk necessary for root gravitropism.
In the root, gravity perception occurs in columella cells, where the sedimentation of statoliths (starch-filled plastids) triggers a range of molecular events regulated by individual and interacting activities of plant hormones (Sack et al., 1985; Kiss et al., 1989; Ottenschläger et al., 2003). For instance, the creation of an asymmetric auxin gradient is instrumental in the transduction of the signal from the columella to the elongation zone, where the asymmetric growth response occurs (Swarup et al., 2005). The gaseous hormone ethylene inhibits the elongation of cells located on the lower side of the gravistimulated root (Aloni et al., 2006). Finally, auxin-ethylene cross talk is involved in the regulation of root penetration into the soil, a process that implies root gravitropism (Santisree et al., 2011). However, the mechanisms underlying auxin-ethylene cross talk in gravitropism remain to be resolved.
Auxin gradients are involved in diverse developmental processes such as embryogenesis, organogenesis, tissue patterning, and tropisms (Vanneste and Friml, 2009). Homeostasis of the most prominent endogenous plant auxin, indole-3-acetic acid (IAA), is controlled through de novo biosynthesis and degradation, the formation of inactive IAA conjugates, and transport. The establishment of auxin gradients occurs through active auxin transport driven by networks of AUXIN-RESISTANT1 (AUX1)/LIKE-AUX1 influx carriers (Bennett et al., 1996; Parry et al., 2001; Yang et al., 2006; Swarup et al., 2008), PIN-FORMED (PIN) efflux carriers (Gälweiler et al., 1998; Petrásek et al., 2006), PIN-LIKES (Barbez et al., 2012), and ATP BINDING CASSETTE B-type transporters (Geisler and Murphy, 2006; Lewis et al., 2009). The auxin-induced response relies on the signaling machinery composed of the receptors TRANSPORT INHIBITOR RESPONSE1/AUXIN F-BOXES (TIR1/AFBs) and the transcriptional repressors AUXIN/INDOLE-3-ACETIC ACID (AUX/IAAs). High levels of auxin activate TIR1/AFBs, which mediate the auxin-dependent proteasomal degradation of the repressors, subsequently allowing the activation of the auxin response transcription factor (ARF) family, the activity of which would otherwise be blocked by AUX/IAA proteins (Salehin et al., 2015).
During the gravity response, auxin is redistributed asymmetrically to the lower side of the curving root tip (Ottenschläger et al., 2003; Band et al., 2012). This process is initiated by PIN3 and PIN7 efflux facilitators in the columella (Friml et al., 2002; Nacry et al., 2005; Kleine-Vehn et al., 2010) and is promoted further by AUX1 influx and PIN2 efflux carriers that catalyze auxin transport from columella cells to the epidermal cells of the elongation zone (Müller et al., 1998; Swarup et al., 2001, 2005; Ottenschläger et al., 2003), where ARF7/NHP4 and ARF19 are believed to activate the transcriptional module of the auxin response that ultimately causes the inhibition of cell elongation on the lower side of the root (Takahashi et al., 2009). Consistent with previous findings, loss of function of the AUX1 (Bennett et al., 1996) or PIN2 gene results in agravitropic root growth (Luschnig et al., 1998; Müller et al., 1998; Ditengou et al., 2018), while the root response to gravity is impaired in the arf7 arf19 double mutant (Okushima et al., 2005; Weijers et al., 2005).
Several lines of evidence suggest that auxin-ethylene cross talk, which occurs at the level of biosynthesis, transport, and signaling, regulates important developmental processes such as the asymmetric growth of the hypocotyl hook of etiolated seedlings, root elongation, and root hair development (Stepanova and Alonso, 2005; Stepanova et al., 2007). Ethylene induces auxin biosynthetic genes, such as WEAK ETHYLENE INSENSITIVE2 (WEI2)/ANTHRANILATE SYNTHASE α1 (ASA1), WEI7/ASB1, WEI8/TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1)/TRANSPORT INHIBITOR RESPONSE2 (TIR2), and its homolog TAR1 (Stepanova et al., 2005, 2008), and stimulates local auxin biosynthesis at the root tip (Růzicka et al., 2007). The promotive effect of ethylene on both rootward and shootward auxin transport is consistent with its action in up-regulating several genes encoding auxin transporters, including PIN1, PIN2, PIN4, and AUX1 (Růzicka et al., 2007; Negi et al., 2008; Vandenbussche et al., 2010; Lewis et al., 2011; Muday et al., 2012). In turn, auxin influences ethylene production through the induction of 1-AMINOCYCLOPROPANE-1-CARBOXYLATE SYNTHASE (ACS) genes, which are responsible for the biosynthesis of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC; Woeste et al., 1999; Tsuchisaka and Theologis, 2004). Interestingly, Stepanova et al. (2007) have shown that, in the aux1 mutant, some auxin-insensitive genes are regulated by ethylene, while inversely, in the ethylene insensitive2 (ein2) mutant lacking the central regulator of ethylene signaling, some ethylene-insensitive genes are sensitive to auxin. In addition, several auxin-related mutants have been described to be ethylene insensitive, whereas most ethylene mutants are sensitive to auxin, suggesting that ethylene acts through auxin (Vandenbussche et al., 2012). In the root transition zone, the ethylene response mediated by the transcription factor EIN3 requires high auxin activity (Stepanova et al., 2007), and EIN3 protein accumulation is enhanced by auxin, seemingly through the repression of EIN3-BINDING F-BOX1 (EBF1)- and EBF2-mediated EIN3 degradation (He et al., 2011). However, how ethylene and auxin synergistically impact the asymmetric growth of the gravitropic root remains largely unknown.
Recently, it has been suggested that para-aminobenzoic acid (PABA; 4-aminobenzoic acid) is a novel plant growth regulator (Crisan et al., 2014). PABA is a cyclic nonprotein amino acid produced in plants and microorganisms, including human intestinal bacteria. PABA is a critical precursor in the biosynthesis of folates, which are essential cofactors involved in the biosynthesis of key metabolic compounds such as purines, certain amino acids, and plant hormones (Hanson and Gregory, 2011). The generic term folate refers to a family of compounds derived from tetrahydrofolate, which is a tripartite molecule consisting of pterin (6-hydroxymethyldihydropterin), PABA, and Glu (Supplemental Fig. S1A). PABA is synthesized in plastids from chorismate in two steps catalyzed by the bifunctional GLUTAMINE AMIDOTRANSFERASE-AMINODEOXYCHORISMATE SYNTHASE (GAT-ADCS) and AMINODEOXYCHORISMATE LYASE (Basset et al., 2004a; Camara et al., 2011). Among the different intermediates of the folate pathway, PABA is the only one that is glucosylated (Quinlivan et al., 2003), a process catalyzed by the UDP-GLYCOSYL TRANSFERASE75B1 (UGT75B1; Eudes et al., 2008). More than 80% of total PABA is glucosylated and stored in vacuoles and does not contribute to folate synthesis (Quinlivan et al., 2003; Eudes et al., 2008).
In this report, we show that PABA promotes the root gravitropism of wild-type Arabidopsis (Arabidopsis thaliana) plants in an ethylene-dependent manner. Remarkably, PABA suppresses the root-coiling phenotype of the auxin transport mutant pin2 and the root sinusoidal growth of the auxin biosynthetic double mutant wei8wei2 but not the root-coiling phenotype of the pin2wei8wei2 triple mutant. This finding reveals the presence of an unsuspected reciprocal compensation between auxin transport and local auxin biosynthesis machinery. Our data also show that PABA-promoted root gravitropism is due to PABA-promoted root asymmetric growth in an ARF7- and ARF19-dependent manner. The PABA-biosynthetic gene GAT-ADCS is expressed at the root tip, suggesting the spatial regulation of PABA production, while manipulating endogenous free PABA levels by modulating the expression of its neutralizing enzyme, UGT75B1, impacts the root graviresponse. Overall, these data suggest that endogenous free PABA levels play a crucial role in modulating the auxin-ethylene cross talk necessary for root gravitropism.
RESULTS
PABA Exhibits an Innate, Dose-Dependent, Folate-Independent Activity on Root Gravitropism
PABA may act as a growth regulator of Arabidopsis roots and inhibits root gravitropism when applied at a high concentration (Crisan et al., 2014). To gain insights into how PABA modulates Arabidopsis root growth, 6-d-old Arabidopsis seedlings were grown in medium containing increasing concentrations of PABA (from 25 to 400 µm) in petri dishes tilted with an angle of 60° from the vertical axis (Fig. 1; Supplemental Fig. S1B). Compared with the control, root growth was repressed significantly by PABA starting from a concentration of 100 µm (Supplemental Fig. S1, B and C). Strikingly, PABA also gradually suppressed the root waving in a concentration-dependent manner (arrowheads in Supplemental Fig. S1B). Root waving is a developmental response consisting of sinusoidal (oscillatory) growth due to the regular periodic change in the direction of the root tip (Roux, 2012). This phenomenon occurs when Arabidopsis plants grow on vertically set hard-agar tilted plates and has been suggested to be regulated by touch response, gravitropic response, and an asymmetric auxin distribution (Okada and Shimura, 1990; Thompson and Holbrook, 2004; Paul et al., 2012).
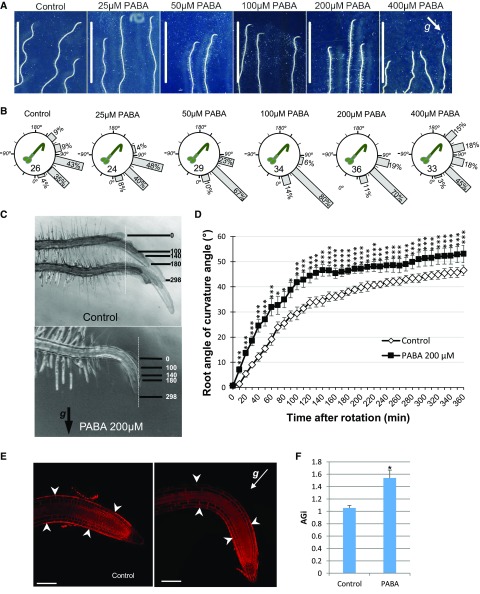
Figure 1.
