Abstract
Omalizumab was the first, and for a long time the only available monoclonal antibody for the add-on treatment of severe allergic asthma. In particular, omalizumab selectively targets human immunoglobulin (Ig)E, forming small-size immune complexes that inhibit IgE binding to its high- and low-affinity receptors. Therefore, omalizumab effectively blunts the immune response in atopic asthmatic patients, thus significantly improving the control of asthma symptoms and successfully preventing disease exacerbations. These very positive effects of omalizumab make it possible to drastically decrease both referrals to the emergency room and hospitalizations for asthma exacerbations. Such important therapeutic actions of omalizumab have been documented by several randomized clinical trials, and especially by more than 10 years of real-life experience in daily clinical practice. Omalizumab can also interfere with airway remodelling by inhibiting the activation of IgE receptors located on structural cells such as bronchial epithelial cells and airway smooth muscle cells. Moreover, omalizumab is characterized by a very good safety and tolerability profile. Hence, omalizumab represents a valuable therapeutic option for the add-on biological treatment of severe allergic asthma.
Keywords: IgE, omalizumab, severe allergic asthma
Introduction
Asthma is a very common chronic obstructive disease of the airways, originating from complex interactions between genetic and environmental factors, and heterogeneously featured by several different phenotypes/endotypes characterized by various patterns of bronchial inflammation and remodelling.1–6 Most adults and children with asthma can obtain quite a good control of their disease using inhaled therapies, including corticosteroids and β2-adrenergic agonists, eventually integrated by the addition of other inhaled bronchodilators such as anticholinergics and oral drugs like leukotriene inhibitors.7–9 However, despite the optimization of standard therapies, patients with severe asthma, who can amount to 5–10% of the global population of asthmatic patients, may need an adjunctive biological treatment because of the recurrence of disease exacerbations associated with an inadequate control of respiratory symptoms.10,11
In particular, the anti-immunoglobulin (Ig)E humanized antibody, omalizumab, was the first, and for a long time the only biological drug available in clinical practice for the add-on therapy of uncontrolled asthma.12–14 The therapeutic efficacy of omalizumab depends on the key pleiotropic functions exerted by IgE in the pathobiology of allergic asthma.15,16 In fact, the main hallmark of allergic asthma is the remarkably increased production of IgE directed against inhaled antigens, defined as atopy.17,18 In atopic patients, many pathogenic events involved in the development of asthma are mediated by the activation of high-affinity and low-affinity IgE receptors located on the surface of both immune-inflammatory and airway structural cells.17,18 By activating their specific receptors, IgEs implement a complex pathobiologic network involving mast cells, basophils, eosinophils, dendritic cells, bronchial epithelial cells and airway smooth muscle cells. This IgE-operated cellular array can be shattered by omalizumab via neutralization of all IgE bioactivities.13,19 The main clinical and functional outcomes of the pharmacological mechanism of action of omalizumab include a significant decrease in the number and severity of asthma exacerbations, better symptom control, and an improvement of airflow limitation.13,19 The add-on treatment of severe allergic asthma with omalizumab thus represents an excellent example of precision medicine consisting of a biological therapy directed against a molecular target, namely IgE, which is overexpressed in atopic patients and plays a prominent role in disease pathophysiology. Therefore, the use of omalizumab in asthma treatment makes it possible to realize a targeted therapy addressed towards a specific phenotype, such as allergic asthma, driven by an IgE-mediated underlying endotype.20 Because omalizumab is currently the only available anti-IgE therapeutic agent, it plays a unique role of the first-choice biologic drug for the add-on treatment of severe allergic asthma.
On the basis of such considerations, the aim of this review article is to outline the pathogenic role of IgE in allergic asthma, and to discuss the mechanism of action as well as the clinical effects of the anti-IgE monoclonal antibody, omalizumab.
Role of IgE in allergic asthma
In patients with atopic asthma, production of IgE takes place in lymph nodes and airway mucosa as a consequence of interleukin (IL)-4-dependent Ig class switching, which empowers B-cells to synthesize this specific antibody subtype.21,22 In particular, IL-4 triggers the maturation of naïve B-lymphocytes into IgE-secreting plasma cells, and then memory B-cells develop that produce very large amounts of allergen-specific IgE. The molecular structure of IgE includes two variable fragments (Fab) which interact with specific antigens, and a constant region (Fc) that binds to IgE receptors. Overall, the IgE molecule is made of two identical light chains, each including a variable (VL) and a constant (CL) domain, paired with two identical heavy chains, each constituted by a variable portion consisting of a unique domain (VH), and by a constant fragment including four domains (Cε1, Cε2, Cε3, Cε4). In allergic diseases the pathogenic role of IgE depends on its binding, via the two Cε3 domains, to high-affinity (FcεRI) and low-affinity (FcεRII/CD23) receptors expressed by many different cells.
