MdMYB88 and MdMYB124 are critical for apple (Malus × domestica) tolerance to drought by mediating root architecture, xylem vessel development, and cell wall deposition.
Abstract
Water deficit is one of the main limiting factors in apple (Malus × domestica Borkh.) cultivation. Root architecture plays an important role in the drought tolerance of plants; however, research efforts to improve drought tolerance of apple trees have focused on aboveground targets. Due to the difficulties associated with visualization and data analysis, there is currently a poor understanding of the genetic players and molecular mechanisms involved in the root architecture of apple trees under drought conditions. We previously observed that MdMYB88 and its paralog MdMYB124 regulate apple tree root morphology. In this study, we found that MdMYB88 and MdMYB124 play important roles in maintaining root hydraulic conductivity under long-term drought conditions and therefore contribute toward adaptive drought tolerance. Further investigation revealed that MdMYB88 and MdMYB124 regulate root xylem development by directly binding MdVND6 and MdMYB46 promoters and thus influence expression of their target genes under drought conditions. In addition, MdMYB88 and MdMYB124 were shown to regulate the deposition of cellulose and lignin root cell walls in response to drought. Taken together, our results provide novel insights into the importance of MdMYB88 and MdMYB124 in root architecture, root xylem development, and secondary cell wall deposition in response to drought in apple trees.
Water scarcity is a threat to agriculture and human societies. Roots have historically been considered the primary option for plants, including fruit trees, to adapt to water deficits (Vadez et al., 2014). Since roots are the primary interface between plants and surrounding soil to facilitate water uptake, logically roots are the answer to solve issues that arise with water deficits. Despite this knowledge, roots, especially fruit tree root systems, are poorly characterized due to a lack of phenotyping methods. Hence, the molecular and cellular mechanisms underlying root responses to drought stress are poorly understood.
Roots are likely to respond to environmental stresses by co-opting root development. Therefore, the genetic control of root development and root architecture under environmental stress conditions will facilitate our understanding of root system responses to stress (Taylor-Teeples et al., 2015). Under water-limited conditions, shoot growth is inhibited, but roots have the ability to continue to elongate, resulting in an increased root-to-shoot ratio aiding in the adaption to water deficits (Sharp et al., 2004; Yamaguchi and Sharp, 2010). In wheat (Triticum aestivum) , a 50% increase in root-to-shoot ratio is observed under drought stress (Rauf et al., 2007). Currently, a number of genes have been identified as root architecture modulators in response to drought stress. DEEPER ROOTING1 (DRO1) is considered a major quantitative trait locus for deep rooting in rice (Oryza sativa; Uga et al., 2011). Encoding for a membrane-associated protein, DRO1 improves drought avoidance by controlling root angle (Uga et al., 2013). In addition, overexpression of DRO1 homologs in Arabidopsis promotes steeper lateral root angles, whereas in peach it results in deeper-rooting phenotype (Guseman et al., 2017). Other genes and quantitative trait loci responsible for root architecture under drought conditions have been identified in various plant species including rice, wheat, soybean (Glycine max), and Arabidopsis (Arabidopsis thaliana; Yue et al., 2006; Koevoets et al., 2016; Kulkarni et al., 2017; Ye et al., 2018).
Secondary cell walls, such as those found in xylem, fibers, and anther cells, consist of cellulose, hemicellulose, and lignin. Secondary cell walls provide mechanical support for plant growing bodies and are responsible for long-distance transportation of water and nutrients. Over the years, great progress has been made in understanding the impact of drought stress on secondary cell wall structure and dynamics. In response to drought stress, cellulose biosynthesis is shown to decrease in Arabidopsis, tobacco (Nicotiana benthamiana) suspension cells, grape leaves (Vitis vinifera), and wheat roots, but increase in cotton (Gossypium hirsutum; Le Gall et al., 2015). Increased lignification is a common response to biotic and abiotic stress (Moura et al., 2010), as observed in ryegrass (Lolium perenne) (Lee et al., 2012), watermelon roots (Citrullis vulgaris; Yoshimura et al., 2008), white clover leaf (Trifolium repens; Lee et al., 2007), and Leucaena leucocephala (Srivastava et al., 2015). Xyloglycan typically develops a pattern similar to lignin in response to drought (Le Gall et al., 2015). Moreover, modification of the cell wall architecture can enhance plant growth under drought conditions. For example, overexpression of the drought-responsive AP2/ERF transcription factor OsERF71 elevates expression of lignin biosynthetic genes, as well as lignification in rice roots, thus increasing rice tolerance to water deficiency (Lee et al., 2016). Mutation of IRX14 and IRX14-LIKE, two closely related glycosyltransferases, causes a decrease in xylem levels and an increase in the drought tolerance of Arabidopsis (Keppler and Showalter, 2010).
Adjustments of the xylem conducting system are important for plants to maximize their water uptake capability and adapt to drought stress (Sperry et al., 2002; Maseda and Fernández, 2006). The xylem-conducting system is composed of a vessel network spanning from roots to leaves, supplying water and nutrients to the aboveground. The number and diameter of the xylem vessels within the network determine the overall conductivity. Xylem diameter is a major factor determining hydraulic conductivity because of the fourth-power relationship described by the Hagen-Poiseuille law (Tyree et al., 1994). Therefore, even a minor difference in the mean diameter of vessels will lead to a significant difference in hydraulic conductivity. Previous research showed that increasing the number of metaxylem vessels in roots can enhance the drought resistance and seed yield in soybean (Prince et al., 2017). Moreover, xylem cavitation resistance can be used as a relevant criterion for screening drought resist species in Prunus, indicating that xylem cavitation, which is related to xylem structure (Guet et al., 2015), is highly related to the drought resistance of Prunus (Cochard et al., 2008).
Formation of secondary cell walls is a complicated process, which requires the coordinated regulation of genes involved in secondary cell wall biosynthesis. In Arabidopsis, biosynthesis of secondary cell walls is mediated by a transcriptional network encompassing a number of NAC and MYB transcription factors, including NST3/ANAC012/SND1, VND6, VND7, PHB, MYB46, MYB83, MYB103, and others (Ko et al., 2014). Of these transcription factors, MYB46 and its paralog MYB83 function as a master switch (Ko et al., 2014). Direct upstream regulators of MYB46/MYB83 expression include SND1, VND6, and VND7 (Zhong et al., 2007a; Ohashi-Ito et al., 2010; Yamaguchi et al., 2011). In addition, MYB46/MYB83 directly regulates genes associated with biosynthesis of secondary cell wall components (Zhong and Ye, 2012; Kim et al., 2013; Ko et al., 2014).
MYB88 and its paralog FOUR LIPS (FLP/MYB124) are known to regulate the development of guard mother cell proliferation, drought stress tolerance, lateral root development, root gravitropism, and female reproductive development in Arabidopsis (Xie et al., 2010a, 2010b; Lai et al., 2005; Makkena et al., 2012; Chen et al., 2015; Wang et al., 2015). We previously demonstrated that MdMYB88 and MdMYB124 are two positive regulators for apple (Malus × domestica Borkh.) freezing tolerance (Xie et al., 2018). In this study, we characterized their roles in modulating root architecture, root hydraulic conductivity, root xylem development, and secondary cell wall deposition under long-term drought conditions in apple trees.
RESULTS
MdMYB88 and MdMYB124 Positively Regulate Root Architecture under Long-Term Drought Stress
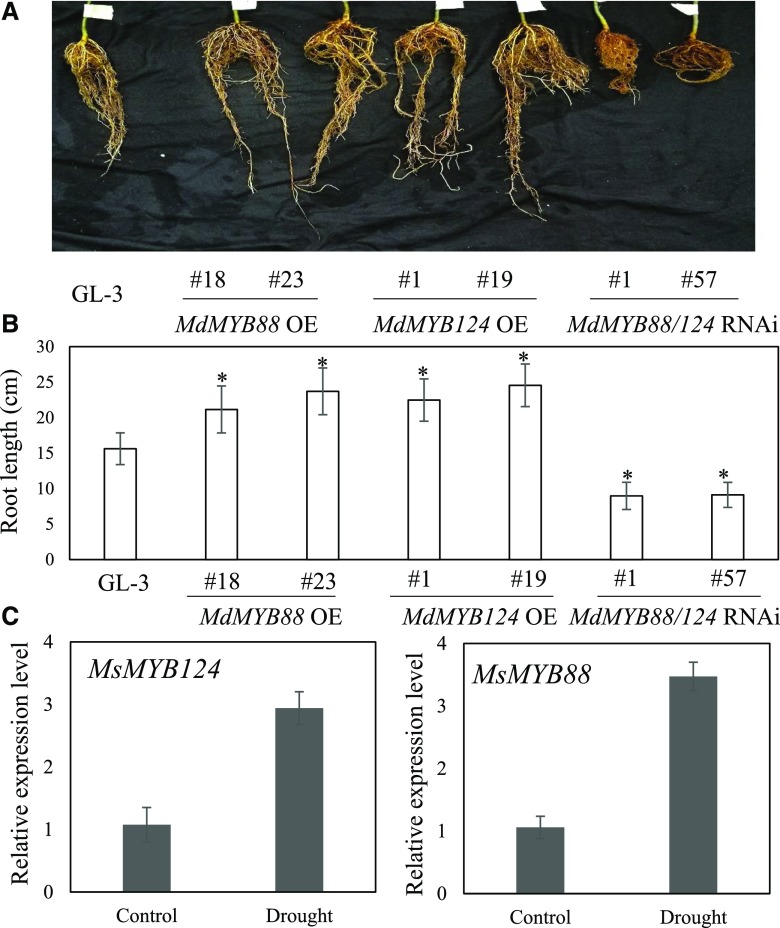
We previously found that MdMYB88 and its paralog MdMYB124 are dominantly expressed in roots of apple trees (Xie et al., 2018). To further investigate their roles in root development, roots of 7-month-old nontransgenic and transgenic apple plants we generated before (Xie et al., 2018) were examined. MdMYB88 and MdMYB124 were simultaneously silenced because the sequences of MdMYB88 and MdMYB124 are so similar that we cannot silence only one of them by RNAi approach. As shown in Figure 1, A and B, plants overexpressing MdMYB88 or MdMYB124 showed vigorous adventitious roots, as determined by adventitious root length. MdMYB88/124 RNAi plants had weak adventitious root systems, as compared with that of nontransgenic GL-3 plants, indicating potential roles for MdMYB88 and MdMYB124 in apple root development. Considering the important roles of roots in drought tolerance, we examined expression of both genes in apple roots in response to drought. Gene expression analysis revealed that MdMYB88 and MdMYB124 were induced slightly in the roots of Malus sieversii under simulated drought conditions, indicating their potential participation in drought tolerance (Fig. 1C). We also tested expression of other MdMYBs, which displayed higher sequence similarity with MdMYB88 and MdMYB124, in MdMYB88/124 RNAi plants and found none of these genes were disrupted in their expression (Supplemental Fig. S1A). These results suggest that weak adventitious roots in MdMYB88/124 RNAi plants are due to disrupted expression of MdMYB88 and MdMYB124, but not other MdMYBs.