Impact of PABA on root gravitropism. A, Six-day-old Arabidopsis seedlings were grown on solid Arabidopsis growth medium (Control) or supplemented with increasing concentrations of PABA (25–400 µm) and rotated by 135° for 4 h. PABA impacts on root gravitropism in a dose-dependent manner. g indicates the gravity vector (white arrow). Bars = 1 cm. B, Quantification of root gravitropism of plants presented in A. Root gravitropism is stimulated by low concentrations of PABA (25–200 µm), while high concentrations (from 400 µm) are inhibitory. Gravistimulated roots were assigned to one of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the percentage of seedlings showing the respective direction of root growth. Numbers inside the circles indicate the number of plants for each treatment. C and D, Root curvature angle over time after rotating at 90° of Arabidopsis seedlings grown in the presence or absence of 200 µm PABA. C, Snapshots of Arabidopsis roots grown on control or PABA medium, taken at 0, 100, 140, 180, and 298 min after gravistimulation. g indicates the gravity vector (black arrow). Dashed white lines indicate the position of the root apex at time 0. D, PABA accelerates the root curvature angle over time. Data are shown as means ± se (n = 5). P values are based on Student’s t tests: P < 0.0001 (****), P < 0.001 (***), and P < 0.05 (*). E and F, Determination of the root asymmetric gravitropic index (AGi). E, Arabidopsis seedlings gravistimulated at 135° for 4 h in the absence or the presence of 100 µm PABA. Roots were stained with the plasma membrane marker FM4-64 (red). g indicates the gravity vector (white arrow). Bars = 100 µm. F, Quantification of root AGi as the ratio between the average size of transition and elongation zone (TZ-EZ) epidermal cells of the upper side over the lower side of the curving root (located between the white arrowheads in E). Data are shown as means ± se (n = 10). The star indicates statistical significance (Student’s t test, P < 0.05).
To test whether PABA suppresses root oscillatory patterns through modulating root gravitropism, plates were rotated at an angle of 135° relative to the Earth’s gravity vector for 4 h and their graviresponse was quantified (Fig. 1, A and B). Already at 25 µm PABA, a concentration that does not inhibit root growth (Supplemental Fig. S1, B and C), we quantified a higher percentage of root tips curved at angles between 30° and 90° compared with the control (88% versus 78%), with 0° representing gravity recovery, with the roots growing downward aligned with the Earth’s gravitational field. From 50 to 200 µm, PABA strongly promoted the root gravity response, with root tips reoriented at angles between 30° and 60°. However, at 400 µm PABA, root gravitropism was inhibited, and the angles of curvature were distributed between 60° and 150° (Fig. 1B). These results indicate that root gravitropism is promoted by low (from 25 to 200 µm) but repressed by higher (from 400 µm) concentrations of PABA. Moreover, these data show that root gravitropism is stimulated by PABA at concentrations that do not normally affect root growth (25–50 µm).
As PABA was proposed to exert an auxin-like activity on root development (Crisan et al., 2014), PABA and auxin (IAA) activities on root growth and root gravitropism were compared. Exogenously applied IAA repressed root growth and root gravitropism in a concentration-dependent manner (Bucher and Pilet, 1983; Eliasson et al., 1989). When compared with untreated roots, both 0.05 µm IAA and 100 µm PABA comparably inhibited root growth; however, root gravitropic curvature was inhibited by IAA, while it was promoted by PABA (Supplemental Fig. S2, B and C). These data suggest that PABA and exogenously applied auxin have distinct activities on root gravitropism.
To test whether the effect of PABA on root gravitropism is related to its role as a folate precursor, plants were grown for 6 d on medium supplemented with 200 µm PABA or 200 µm 5-formyltetrahydrofolic acid (5-FTHF), a natural stable derivative that is readily converted to metabolically active folates once incorporated into cells (Camara et al., 2012). PABA, but not 5-FTHF, suppressed the waving of roots, suggesting that PABA and 5-FTHF may have distinct activities on root gravitropism (Supplemental Fig. S3A). To further evaluate the impact of PABA and 5-FTHF on root gravitropic curvature, 5-d-old plants grown on control medium were transferred onto fresh medium supplemented with PABA or 5-FTHF for 24 h and then gravistimulated for 4 h (Supplemental Fig. S3B). Roots treated with 200 μm PABA exhibited enhanced root gravitropism (all root tips curved at angles from 0° to 90°, with the majority of roots curving at angles from 30° to 60°), while 1 mm PABA severely perturbed the root graviresponse (the majority of root tips curved at angles of 60° to 150° for 1 mm PABA; Supplemental Fig. S3B). In contrast, the majority of root tips treated with both 200 µm or 1 mm 5-FTHF displayed gravitropic angles similar to those found in control roots (Supplemental Fig. S3B). Taken together, these data indicate that PABA has an innate activity on root gravitropism that is independent of its canonical role as a folate precursor.
To gain more insight into the kinetics of root gravitropism, we videotaped the gravitropic response of 90°-reoriented Arabidopsis seedlings grown in the presence or absence of PABA. Plants were grown in a bicompartmented petri dish (90 mm diameter) in Arabidopsis growth medium supplemented or not with 200 µm PABA. Roots displayed an accelerated curvature on PABA medium (Fig. 1, C and D; Supplemental Video S1). Already after 10 min, roots of plants grown on PABA curved at 7° versus only 1° for controls, and this difference increased over time (Fig. 1C). This result suggested that asymmetric growth between the upper and lower side of the root was enhanced. This presumption was confirmed by the quantification of the asymmetric growth between the upper and lower sides of the curving root (Fig. 1, E and F). We calculated the AGi as the ratio between the average size of epidermal cells of the upper side over the lower side of the curving root. The AGi of roots grown on PABA (1.53 ± 0.12) was significantly greater than that of control roots (1.05 ± 0.04), strongly suggesting that PABA promotes asymmetric root growth (Fig. 1F).
PABA Promotes an Asymmetric Auxin Response at the Root Tip
During the gravity response, the asymmetric distribution of auxin between the two sides of the gravistimulated root is crucial for root gravitropism (Müller et al., 1998; Ottenschläger et al., 2003) and occurs before the first sign of root bending (Band et al., 2012). To determine whether PABA impacts the auxin response at the root tip, we used both plants expressing the synthetic auxin-sensitive DR5rev promoter driving the expression of a nucleus-localized yellow fluorescent protein (YFP; pDR5rev::3XVENUS-N7; Heisler et al., 2005) and an endoplasmic reticulum-localized GFP (pDR5rev::GFP; Ottenschläger et al., 2003). DR5rev expression was restricted to quiescent center and columella cells in pDR5rev::GFP plants grown in control medium (Ottenschläger et al., 2003; Fig. 2A, time point 0 h, top row; Supplemental Fig. S4), while in pDR5rev::3XVENUS-N7 seedlings grown on 100 μm PABA or pDR5:DR5-GFP plants grown on 200 µm PABA, the overall auxin response appeared to be increased, with the auxin response signal extending from columella to lateral root cap (LRC) cells (Fig. 2A, time point 0 h, bottom row; Supplemental Fig. S4), suggesting that auxin responses are stimulated by PABA in these tissues. After 3 h of gravistimulation, plants grown on control medium exhibited an asymmetric activation of pDR5rev::3XVENUS-N7 in columella and LRC cells of the lower side of the root (Fig. 2A). The signal became visible in epidermal cells after 4 h (Fig. 2A). In comparison, in PABA-treated roots, in addition to the DR5rev signal observed in columella and LRC cells, PABA also induced a significant asymmetric auxin response in epidermal cells of the root TZ-EZ already after 3 h (Fig. 2, A and B). After 5 h, the DR5rev signal also was visible in cortex cells (Fig. 2, A and B). These observations suggest that PABA promotes the gravitropic root curvature through an enhancement of the asymmetric auxin response to the lower side of the root.
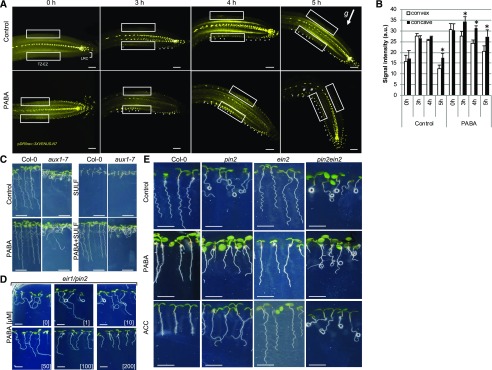
Figure 2.
PABA modulates auxin distribution and rescues the agravitropic root phenotype of the pin2 mutant in an ethylene-dependent manner. A, PABA promotes the asymmetric auxin gradient in 135° gravistimulated roots. Seedlings expressing a nucleus-targeted pDR5rev::3XVENUS-N7 auxin response marker were grown in the absence or presence of 100 µm PABA and gravistimulated for 0, 3, 4, and 5 h. Boxed areas represent the TZ-EZ and LRC cells. Stars indicate asymmetric auxin distribution in the LRC and in the elongation zone. Hash tags indicate DR5 signal in cortex cells. The white arrow indicates the direction of the gravitational vector (g). Bars = 20 µm. B, Quantification of VENUS signal at the upper and lower sides from the root elongation zone cells (boxed areas in A). VENUS signal was quantified in 13 ± 2 epidermal cells of the TZ-EZ starting from the last proximal root cap cell (the same area as in Fig. 1F). Data are shown as means ± se (n = 10). Asterisks indicate statistical significance (P < 0.05 based on Student’s t test). a.u., Arbitrary units. C, Root phenotypes of the aux1 mutant grown on 100 µm PABA or 100 µm of the folate inhibitor sulfanilamide (SULF), alone or in combination, to show that PABA is taken up by aux1 roots but does not rescue the agravitropic aux1 root phenotype. Bars = 1 cm. D, Root graviresponse of 7-d-old pin2 seedlings grown on control medium (0) or in medium supplemented with 1, 10, 50, 100, and 200 µm PABA. pin2 root coiling is suppressed by PABA in a dose-dependent manner. Bars = 0.5 cm. E, Root phenotypes of 7-d-old seedlings of Columbia-0 (Col-0), pin2, ein2, and pin2ein2 mutants on control, 200 µm PABA, and 0.5 µm ACC treatment. Bars = 0.5 cm.