FcεRIs located on mast cells and basophils have a tetrameric structure consisting of one α, one β and two γ subunits (αβγ2), whereas FcεRIs expressed by eosinophils, monocytes/macrophages, myeloid and plasmacytoid dendritic cells, and also by structural cells like bronchial epithelial cells and airway smooth muscle cells, are αγ2 trimers lacking the β subunit.15,16,23,24 FcεRIs bind to IgE through the two extracellular domains of the α chain, that interact with the two Cε3 domains of IgE, whilst the intracellular β- and γ-subunits are engaged in signalling functions. On mast cell and basophil surfaces, antigenic epitopes promote the bridging of two contiguous IgE molecules already anchored on their FcεRI receptors (cross-linking). The resulting dimerization of adjacent FcεRIs induces the activation of a complex signalling network leading to the release of preformed granule-associated mediators (histamine, tryptase, chymase and heparin), as well as to the secretion of newly formed autacoids (cysteinyl leukotrienes C4-D4 and prostaglandin D2) and to the synthesis of many cytokines, chemokines and growth factors including IL-3, IL-4, IL-5, IL-6, IL-8, IL-13, RANTES, and granulocyte macrophage colony-stimulating factor (GM-CSF).25 Such cellular events trigger early and late asthmatic reactions manifested by patients with allergic asthma upon exposure to inhaled antigens.26 The early-phase reaction occurs in a few minutes after cross-linking of antigen/IgE/FcεRI complexes, and is mainly mediated by the contractile response of airway smooth muscle cells elicited by bronchoconstrictive agents released upon mast cell degranulation. The late-phase reaction takes place some hours after exposure to inhaled antigens and features airway smooth muscle contraction and bronchial inflammatory changes induced by cytokines and chemokines responsible for eosinophil activation and infiltration. Such effects are mostly evoked by antigen-mediated IgE aggregation, followed by mast cell stimulation. However, the activation of mast cells can be also promoted by monomeric IgE, regardless of antigen-dependent cross-linking. In fact, monomeric IgE molecules have the property of triggering the release of histamine and leukotrienes from mast cells. Moreover, monomeric IgE prolong the survival of mast cells by stimulating them to produce IL-6.27,28 Such effects are further potentiated by mast cell exposure to IL-4.29
FcεRII/CD23 receptors are featured by a C-type (calcium-dependent) lectin configuration including a globular domain, which binds IgE and is associated with a long stalk chain that induces the constitution of a trimeric receptor complex. The latter is characterized by a slightly lower affinity for IgE when compared with FcεRI.30,31 FcεRII receptors include two different forms named CD23a and CD23b, arising from alternative splicing of the CD23 gene. CD23a is constitutively present on B-cells; CD23b expression, induced by IL-4, is detectable on eosinophils, antigen presenting cells, and human bronchial epithelial cells.31,32
IgE plays a key role in the regulation of FcεRI/FcεRII expression. In mice lacking IgE, FcεRIs and FcεRIIs are down-regulated on mast cells/basophils and B-lymphocytes, respectively; expression of these receptors can indeed be restored by IgE supplementation.33–35 Similar observations were also reported in humans, who are characterized by a close relationship between blood IgE level and FcεRI expression. In this regard, it is noteworthy that free receptors are internalized, whereas they persist on the cell surface when bound to their specific ligands; this implies that IgE exerts a valid protective effect against internalization and proteolytic degradation of IgE receptors.36,37 By inducing FcεRI expression, IgE makes mast cells more responsive to antigens and thereby, can more effectively stimulate the secretion of inflammatory mediators.34 Thus, IgE-dependent upregulation of FcεRIs is responsible for a marked amplification of the allergic cascade. Furthermore, mast cell/basophil expression of FcεRIs is induced by monomeric IgE, independently of antigen cross-linking.38 In addition to mast cells and basophils, a direct relationship between FcεRI expression and IgE serum counts was also detected in monocytes and dendritic cells.39,40 Moreover, IgE binding to FcεRII/CD23 prevents receptor cleavage, thus promoting the stabilization of FcεRII coupled to IgE.41 On the contrary, unoccupied FcεRIIs are susceptible to receptor downregulation.42
It is not only mast cell degranulation and the subsequent development of early and late asthmatic responses that are triggered by IgE interactions with their receptors, but further pathogenic actions occurring in atopic asthmatic patients are dependent on activation of FcεRIs and FcεRIIs located within the airways on many different immune-inflammatory and structural cells.
For instance, the binding of IgE to FcεRIs expressed by dendritic cells implements key bioactivities, including the facilitation of antigen capture, elaboration and presentation to T-lymphocytes.15,17,43 Moreover, upon IgE binding, dendritic cells increase their production of the chemokine CCL28, which specifically interacts with the CCR10 receptor expressed by Th2 lymphocytes, thus recruiting these cells into the airways.44,45 Furthermore, IgE-operated activation of FcεRIs located on plasmacytoid dendritic cells suppresses the production of antiviral proteins such as interferons.46
Eosinophils express both FcεRI and FcεRII receptors, whose stimulation by IgE is involved in several different functions.47–49 In allergic patients with bronchial eosinophilia, FcεRIs expressed by blood and tissue eosinophils are characterized by either a trimeric or a tetrameric structure. High expression levels of FcεRI/FcεRIIs seem to be correlated with eosinophilic infiltration of the airways. In addition, IgE exerts an anti-apoptotic function on eosinophils, thus prolonging their survival.50
With regard to bronchial resident cells, IgE receptors can be detected at the level of epithelial and smooth muscle cells. In particular, both FcεRIs and FcεRIIs are expressed by bronchial epithelial cells.23,32 FcεRIIs behave as carriers of either IgE or IgE-antigen complexes through the airway epithelial layer.51,52 Moreover, it is also possible that IgE stimulates bronchial epithelial cells to synthesize and release growth factors involved in airway remodelling, such as transforming growth factor (TGF)-β.13
FcεRIs are also expressed by bronchial smooth muscle cells.53 In patients with asthma, the binding of IgE to these receptors is responsible for cell proliferation, as well as for the production of proinflammatory agents and proteins of the extracellular matrix. Indeed, it has been reported that IgE is able to induce the synthesis of collagens I and III by airway smooth muscle cells.54 Therefore, in patients with atopic asthma IgE exerts key functions also in regard to the pathobiology of airway remodelling.