Figure 1.
Root morphology of transgenic plants with altered MdMYB88 and MdMYB124 expression and MsMYB88 and MsMYB124 expression level changes in response to drought. A, Root morphology of nontransgenic plants (GL-3), MdMYB88 or MdMYB124 overexpression plants (OE), and MdMYB88/124 RNAi plants. B, Quantitation of adventitious root length of the plants shown in A. Data are means ± sd (n = 5). One-way ANOVA (Tukey test) was performed, and statistically significant differences are indicated by *P < 0.05. C, Relative expression level of MsMYB88 and MsMYB124 in M. sieversii roots under 20% PEG8000 treatment for 0 or 6 h. Data are means ± sd (n = 3).
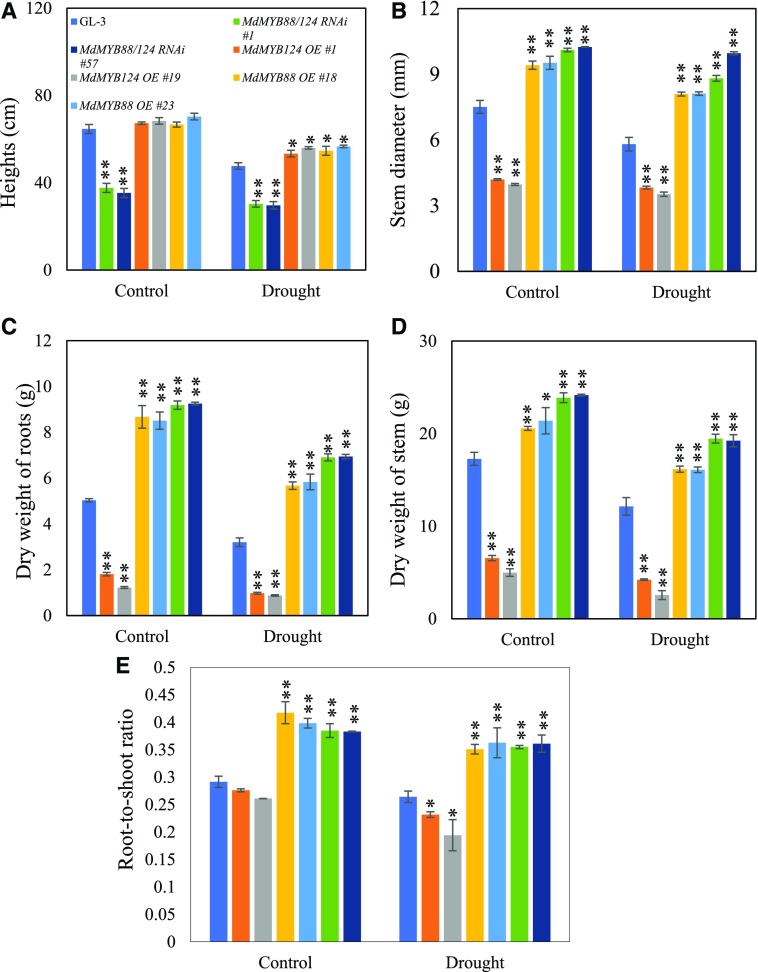
To further explore the roles of MdMYB88 and MdMYB124 in root development under drought, we applied long-term drought treatment on transgenic and nontransgenic plants (Supplemental Fig. S1B). As shown in Figure 2, drought treatment significantly affected plant height, stem diameter, dry weight of shoots, dry weight of roots, and root-to-shoot ratio. After 2 months of drought stress, MdMYB88/124 RNAi plants were much shorter, whereas MdMYB88 or MdMYB124 overexpression plants were taller, when compared to the height of GL-3 plants (Fig. 2A). The stems of MdMYB88/124 RNAi plants were much thinner than those of GL-3 plants under drought. Overexpression of MdMYB88 or MdMYB124 increased stem diameter compared to that in the control after drought (Fig. 2B). Dry weight of shoots and roots in MdMYB88/124 RNAi plants were clearly lower than that of GL-3 plants, resulting in a lower root-to-shoot ratio in MdMYB88/124 RNAi plants under drought stress (Fig. 2, C–E). Consistently, MdMYB88 or MdMYB124 overexpression plants had a higher root-to-shoot ratio than that of GL-3 plants in response to long-term drought stress, proportional to the relatively higher dry weight of shoots and roots under drought (Fig. 2, C–E). These data suggest that MdMYB88 and MdMYB124 positively regulate the drought tolerance of apple roots, at least in part, by mediating root architecture.
Figure 2.
Quantitation of morphological traits of GL-3, MdMYB88, or MdMYB124 overexpression plants, and MdMYB88/124 RNAi plants under long-term drought conditions. A, Plant height. B, Stem diameter. C, Dry weight of roots. D, Dry weight of stem. E, Root-to-shoot ratio. Plants were subjected to long-term drought stress for 2 months in a greenhouse. Data are means ± sd (n = 9). One-way ANOVA (Tukey test) was performed, and statistically significant differences are indicated by *P < 0.05 or **P < 0.01.
MdMYB88 and MdMYB124 Regulate Hydraulic Conductivity of Apple Roots under Long-Term Drought Conditions
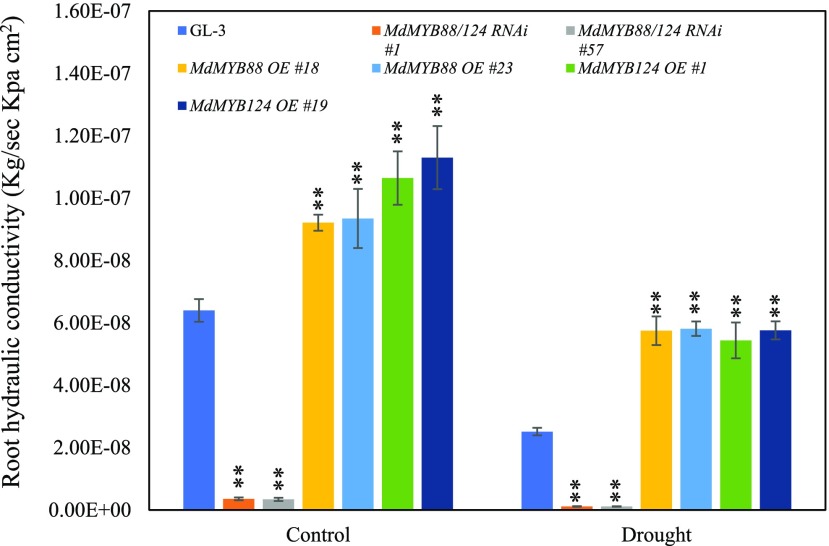
Two fundamental capabilities of roots are supporting shoot components and transporting water and mineral elements to shoots (Warren et al., 2015). Under drought stress, hydraulic conductivity, an indicator of the ability to transport water, decreases in both roots and shoots (Moshelion et al., 2015). Changed root morphology of transgenic plants under drought stress prompted us to examine their root hydraulic conductivity in response to drought. After 2-month exposure to drought conditions, root hydraulic conductivity as measured by high pressure flow matter (HPFM) was reduced remarkably (Fig. 3). Compared with GL-3 plants, roots of MdMYB88/124 RNAi plants had a much lower hydraulic conductivity, whereas MdMYB88 or MdMYB124 overexpression plants had a clearly higher root hydraulic conductivity (Fig. 3; Supplemental Fig. S2). These data are suggestive of a stronger water transportation ability with MdMYB88 or MdMYB124 overexpression under long-term drought stress. We also measured the shoot hydraulic conductivity of the plants tested above and found that, similar to root hydraulic conductivity, shoot hydraulic conductivity of MdMYB88/124 RNAi plants was much lower than that of GL-3 plants under drought stress (Supplemental Fig. S3). Consistently, MdMYB88 or MdMYB124 overexpression plants had a higher shoot hydraulic conductivity than that of GL-3 plants in response to drought stress (Supplemental Fig. S3).
Figure 3.
Root hydraulic conductivity of GL-3, MdMYB88, or MdMYB124 overexpression plants and MdMYB88/124 RNAi plants under long-term drought conditions. Plants were subjected to long-term drought stress for 2 months in a greenhouse. Data are means ± sd (n = 9). One-way ANOVA (Tukey test) was performed, and statistically significant differences are indicated by **P < 0.01.