PABA Complements the Agravitropic Root Phenotype of the pin2 Mutant and Requires Ethylene in an EIN2-Dependent Manner
In Arabidopsis, AUX1 influx or PIN2 efflux carriers are pivotal for the flow of auxin from columella cells to epidermal cells of the elongation zone via LRC cells (Müller et al., 1998; Swarup et al., 2001, 2005; Ottenschläger et al., 2003), and both aux1 and pin2 mutations result in a characteristic agravitropic root-coiling phenotype (Maher and Martindale, 1980; Bennett et al., 1996; Luschnig et al., 1998; Müller et al., 1998). Because PABA promotes root gravitropism, we addressed the question of whether the effects of PABA activity on root gravitropism require the transport activities of AUX1 and/or PIN2 using aux1-7 and pin2 (eir1-1) mutants. aux1-7 plants grown vertically on medium supplemented with PABA exhibited an agravitropic root-coiling phenotype (Fig. 2C), indicating that AUX1 is required for PABA-promoted root gravitropism and that PABA, as a weak acid, may be a substrate of AUX1-mediated transport. To test this assumption, aux1-7 seedlings were grown in the presence of PABA and sulfanilamide (a PABA agonist and a strong inhibitor of the folate pathway; Supplemental Fig. S3C) separately or in combination. As expected, the growth of wild-type and aux1-7 plants was severely impaired by sulfanilamide, but when PABA and sulfanilamide were applied simultaneously, wild-type and aux1-7 plants developed normally (Fig. 2C). This finding indicates that PABA incorporation into cellular metabolic processes occurred normally and that the AUX1 transporter is not crucial for PABA uptake. Nevertheless, these results suggest that the effect of PABA on root gravitropism requires AUX1-mediated auxin influx. In contrast to the aux1-7 mutant, the eir1/pin2 root-coiling phenotype was reversed by PABA in a concentration-dependent manner (Fig. 2D). The positive effect of PABA could be observed at a concentration as low as 50 µm, and at 200 µm PABA, the eir1/pin2 root-coiling phenotype was suppressed (Fig. 2D).
Exogenous application of flavonoids has been shown to be sufficient to partially restore pin2 root gravitropism (Santelia et al., 2008). Therefore, we tested whether PABA promotes root gravitropism in a flavonoid-dependent manner. We investigated the effect of PABA on the agravitropic and flavonoid-deficient mutant transparent testa4 (tt4), which lacks the chalcone synthase in flavonoid biosynthesis (Koornneef et al., 1982). When grown on 100 μm PABA, the altered root gravitropic phenotype of the tt4 mutant was suppressed (Supplemental Fig. S5), suggesting that PABA-promoted root gravitropism does not rely on the flavonoid pathway.
It is noteworthy that the roots of light-grown aux1 and pin2 seedlings have been reported to display different sensitivities toward ethylene (Stepanova et al., 2007; Lewis et al., 2011). Huang et al. (2013) also showed that the ethylene precursor ACC reversed the gravitropic defects of the ethylene biosynthesis mutant acs7, suggesting that ethylene levels positively regulate root gravitropism. In line with this, 1 µm ACC was shown to stimulate the root graviresponse of the Arabidopsis ecotype Wassilewskija (Huang et al., 2013).
We examined whether the PABA-stimulated root gravitropism in the pin2/eir1 mutant involves the ethylene pathway. pin2 was crossed with the ethylene signaling mutant ein2, known to have a wild-type-like gravity response (Buer et al., 2006; Ikeda et al., 2009), and the growth of the double mutant pin2ein2 was compared with that of the pin2 single mutant on medium supplemented either with PABA or ACC, the immediate precursor of ethylene biosynthesis. Remarkably, similar to PABA, ACC treatment also suppressed the pin2 root-coiling phenotype, indicating that ethylene and PABA might activate similar signaling pathways to restore the pin2 root gravitropic response (Fig. 2E). Consistent with this hypothesis, both PABA and ACC failed to prevent the root-coiling phenotype of the pin2ein2 double mutant (Fig. 2E), suggesting that the suppression of the pin2 root-coiling phenotype by both PABA and ethylene requires the EIN2-mediated signaling pathway. However, despite the fact that the pin2 root-coiling phenotype was suppressed by PABA and ACC, we noted that pin2 roots remained slightly agravitropic. To test whether PABA or ACC really affected the pin2 gravitropic response, Col-0 and pin2 roots grown on control, ACC-, or PABA-supplemented medium were rotated at 135° for 4 and 12 h (a time point at which significant pin2 root curvature angles were quantifiable). As expected, after 4 h, both PABA and ACC promoted root gravitropism of wild-type plants (Fig. 3A), and after 12 h, they partially restored the pin2 gravitropic response (Fig. 3, B and C).
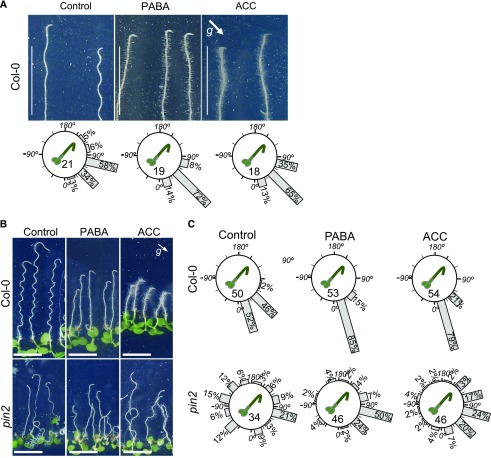
Figure 3.
PABA activity intersects with the ethylene pathway. A, Top, 6-d-old Arabidopsis seedlings were grown on solid Arabidopsis growth medium (Control) or supplemented with 200 µm PABA or 0.5 µm ACC and rotated by 135° for 4 h. The white arrow indicates the gravity vector (g). Bars = 1 cm. Bottom, Quantification of root gravitropism of plants presented in A. Gravistimulated roots were assigned to one of the 12 30° sectors on the gravitropism diagram. The length of each bar represents the percentage of seedlings showing the respective direction of root growth. Numbers inside the circles indicate the number of plants for each genotype. B, Root phenotypes of 7-d-old seedlings of Col-0 and pin2 mutant on control, 200 µm PABA, and 0.5 µm ACC treatment. The white arrow indicates the direction of the gravity vector (g). Bars = 0.5 cm. C, Quantification of root gravitropism of wild-type and pin2 seedlings (with roots pulled straight) gravistimulated at 135° for 12 h.
The PABA-ethylene interaction in gravistimulated seedlings was studied further in the presence of the ethylene biosynthesis and signaling inhibitors aminoethoxyvinylglycine (AVG) and silver nitrate (AgNO3), respectively. When applied alone, AVG and AgNO3 delayed root bending and affected PABA-promoted root gravitropism (Fig. 4A). The combined application of AVG and AgNO3 completely suppressed the inductive effect of PABA on root curvature. Next, we tested whether the PABA-promoted lateral auxin gradient at the tip of the bending root also might depend on ethylene. When applied separately, AVG and AgNO3 prevented asymmetric DR5rev::GFP expression on the lower side of the gravitropic root, but this negative effect was reversed by PABA treatment, resulting in a significantly increased DR5rev::GFP signal in the LRC and in the elongation zone of the concave side of the root (Fig. 4, B and C). Importantly, when ethylene inhibitors were applied together, they failed to prevent the PABA-promoted asymmetric auxin response in LRC cells on the lower side of the root, while they prevented the induction of the auxin response in the TZ-EZ, as observed for seedlings treated with PABA alone (Fig. 4, B and C). Taken together, these results suggest that PABA requires both ethylene biosynthesis and signaling to promote the asymmetric auxin response in the TZ-EZ, but not in the LRC, during root gravitropism.
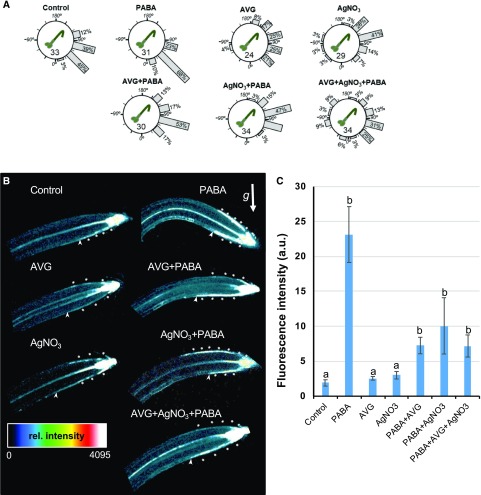
Figure 4.