Mechanism of action of omalizumab
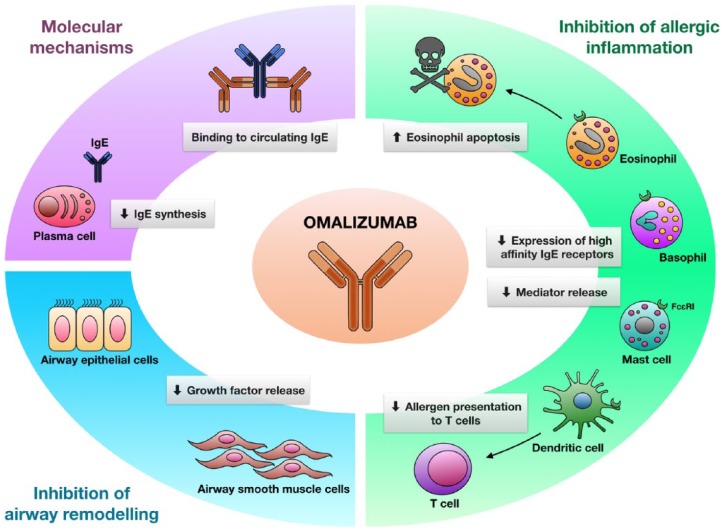
Omalizumab is an anti-human IgE monoclonal antibody developed by means of hybridoma technology.55 In particular, being a humanized antibody that includes only 5% or less of murine residues corresponding to the complementarity determining regions of the original mouse antibody, omalizumab is characterized by a very low risk of eliciting immune reactions against nonself antigenic determinants.56,57 Omalizumab selectively binds to the two Cε3 domains of human IgE, thus forming trimeric or hexameric IgE/anti-IgE immune complexes (Figure 1).58 The very limited dimensions of these immune complexes, which do not bind complement, make them soluble and unable to precipitate, thereby being readily removable by the mononuclear phagocytic system.58,59 Moreover, IgE/omalizumab aggregates are characterized by a strong stability thanks to the high affinity of omalizumab for IgE, so that they do not cross vascular walls and accumulate in peripheral blood, as well as into mucosal membranes of upper and lower airways.60
Figure 1.
Mechanism of action and therapeutic effects of omalizumab.
The humanized monoclonal antibody omalizumab binds to free human IgE, thus inducing the generation of immune complexes which impede the interactions between IgE and its receptors. As a consequence, omalizumab inhibits all IgE-dependent cellular events, including mast cell degranulation, basophil expression of high-affinity IgE receptors, facilitation of antigen presentation to T-cells, eosinophil survival and IgE synthesis, as well as release of growth factors from airway epithelial cells and production of extracellular matrix proteins by airway smooth muscle cells. Taken together, these effects result in a clinically significant inhibition of allergic inflammation and airway remodelling.
IG, immunoglobulin.
Because omalizumab specifically interacts with the Cε3 domain, that includes the IgE binding sites for both FcεRIs and FcεRIIs/CD23, this humanized antibody impedes IgE linkage to high-affinity and low-affinity receptors, thus preventing all IgE-mediated cellular events involved in asthma pathobiology.60,61 Hence, at the level of mast cells and basophils, omalizumab inhibits antigen-triggered degranulation (Figure 1), and also prevents the release of eicosanoids and the synthesis of cytokines/chemokines induced by FcεRI activation. In addition, omalizumab is also thought to be capable of abrogating mast cell functions which do not depend on IgE dimerization. Indeed, omalizumab can inhibit the cellular actions mediated by monomeric IgE via signalling mechanisms responsible for a prolongation of mast cell survival, as well as for an enhanced production of mast cell-derived cytokines including IL-4, IL-6, IL-13 and tumour necrosis factor (TNF)-α.38,62 Therefore, via effective interferences with the production of key cytokines and growth factors implicated in mast cell survival, omalizumab causes the apoptotic death of mast cells. Moreover, omalizumab also induces the apoptosis of eosinophils. In fact, omalizumab significantly increases eosinophil staining with annexin V, a very well-known marker of apoptosis. Omalizumab exerts this pro-apoptotic effect by inhibiting the synthesis of GM-CSF, an essential survival agent for eosinophils.63
By impeding the binding of IgE to FcεRIs, omalizumab causes a 97% decrease in the number of these high-affinity receptors expressed on the basophil surface, and this effect is paralleled by an increased threshold of basophil and mast cell activation inducible by antigen challenge.56 In fact, because of the depletion of free IgE caused by omalizumab, empty FcεRI receptors located on the plasma membrane of basophils translocate into the cytoplasm, and are not resynthesized.62 Upon omalizumab binding to blood IgE, the free serum concentrations of the antibody decreases by 96–99%.64 Furthermore, by preventing IgE linkage to FcεRIs present on the cell membrane of dendritic cells, omalizumab neutralizes IgE-dependent facilitation of antigen presentation to T-lymphocytes (Figure 1). Omalizumab also inhibits IgE production by impeding the interaction of the antibody with FcεRII receptors expressed by B-cells specialized in synthesizing IgE. Moreover, omalizumab can also reduce IgE expression by inhibiting mast cell production of IL-4, the main cytokine responsible for the induction of IgE synthesis. Even when complexed with omalizumab, IgE maintains the property of binding allergens, thereby sequestrating them and preventing their linkage to residual IgE eventually bound to mast cell FcεRIs.56 Omalizumab does not bind to IgE linked to FcεRIs, so that this biologic drug cannot trigger degranulation of mast cells and basophils, and is thus not able to induce anaphylaxis.