MdMYB88 and MdMYB124 Mediate Root Xylem Development under Long-Term Drought Conditions
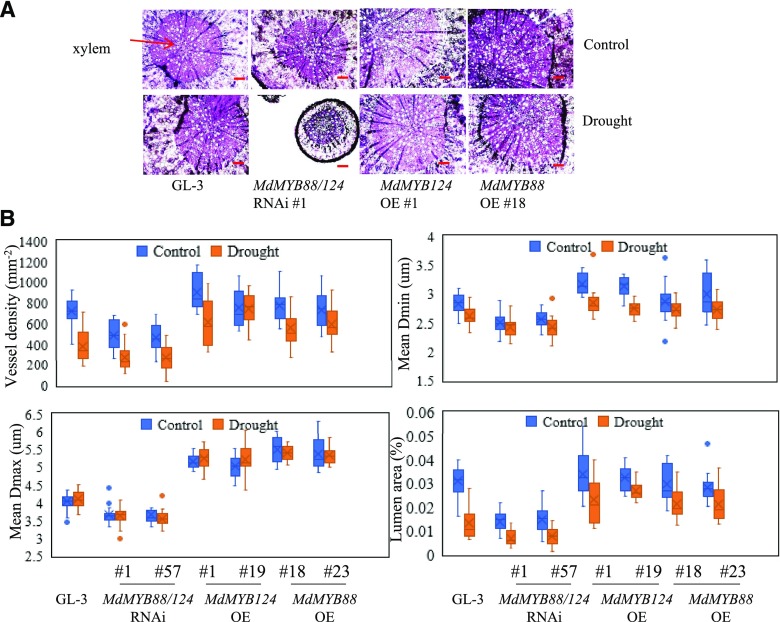
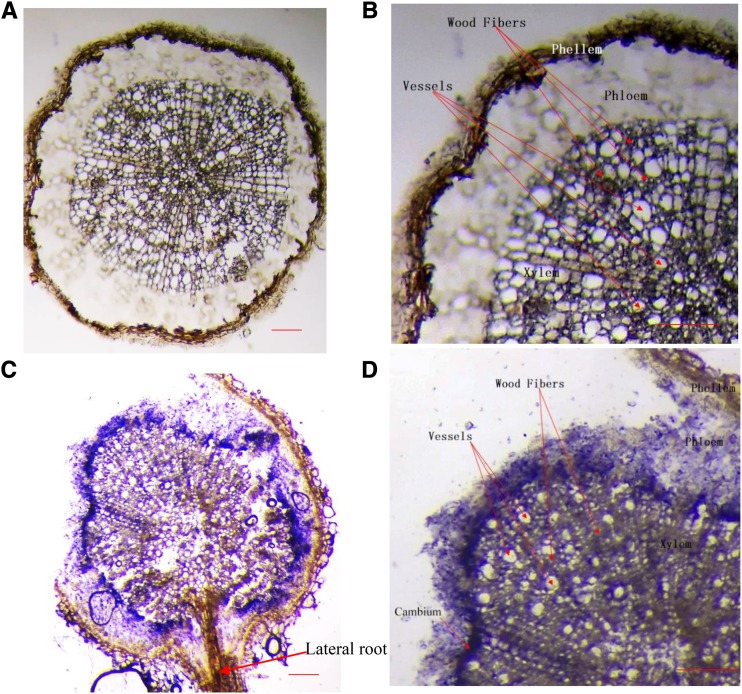
Water is transported from roots to shoots by vessels; vessel embolism and development therefore significantly affect hydraulic conductivity (Olson et al., 2014). We next asked whether MdMYB88 and MdMYB124 were regulators of root xylem development in response to long-term drought treatment (Fig. 3). We first stained roots of transgenic plants and GL-3 plants with Safranin O under control and drought treatments (Fig. 4A; Supplemental Fig. S4). Obviously, MdMYB88/124 RNAi plants had decreased vessel density in response to drought treatment. In comparison with that in GL-3 plants, MdMYB88 or MdMYB124 overexpression plants had higher vessel density under drought conditions (Fig. 4A; Supplemental Fig. S4). We quantified vessel density, vessel diameter (average length of major axis of vessels [mean Dmin], average length of minor axis of vessels [mean Dmax]), and lumen area (Fig. 4B). As shown in Figure 4B, compared to that in GL-3 plants, vessel density and vessel diameter were lower in MdMYB88/124 RNAi plants under drought conditions, whereas those of MdMYB88 or MdMYB124 overexpression plants displayed greater vessel density and diameter (Fig. 4B). Lumen area was quantified as the ratio of total vessel area compared to xylem area. In response to long-term drought stress, the lumen area was decreased in MdMYB88/124 RNAi plants but increased in MdMYB88 or MdMYB124 overexpression plants when compared to nontransgenic GL-3 plants (Fig. 4B). We also noticed that root phloem thickness was significantly decreased in MdMYB88/124 RNAi plants under drought treatment compared with that in GL-3 plants, indicating that MdMYB88 and MdMYB124 might also regulate phloem development in response to drought (Supplemental Fig. S4B).
Figure 4.
Xylem development in roots of GL-3, MdMYB88, or MdMYB124 overexpression plants and MdMYB88/124 RNAi plants under long-term drought conditions. A, Cross sections of roots from GL-3 and transgenic plants stained with Safranin O. Bars, 100 µm. B, Quantification of root xylem of plants shown in A. Mean Dmax, average length of major axis of vessels; mean Dmin, average length of minor axis of vessels; lumen area, total lumen area, relative to xylem area. n = 10.
MdMYB88 and MdMYB124 Are Predominantly Expressed in Xylem Vessels and Cambium in Apple Roots
Previously, we found that MdMYB88 and MdMYB124 are predominantly expressed in the roots of apple plants (Xie et al., 2018). To specifically investigate the localization of MdMYB88 and MdMYB124 transcripts in roots of apple, we performed an in situ hybridization (Fig. 5). When using a sense probe, only background was detectable (Fig. 5, A and B); however, strong signals were observed in the vessels and cambium of apple roots when using an antisense probe (Fig. 5C). Enlarged images showed that transcripts of MdMYB88 and MdMYB124 were visualized in xylem vessels but not in xylem fiber cells (Fig. 5D). In addition, weak signals were detected in the phloem of apple roots (Fig. 5D).
Figure 5.
Localization of MdMYB88 transcripts in roots of GL-3. A, In situ hybridization of MdMYB88 transcripts using sense probe. B, Enlarged image of A. C, In situ hybridization of MdMYB88 transcripts using antisense probe. D, Enlarged image of C. MdMYB88 transcript in roots is indicated by purple coloring. Bars, 100 µm.
MdMYB88 and MdMYB124 Mediate Expression of MdVND6 and MdMYB46 in Apple Roots under Simulated Drought Conditions
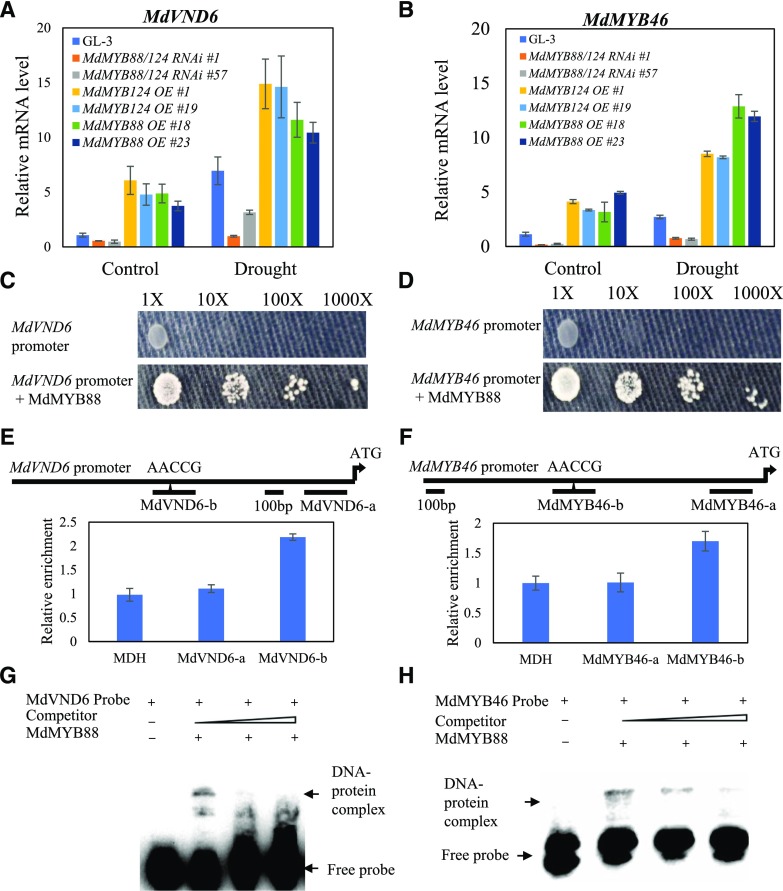
We next asked how MdMYB88 and MdMYB124 regulate xylem vessel development in apple roots. In Arabidopsis, a battery of NAC and MYB genes, including MYB46, VND6, VND7, and SND1, are known to mediate xylem vessel development (Zhong et al., 2007b; Ohashi-Ito et al., 2010; Kim et al., 2013). We then investigated expression of some of these genes in the roots of nontransgenic or transgenic plants under control or simulated drought conditions (Fig. 6, A and B; Supplemental Fig. S5). Reverse transcription quantitative PCR (RT-qPCR) analysis suggested a positive relationship between MdMYB88 and MdMYB124 presence and expression of both MdVND6 and MdMYB46 in the roots of apple under control or drought conditions (Fig. 6, A and B). In contrast, no such relationship was found with MdVND7 and MdSND1 (Supplemental Fig. S5). These data suggest that MdMYB88 and MdMYB124 may regulate root xylem vessel development by mediating expression of MdVND6 and MdMYB46.