PABA activity intersects with the ethylene pathway. A, Distribution of gravitropic angles. Seven-day-old Col-0 seedlings were grown on control medium and medium containing PABA (200 µm), in the absence or presence of the inhibitor of ethylene biosynthesis (AVG; 20 µm) or signaling (AgNO3; 1 µm), applied separately or in combination. Then, seedlings were gravistimulated at 135° for 4 h. Gravistimulated roots were assigned to one of the 12 30° sectors on the gravitropism diagram. The length of each bar represents the percentage of seedlings showing the respective direction of root growth. Numbers inside the circles indicate the number of plants for each genotype. B, Heat map of the asymmetric auxin response at the root tip of 7-d-old DR5rev::GFP seedlings grown as in A. For relative (rel.) intensity, dark and white pixels indicate low and high intensity, respectively; pixel values range from 0 to 4,095. Stars indicate asymmetric auxin responses in the LRC and in the elongation zone. The white arrow indicates the gravity vector (g). C, Quantification of DR5rev::GFP signal in TZ-EZ epidermal cells of the lower side of the curving root. GFP signal was measured in 13 ± 2 epidermal cells starting from the last proximal root cap cell (arrowheads in B). Data are shown as means ± se (n > 8). DR5rev::GFP signal is considered different from the control at P < 0.05. One-way ANOVA with Bonferroni multiple testing corrections was used to attest for the differences between treatment groups. The letters (a and b) indicate independent groups according to one‐way ANOVA. a.u., Arbitrary units.
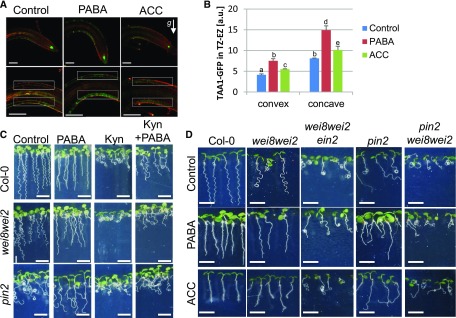
PABA and Ethylene Enhance the Differential Expression of TAA1-GFP on the Concave Side of the Gravitropic Root
Ethylene modulates tissue-specific auxin biosynthesis during apical hook formation through the induction of TAA1/WEI8, an aminotransferase that catalyzes the conversion of the amino acid Trp into the IAA precursor indole-3-pyruvate (Stepanova et al., 2008). This gene was found to be expressed on the concave side during apical hook formation (Stepanova et al., 2008) and on the lower side of gravistimulated roots (Yamada et al., 2009). This finding suggests that, as shown for apical hook formation, TAA1/WEI8 may work downstream of a positive regulatory loop required for root gravitropism (Yamada et al., 2009). To verify whether the effect of PABA activity on root gravitropism involves TAA1/WEI8, we inspected TAA1 expression during gravitropic root curvature in the presence of PABA and ACC using pTAA1::TAA1-GFP transgenic plants (Stepanova et al., 2008). TAA1-GFP expression was more prominent on the lower side of gravistimulated control roots in epidermal cells of the root TZ-EZ (Fig. 5, A and B). Quantification of the GFP signal revealed that, among all treatments (control, PABA, and ACC), the differential expression of TAA1-GFP between the upper and lower sides of the root was significantly higher in PABA-treated plants and, to a lesser extent, in ACC-treated plants (Fig. 5B). These results suggest that PABA and ethylene regulate the tissue-specific stimulation of TAA1 in the TZ-EZ on the concave side of the curving root.
Figure 5.
PABA-promoted root gravitropism requires local auxin biosynthesis. A, pTAA1::TAA1-GFP expression at the root tip of Col-0 seedlings (top row) or at the gravity-induced curvature (bottom row) on control, 200 µm PABA-, or 0.5 µm ACC-supplemented medium. Plants were gravistimulated by rotating the plate at 135° for 4 h. The white arrow indicates the direction of the gravity vector (g). Roots were stained with the plasma membrane marker FM4-64 (red). Bars = 100 µm. B, Quantification of the GFP fluorescence in the root TZ-EZ (boxed areas in A) of Col-0 seedlings expressing pTAA1::TAA1-GFP grown and gravistimulated as in A. Data are shown as means ± se of arbitrary units (a.u.; n > 20). GFP signal is considered different from the control (P < 0.05). One-way ANOVA with Bonferroni multiple testing corrections was used to attest to the differences between treatment groups. The letters (a, b, c, d, and e) indicate independent groups according to one‐way ANOVA. C, Seven-day-old seedlings of Col-0, wei8wei2, and pin2 were grown in the absence (Control) and presence of PABA (200 µm) or of the auxin biosynthesis inhibitor l-kynurenine (Kyn; 1 µm), separately or in combination. Bars = 0.5 cm. D, Seven-day-old seedlings of Col-0, wei8wei2, wei8wei2ein2, pin2, and pin2wei8wei2 were grown on control, 200 µm PABA-, and 0.5 µm ACC-supplemented medium. Bars = 1 cm.
PABA Activates Ethylene-Dependent Cross-Compensation between Auxin Efflux and Biosynthesis
The activity of TAA1/WEI8 and its homologs, TARs, can be competitively inhibited by l-kynurenine, an alternate substrate that selectively binds to the substrate-binding pocket (He et al., 2011). l-Kynurenine prevents ethylene-induced auxin biosynthesis, since it abolishes auxin-mediated nuclear accumulation of the key ethylene-related transcription factor EIN3 (He et al., 2011). To determine whether local auxin biosynthesis intersects with PABA-promoted root gravitropism, we checked the root phenotype of the auxin biosynthetic double mutant wei8wei2 (Stepanova et al., 2008), which is allelic to the agravitropic root tir7tir2 double mutant (Yamada et al., 2009), as well as the pin2 mutant exposed to l-kynurenine alone or in combination with PABA. In the presence of l-kynurenine, 6-d-old wild-type plants displayed a coiling root phenotype, which was partially reversed by PABA (Fig. 5C, top row), suggesting that PABA may activate other components of the auxin biosynthesis pathway. Interestingly, the exaggerated root oscillatory pattern of the wei8wei2 mutant was suppressed by exogenous PABA and enhanced further by l-kynurenine, resulting in a severe root-coiling phenotype (Fig. 5C, middle row). Similarly, l-kynurenine exacerbated the pin2 root phenotype, while PABA slightly counteracted this effect (Fig. 5C), suggesting that PIN2-driven auxin transport and WEI8/WEI2-mediated auxin biosynthesis may cooperate to promote root gravitropism. Taken together, these results strongly suggest that PABA-mediated pin2 root gravitropism recovery occurs via PABA-induced local auxin biosynthesis.
We examined the relevance of local auxin biosynthesis in the cross talk between auxin and PABA and further addressed whether stimulated root gravitropism in the wei8wei2 mutant also required EIN2 by generating a wei8wei2ein2 triple mutant (Fig. 5D). On control medium, the exaggerated waving of the wei8wei2 root was enhanced further in the wei8wei2ein2 triple mutant, resulting in a root-coiling phenotype that persisted in the presence of PABA or ACC (Fig. 5D). This finding suggests a synergy between WEI8/WEI2-mediated auxin biosynthesis and ethylene signaling during the root response to gravitropism and demonstrates that EIN2-mediated ethylene signaling is involved in the PABA-stimulated root gravitropism of the wei8wei2 mutant.
Next, to reveal the extent to which auxin biosynthesis and efflux interact at the root tip, the pin2 and wei8wei2 mutations were combined, and the root-coiling phenotype of the pin2wei8wei2 triple mutant was analyzed. The pin2wei8wei2 root-coiling phenotype persisted in both ACC and PABA treatments (Fig. 5D). Hence, since the pin2 root-coiling phenotype and the sinusoidal growth of the wei8wei2 mutant were suppressed individually by PABA and ACC, this result suggests that both substances might activate a reciprocal compensation between PIN2-driven auxin transport and local WEI8/WEI2-mediated auxin biosynthesis machinery, and this cross-compensatory mechanism is clearly dependent on the ethylene signaling pathway.
PABA-Promoted Asymmetric Root Growth Requires ARF7 and ARF19
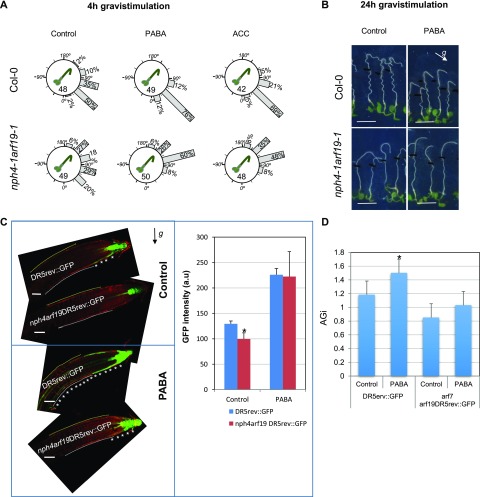
The ARF7/NPH4 transcription factor mediates the auxin response required for asymmetric growth during hypocotyl and root phototropism (Harper et al., 2000; Okushima et al., 2005). Together with ARF7, ARF19 participates in auxin signaling but also plays a critical role in ethylene responses in Arabidopsis roots (Li et al., 2006). Consistent with this finding, the gravitropic response was strongly impaired in the nph4-1arf19-1 double mutant (Okushima et al., 2005; Li et al., 2006). To test whether PABA- or ethylene-induced root gravitropism requires ARF7 and ARF19, nph4-1arf19-1 plants were grown on PABA or ACC and gravistimulated by rotating plates with an angle of 135° for 4 or 24 h. Neither PABA nor ACC restored normal nph4-1arf19-1 gravitropic growth after 4 or 24 h (Fig. 6, A and B). These results indicate that ARF7 and ARF19 transcription factors are required for both PABA- and ethylene-induced root gravitropism.
Figure 6.