Because of the competitive mechanism of action of omalizumab with regard to its interaction with the Cε3 domains of IgE, it is necessary that omalizumab overcomes IgE numbers in a ratio within a range of 7:1–15:1.65 Hence, the dosage of omalizumab needs to be based on both blood IgE levels and body weight. If correctly dosed, after a single subcutaneous injection, omalizumab induces an 84–99% reduction in serum free IgE concentration.66
Add-on therapy of severe asthma with omalizumab
Omalizumab was initially shown to have the property of inhibiting both early and late asthmatic responses elicited by inhaled antigens.67 Later on, patients with moderate to severe asthma were enrolled in many randomized controlled trials.68–73 These studies demonstrated that an add-on treatment with omalizumab significantly attenuated asthma symptoms and decreased asthma exacerbations, hospital admissions, access to emergency rooms, unscheduled visits and intake of systemic glucocorticoids.10,13,74
Specifically, Busse and colleagues, Solér and colleagues and Holgate and colleagues observed that omalizumab significantly reduced asthma exacerbations in inadequately controlled allergic asthmatic patients.68–70 Furthermore, Vignola and colleagues showed that a treatment with omalizumab for 28 weeks, carried out in patients with moderate to severe asthma and allergic rhinitis, induced significant improvements in the Asthma Quality of Life Questionnaire (AQLQ) and the Rhinitis Quality of Life Questionnaire scores;71 such findings are very relevant because of the clinical importance of allergic rhinitis as a highly frequent asthma comorbidity. In this regard, Tsabouri and colleagues performed a meta-analysis including 11 trials undertaken in uncontrolled allergic rhinitis patients, thus demonstrating that omalizumab reduced the clinical manifestations of rhinitis and the need for rescue therapy.75
In the Investigation of Omalizumab in Severe Asthma Treatment (INNOVATE) study, Humbert and colleagues enrolled 419 atopic patients with severe allergic asthma, susceptible to frequent exacerbations and exhibiting a remarkable airway obstruction [baseline forced expiratory volume in 1 s (FEV1) ⩾ 40 < 80% predicted].73 In patients receiving a 28-week add-on treatment with omalizumab, significant reductions in disease exacerbations, accesses to emergency care and consumption of oral corticosteroids were recorded. Moreover, when compared with patients treated with placebo, patients in the intervention group experienced marked benefits with regard to quality of life and peak expiratory flow.73 Subsequently, 850 patients suffering from asthma and aged from 12 to 75 years were included by Hanania and colleagues in a study aimed to compare the effects of omalizumab versus placebo after 48 weeks of add-on therapy. This trial showed that omalizumab was able to improve asthma symptoms and decrease disease exacerbations; at the same time, the anti-IgE treatment lowered the requirement for rescue medications.76 A further important randomized controlled study, involving 419 allergic patients with moderate to severe asthma, was conducted by Busse and colleagues in inner city children, adolescents and young adults during 60 weeks of add-on treatment with omalizumab; the main results included relevant benefits with regard to control of asthma symptoms and reduction of seasonal disease exacerbations, and also a significant decrease in inhaled corticosteroid intake.77 An interesting meta-analysis performed by Rodrigo and colleagues and including eight controlled trials, published from 2001 to 2009 and comprising about 3000 patients, strengthened the demonstration of the marked efficacy of omalizumab in school-aged children, adolescents and adults with moderate to severe persistent allergic asthma.78 The decreases in exacerbation rate and consumption of oral corticosteroids represented the primary findings of the above mentioned meta-analysis; the secondary outcomes referred to pulmonary function, use of as-needed bronchodilators, asthma symptom score and overall AQLQ score. Furthermore, it has been recently shown that the clinical improvements regarding better symptom control and lower exacerbation risk can be maintained during long-term treatment with omalizumab.79
Such important results, referring to placebo-controlled studies, have been also confirmed and expanded by a very large body of real-life investigations carried out worldwide,80–93 which have been systematically reviewed by Abraham and colleagues and by Alhossan and colleagues.94,95 These authors have clearly shown that many patients with severe allergic asthma can be defined as ‘good’ or ‘excellent’ responders to omalizumab with regard to decreases in disease exacerbations, hospital admissions, and overall intake of both oral and inhaled corticosteroids, as well as with regard to improvements in asthma symptoms, pulmonary function, and quality of life. These very positive results have been confirmed also by real-life studies performed in severe asthmatic children.96,97 The relevant benefits obtained in terms of symptom control and lung function, evaluated as significant increases in asthma control test (ACT) score and FEV1, respectively, persist and progressively improve during long-term (1–4 years) treatment with omalizumab.93 Such remarkable improvements including very important clinical, functional, and corticosteroid-sparing effects, associated with a good safety and tolerability profile, have been shown to persist after 7 and even 9 years of anti-IgE therapy.98–100 In addition to corroborating these convincing findings, further data have been obtained by real-life studies. In particular, it has been shown that omalizumab efficacy can be affected by relevant asthma comorbidities, such as chronic rhinitis and sinusitis, nasal polyposis, obesity, gastro-oesophageal reflux and abnormal sensitivity to aspirin. Indeed, Novelli and colleagues reported that, in comparison with patients without comorbidities, asthmatics with comorbidities under treatment with omalizumab were characterized by significantly lower measures of asthma control (ACT score) and lung function (FEV1), associated with higher levels of fractional exhaled nitric oxide (FeNO) and peripheral blood eosinophils.90 Therefore, omalizumab represents a key additional therapeutic strategy, aimed to integrate the standard treatment of severe allergic asthma; however, it is also critical to adequately manage comorbidities because they crucially contribute to the lack of asthma control.90 Moreover, these authors reported that omalizumab was very effective along a broad age range (18 to ⩾65 years), but the therapeutic effectiveness resulted to be greater in younger people.91