Figure 6.
MdMYB88 and MdMYB124 regulate MdMYB46 and MdVND6 expression by directly targeting their promoters. A and B, Expression level of MdVND6 and MdMYB46 in roots of GL-3, MdMYB88 or MdMYB124 overexpression plants, and MdMYB88/124 RNAi plants in response to drought stress. Plants were subjected to 20% PEG8000 for 0 or 6 h. Data are means ± sd (n = 3). C and D, Yeast one-hybrid analysis of interaction between MdMYB88 and MdVND6 (C) and MdMYB46 (D) promoters. AbA concentration is 500 ng/mL. E and F, ChIP-qPCR analysis of MdVND6 (E) and MdMYB46 (F) binding by MdMYB88 and MdMYB124. MDH is the negative control, also serves as the reference gene. Fragments MdVND6-a and MdMYB46-a serve as negative controls in E and F, respectively. Fragments MdVND6-b and MdMYB46-b both contain cis-element of AACCG. Data are means ± sd (n = 3). G and H, EMSA analysis of MdMYB88-His binding to the promoter region of MdVND6 (G) and MdMYB46 (H). Arrowheads indicate protein-DNA complex or free probe.
MdMYB88 and MdMYB124 Directly Target MdVND6 and MdMYB46 Promoters
Previously, we identified one binding site of MdMYB88 and MdMYB124 using chromatin immunoprecipitation qPCR (ChIP-qPCR) and EMSA analyses: AACCG (Xie et al., 2018). Regulation of MdVND6 and MdMYB46 expression by MdMYB88 and MdMYB124 under control and drought conditions prompted us to analyze MdVND6 and MdMYB46 promoter sequences. As expected, a cis-element of AACCG in the promoter region of MdVND6 and MdMYB46 was discovered (Supplemental Fig. S6). By performing yeast one-hybrid (Y1H) analysis, direct binding of MdMYB88 to both promoters was detected (Fig. 6, C and D). ChIP-qPCR analysis was then completed to further determine this direct binding in planta. Our results demonstrated MdMYB88 and MdMYB124 to be capable of binding to the AACCG site in promoters of MdVND6 and MdMYB46 (Fig. 6, E and F). EMSA analysis further confirmed MdMYB88 to directly target MdVND6 and MdMYB46 promoters (Fig. 6, G and H).
MdMYB88 and MdMYB124 Regulate Cellulose and Lignin Deposition in the Roots of Apple in Response to Long-Term Drought Conditions
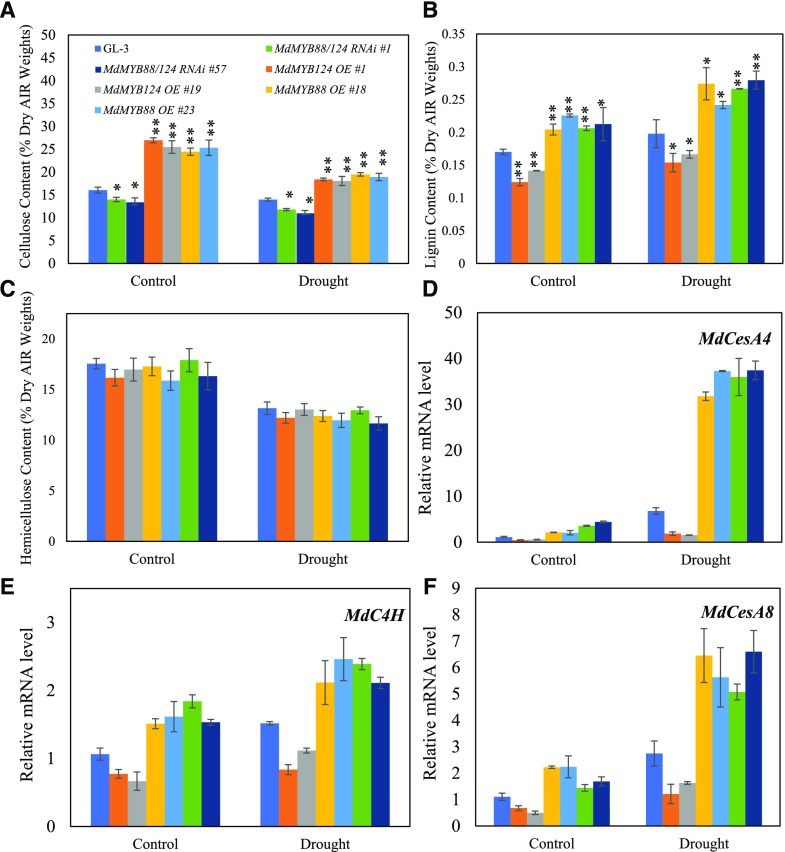
In Arabidopsis, MYB46 is a master regulator for secondary wall-associated cellulose accumulation (Kim et al., 2013). Furthermore, VND6 is a key regulator for xylem vessel differentiation, programmed cell death, and secondary wall formation (Ohashi-Ito et al., 2010; Yamaguchi et al., 2010). Direct regulation of MdVND6 and MdMYB46 by MdMYB88 and MdMYB124 suggests that, in response to long-term drought stress, MdMYB88 and MdMYB124 may participate in the biosynthesis of secondary cell wall components. We then first examined contents of cellulose, lignin, and hemicellulose in roots of transgenic and nontransgenic plants under control or drought conditions. After 2-month drought treatment, MdMYB88/124 RNAi plants accumulated less cellulose and lignin compared with that in GL-3 plants. Under control conditions, MdMYB88 and MdMYB124 expression was positively associated with cellulose and lignin accumulation (Fig. 7, A and B). Consistently, roots of plants overexpressing MdMYB88 or MdMYB124 contained more cellulose and lignin content under control or drought conditions than that of nontransgenic GL-3 plants (Fig. 7, A and B). MdMYB88 and MdMYB124 did not regulate accumulation of hemicellulose in the roots under control or long-term drought conditions (Fig. 7C).
Figure 7.
Content of cellulose, lignin, hemicellulose, and expression level of genes associated with secondary cell wall biosynthesis in roots of GL-3, MdMYB88, or MdMYB124 overexpression plants and MdMYB88/124 RNAi plants under drought conditions. A to C, Contents of cellulose (A), lignin (B), and hemicellulose (C). Plants were subjected to long-term drought stress for 2 months in a greenhouse. Data are means ± sd (n = 9). One-way ANOVA (Tukey test) was performed, and statistically significant differences are indicated by *P < 0.05 or **P < 0.01. D to F, Relative expression levels of MdCesA4 (D), MdCesA8 (E), and MdC4H (F). Plants were subjected to 20% PEG8000 for 0 or 6 h. Data are means ± sd (n = 3).
In Arabidopsis, CELLULOSE SYNTHASE A4, A7, and A8 (CesA4, CesA7, CesA8), CINNAMATE 4-HYDROXYLASE (C4H), PHE AMMONIA LYASE 1 (PAL1), 4-COUMARATE:COA LIGASE 1 (4CL), ACAULIS 5 (ACL5), XYLEM CYSTEINE PEPTIDASE 1 (XCP1), and IRREGULAR XYLEM 9 (IRX9) ) are responsible for the biosynthesis of cellulose, lignin, and hemicellulose. We thus examined expression levels of these genes in roots of transgenic and nontransgenic plants under control or drought conditions. We found that expression levels of MdCesA4, MdCesA8, and MdC4H were decreased in MdMYB88/124 RNAi plants as compared with that in nontransgenic GL-3 plants under control and drought conditions (Fig. 7, D–F). Consistently, the expression levels of these three genes were significantly elevated in plants overexpressing MdMYB88 or MdMYB124 under drought and control conditions. No variation in expression of MdIRX9, MdPAL1, Md4CL1, MdACL5, or MdXCP1 was detected under any conditions (Supplemental Fig. S7).
DISCUSSION
In this study, transgenic apple plants were used to characterize the roles of MdMYB88 and MdMYB124 in drought tolerance of apple trees through the modulation of root xylem development under long-term drought stress. Previously, Xie et al. (2010b) found that Arabidopsis MYB88 and FLP positively regulate plant tolerance to drought stress, as determined by water loss of Arabidopsis leaves and plant survival rate. However, the mechanisms behind the responses in perennial trees to long-term drought stress, specifically changes to tree roots, remains unclear.
In this research, MdMYB88 and MdMYB124 were found to be positive regulators of drought tolerance in apple roots. First, when compared to that in nontransgenic GL-3 control plants, transgenic plants overexpressing MdMYB88 or MdMYB124 had higher root-to-shoot ratios under long-term drought stress. In contrast, MdMYB88/124 RNAi plants had lower root-to-shoot ratios in response to long-term drought stress compared to that in control plants (Fig. 2). Root-to-shoot ratio is often considered an indicative measurement of plant tolerance to drought stress (Xu et al., 2016). Second, root hydraulic conductivity in MdMYB88 or MdMYB124 overexpression plants was higher than that of GL-3 plants, whereas MdMYB88/124 RNAi plants had lower root hydraulic conductivity compared with that in GL-3 plants under long-term drought stress (Fig. 3). Root hydraulic conductivity represents the capability to transport water from the surrounding soil under drought stress; thus, higher root hydraulic conductivity often indicates greater potential water transport from the soil through the root (Melchior and Steudle, 1993; Gambetta et al., 2013; Olaetxea et al., 2015). Third, preliminary data collected from MdMYB88/124 RNAi plants, performed in 2016, also obtained similar results (Supplemental Figs. S8–S11). The long-term consistency of these data are indicative of the reproducibility of these findings. Fourth, shoot hydraulic conductivity also plays critical roles in plant drought tolerance (Faustino et al., 2015; Zhang et al., 2018). MdMYB88 and MdMYB124 were found to be positively associated with shoot hydraulic conductivity, in response to long-term drought conditions, further supporting the conclusion that MdMYB88 and MdMYB124 positively regulate apple root drought tolerance (Supplemental Fig. S3). The positive association of MdMYB88 and MdMYB124 in apple root adaptations under drought stress is consistent with previous findings by Xie et al. (2010b), suggesting MYB88 and MYB124 may have conserved roles across plants species.