ARF7 and ARF19 are required for PABA-promoted root gravitropism. A and B, Seedlings were grown for 5 d on control medium and transferred to control medium supplemented or not with 200 µm PABA or 0.5 µm ACC for 24 h, then gravistimulated by rotating the plate at 135° for 4 or 24 h. A, Quantification of the root graviresponse after 4 h of gravistimulation. B, Root curvature after 24 h of gravistimulation. The black marks indicate the positions of the root apex after plant transfer. The white arrow indicates the direction of the gravity vector (g). C, Left, auxin response in wild-type (DR5rev::GFP) and in arf7arf19 (nph4-1arf19-1DR5rev::GFP) seedlings grown and gravistimulated by rotating the plate at 135° for 4 h as in A. White stars indicate the presence of the GFP signal in the LRC and/or TZ-EZ cells. Note the absence of DR5 signal in LRC cells of the control arf7arf19 root or in TZ-EZ cells of both control and PABA-treated roots. The black arrow indicates the direction of the gravity vector (g). Right, quantification of the GFP intensity of plants grown and gravistimulated as in C. a.u., Arbitrary units. D, Determination of the root AGi of plants shown in C. The data in C (right) and D are shown as means ± se (n = 15). Roots in C were stained with the plasma membrane marker FM4-64 (red). Black stars indicate significantly different GFP signal intensities between DR5rev::GFP and nph4-1arf19-1DR5rev::GFP roots (Student’s t test, P < 0.05). Bars = 1 cm (B) and 100 µm (C).
To determine whether ARF7 and ARF19 mediate the PABA-enhanced asymmetric auxin response, we generated the nph4-1arf19-1DR5rev::GFP line and examined the auxin response after 4 h of root gravistimulation. On control medium, in comparison with the wild type, the DR5rev::GFP signal was reduced significantly in nph4-1arf19-1DR5rev::GFP roots, and almost no GFP signal was visible in LRC cells on the lower side (asterisks in Fig. 6C). In the presence of PABA, the overall DR5rev::GFP signal intensity was not reduced significantly in nph4-1arf19-1 root tips when compared with the wild-type, except in LRC cells, and it was never detected in the TZ-EZ (Fig. 6, C and D). Taken together, these results demonstrate that ARF7 and ARF19 mediate the PABA-promoted auxin response in both LRC cells and the TZ-EZ during root gravitropism.
To verify whether PABA promotes asymmetric growth in an ARF7/ARF19-dependent manner, we calculated the AGi of gravistimulated nph4-1arf19-1DR5rev::GFP plants (Fig. 6, C and D). The AGi of nph4-1arf19-1DR5rev::GFP roots grown on PABA (1.03 ± 0.04) was not significantly different from that of nph4-1arf19-1DR5rev::GFP control roots (0.85 ± 0.1), confirming that ARF7 and ARF19 are crucial for PABA-promoted asymmetric root growth. Then, we analyzed the localization of ARF7/NPH4 during the root response to gravity in nph4arf19 (arf7arf19) plants expressing the pARF7::ARF7-GFP construct (Fig. 7; Ito et al., 2016). In contrast to the arf7arf19 mutant, nph4arf19pARF7::ARF7-GFP plants responded to gravity, suggesting that, as was shown for lateral root development (Ito et al., 2016), this construct also could partially rescue the arf7arf19 gravitropic phenotype (compare Figs. 6C and 7A). In control roots, ARF7-GFP signal was visible in nuclei of the LRC, in epidermal cells of the elongation zone (asterisks in Fig. 7, A–C), and weakly detectable in the transition zone and meristematic cells. In the presence of PABA, the expression pattern of pARF7::ARF7-GFP was similar to that found in the control roots, except in the meristematic cells on the concave side of the root, where the nuclear ARF7-GFP signal was now clearly detected (Fig. 7, D–F). Quantification of the GFP signal in TZ-EZ epidermal cells of the upper and lower sides of the root confirmed that, compared with control plants, ARF7-GFP localized significantly to the lower side of the root in PABA-treated plants (Fig. 7G). The asymmetric expression of ARF7 suggests that it may regulate genes that control cell elongation in this area.
Figure 7.
Auxin signaling during root gravitropism. Subcellular localization of ARF7-GFP is shown in roots after 4 h of gravistimulation at 135°. A, pARF7::ARF7-GFP expression in control root. B, TZ-EZ of the root (white box in A). C, Root meristem (blue box in A). D, pARF7::ARF7-GFP expression in 200 µm PABA-treated root. E, TZ-EZ (white box in D). F, Root meristem (blue box in D). White asterisks indicate ARF7-GFP signal. Roots were stained with the plasma membrane marker FM4-64 (red). Bars = 100 µm (A and D) and 50 µm (B, C, E, and F). G, Quantification of ARF7-GFP fluorescence in TZ-EZ epidermal cells of the upper and lower sides of the root. Box plots show median and 75th percentile values calculated from 10 independent images per genotype. P values are based on Student’s t tests, P < 0.001 (***); n.s, not significant. a.u., Arbitrary units.
The Regulation of Free Endogenous PABA Levels Impacts Root Gravitropism
To investigate the importance of plant endogenous PABA, we identified sites of PABA synthesis by analyzing the expression pattern of GAT-ADCS (Basset et al., 2004a) in planta by fusing the promoter of GAT-ADCS to the GUS reporter gene (Jefferson et al., 1987). In shoots, GAT-ADCS (pADCS::GUS) was expressed in cotyledons and leaves (mainly in the vasculature and stomata) as well as in the shoot apical meristem. GAT-ADCS expression was detected at the root-shoot junction and along the root vasculature (Supplemental Fig. S1E). At the root tip, GAT-ADCS was expressed in the quiescent center and LRC cells (indicated by a pound sign in Supplemental Fig. S1F, left). When plants were stained for a longer time, the GUS signal could be detected in the epidermis and cortex (white and dark asterisks in Supplemental Fig. S1F, right).
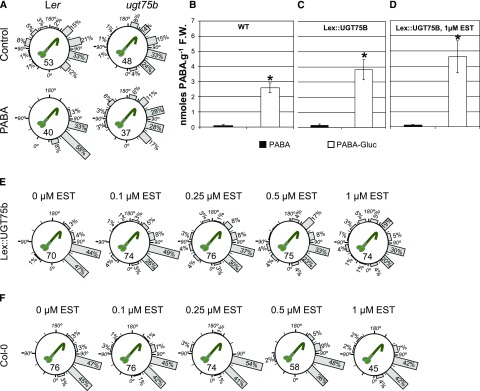
A major regulatory step controlling endogenous levels of active PABA is its inactivation by conjugation to Glc (Eudes et al., 2008). The glycosylation of PABA is catalyzed by UGT75B1, and the resulting PABA-Glc conjugate is stored in the vacuole (Supplemental Fig. S1D; Eudes et al., 2008). To test whether the regulation of free versus glycosylated endogenous PABA is involved in root gravitropism, we first analyzed the gravitropic response of the ugt75b loss-of-function mutant, which accumulates significantly more free PABA than the wild type (Eudes et al., 2008). When gravistimulated, roots of ugt75b plants responded faster than those of the wild type: 60% of ugt75b root tips were positioned between 0° and 90°, while only 45% of wild-type roots had reached that position (Fig. 8A). However, increasing levels of endogenous free PABA by growing ugt75b plants in the presence of exogenous PABA delayed their gravitropic response (Fig. 8A), suggesting that, as expected, high levels of free PABA were detrimental for the root graviresponse.
Figure 8.
UGT75B1 regulates endogenous free PABA levels and the root gravity response. A, Distribution of gravitropic angles of 7-d-old seedlings of wild-type Landsberg erecta and the ugt75b mutant grown on control and 200 µm PABA medium and gravistimulated by rotating the plate at 135° for 4 h. B to D, Quantification of PABA in planta. Each value is the average of three independent experiments ± sd. Asterisks indicate significant differences from the control by Student’s t test (P < 0.01). B, Wild-type (WT; Col-0) plants grown on control medium. C, Lex::UGT75B plants grown on control medium. D, Lex::UGT75B plants grown on medium supplemented with 1 µm β-estradiol (EST). F.W., Fresh weight. E, Distribution of gravitropic angles of 7-d-old Lex::UGT75B seedlings grown on medium containing different concentrations of β-estradiol and gravistimulated as in A. F, Distribution of gravitropic angles of 7-d-old wild-type (Col-0) seedlings grown on medium containing different concentrations of β-estradiol and gravistimulated as in A.
Next, to artificially reduce the endogenous levels of free PABA, we generated stable conditional lines differentially overexpressing UGT75B1. The induction of UGT75B1 (Lex::UGT75B1) with β-estradiol resulted in a 2-fold increase in PABA-Glc compared with the wild type (Fig. 8, B–D). Note that the noninduced UGT75B1 (Fig. 8C) also displayed an increased level of PABA-Glc (approximately 1.5-fold the amount detected in the wild type). This result is consistent with the reported leakiness of the β-estradiol-inducible promoter (Kubo et al., 2013). Due to the low initial level of free PABA, which was barely detectable and represented less than 5% of the overall PABA content in the plant, it was not possible in these experiments to determine any significant decrease within the free PABA pool. However, considering that the increase in PABA-Glc resulting from the overexpression of UGT75B1 required the contribution of the free pool, this pool might be less available for other metabolic purposes. Furthermore, a gradual reduction of endogenous free PABA by the application of increasing concentrations of β-estradiol (from 0 to 1 µm) resulted in a disturbed root gravitropic response (note the β-estradiol concentration-dependent reduction of roots displaying gravitropic angles positioned between 30° and 90°), while wild-type plants were affected only marginally (Fig. 8, E and F). These gravitropic defects could be abolished by an exogenous application of 200 µm PABA and by 1 mm 5-FTHF (Supplemental Fig. S6). The percentage of root tips (3%) that reoriented at angles between 0° and 30° in the control Lex::UGT75B1 plants induced with β-estradiol increased in the presence of PABA (13%), versus 4% in 5-FTHF. Taken together, these results support the hypothesis that the levels of endogenous UGT75B1 activity, which presumably control the level of free PABA, affect the root response to gravity.