A very careful screening of asthmatic patients potentially eligible to anti-IgE therapy with omalizumab is mandatory in the management of severe allergic asthma. In our real-life daily clinical practice, the most successful results can be achieved by adding omalizumab to the treatment of severe allergic asthma characterized by inadequate disease control, as well as by recurrent exacerbations and oral glucocorticoid-dependence. In these patients, we frequently observe drastic decreases in asthma exacerbations, associated with marked reductions of oral corticosteroid intake, relevant improvements in lung function featured by notable increases in both FEV1 and FEV1/forced vital capacity ratio, and also with dramatic drops of peripheral blood eosinophil counts.85 Indeed, the EXTRA study has shown that high levels of peripheral blood eosinophils, together with the overexpression of other two biomarkers such as FeNO and serum periostin, can be considered as reliable indicators of type-2 asthmatic inflammation, capable of predicting a good therapeutic effect of omalizumab consisting of marked reductions in asthma exacerbation rates.101,102 However, a very recent retrospective real-life study demonstrated, in patients with severe allergic asthma, that the therapeutic response to omalizumab was similar in patients with relatively high (⩾300 cells/µl of blood) or low (<300 cells/µl of blood) pretreatment blood eosinophil numbers.103 As an add-on biological therapy, omalizumab might be also administered concomitantly with allergen-specific immunotherapy (AIT). In fact, the results of preliminary studies suggest that omalizumab can improve AIT efficacy and safety.104–107
Omalizumab has been shown not only to effectively attenuate airway inflammation, but also to potentially blunt bronchial remodelling, which particularly characterizes severe asthma (Figure 1). Indeed, omalizumab can reduce TGF-β synthesis by bronchial epithelial cells, thus possibly interfering with airway remodelling. In this regard, Huang and colleagues investigated the effects of omalizumab in cultures of bronchial epithelial cells, stimulated by IL-1β or ragweed allergen and grown in a medium including the serum collected from an atopic patient sensitized to dust mite.108 These authors observed that omalizumab inhibited the production of proinflammatory cytokines and TGF-β, thereby preventing airway fibrosis.108 Moreover, Zietkowski and colleagues reported that omalizumab significantly lowered the concentration of endothelin (ET)-1 in the exhaled breath condensate of allergic patients with severe asthma;109 ET-1 is a powerful peptide synthesized by activated endothelial cells, airway epithelial cells and mast cells, which induces fibrosis of the bronchial subepithelial layer and proliferation of airway smooth muscle cells. Collagen production by airway smooth muscle cells can be also inhibited by omalizumab.54 Furthermore, Hoshino and Ohtawa showed that the chest computed tomography (CT) of patients treated for 16 weeks with omalizumab was characterized by a decrease of bronchial wall thickness, paralleled by a coexistent expansion of airway caliber.110 These results were corroborated by Tajiri and colleagues, who demonstrated that omalizumab significantly reduced airway wall thickness, evaluated on CT scans obtained from severe asthmatics before and after 48 weeks of treatment.111 Such imaging findings are consistent with the histological evidence of a positive effect of omalizumab on airway remodelling. Indeed, Riccio and colleagues collected bronchial biopsies from patients with severe allergic asthma before and after 1 year of treatment with omalizumab, and they observed a decreased thickness of the bronchial epithelial reticular basement membrane (RBM), associated with a reduced eosinophilic infiltration.112 Nevertheless, not all patients exhibited a decreased RBM thickness. In particular, the patients enrolled in this study can be subdivided in responders and nonresponders to the anti-remodelling action of anti-IgE therapy on the basis of galectin-3 expression in bronchial samples. In fact, only the responder group was characterized by high expression levels of galectin-3, when compared with nonresponders;113 galectin-3 could thus be considered as a reliable predictor of patient response to the effects of omalizumab on airway remodelling.
According to some trials, omalizumab can surprisingly be effective also in nonallergic asthmatics. In this regard, de Llano and colleagues reported that a 2-year course of add-on therapy with omalizumab induced significant improvements in both ACT score and Global Evaluation of Treatment Effectiveness scale in nonallergic asthmatic patients.114 Moreover, a real-life trial which enrolled both allergic and nonallergic asthmatics was carried out by Grimaldi-Bensouda and colleagues, who found that omalizumab elicited a marked reduction of hospitalizations and emergency department visits regardless of the atopic status.88 Furthermore, in patients with nonatopic asthma Garcia and colleagues performed a randomized controlled study which showed that omalizumab, when compared with placebo, was able to lower the frequency of asthma exacerbations and to induce also a significant FEV1 increase and a sharp decrease of FcεRI expression on basophils and plasmacytoid dendritic cells (pDC).115 Such experimental findings prompted Lommatzsch and colleagues to hypothesize at least two possible explanations for the unexpected effects of omalizumab in nonallergic asthma.116 The first hypothesis implies that, even if skin prick tests resulted to be negative and allergen-specific serum IgE-antibodies were not detected, these apparently nonallergic patients could be sensitized to unrecognized allergens responsible for a local sensitization process triggering an immune response confined within the airways, with no systemic spreading. The second, more fascinating hypothesis, is based on the potential ability of omalizumab to restore the antiviral protective action of pDCs, which can be defective in both allergic and nonallergic asthmatic patients. Indeed, upon FcεRI activation by anti-allergen, as well as by antiviral and antibacterial IgE, pDCs lose their ability to produce interferons, that might be reinstated by omalizumab. Therefore, by neutralizing the deleterious impact of IgE on the innate antiviral function of pDCs, omalizumab could reduce the exacerbations induced by respiratory viruses. This second hypothesis has been strengthened by the results of the Preventative Omalizumab or Step-up Therapy for Fall Exacerbations (PROSE) study, carried out by Teach and colleagues, who showed that omalizumab significantly potentiated the production of interferon (IFN)-α by inner city asthmatic children in response to rhinovirus infection;117 higher IFN-α increases were associated with lower numbers of fall asthma exacerbations after a preseasonal treatment with omalizumab, started 4–6 weeks before the return to school and ended 90 days after the school beginning date.