MdMYB88 and MdMYB124 were slightly induced by simulated drought in roots of M. sieversii; however, this should not indicate a weak role of them in apple drought tolerance. Genome-wide expression profile of MdMYBs by Cao et al. (2013) showed lower expression levels of all inducible MdMYBs (∼2- to 6-fold) by simulated drought, indicating that MdMYBs are likely to be expressed at a lower level under simulated drought conditions. We selected 10 MdMYB genes, which showed relatively higher expression level in study of Cao et al. (2013), to examine expression of them in M. sieversii roots under simulated drought conditions. Consistent with the results by Cao et al. (2013), we found that all these 10 up-regulated MdMYB genes were not highly induced by simulated drought stress (Supplemental Fig. S12), indicating that MdMYB transcription factors cannot be expressed at higher levels in response to simulated drought treatment. Thus, it is not surprised that expression level of MdMYB88 and MdMYB124 was not high in M. sieversii roots under simulated drought treatment. In addition, Cao et al. (2013) found that MdMYB121, which was also slightly induced by simulated drought (∼2 fold), plays a positive role in tomato drought tolerance.
Many factors affect root hydraulic conductivity, and one of the important factors is vessel (Bramley et al., 2009; Hajek et al., 2014; Brunner et al., 2015). Regulation of root hydraulic conductivity during long-term drought conditions by MdMYB88 and MdMYB124 is primarily the result of root xylem developmental modulation by expression level of MdMYB88 and MdMYB124. Although in seriously suberized roots water is predominantly absorbed by unsuberized fine roots (Kramer and Boyer, 1995) and radio water flow in roots is regulated mainly by aquaporin (Steudle, 2000), xylem vessels are still an important participator of axial hydraulic conductivity (Melchior and Steudle, 1993; Schuldt et al., 2013; Hajek et al., 2014). Through these relationships, additional relationships between vessel density, vessel diameter, and hydraulic conductivity were discovered. It is often believed that larger vessel density and diameter indicate higher root hydraulic conductivity (Syvertsen and Graham, 1985; Vasconcellos and Castle, 1994; Zhang et al., 2018). In MdMYB88/124 RNAi plants, xylem vessel development of roots was disrupted; as a result, plants displayed lower vessel density and vessel diameter under long-term drought stress, compared with GL-3 plants (Fig. 4). Lower vessel density and vessel diameter contributed to a lower root hydraulic conductivity in MdMYB88/124 RNAi plants under drought conditions. In contrast, plants overexpressing MdMYB88 or MdMYB124 had higher vessel density and vessel diameter, resulting in a higher root hydraulic conductivity under drought conditions. In addition, less developed roots of MdMYB88/124 RNAi lines led to lower root dry weight (Fig. 2) and root surface area, potentially resulting in a decreased area of unsuberized fine roots and lower root hydraulic conductivity under drought conditions. Cellulose and lignin are also responsible for xylem conductivity. In poplar (Populus spp.), reduced lignin content impairs xylem conductivity and growth efficiency (Voelker et al., 2011). Mutation of ESK1 in Arabidopsis results in the reduced cellulose content, leading to the collapsed xylem vessels and thus lower xylem hydraulic conductivity (Lefebvre et al., 2011). However, xylem conductivity is not directly regulated by cellulose or lignin but instead by vessel development (Lefebvre et al., 2011; Voelker et al., 2011). In our results, lower hydraulic conductivity in MdMYB88/124 RNAi plants should not be a direct result of lower content of cellulose and lignin but through disrupted vessel development. Moreover, MdMYB88 and MdMYB124 were predominantly expressed in root xylem vessels, but not in root xylem fibers, as determined by in situ hybridization (Fig. 5), further supporting MdMYB88 and MdMYB124 as two positive regulators of root hydraulic conductivity through the modulation of root xylem vessel development.
Root cross section analysis and in situ hybridization also suggested that MdMYB88 and MdMYB124 regulate phloem development (Fig. 5; Supplemental Fig. S4). Phloem does not participate in water conduction directly but can regulate primary root growth as a source of water in maize (Wiegers et al., 2009). Phloem also functions as a capacitance to buffer the pulse of xylem water conduction under drought stress (Pfautsch and Adams, 2013; Pfautsch et al., 2015). Under drought stress, the ability of developing secondary phloem will increase in wooden plants (Robert et al., 2011). Thus, the difference in phloem between MdMYB88/124 RNAi plants, MdMYB88 and MdMYB124 overexpression plants, and GL-3 plants may modulate hydraulic conductivity by regulating root growth.
In Arabidopsis, MYB88 and FLP also participate in the regulation of root gravitropism (Wang et al., 2015) and lateral root development (Lei et al., 2015). Strong expression of MYB88 or FLP has been detected in developing xylem cells (Lei et al., 2015), consistent with our in situ hybridization results as seen in Figure 5. Predominant expression of MdMYB88 and MdMYB124 in root xylem vessels, but not in fibers, also explained why MdSND1 required for secondary cell wall deposition in fiber cells was not modulated by MdMYB88 and MdMYB124 under control or drought conditions (Supplemental Fig. S5).
Root xylem development is regulated by genes including MYB46, VND6, and VND7 on a molecular level in Arabidopsis (Zhong et al., 2007a; Yamaguchi et al., 2010; Kim et al., 2013). Among these, MYB46, an R2R3 MYB transcription factor, is the hub for the regulation of xylem vessel development (Kim et al., 2013). VND6 and VND7, two NAC-domain-containing proteins in Arabidopsis, regulate root xylem vessel differentiation by direct regulation of MYB46 (Ohashi-Ito et al., 2010; Yamaguchi et al., 2011). Furthermore, an Y1H approach revealed key upstream factors of VND6, VND7, MYB46, cellulose-, hemicellulose-, and lignin-associated genes in Arabidopsis: ARABIDOPSIS THALIANA HOMOLOG OF E2F C (E2Fc) (Taylor-Teeples et al., 2015). In this study, MdMYB88 and MdMYB124 were found to regulate root xylem vessel development by directly modulating expression levels of MdVND6 and MdMYB46 in response to drought conditions. First, we found that MdMYB88 and MdMYB124 positively controlled the expression of MdVND6 and MdMYB46 in response to drought stress (Fig. 6, A and B). Secondly, EMSA, ChIP-qPCR, and Y1H results supported evidence of direct binding of MdMYB88 (EMSA, Y1H, ChIP-qPCR) and MdMYB124 (ChIP-qPCR) to the promoter regions of MdVND6 and MdMYB46 (Fig. 6, C–H). However, we did not find any recognition sites of MdMYB88 and MdMYB124 in the MdVND7 promoter, indicating that MdMYB88 and MdMYB124 may not directly regulate MdVND7 in apple roots. This was also consistent with our observation that MdMYB88 and MdMYB124 did not regulate expression of MdVND7 in apple roots under control or drought conditions (Supplemental Fig. S5).
MdMYB88 and MdMYB124 were found to be two positive regulators of genes responsible for deposition of cellulose and lignin, including MdCesA4, MdCesA8, and MdC4H, in response to drought stress and thus cellulose and lignin accumulation under long-term drought (Fig. 7). Cellulose is the most abundant polysaccharide in plants. Previous research has suggested that modulation of the architecture of secondary cell walls might be one of the mechanisms of plant adaptation to drought stress (Lee et al., 2016). Therefore, modulation of drought tolerance through MdMYB88 and MdMYB124 might be due to regulation of cellulose content in apple roots under drought conditions. Increased lignification is a common response to drought stress (Moura et al., 2010). Hence, elevated lignification in plant roots overexpressing MdMYB88 or MdMYB124 while under drought conditions may reflect specific adaptation strategies to drought stress. Furthermore, decreased lignin levels in the roots of MdMYB88/124 RNAi plants under drought conditions may explain why these plants were more sensitive to drought.