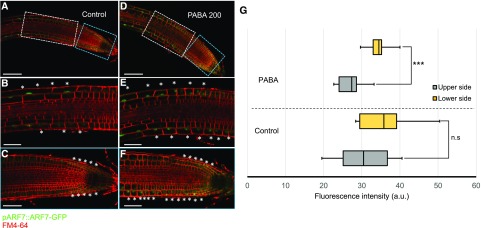
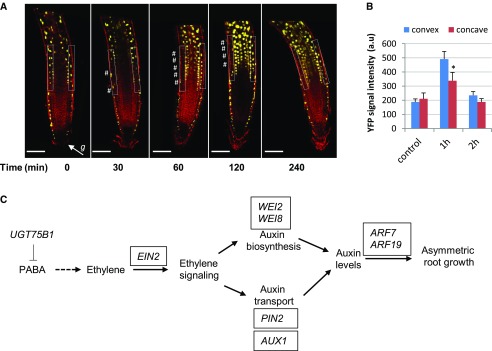
To precisely identify the tissues in which PABA might be conjugated to Glc, we investigated the expression pattern of the UGT75B1 gene. The promoter of UGT75B1 was fused to the gene encoding a nucleus-targeted YFP (NLS3xYFP; Sarkar et al., 2007). In nongravistimulated roots of vertically grown seedlings, UGT75B1 is expressed in the LRC, in the epidermis of the transition zone, and in the epidermis, cortex, and pericycle of the elongation zone (Fig. 9A). Expression analysis revealed a time-dependent gravity-regulated UGT75B1 expression (Fig. 9A). Hence, 1 h after gravistimulation, pUGT75B1::NLS3xYFP expression in the elongation zone was lower in epidermal cells on the lower side of the root than in the epidermal cells on the upper side of the root (Fig. 9, A and B). This modification was transient because similar pUGT75B1::NLS3xYFP expression levels were restored on both sides after 4 h (Fig. 9A). This result suggests a dynamic and differential conjugation of PABA during root gravitropism.
Figure 9.
Gravity modulates UGT75B1 tissue-specific expression. A, pUGT75B1::NLS3xYFP expression pattern (yellow) showing the differential expression between the upper and lower sides of the gravitropic root over time after 135° gravistimulation. White hash tags indicate down-regulation of pUGT75B1::NLS3xYFP. The white arrow indicates the gravity vector (g). Roots were stained with the plasma membrane marker FM4-64 (red). Bars = 100 µm. B, YFP intensity in TZ-EZ cells (boxed areas in A) of 6-d-old pUGT75B1::NLS3xYFP seedlings that were grown on control medium and gravistimulated for 1 and 2 h by 135°. The data are shown as means ± se (n = 10). The asterisk indicates a statistically significant difference in YFP signal intensity between the convex and concave sides. a.u., Arbitrary units. C, Model of PABA-mediated root gravitropism. Solid arrows indicate known ethylene-auxin interactions; the dashed arrow indicates an anticipated metabolic step.
DISCUSSION
During the course of evolution, plants have become able to respond very sensitively to gravity. Although auxin is accepted as the central component of the root graviresponse, other plant hormones are required (Philosoph-Hadas et al., 2005). How interactions among plant hormones are mechanistically orchestrated in this context is not fully understood. In this study, we used a combination of genetic, molecular, and pharmacological approaches to demonstrate that the folate precursor PABA is an important node that connects the auxin and ethylene pathways during the root response to gravity. We showed that PABA positively regulates root gravitropism and provided evidence that this activity occurs independent of the role of PABA as a precursor of folates.
PABA Activity Intersects with the Auxin and Ethylene Pathways
Here, we provide evidence that the effect of PABA on the root’s response to gravity is distinct from the effect of auxin. IAA inhibits root gravitropism, whereas PABA promotes this process. Our data show that the impact of both chemicals on root gravitropism is not related to their impact on overall root growth. When applied at concentrations that inhibit root growth to comparable extents, only PABA promotes gravitropic root curvature.
The ethylene precursor ACC has been reported either to inhibit (Buer et al., 2000, 2006) or promote (Chadwick and Burg, 1970; Eliasson et al., 1989; Huang et al., 2013) root gravitropism. Buer et al. (2006) reported that root gravitropism of Arabidopsis Col-0 was inhibited by 2.5 µm ACC, whereas Huang et al. (2013) showed that 1 µm ACC stimulates the root graviresponse in the Wassilewskija ecotype. In line with the latter finding, Huang et al. (2013) also reported that ACC could reverse root gravitropic defects of the ethylene biosynthesis mutant acs7, giving weight to the claim that, at certain concentrations, ethylene can positively regulate root gravitropism. In this study, a gravitropic root response was stimulated in Col-0 seedlings by medium supplemented with 0.5 µm ACC, a concentration that clearly suppresses root oscillations and pin2 root coiling and also promoted root graviresponse.
Intriguingly, the PABA effect on the root graviresponse is very similar to that of ethylene. First, PABA-promoted root gravitropism is suppressed when the effect of ethylene is blocked by inhibitors of its biosynthesis (AVG) and perception (AgNO3). Second, both PABA and low doses of the ethylene precursor ACC promote root gravitropism. Third, both chemicals reverse the root agravitropic phenotype of light-grown auxin transport mutant pin2 and auxin biosynthesis mutant wei8wei2 seedlings, and this effect also is abolished when the ethylene pathway is impaired. Altogether, these observations demonstrate that ethylene is required for the stimulatory effect of PABA on root gravitropism when either PIN2 or WEI8WEI2 is missing.
Next, it is well established that the asymmetric auxin response during the root gravitropic response drives root asymmetric growth and is facilitated by the coordinated activity of auxin influx (AUX1) and efflux (PIN2) carriers within the root apex (Müller et al., 1998; Rashotte et al., 2001; Ottenschläger et al., 2003). Therefore, in addition to the pin2 mutant, we also used the aux1 mutant to gain insights into how PABA modulates root gravitropism. aux1 roots were resistant to the PABA-stimulated graviresponse, although they clearly took up PABA. This finding is probably due to the critical role of AUX1 in the columella, LRC, and epidermal cells, where it facilitates the cell-to-cell auxin movement necessary for root curvature (Swarup et al., 2005). However, since PABA suppressed the root-coiling phenotype and could partially reverse the root agravitropic phenotype of the pin2 mutant, this suggests that PABA activates molecular mechanisms that compensate for the loss of function of PIN2. For instance, we showed that the PABA-mediated restoration of pin2 root gravitropism requires local auxin biosynthesis. Accordingly, we found that PABA promotes the gravitropic response of wild-type Arabidopsis roots through asymmetric, cell-specific TAA1-mediated auxin biosynthesis, an observation that is consistent with the increased auxin response on the concave side of gravistimulated PABA-treated roots. These results also are consistent with the ability of ethylene inhibitors to prevent the asymmetric auxin response during root gravitropism, confirming that endogenous ethylene regulates auxin gravitropic gradients, as suggested previously (Růzicka et al., 2007). However, an asymmetric auxin gradient, albeit weak, was still visible in LRC cells following the simultaneous application of PABA and ethylene inhibitors, suggesting that, in addition to ethylene, PABA also may activate other pathways.
PABA Activates Ethylene-Dependent Cross-Compensation between Auxin Transport and Biosynthesis
It has been suggested that a tissue-specific positive regulatory loop between auxin biosynthesis and ethylene is required for root gravitropism (Stepanova et al., 2005). Here, we demonstrated that PABA activates ethylene-dependent cross-compensation between auxin transport and the auxin biosynthetic machinery. This finding is supported by the fact that the root gravitropic response of the auxin biosynthesis wei8wei2 double mutant (Stepanova et al., 2008) and the auxin transport mutant pin2 was individually stimulated by PABA, whereas the pin2wei8wei2 triple mutant was insensitive. This finding also indicated that PIN2 and WEI8/WEI2 had overlapping roles in modulating the root gravity response. A similar genetic interaction between TAA1-mediated auxin biosynthesis and auxin transport has been reported in Arabidopsis, in which the elongation of cells in the stem was shown to be dependent on leaf-synthesized auxin (Tao et al., 2008). In the root, the synergistic interaction occurs locally, at the site of auxin biosynthesis, where it intersects with the ethylene pathway. In agreement with this observation, pin2ein2 and wei8wei2ein2 mutants were insensitive, indicating that pin2 and wei8wei2 sensitivity to PABA is dependent on ethylene signaling. These results also strongly substantiate EIN2 as a key component in the cross talk among auxin, ethylene, and PABA when either PIN2 or WEI8/WEI2 function is lacking. However, since the ein2-5 mutant normally responds to gravistimulation, it suggests that EIN2 is dispensable for the root gravity response in wild-type Arabidopsis (Buer et al., 2006). Taken together, our data reveal a contextual role of EIN2 during root gravitropism when either shootward auxin transport or local auxin biosynthesis is defective.
The influence of EIN2 on the auxin pathway through the mediation of ethylene-induced up-regulation of the expression of the auxin biosynthesis genes WEI2 and WEI7 in the root meristem has been documented (Stepanova et al., 2007). These findings clearly suggest that EIN2 mediates the up-regulation of WEI7, but it also might be involved in the induction of other auxin biosynthetic genes, including YUCCA gene family members, which encode flavin monooxygenases (Zhao et al., 2001) and function downstream of TAA1/WEI8/TARs (Stepanova et al., 2011; Won et al., 2011). Accordingly, PABA stimulates cell-specific TAA1 expression (see above). This finding is consistent with the stronger auxin response on the concave side of gravistimulated PABA-treated roots. Therefore, in wei8wei2 and pin2 mutants, EIN2-mediated auxin biosynthesis might compensate for the perturbed auxin levels in these mutants. However, it is possible that PABA also might recruit other PIN proteins or activate alternative transport machinery that can substitute for PIN2. For instance, the flavonoid-mediated rescue of the pin2 gravitropic response correlates with an asymmetric distribution of PIN1 and the partial formation of lateral auxin gradients (Santelia et al., 2008), although our data show that PABA reverses the root gravitropic defects of the flavonoid-deficient tt4 mutant, suggesting that PABA acts downstream or independently of the flavonoid-mediated gravity response.