A crucial clinical aspect regarding the use of omalizumab refers to the duration of anti-IgE treatment. In particular, because the therapeutic effects of omalizumab are dependent on its persistent occupancy of the Cε3 domain of the constant portion of IgE, it could be argued that the administration of this drug should occur continuously, without interruptions. In fact, discontinuation of a long-term treatment with omalizumab is often followed within a few months by an increased serum concentration of free IgE, associated with a recrudescence of asthma symptoms and exacerbations, as well as with an enhanced intensity of immune responses triggered by allergens and evaluated through a skin prick test.57,118 Nonetheless, Nopp and colleagues performed a small-size withdrawal trial which enrolled 18 patients, treated for 6 years with omalizumab. These authors reported that the suspension of anti-IgE treatment was associated, in 12 of 18 participants, with the persistence for at least further 3 years of the therapeutic effects induced by omalizumab, including a better control of asthma symptoms and a relevant improvement of lung function.119 This report is consistent with subsequent findings, obtained by Baena-Cagnani and colleagues in some Argentinian children with asthma, who were completely free of respiratory symptoms during the first 3 years of follow up after interruption of anti-IgE treatment.120 Hence, these authors speculated that omalizumab might change the natural history of severe asthma thanks to its potential positive effects on airway remodelling. By contrast, Kuprys-Lipinska and Kuna reported that 9 out of 11 Polish adult patients experienced a relapse of asthma exacerbations during the first 5 months after forced withdrawal of omalizumab treatment due to modified reimbursement policies.121 Moreover, a worsening of Asthma Control Questionnaire and AQLQ scores was recorded. In order to explain the apparently discordant results of these studies, it should be kept in mind that the patients enrolled in such trials belonged to different age groups. Therefore, it can be argued that omalizumab might modify the natural history of asthma in younger, but not in older patients. Anyway, further studies are required to assess the potential impact of omalizumab on the pathobiologic evolution of severe asthma. Due to these considerations, the decision of interrupting anti-IgE therapy after many years of add-on treatment is a crucial point, which should be carefully pondered with particular regard to adult patients with severe uncontrolled asthma.122 Indeed, the recent Xolair Persistency Of Response after long-term Therapy (XPORT) study, performed in adult asthmatics receiving long-term treatment with omalizumab, demonstrated that continuation of anti-IgE therapy resulted in sustained symptom control and persistent decrease in exacerbation risk, whereas discontinuation of omalizumab was associated with significant increases in both free blood IgE levels and FcεRI basophil expression.79
Safety and tolerability of omalizumab
Omalizumab is characterized by a very good profile of safety and tolerability. In fact, the most frequent adverse events include local reactions confined to the sites of drug injection. Headache, nausea, or fatigue have also been occasionally reported. Anyway, the global pattern of side effects and adverse events related to omalizumab is quite similar to that one occurring in placebo-treated patients.123 Although omalizumab is a nonanaphylactogenic antibody, anaphylactic and anaphylactoid events have been sporadically observed, but such reactions occur very rarely.124 Furthermore, antibodies against omalizumab are not usually detected in patients undergoing add-on treatment with this drug.
Several years ago, a slight increase in malignancies was reported in asthmatic patients receiving omalizumab, when compared with patients treated with placebo.66 However, no difference in cancer incidence was found between the general population and patient groups under biological therapy with omalizumab.125,126
Some cases of Churg–Strauss syndrome have also been seldom reported in association with omalizumab treatment.127–129 However, reasonable doubts exist with regard to the possibility that a Churg–Strauss syndrome can be a true consequence of omalizumab therapy, rather than a pre-existing condition probably unveiled by the progressive tapering and the subsequent cessation of systemic corticosteroid treatment, made possible by the omalizumab treatment itself. Curiously, omalizumab has also been suggested as a potential therapy of Churg–Strauss vasculitis.130
Although IgEs are the antibodies responsible for immune protection against parasitic infestations, the occurrence of these infections seems to be quite rare during biological therapy with omalizumab. Nevertheless, because some reports suggested that treatment with omalizumab can be associated with a slight increase in the prevalence of helminthic infestations,131 patients living in or moving to regions where these parasitic infections are endemic should be counselled about such a potential risk.
Omalizumab appears to be safe also for the cardiovascular system. Indeed, a careful analysis of eight controlled trials comprehensively including more than 3000 participants, showed that the omalizumab-related cardiovascular risk was similar to that one observed in patients undergoing treatment with placebo.78 However, the postmarketing Epidemiologic Study of Xolair: Evaluating Clinical Effectiveness and Long-term Safety in Patients with Moderate to Severe Asthma (EXCELS) observational cohort study did not rule out the possibility of increased cardiovascular and cerebrovascular risks among patients treated with omalizumab.132 Anyway, the same authors of the EXCELS study carefully analyzed the pooled data referring to 25 randomized controlled trials and 2 extension studies, comprehensively including 3342 patients receiving omalizumab and 2895 participants treated with placebo.133 They concluded that the occurrence of serious arterial thrombotic events, referring to cardiovascular death, myocardial infarction, ischemic stroke, transient ischemic attack and unstable angina, was similar between the two groups.