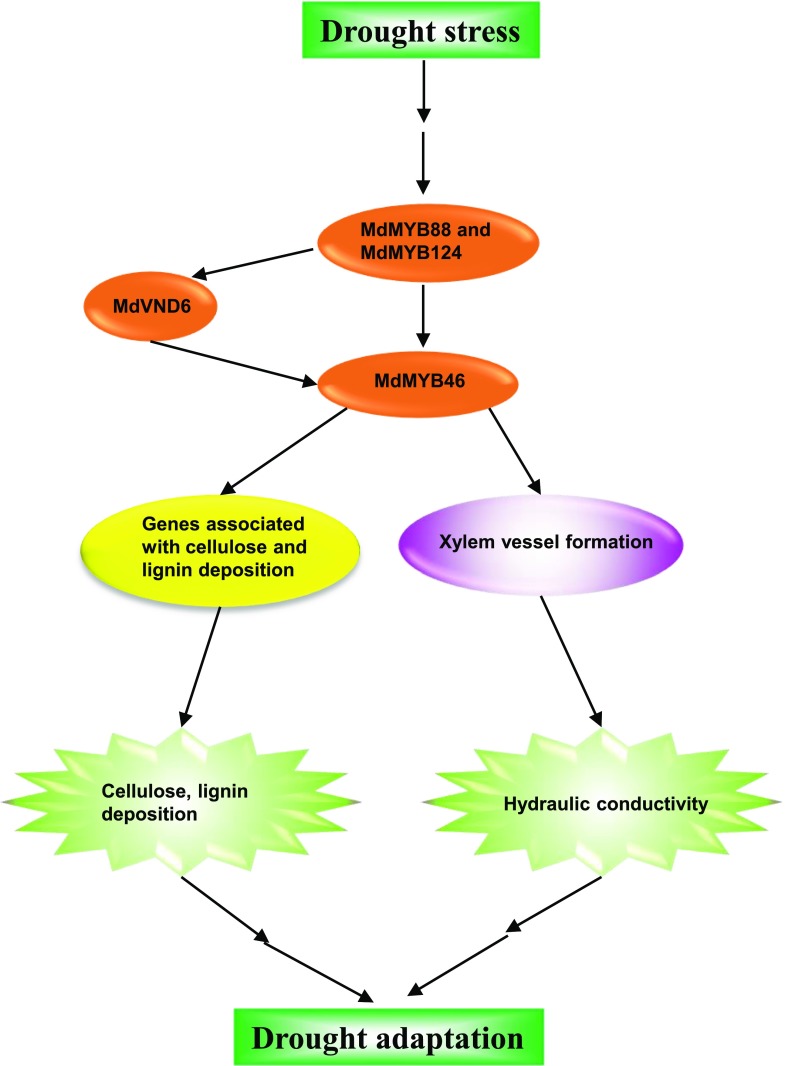
In summary, drought stress in apple roots activates MdMYB88 and MdMYB124, which then directly target and induce the expression of MdVND6 and MdMYB46. Up-regulated MdMYB46 enhances drought tolerance by regulating root xylem vessel formation, which results in a greater hydraulic conductivity and thus increases drought tolerance. Increased expression of MdMYB46 also activates its downstream genes associated with cellulose and lignin biosynthesis, resulting in cellulose and lignin deposition, as well as drought adaptation (Fig. 8).
Figure 8.
A model for drought adaptation mediated by MdMYB88 and MdMYB124 in apple roots. In apple roots, drought stress activates MdMYB88 and MdMYB124, which then directly target the promoters of MdVND6 and MdMYB46 and induce their expression. Up-regulated MdMYB46 expression enhances drought tolerance by regulating root xylem vessel formation, which results in a greater hydraulic conductivity and thus drought adaptation. Increased expression of MdMYB46 also activates downstream genes associated with cellulose and lignin biosynthesis, resulting in cellulose and lignin deposition as well as drought adaptation.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Stress Treatment
For long-term drought treatment, 18 tissue-cultured GL-3 (from Royal Gala [Malus × domestica] seedlings with high regeneration capacities [Dai et al., 2013]) or transgenic MdMYB88/124 RNAi or overexpression plants (Xie et al., 2018), were rooted and transferred to pots (30 cm × 18 cm) filled with equal parts of local loess sand and wormcast medium. Pots were placed in a greenhouse under natural illumination, with a temperature of 20°C–35°C and humidity of 35%–55%. In July, seedlings of each line were divided into a well-watered group (n = 9) and long-term drought group (n = 9). Seedlings of the well-watered group were irrigated daily to maintain field capacity of 75%–85%; seedlings of the long-term drought group were daily irrigated to maintain a field capacity of 45%–55%. Both treatments lasted for 2 months. At the end of treatment, roots were harvested for morphology and vessel analysis.
For RT-qPCR, 3-month-old Malus sieversii seedlings, transgenic plants, and nontransgenic GL-3 plants, were transferred into plastic containers containing 20 L of Hoagland solution for an additional month. M. sieversii is a drought-tolerant wild species (Liu et al., 2012). All plants were hydroponically cultured in a growth chamber with a temperature of 25°C, illuminance of 4,000 lx, and humidity of 50%–75%. Plants were then treated with 20% (w/v) PEG6000 (Sigma) for 0 h or 6 h. At the end of each treatment, roots were washed and snap frozen with liquid nitrogen. Samples were stored at −80°C until RT-qPCR analysis. Primers used are listed in Supplemental Table S1.
Root Morphology Analysis
Shoot height, diameter of the stem, dry weight of roots, and dry weight of shoots were measured directly after harvesting. Total root length, root surface area, root volume, and average diameter were measured using a Winrhizo 2002 (Regent Corporation, Canada). Five biological replicates were performed for each measurement.
Measurement of Root or Shoot Hydraulic Conductivity
Hydraulic conductivity of roots and shoots of both transgenic and nontransgenic plants was performed with an HPFM (Dynamax, Houston) as described by Tyree et al. (1994) and Wei et al. (1999) with modifications. In brief, after drought treatment, plants were cut into two sections at 2 cm above ground. Sections were then soaked in de-gassed water and connected to HPFM. Root hydraulic conductivity was measured using a transient method, whereas shoot hydraulic conductivity was measured with a quasi steady-state method in accordance with the HPFM manual.
Root Xylem Vessel Analysis
Roots with diameters of 0.5 to 2 mm were selected for vessel analysis. Five root segments of each plant were fixed in FAA stationary solution (5% [v/v] formalin, 5% [v/v] acetic acid, and 90% [v/v] ethyl alcohol) for 24 h, then transferred into 18% (v/v) ammonia at 65°C for 90 min for dissociation. The root segments were subsequently dehydrated in a graded ethyl alcohol series for 3 h (30%, 50%, 75%, 85%, 95%, and 100% twice, v/v). Transparent roots obtained from sequential xylene treatment were embedded in paraffin; embedded blocks were sectioned with a rotary microtome (RM2125RTS, Leica, Germany) and observed with a light microscope (80i, Nikko, Japan). Photos were taken with a digital camera (CFI60, Nikko, Japan) mounted on the microscope and analyzed with Image J software .
The theoretical maximum hydraulic conductivity was calculated with the equation described by Hagen-Poiseuille’s law (Eq. 1). Since the cross section of the vessel was ellipse, a modified Equation 2 was used for the calculation of hydraulic conductivity of apple roots (Nobel, 2005).
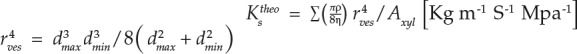 |
where ρ is the density of water at 20°C (998.205kg/m3), η is the viscosity of water at 20°C (1.002 × 10−9 Mpa s), rves is the vessel radius, Axyl is the area of specific root xylem, and Dmax and Dmin are the major and minor axis of vessel, respectively.
In situ Hybridization in Apple Roots
Harvested roots of GL-3 with a diameter of 0.5 mm were cut into 1-mm segments and fixed immediately in 4% (w/v) paraformaldehyde in 0.1 m phosphate-buffered saline buffer (pH = 7) for 4 h at room temperature and then overnight at 4°C. Root segments were then rinsed three times with water, dehydrated in a graded ethanol series (75%, 85%, 95%, and 100%, v/v), embedded in paraffin, and then processed into 10-µm sections using a microtome (RM2125RTS, Leica, Germany). Finally, sections were collected onto po-Lys slides, dried on a hot plate at 45°C for 3 h, and then overnight at 37°C, for complete drying. Prepared slides were store at −80°C until used.
cDNA fragment of MdMYB88 and MdMYB124 was cloned into a pST19 vector with the primers listed in Supplemental Table S1, resulting in MdMYB88/124-pST19. MdMYB88/124-pST19 plasmid DNA was transcribed in vitro and labeled with DIG (Digoxin) using a DIG labeling kit (Roche, Switzerland). For hybridization, the probes were hydrolyzed in carbonate buffer (0.04 mm NaHCO3 and 0.06 mm Na2CO3) to 100- to 150-bp fragments, precipitated in 70% (v/v) ethanol, and dissolved in DEPC (Diethylpyrocarbonate)-treated water to a final concentration of 1 ng/μL. In situ hybridization was performed as described by Omori et al. (2009).
Yeast One-Hybrid Analysis
Yeast one-hybrid was performed using MATCHMAKER One-Hybrid System (Clonetech, USA). MdMYB46 and MdVND6 promoters were individually cloned into pABAi vectors, resulting in MdMYB46-pABAi and MdVND6-pABAi plasmids. Plasmids were then transformed into yeast strain Y1H Gold after linearization. Positive clones were used to determine the Aureobasidin A (AbA) concentration due to growth restraints of positive clones on SD (Synthetic Dropout) medium without uracil.
Full-length CDS (coding sequence) of MdMYB88 was cloned into pGADT7 vector to form MdMYB88-pGADT7, which was then transformed into Y1H Gold competent cells carrying MdMYB46-PAbAi or MdVND6-PAbAi. Cell growth was observed on SD medium without Leu supplemented with AbA.
EMSA and ChIP-qPCR
EMSA and ChIP-qPCR were performed as described by Xie et al. (2018). Probes used for EMSA are listed in Supplemental Table S1.
Quantification of Cellulose, Lignin, and Hemicellulose in Apple Roots
Following root morphology analysis, dried roots from all plants were smashed with a pulverizer. Alcohol was added to prepare alcohol insoluble residues (AIR) of all roots as described by Merali et al. (2013). The cellulose content was measured with AIR, utilizing the anthrone method described by Ondiaka et al. (2015). The lignin content was determined with AIR using the acetyl bromide method described by Brinkmann et al. (2002).