PABA-Promoted Root Asymmetric Growth and Root Gravitropism Are Dependent on ARF7 and ARF19
The auxin response factors ARF7/NPH4 and ARF19 are transcriptional activators of auxin-responsive genes involved in numerous auxin-mediated developmental processes (Fukaki et al., 2005; Okushima et al., 2007; Narise et al., 2010; Ito et al., 2016). ARF7 and ARF19 are involved in the differential growth responsible for hypocotyl hook formation in etiolated seedlings (Stowe-Evans et al., 1998; Tatematsu et al., 2004). Moreover, the arf7arf19 double mutant displays a reduced root gravitropic response (Okushima et al., 2005; Weijers et al., 2005), indicating that ARF7 and ARF19 are indispensable for root gravitropism. This finding was confirmed by the observation that PABA and ACC did not remediate the defective root gravitropism of the nph4arf19 mutant, suggesting that PABA signal transduction recruits the canonical auxin signaling pathway. Consistent with this scenario, ARF7 is expressed asymmetrically in PABA-treated roots after gravistimulation, and this pattern is maintained in epidermal cells in the TZ-EZ, where, presumably, it regulates the expression of genes that control cell elongation.
Because high DR5rev expression still is visible in columella cells of PABA-treated arf7arf19 roots, we can conclude that, in this zone, PABA may act through other ARFs or in an ARF-independent manner. However, in the root elongation zone, on the concave side, where root gravitropic curvature occurs, ARF7 and ARF19 are essential for the PABA-promoted asymmetric auxin response. This observation is consistent with the observation that the nph4arf19 mutant lacks the capacity for differential growth in epidermal cells in the TZ-EZ, where the expression of IAA14 (Ditengou et al., 2008), which encodes the direct transcriptional repressor of ARF7 and ARF19, may sustain an auxin-dependent transcriptional module that regulates the asymmetric growth response.
UGT75B1 Regulates the Interaction among PABA, Ethylene, and Auxin
UGT75B1 and GAT-ADCS both are expressed in root epidermis and LRC cells, suggesting that the regulation of free PABA levels in these tissues might be of physiological importance. PABA is a weak acid and, therefore, is able to enter cells in its uncharged form (Quinlivan et al., 2003). Thus, PABA could be redistributed via diffusion. When PABA is conjugated to Glc, the resulting PABA-Glc is stored in vacuoles (Eudes et al., 2008), causing the cytosol to be depleted of free PABA. The perturbed root gravitropism observed in plants overexpressing UGT75B1 is likely a consequence of the depletion of active free PABA, as these gravity defects could be fully suppressed by applying exogenous PABA, suggesting that UGT75B1 may act as a negative regulator of root gravitropism. This statement is supported by the spatiotemporal expression of UGT75B1, which is maintained on the upper side but decreased in a time-dependent manner on the lower side of the gravitropic curving root. Consequently, free PABA is expected to increase on the concave side of the curving root, where it would modulate TAA1 expression and auxin biosynthesis. PABA might regulate the positive regulatory loop between auxin biosynthesis and ethylene (Swarup et al., 2007; Zheng et al., 2013), which would ultimately result in ARF7/ARF19-mediated auxin activity effects on cell elongation and asymmetric growth promotion.
In conclusion, our data lead to a model positioning PABA and its esterifying enzyme, PABA-glycosyltransferase UGT75B1, upstream of the ethylene pathway, through which PABA regulates the asymmetric auxin response and local auxin biosynthesis, which are fundamental for the root gravity response (Fig. 9C). The fact that PABA suppresses the oscillatory pattern of the root suggests that it probably uncouples the root gravity response (sensu stricto) and perturbations due to root-surface interactions. Moreover, our data suggest that PABA regulates the root curvature during the graviresponse in two steps in two distinct zones: in LRC cells and in epidermal cells of the TZ-EZ. In LRC cells, PABA activity is mediated only partially by ethylene, since DR5-asymmetric distribution, albeit reduced, still is visible. In contrast, there seems to be a stronger dependence of PABA activity on ethylene-mediated pathways in the TZ-EZ.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Apart from ugt75b (GT6017) and tt4 in the Landsberg erecta background, all genetic backgrounds were from the Arabidopsis (Arabidopsis thaliana) ecotype Col-0. DR5rev::GFP (Friml et al., 2003), ein2-1 (Guzmán and Ecker, 1990; Alonso et al., 1999), eir1-1/pin2 (Luschnig et al., 1998), nph4-1arf19-1 (Okushima et al., 2005), aux1-7 (Marchant and Bennett, 1998), and pin2ein2/pin2ein2, generated by crossing SALK_091142 and SALK_086500 T-DNA lines (Ikeda et al., 2009), were kindly provided by Markus Grebe (Umeå Plant Science Centre, Umeå University); pDR5rev::3XVENUS-N7 (Heisler et al., 2005) was a gift from M. Heisler (California Institute of Technology); pTAA1::TAA1-GFP and wei8-1wei2-1 (Stepanova et al., 2008) were donated by José M. Alonso (North Carolina State University); and pARF7::ARF7-GFP/nph4-1arf19-1 was a gift from H. Fukaki (Department of Botany, Kobe University). Furthermore, we generated the introgression lines nph4-1arf19-1DR5rev::GFP, pin2wei8-1wei2-1, and ein2-1wei8-1wei2-1 by crossing. tt4-1 (N85) seeds were obtained from the Nottingham Arabidopsis Stock Centre (NASC). The newly generated lines were isolated by selective phenotyping, PCR-based genotyping, and/or fluorescence validation. Seeds were surface sterilized (5% [w/v] calcium-hypochloride and 0.02% [v/v] Triton X-100 in 80% [v/v] ethanol) and rinsed three times with 80% (v/v) ethanol. For all experiments, prior to gravistimulation, seedlings were grown vertically (in petri dishes tilted with an angle of 60° from the vertical axis) on solid 1.3% (w/v) agar Arabidopsis medium, which consisted of a 0.5× basal salt Murashige and Skoog medium supplemented with 1% Suc and 5 mm MES (pH 5.8) in a climatic cabinet (21°C, long-day conditions [16 h of light/8 h of dark], and 70% humidity).
Quantification of Root Gravitropism
For long-term treatments, Arabidopsis seedlings were grown vertically for 5 to 7 d (as indicated in the figure legends) in medium supplemented with PABA, 5-FTHF, ACC, or IAA, alone or in combination with inhibitors, and gravistimulated (for details, see figure legends) by rotating petri dishes at an angle of 135° relative to the Earth’s gravity vector for 4 h. For short-term treatments, plants were grown for 5 d on control medium and transferred for 24 h to fresh medium supplemented with PABA (for details, see figure legends) and gravistimulated as above. Quantification of root curvature angles was performed according to Petrásek et al. (2006). Gravistimulated roots were assigned to one of the 12 30° sectors on the gravitropism diagram. The length of each bar represents the percentage of seedlings showing the respective direction of root tip growth. Root tip angles from the vertical plane were measured using ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/ij).
Quantification of PABA
Free and total PABA determinations were performed essentially according to Camara et al. (2012). For a detailed protocol, see Supplemental Methods S1.
Video
A time-lapse movie was created of 6-d-old Arabidopsis seedlings grown in a bicompartmented petri dish (90 mm diameter) in Arabidopsis growth medium supplemented or not with 200 µm PABA. Plants were gravistimulated for 6 h, and a single image was recorded every 2 min using a Keyence VHX digital microscope.
Construction of Plasmids
Plasmids and the modifications applied are listed in Supplemental Tables S1 and S2. Plasmids were constructed according to Sambrook et al. (1989) with Gateway cloning procedures (Invitrogen). For a detailed protocol, see Supplemental Methods S1.
Plant Scanning and Microscopy
Seedlings were imaged with a flatbed CanonScan 9950F scanner while growing on a plastic petri plate. Histological detection of GUS activity and plant preparation for microscopy were performed according to Ditengou et al. (2008). For light microscopy, samples were observed with a Zeiss Axiovert 200M MOT device (Carl Zeiss MicroImaging) for high-magnification images. Low-magnification views were obtained with a Zeiss Stemi SV11 Apo stereomicroscope (Carl Zeiss MicroImaging), viewed under differential interference contrast optics. Plants expressing fluorescent proteins were stained with 5 µm FM4-64 (a lipophilic probe that binds to cell plasma membranes) and analyzed with a Zeiss LSM 5 DUO scanning microscope and an AZ-C1 Macro Laser Confocal Microscope from Nikon. To simultaneously monitor GFP, YFP, and FM4-64 fluorescence, we used multitracking in-frame mode, and the emission was separated using the META spectral analyzer online unmixing feature. Images were extracted and analyzed with Zen2009 software (Carl Zeiss MicroImaging) and Imaris 7.4.0 (Bitplane). All images were assembled using Microsoft PowerPoint 2016.
Quantification of the Root AGi
To quantify the gravitropic root AGi, wild-type Arabidopsis seedlings grown for 6 d in the presence or absence of PABA were gravistimulated for 4 h and then stained with 10 µm FM4-64 for 5 min. After a quick rinse, the roots were scanned with a Nikon C1 confocal microscope, and the lengths of 13 ± 2 TZ-EZ epidermal cells of the upper and lower sides of the root were measured in images using ImageJ software (National Institutes of Health). The AGi is the ratio between the average cell sizes of the upper side over the lower side of the curving root.
Quantification of GFP or YFP Signals
GFP or YFP quantifications were done by selecting a region of interest including 13 ± 2 TZ-EZ epidermal cells, starting from the last proximal root cap cell. Fluorescence intensity was measured using ImageJ software.
Accession Numbers
The sequence data from this article can be found in the GenBank/EMBL database and the Arabidopsis Genome Initiative database under the following accession numbers: At5g20730 (ARF7/NPH4), At1g19220 (ARF19), At2g38120 (AUX1), At5g03280 (EIN2), At2g28880 (GAT-ADCS), At5g57090 (PIN2/EIR1), At1g05560 (UGT75B1), At1g70560 (TAA1/WEI8), At5g13930 (TT4), and At5g05730 (WEI2/ASA1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. PABA impact on root waving and visualization of PABA production and catabolism sites.