When compared with controls, no difference in the rate of spontaneous abortions was detected in pregnant women treated with omalizumab.134 In addition, no increases in the numbers of major congenital abnormalities and small-size newborns for gestational age have been reported with regard to children born from women treated with omalizumab.135
Current positioning of omalizumab as an anti-asthma biological therapy
Within the context of add-on biological treatments for severe uncontrolled asthma, anti-IgE and anti-IL-5/IL-5 receptor monoclonal antibodies are currently available worldwide.136–141 Therefore, a careful characterization of patient features is needed in order to select the best therapeutic option for each patient. In particular, clinical and functional aspects, allergy tests, IgE serum levels, and blood eosinophil counts are essential to make the right drug choice. In this regard, patients with severe allergic asthma are natural candidates for an add-on treatment with omalizumab.136 For nonallergic severe asthmatic patients with refractory blood eosinophilia, there is a clear indication for the use of either anti-IL-5 drugs (mepolizumab or reslizumab), or the IL-5 receptor blocker benralizumab. Atopic patients with blood eosinophilia are potentially eligible for both anti-IgE and anti-IL-5/IL-5 receptor therapies; however, when compared with the latter treatment options, the preference for omalizumab can be reliably based on a very large body of evidence, especially including more than a decade of real-life, postmarketing efficacy and safety studies. Finally, for children aged between 6 and 12 years, obviously satisfying the prescribing criteria for omalizumab, anti-IgE treatment is currently the only biological therapeutic choice.136
Conclusion
The availability of omalizumab in real-life treatment of allergic asthma has represented a remarkable advance for the therapeutic control of the most severe phenotypes of this widespread disease. Indeed, omalizumab was the first, and until a short time ago the only biologic drug approved for the add-on treatment of severe asthma. Randomized controlled trials, and especially real-life studies have shown the therapeutic effectiveness of omalizumab in order to prevent asthma exacerbations, as well as to improve respiratory symptoms and quality of life. Such very positive clinical effects of omalizumab depend on its marked ability to dampen allergic airway inflammation and to inhibit bronchial remodelling. This excellent therapeutic profile is associated with a high degree of safety and tolerability. Therefore, omalizumab has been, is, and will be a valuable add-on therapeutic option for allergic asthmatic patients, characterized by severe type-2 airway inflammation leading to inadequate asthma control. Indeed, omalizumab holds a key position within the current context of biological therapies for severe allergic asthma. However, in order to better elucidate the eventual therapeutic differences existing between omalizumab and other biologics such as anti-IL-5/anti-IL-5 receptor drugs, as well as anti-IL-4 receptor molecules, head-to-head comparisons should be carried out. These comparative trials could thus contribute to provide an informed guidance to a more personalized, patient-oriented approach, when prescribing an add-on biological treatment for severe asthma.142
Table 1.
Add-on treatment of asthma with omalizumab: selection of real-life studies.
| Authors | Duration | Main outcomes |
|---|---|---|
| Molimard and colleagues80 | 5 months | Fewer exacerbations, emergency department visits and hospitalizations. |
| Korn and colleagues81 | 6 months | Fewer exacerbations, nocturnal symptoms, unscheduled visits and hospitalizations. |
| Brusselle and colleagues82 | 1 year | Fewer exacerbations, lesser healthcare use, improvement in asthma-related quality of life. |
| Cazzola and colleagues83 | At least 4 months | Fewer exacerbations and hospitalizations, reduced use of control medications. |
| Pace and colleagues98 | 7 years | Fewer exacerbations, better symptom control, improvement in lung function. |
| Pelaia and colleagues85 | 40 weeks | Fewer exacerbations and blood eosinophils, improvement in lung function. |
| Tzortzaki and colleagues87 | 4 years | Fewer exacerbations, improvements in asthma control and lung function. |
| Novelli and colleagues90 | 32 months (median) | Fewer exacerbations, better asthma control in patients without comorbidities. |
| Lopez-Tiro and colleagues89 | 3 years | Fewer emergency room visits and hospitalizations, improvement in lung function. |
| Licari and colleagues96 | 1 year | Fewer exacerbations and hospitalizations, corticosteroid-sparing effect. |
| Pitrez and colleagues97 | At least 6 months | Better asthma control, fewer hospitalizations, corticosteroid-sparing effect. |
| Menzella and colleagues99 | 9 years | Fewer exacerbations, better symptom control, improvement in lung function. |
| Di Bona and colleagues100 | 9 years | Very good profile of safety and tolerability. |
| Al-Ahmad and colleagues93 | 4 years | Better asthma control, sparing effects on both inhaled and oral corticosteroids. |
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Girolamo Pelaia  https://orcid.org/0000-0001-9288-8913
https://orcid.org/0000-0001-9288-8913
Contributor Information
Corrado Pelaia, Department of Medical and Surgical Sciences, University ‘Magna Græcia’ of Catanzaro, Catanzaro, Italy.
Cecilia Calabrese, Department of Cardio-Thoracic and Respiratory Sciences, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Rosa Terracciano, Department of Health Sciences, University ‘Magna Græcia’ of Catanzaro, Catanzaro, Italy.