Hemicellulose extraction was performed as described by Mortimer et al. (2015) with modifications. Five to fifteen milligrams AIR was transferred to a 1.5-mL tube and dissolved with 400 μL 4 m NaOH at room temperature for 1 h. After centrifugation at 5,000g for 10 min, pellets were discarded, the supernatant was transferred to a new tube, and the sample was neutralized with 4 m HCl. Hemicellulose was precipitated by adding ethanol to a final concentration of 90% (v/v). After centrifugation at 5,000g for 10 min, pellets were washed three times with 70% (v/v) ethanol and once with absolute ethanol. The pellets were then dried overnight at 60°C before assessing the hemicellulose content using the previously described anthrone method (Ondiaka et al., 2015).
RNA Extraction and RT-qPCR Analysis
RNA extraction was carried out as described by Xie et al. (2018). The RT-qPCR analysis was performed according to Guan et al. (2013). Primers used are listed in Supplemental Table S1.
Statistical Analysis
Unless noted otherwise, data are reported as the mean ± sd. Statistical significance was determined though one-way ANOVA (Tukey’s test) analysis using SPSS (version 21.0, USA). Variations were considered significant if P < 0.05 or 0.01.
Accession Numbers
Sequence data used in this article can be found in the GenBankwith thefollowing accession numbers KY569647 (MdMYB88), KY569648(MdMYB124), XP_008376439.1 (MdVND6), XP_008363629.1 (MdMYB46), XP_008380992.1 (MdVND7), NP_001280877.1 MdSND1), XP_008348984.1 (MdCesA4), XP_008383611.(1MdCesA8 ), NP_001281035.1 (MdC4H), KY359347 (Md4CL1 ), XP_008387584.1 (MdPAL1), XP_008382503.1 (MdXCP1), and XP_008393603.1 (MdACL5).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression of MdMYB86-like, MdMYB40, and MdMYB6 in GL-3 control and MdMYB88/124 RNAi plants and plant morphology of GL-3 control, MdMYB88, or MdMYB124 overexpression, and MdMYB88/124 RNAi plants under control or long-term drought conditions.
Supplemental Figure S2. The relationship between flow rate and pressure of roots of GL-3 control, MdMYB88 or MdMYB124 overexpression, and MdMYB88/124 RNAi plants under control or long-term drought conditions.
Supplemental Figure S3. Shoot hydraulic conductivity of GL-3 control, MdMYB88 or MdMYB124 overexpression, and MdMYB88/124 RNAi plants under control or long-term drought conditions.
Supplemental Figure S4. Cross section analysis of roots from GL-3 control and transgenic plants after drought stress.
Supplemental Figure S5. Expression level of MdVND7 and MdSND1 in roots of GL-3 control, MdMYB88 or MdMYB124 overexpression, and MdMYB88/124 RNAi plants in response to drought stress.
Supplemental Figure S6. Analysis of MdMYB46 and MdVND6 promoter sequences.
Supplemental Figure S7. Expression level of MdPAL1, Md4CL1, MdIRX9, MdXCP1, and MdACL5 in roots of GL-3 control, MdMYB88 or MdMYB124 overexpression, and MdMYB88/124 RNAi plants in response to drought.
Supplemental Figure S8. Dry weight of shoots, shoot height, dry weight of roots, and root-to-shoot ratio in roots of GL-3 control and MdMYB88/124 RNAi plants under control and long-term drought conditions.
Supplemental Figure S9. Root hydraulic conductivity of GL-3 control and MdMYB88/124 RNAi lines in response to long-term drought stress.
Supplemental Figure S10. Cross sections of roots from GL-3 control and MdMYB88/124 RNAi roots under control and long-term drought conditions.
Supplemental Figure S11. Root vessel development of GL-3 control and MdMYB88/124 RNAi plants under control and long-term drought conditions.
Supplemental Figure S12. Expression of MdMYB genes in M. sieversii roots under 20% PEG8000 treatment for 0 or 6 h.
Supplemental Table S1. Primers used in this study.
Acknowledgments
We thank Dr. Dongmei Yang for her assistance in measurement of hydraulic conductivity. We thank Dr. Zhihong Zhang from Shenyang Agricultural University for providing tissue-cultured GL-3 plants. This work was supported by the National Natural Science Foundation of China (31622049 and 31572106), the Key Program of the National Natural Science Foundation of China (31330068), the Program of Sci-Tech Star of Shaanxi (2015kjxx14), and startup funding (Z111021402) from Northwest A&F University. Q. Guan is also supported by the Thousand Talents Plan of China.
Footnotes
This work was supported by the National Natural Science Foundation of China (31622049 and 31572106), the Key Program of the National Natural Science Foundation of China (31330068), the Program of Sci-Tech Star of Shaanxi (2015kjxx14), and startup funding from Northwest A&F University (Z111021402). Q. Guan is also supported by the Thousand Talents Plan of China.
Articles can be viewed without a subscription.
References
- Bramley H, Turner NC, Turner DW, Tyerman SD (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol 150: 348–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann K, Blaschke L, Polle A (2002) Comparison of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J Chem Ecol 28: 2483–2501 [DOI] [PubMed] [Google Scholar]
- Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZH, Zhang SZ, Wang RK, Zhang RF, Hao YJ (2013) Genome wide analysis of the apple MYB transcription factor family allows the identification of MdoMYB121 gene confering abiotic stress tolerance in plants. PLoS One 8: e69955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liu Y, Maere S, Lee E, Van Isterdael G, Xie Z, Xuan W, Lucas J, Vassileva V, Kitakura S, Marhavý P, Wabnik K, et al. (2015) A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development. Nat Commun 6: 8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Barigah ST, Kleinhentz M, Eshel A (2008) Is xylem cavitation resistance a relevant criterion for screening drought resistance among Prunus species? J Plant Physiol 165: 976–982 [DOI] [PubMed] [Google Scholar]
- Dai H, Li W, Han G, Yang Y, Ma Y, Li H, Zhang Z (2013) Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci Hortic (Amsterdam) 164: 202–208 [Google Scholar]
- Faustino LI, Moretti AP, Graciano C (2015) Fertilization with urea, ammonium and nitrate produce different effects on growth, hydraulic traits and drought tolerance in Pinus taeda seedlings. Tree Physiol 35: 1062–1074 [DOI] [PubMed] [Google Scholar]
- Gambetta GA, Fei J, Rost TL, Knipfer T, Matthews MA, Shackel KA, Walker MA, McElrone AJ (2013) Water uptake along the length of grapevine fine roots: developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiol 163: 1254–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q, Wu J, Zhang Y, Jiang C, Liu R, Chai C, Zhu J (2013) A DEAD box RNA helicase is critical for pre-mRNA splicing, cold-responsive gene regulation, and cold tolerance in Arabidopsis. Plant Cell 25: 342–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guet J, Fichot R, Lédée C, Laurans F, Cochard H, Delzon S, Bastien C, Brignolas F (2015) Stem xylem resistance to cavitation is related to xylem structure but not to growth and water-use efficiency at the within-population level in Populus nigra L. J Exp Bot 66: 4643–4652 [DOI] [PubMed] [Google Scholar]
- Guseman JM, Webb K, Srinivasan C, Dardick C (2017) DRO1 influences root system architecture in Arabidopsis and Prunus species. Plant J 89: 1093–1105 [DOI] [PubMed] [Google Scholar]
- Hajek P, Leuschner C, Hertel D, Delzon S, Schuldt B (2014) Trade-offs between xylem hydraulic properties, wood anatomy and yield in Populus. Tree Physiol 34: 744–756 [DOI] [PubMed] [Google Scholar]
- Keppler BD, Showalter AM (2010) IRX14 and IRX14-LIKE, two glycosyl transferases involved in glucuronoxylan biosynthesis and drought tolerance in Arabidopsis. Mol Plant 3: 834–841 [DOI] [PubMed] [Google Scholar]
- Kim WC, Ko JH, Kim JY, Kim J, Bae HJ, Han KH (2013) MYB46 directly regulates the gene expression of secondary wall-associated cellulose synthases in Arabidopsis. Plant J 73: 26–36 [DOI] [PubMed] [Google Scholar]
- Ko JH, Jeon HW, Kim WC, Kim JY, Han KH (2014) The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann Bot 114: 1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JT, Testerink C (2016) Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7: 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PJ, Boyer JS (1995) Water Relations of Plants and Soil. Academic Press, San Diego, CA [Google Scholar]
- Kulkarni M, Soolanayakanahally R, Ogawa S, Uga Y, Selvaraj MG, Kagale S (2017) Drought response in wheat: key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front Chem 5: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LB, Nadeau JA, Lucas J, Lee EK, Nakagawa T, Zhao L, Geisler M, Sack FD (2005) The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. Plant Cell 17: 2754–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Kim KY, Jung WJ, Avice JC, Ourry A, Kim TH (2007) Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J Exp Bot 58: 1271–1279 [DOI] [PubMed] [Google Scholar]
- Lee BR, Muneer S, Jung WJ, Avice JC, Ourry A, Kim TH (2012) Mycorrhizal colonization alleviates drought-induced oxidative damage and lignification in the leaves of drought-stressed perennial ryegrass (Lolium perenne). Physiol Plant 145: 440–449 [DOI] [PubMed] [Google Scholar]
- Lee DK, Jung H, Jang G, Jeong JS, Kim YS, Ha SH, Do Choi Y, Kim JK (2016) Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol 172: 575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Fortabat MN, Ducamp A, North HM, Maia-Grondard A, Trouverie J, Boursiac Y, Mouille G, Durand-Tardif M (2011) ESKIMO1 disruption in Arabidopsis alters vascular tissue and impairs water transport. PLoS One 6: e16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants (Basel) 4: 112–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Lee E, Keerthisinghe S, Lai L, Li M, Lucas JR, Wen X, Ren X, Sack FD (2015) The FOUR LIPS and MYB88 transcription factor genes are widely expressed in Arabidopsis thaliana during development. Am J Bot 102: 1521–1528 [DOI] [PubMed] [Google Scholar]