Supplemental Figure S2. Impact of PABA and auxin on root growth and root gravitropism.
Supplemental Figure S3. Impact of PABA and 5-FTHF on root gravitropism.
Supplemental Figure S4. PABA promotes the auxin response.
Supplemental Figure S5. PABA rescues the tt4 mutant’s gravity defects.
Supplemental Figure S6. PABA rescues gravity defects in β-estradiol-induced LEX::UGT75B1.
Supplemental Table S1. List of primers used in this study.
Supplemental Table S2. List of vectors and cloning strategy.
Supplemental Video S1. Arabidopsis root gravitropism on control and PABA medium.
Supplemental Methods S1. Supplemental Materials and Methods
Supplemental References S1. Supplemental References
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This work could not have been accomplished without the help of colleagues, collaborators, and friends who provided support, suggestions, and materials. We gratefully acknowledge excellent technical support from Beata Ditengou and Katja Rapp.
Footnotes
This work was supported by the Baden-Württemberg Stiftung, Bundesministerium für Bildung und Forschung (BMBF SYSBRA, SYSTEC, Microsystems), the Excellence Initiative of the German Federal and State Governments (EXC 294), Deutsche Forschungsgemeinschaft (DFG SFB 746, INST 39/839,840,841), the Deutsches Zentrum für Luft und Raumfahrt (DLR 50WB1022), and the Deutscher Akademischer Austauschdienst (DAAD).
Articles can be viewed without a subscription.
References
- Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 97: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Péret B, et al. (2012) Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci USA 109: 4668–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E, Kubeš M, Rolčík J, Béziat C, Pěnčík A, Wang B, Rosquete MR, Zhu J, Dobrev PI, Lee Y, et al. (2012) A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485: 119–122 [DOI] [PubMed] [Google Scholar]
- Basset GJC, Quinlivan EP, Ravanel S, Rébeillé F, Nichols BP, Shinozaki K, Seki M, Adams-Phillips LC, Giovannoni JJ, Gregory JF III, et al. (2004a) Folate synthesis in plants: the p-aminobenzoate branch is initiated by a bifunctional PabA-PabB protein that is targeted to plastids. Proc Natl Acad Sci USA 101: 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset GJC, Ravanel S, Quinlivan EP, White R, Giovannoni JJ, Rébeillé F, Nichols BP, Shinozaki K, Seki M, Gregory JF III, et al. (2004b) Folate synthesis in plants: the last step of the p-aminobenzoate branch is catalyzed by a plastidial aminodeoxychorismate lyase. Plant J 40: 453–461 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Bucher D, Pilet PE (1983) Auxin effects on root-growth and ethylene production. Experientia 39: 493–494 [Google Scholar]
- Buer CS, Masle J, Wasteneys GO (2000) Growth conditions modulate root-wave phenotypes in Arabidopsis. Plant Cell Physiol 41: 1164–1170 [DOI] [PubMed] [Google Scholar]
- Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140: 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara D, Richefeu-Contesto C, Gambonnet B, Dumas R, Rébeillé F (2011) The synthesis of pABA: coupling between the glutamine amidotransferase and aminodeoxychorismate synthase domains of the bifunctional aminodeoxychorismate synthase from Arabidopsis thaliana. Arch Biochem Biophys 505: 83–90 [DOI] [PubMed] [Google Scholar]
- Camara D, Bisanz C, Barette C, Van Daele J, Human E, Barnard B, Van der Straeten D, Stove CP, Lambert WE, Douce R, et al. (2012) Inhibition of p-aminobenzoate and folate syntheses in plants and apicomplexan parasites by natural product rubreserine. J Biol Chem 287: 22367–22376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick AV, Burg SP (1970) Regulation of root growth by auxin-ethylene interaction. Plant Physiol 45: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan ME, Bourosh P, Maffei ME, Forni A, Pieraccini S, Sironi M, Chumakov YM (2014) Synthesis, crystal structure and biological activity of 2-hydroxyethylammonium salt of p-aminobenzoic acid. PLoS ONE 9: e101892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, van der Graaff E, Nziengui H, Pinosa F, Li X, Nitschke R, et al. (2008) Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc Natl Acad Sci USA 105: 18818–18823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditengou FA, Gomes D, Nziengui H, Kochersperger P, Lasok H, Medeiros V, Paponov IA, Nagy SK, Nádai TV, Mészáros T, et al. (2018) Characterization of auxin transporter PIN6 plasma membrane targeting reveals a function for PIN6 in plant bolting. New Phytol 217: 1610–1624 [DOI] [PubMed] [Google Scholar]
- Eliasson L, Bertell G, Bolander E (1989) Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol 91: 310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudes A, Bozzo GG, Waller JC, Naponelli V, Lim EK, Bowles DJ, Gregory JF III, Hanson AD (2008) Metabolism of the folate precursor p-aminobenzoate in plants: glucose ester formation and vacuolar storage. J Biol Chem 283: 15451–15459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M (2005) Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J 44: 382–395 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Geisler M, Murphy AS (2006) The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett 580: 1094–1102 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Gregory JF III (2011) Folate biosynthesis, turnover, and transport in plants. Annu Rev Plant Biol 62: 105–125 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. (2011) A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911 [DOI] [PubMed] [Google Scholar]
- Huang SJ, Chang CL, Wang PH, Tsai MC, Hsu PH, Chang IF (2013) A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J Exp Bot 64: 4343–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, Grebe M (2009) Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol 11: 731–738 [DOI] [PubMed] [Google Scholar]
- Ito J, Fukaki H, Onoda M, Li L, Li C, Tasaka M, Furutani M (2016) Auxin-dependent compositional change in Mediator in ARF7- and ARF19-mediated transcription. Proc Natl Acad Sci USA 113: 6562–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177: 198–206 [PubMed] [Google Scholar]
- Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J (2010) Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc Natl Acad Sci USA 107: 22344–22349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Luiten W, Vlaming P, Schram AW (1982) A gene controlling flavonoid-3′-hydroxylation in Arabidopsis. Arabidopsis Information Service 19: 113–115 [Google Scholar]
- Kubo M, Imai A, Nishiyama T, Ishikawa M, Sato Y, Kurata T, Hiwatashi Y, Reski R, Hasebe M (2013) System for stable β-estradiol-inducible gene expression in the moss Physcomitrella patens. PLoS ONE 8: e77356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Wu G, Ljung K, Spalding EP (2009) Auxin transport into cotyledons and cotyledon growth depend similarly on the ABCB19 multidrug resistance-like transporter. Plant J 60: 91–101 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK (2011) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495 [DOI] [PubMed] [Google Scholar]
- Li J, Dai X, Zhao Y (2006) A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol 140: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EP, Martindale SJ (1980) Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem Genet 18: 1041–1053 [DOI] [PubMed] [Google Scholar]
- Marchant A, Bennett MJ (1998) The Arabidopsis AUX1 gene: a model system to study mRNA processing in plants. Plant Mol Biol 36: 463–471 [DOI] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195 [DOI] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138: 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narise T, Kobayashi K, Baba S, Shimojima M, Masuda S, Fukaki H, Ohta H (2010) Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Mol Biol 72: 533–544 [DOI] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y (1990) Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250: 274–276 [DOI] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ (2001) Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. Plant J 25: 399–406 [DOI] [PubMed] [Google Scholar]
- Paul AL, Amalfitano CE, Ferl RJ (2012) Plant growth strategies are remodeled by spaceflight. BMC Plant Biol 12: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Philosoph-Hadas S, Friedman H, Meir S (2005) Gravitropic bending and plant hormones. Vitam Horm 72: 31–78 [DOI] [PubMed] [Google Scholar]
- Quinlivan EP, Roje S, Basset G, Shachar-Hill Y, Gregory JF III, Hanson AD (2003) The folate precursor p-aminobenzoate is reversibly converted to its glucose ester in the plant cytosol. J Biol Chem 278: 20731–20737 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK (2001) Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13: 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux SJ. (2012) Root waving and skewing: unexpectedly in micro-g. BMC Plant Biol 12: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD, Suyemoto MM, Leopold AC (1985) Amyloplast sedimentation kinetics in gravistimulated maize roots. Planta 165: 295–300 [PubMed] [Google Scholar]
- Salehin M, Bagchi R, Estelle M (2015) SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell 27: 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, et al. (2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem 283: 31218–31226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisree P, Nongmaithem S, Vasuki H, Sreelakshmi Y, Ivanchenko MG, Sharma R (2011) Tomato root penetration in soil requires a coaction between ethylene and auxin signaling. Plant Physiol 156: 1424–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Alonso JM (2005) Arabidopsis ethylene signaling pathway. Sci STKE 2005: cm4. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23: 3961–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E (1998) NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol 118: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15: 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol 7: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Miyazawa Y, Fujii N (2009) Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Mol Biol 69: 489–502 [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MV, Holbrook NM (2004) Root-gel interactions and the root waving behavior of Arabidopsis. Plant Physiol 135: 1822–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136: 2982–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrásek J, Zádníková P, Hoyerová K, Pesek B, Raz V, Swarup R, Bennett M, Zazímalová E, Benková E, et al. (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137: 597–606 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vaseva I, Vissenberg K, Van Der Straeten D (2012) Ethylene in vegetative development: a tale with a riddle. New Phytol 194: 895–909 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G (2005) Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 24: 1874–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA 108: 18518–18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Greenham K, Prigge MJ, Jensen PJ, Estelle M (2009) The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol 151: 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16: 1123–1127 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Guo Y, Novák O, Dai X, Zhao Y, Ljung K, Noel JP, Chory J (2013) Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat Chem Biol 9: 244–246 [DOI] [PMC free article] [PubMed] [Google Scholar]