- Liu BH, Cheng L, Ma FW, Liang D, Zou YJ (2012) Influence of rootstock on drought response in young ‘Gale Gala’ apple (Malus domestica Borkh.) trees. J Sci Food Agric 92: 2421–2427 [DOI] [PubMed] [Google Scholar]
- Makkena S, Lee E, Sack FD, Lamb RS (2012) The R2R3 MYB transcription factors FOUR LIPS and MYB88 regulate female reproductive development. J Exp Bot 63: 5545–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseda PH, Fernández RJ (2006) Stay wet or else: three ways in which plants can adjust hydraulically to their environment. J Exp Bot 57: 3963–3977 [DOI] [PubMed] [Google Scholar]
- Melchior W, Steudle E (1993) Water transport in onion (Allium cepa L.) roots (changes of axial and radial hydraulic conductivities during root development). Plant Physiol 101: 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Ho JD, Collins SRA, Le Gall G, Elliston A, Käsper A, Waldron KW (2013) Characterization of cell wall components of wheat straw following hydrothermal pretreatment and fractionation. Bioresour Technol 131: 226–234 [DOI] [PubMed] [Google Scholar]
- Mortimer JC, Faria-Blanc N, Yu X, Tryfona T, Sorieul M, Ng YZ, Zhang Z, Stott K, Anders N, Dupree P (2015) An unusual xylan in Arabidopsis primary cell walls is synthesised by GUX3, IRX9L, IRX10L and IRX14. Plant J 83: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Halperin O, Wallach R, Oren R, Way DA (2015) Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water-use efficiency, growth and yield. Plant Cell Environ 38: 1785–1793 [DOI] [PubMed] [Google Scholar]
- Moura JC, Bonine CA, de Oliveira Fernandes Viana J, Dornelas MC, Mazzafera P (2010) Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol 52: 360–376 [DOI] [PubMed] [Google Scholar]
- Nobel PS. (2005) Physiochemical and environmental plant physiology. Elsevier Academic Press, Netherlands [Google Scholar]
- Ohashi-Ito K, Oda Y, Fukuda H (2010) Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell 22: 3461–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaetxea M, Mora V, Bacaicoa E, Garnica M, Fuentes M, Casanova E, Zamarreño AM, Iriarte JC, Etayo D, Ederra I, et al. (2015) Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids. Plant Physiol 169: 2587–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ME, Anfodillo T, Rosell JA, Petit G, Crivellaro A, Isnard S, León-Gómez C, Alvarado-Cárdenas LO, Castorena M (2014) Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol Lett 17: 988–997 [DOI] [PubMed] [Google Scholar]
- Omori H, Hosokawa M, Shitsukawa N, Murai K, Yazawa S (2009) Screening of chrysanthemum plants with strong resistance to chrsanthemum stunt viroid. Journal of the Japanese Society for Horticultural Science 78: 350–355 [Google Scholar]
- Ondiaka SN, Masinde EW, Koenraadt CJ, Takken W, Mukabana WR (2015) Effects of fungal infection on feeding and survival of Anopheles gambiae (Diptera: Culicidae) on plant sugars. Parasit Vectors 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfautsch S, Adams MA (2013) Water flux of Eucalyptus regnans: defying summer drought and a record heatwave in 2009. Oecologia 172: 317–326 [DOI] [PubMed] [Google Scholar]
- Pfautsch S, Hölttä T, Mencuccini M (2015) Hydraulic functioning of tree stems--fusing ray anatomy, radial transfer and capacitance. Tree Physiol 35: 706–722 [DOI] [PubMed] [Google Scholar]
- Prince SJ, Murphy M, Mutava RN, Durnell LA, Valliyodan B, Shannon JG, Nguyen HT (2017) Root xylem plasticity to improve water use and yield in water-stressed soybean. J Exp Bot 68: 2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf M, Munir MQ, Ul Hassan M, Ahmad M, Afzal M (2007) Performance of wheat genotypes under osmotic stress at germination and early seedling growth stage. Afr J Biotechnol 6: 971–975 [Google Scholar]
- Robert EMR, Schmitz N, Boeren I, Driessens T, Herremans K, De Mey J, Van de Casteele E, Beeckman H, Koedam N (2011) Successive cambia: a developmental oddity or an adaptive structure? PLoS One 6: e16558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt B, Leuschner C, Brock N, Horna V (2013) Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. Tree Physiol 33: 161–174 [DOI] [PubMed] [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot 55: 2343–2351 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ 25: 251–263 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Vishwakarma RK, Arafat YA, Gupta SK, Khan BM (2015) Abiotic stress induces change in Cinnamoyl CoA Reductase (CCR) protein abundance and lignin deposition in developing seedlings of Leucaena leucocephala. Physiol Mol Biol Plants 21: 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. (2000) Water uptake by roots: effects of water deficit. J Exp Bot 51: 1531–1542 [DOI] [PubMed] [Google Scholar]
- Syvertsen JP, Graham JH (1985) Hydraulic conductivity of roots, mineral nutrition and leaf gas exchange of citrus rootstocks. J Am Soc Hortic Sci 110: 865–869 [Google Scholar]
- Taylor-Teeples M, Lin L, de Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, et al. (2015) An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 517: 571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Yang S, Cruiziat P, Sinclair B (1994) Novel methods of measuring hydraulic conductivity of tree root systems and interpretation using AMAIZED (a maize-root dynamic model for water and solute transport). Plant Physiol 104: 189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Okuno K, Yano M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot 62: 2485–2494 [DOI] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Vadez V, Kholova J, Medina S, Kakkera A, Anderberg H (2014) Transpiration efficiency: new insights into an old story. J Exp Bot 65: 6141–6153 [DOI] [PubMed] [Google Scholar]
- Vasconcellos LABC, Castle WS (1994) Trunk xylem anatomy of mature healthy and blighted grapefruit trees on several rootstocks. J Am Soc Hortic Sci 119: 185–194 [Google Scholar]
- Voelker SL, Lachenbruch B, Meinzer FC, Kitin P, Strauss SH (2011) Transgenic poplars with reduced lignin show impaired xylem conductivity, growth efficiency and survival. Plant Cell Environ 34: 655–668 [DOI] [PubMed] [Google Scholar]
- Wang HZ, Yang KZ, Zou JJ, Zhu LL, Xie ZD, Morita MT, Tasaka M, Friml J, Grotewold E, Beeckman T, et al. (2015) Transcriptional regulation of PIN genes by FOUR LIPS and MYB88 during Arabidopsis root gravitropism. Nat Commun 6: 8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD (2015) Root structural and functional dynamics in terrestrial biosphere models--evaluation and recommendations. New Phytol 205: 59–78 [DOI] [PubMed] [Google Scholar]
- Wei C, Tyree MT, Steudle E (1999) Direct measurement of xylem pressure in leaves of intact maize plants. A test of the cohesion-tension theory taking hydraulic architecture into consideration. Plant Physiol 121: 1191–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegers BS, Cheer AY, Silk WK (2009) Modeling the hydraulics of root growth in three dimensions with phloem water sources. Plant Physiol 150: 2092–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Chen P, Yan Y, Bao C, Li X, Wang L, Shen X, Li H, Liu X, Niu C, et al. (2018) An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol 218: 201–218 [DOI] [PubMed] [Google Scholar]
- Xie Z, Li D, Wang L, Sack FD, Grotewold E (2010b) Role of the stomatal development regulators FLP/MYB88 in abiotic stress responses. Plant J 64: 731–739 [DOI] [PubMed] [Google Scholar]
- Xie Z, Lee E, Lucas JR, Morohashi K, Li D, Murray JA, Sack FD, Grotewold E (2010a) Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB FOUR LIPS via direct targeting of core cell cycle genes. Plant Cell 22: 2306–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Burgess P, Zhang X, Huang B (2016) Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J Exp Bot 67: 1979–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sharp RE (2010) Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant Cell Environ 33: 590–603 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Goué N, Igarashi H, Ohtani M, Nakano Y, Mortimer JC, Nishikubo N, Kubo M, Katayama Y, Kakegawa K, et al. (2010) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol 153: 906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Mitsuda N, Ohtani M, Ohme-Takagi M, Kato K, Demura T (2011) VASCULAR-RELATED NAC-DOMAIN7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J 66: 579–590 [DOI] [PubMed] [Google Scholar]
- Ye H, Roorkiwal M, Valliyodan B, Zhou L, Chen P, Varshney RK, Nguyen HT (2018) Genetic diversity of root system architecture in response to drought stress in grain legumes. J Exp Bot 69: 3267–3277 [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008) Programmed proteome response for drought avoidance/tolerance in the root of a C(3) xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 49: 226–241 [DOI] [PubMed] [Google Scholar]
- Yue B, Xue W, Xiong L, Yu X, Luo L, Cui K, Jin D, Xing Y, Zhang Q (2006) Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172: 1213–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Srivastava AK, Pan Y, Bai J, Fang J, Shi H, Zhu JK (2018) Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol 176: 2082–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye ZH (2012) MYB46 and MYB83 bind to the SMRE sites and directly activate a suite of transcription factors and secondary wall biosynthetic genes. Plant Cell Physiol 53: 368–380 [DOI] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH (2007a) The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 19: 2776–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH (2007b) Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta 225: 1603–1611 [DOI] [PubMed] [Google Scholar]