Francesco de Blasio, Respiratory Medicine and Pulmonary Rehabilitation Section, Clinic Center Private Hospital, Naples, Italy; Department of Medicine and Health Sciences ‘V. Tiberio’, University of Molise, Campobasso, Italy.
Alessandro Vatrella, Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy.
Girolamo Pelaia, Department of Medical and Surgical Sciences, University ‘Magna Græcia’ of Catanzaro, Catanzaro, Italy; Campus Universitario ‘Salvatore Venuta’, Viale Europa – Località Germaneto, Catanzaro, 88100, Italy.
References
- 1. Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet 2018; 391: 783–800. [DOI] [PubMed] [Google Scholar]
- 2. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. [DOI] [PubMed] [Google Scholar]
- 3. Carr TF, Zeki AA, Kraft M. Eosinophilic and non-eosinophilic asthma. Am J Respir Crit Care Med 2018; 197: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holgate ST, Wenzel S, Postma DS, et al. Asthma. Nat Rev Dis Primers 2015; 1: 15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm 2015; 2015: 879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ray A, Oriss TB, Wenzel SE. Emerging molecular phenotypes of asthma. Am J Physiol Lung Cell Mol Physiol 2015; 308: L130–L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL (GOAL) study. Am J Respir Crit Care Med 2004; 170: 836–844. [DOI] [PubMed] [Google Scholar]
- 8. Fanta CH. Drug therapy: asthma. N Engl J Med 2009; 360: 1002–1014. [DOI] [PubMed] [Google Scholar]
- 9. Vitale C, Maglio A, Pelaia C, et al. Long-term treatment in pediatric asthma: an update on chemical pharmacotherapy. Expert Opinion Pharmacother 2017; 18: 667–676. [DOI] [PubMed] [Google Scholar]
- 10. Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov 2012; 11: 958–972. [DOI] [PubMed] [Google Scholar]
- 11. Viswanathan RK, Busse WW. Biologic therapy and asthma. Semin Respir Crit Care Med 2018; 39: 100–114. [DOI] [PubMed] [Google Scholar]
- 12. Humbert M, Busse W, Hanania NA, et al. Omalizumab in asthma: an update on recent developments. J Allergy Clin Immunol Pract 2014; 2: 525–536. [DOI] [PubMed] [Google Scholar]
- 13. Pelaia G, Vatrella A, Busceti MT, et al. Anti-IgE therapy with omalizumab for severe asthma: current concepts and potential developments. Curr Drug Targets 2015; 16: 171–178. [DOI] [PubMed] [Google Scholar]
- 14. Pelaia G, Canonica GW, Matucci A, et al. Targeted therapy in severe asthma today: focus on immunoglobulin E. Drug Des Devel Ther 2017; 11: 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol 2008; 8: 205–217. [DOI] [PubMed] [Google Scholar]
- 16. Dullaers M, De Bruyne R, Ramadani F, et al. The who, where and when of IgE in allergic airway disease. J Allergy Clin Immunol 2012; 129: 635–645. [DOI] [PubMed] [Google Scholar]
- 17. Froidure A, Mouthuy J, Durham SR, et al. Asthma phenotypes and IgE responses. Eur Respir J 2016; 47: 304–319. [DOI] [PubMed] [Google Scholar]
- 18. Hentges F, Leonard C, Arumugam K, et al. Immune responses to inhalant mammalian allergens. Front Immunol 2014; 5: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pelaia G, Renda T, Romeo P, et al. Omalizumab in the treatment of severe asthma: efficacy and current problems. Ther Adv Respir Dis 2008; 2: 409–421. [DOI] [PubMed] [Google Scholar]
- 20. Chung KF. Precision medicine in asthma: linking phenotypes to targeted treatments. Curr Opin Pulm Med 2018; 24: 4–10. [DOI] [PubMed] [Google Scholar]
- 21. Takhar P, Corrigan CJ, Smurthwaite L, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol 2007; 119: 213–218. [DOI] [PubMed] [Google Scholar]
- 22. Altin J, Shen C, Liston A. Understanding the genetic regulation of IgE production. Blood Rev 2010; 24: 163–169. [DOI] [PubMed] [Google Scholar]
- 23. Campbell AM, Vachier I, Chanez P, et al. Expression of the high-affinity receptor for IgE on bronchial epithelial cells of asthmatics. Am J Respir Cell Mol Biol 1998; 19: 92–97. [DOI] [PubMed] [Google Scholar]
- 24. Gounni AS, Wellemans V, Yang J, et al. Human airway smooth muscle cells express the high affinity receptor for IgE (FcεRI): a critical role of FcεRI in human airway smooth muscle cell function. J Immunol 2005; 175: 2613–2621. [DOI] [PubMed] [Google Scholar]
- 25. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol 2006; 6: 218–230. [DOI] [PubMed] [Google Scholar]
- 26. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 2008; 38: 872–897. [DOI] [PubMed] [Google Scholar]
- 27. Cruse G, Kaur D, Yang W, et al. Activation of human lung mast cells by monomeric immunoglobulin E. Eur Respir J 2005; 25: 858–863. [DOI] [PubMed] [Google Scholar]
- 28. Cruse G, Cockerill S, Bradding P. IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC Immunology 2008; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuda K, Piliponsky AM, Iikura M, et al. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J Allergy Clin Immunol 2005; 116: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 30. Hibbert RG, Teriete P, Grundy GJ, et al. The structure of human CD23 and its interactions with IgE and CD21. J Exp Med 2005; 202: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Acharya M, Borland G, Edkins AL, et al. CD23/FcεRII: molecular multi-tasking. Clin Exp Immunol 2010; 162: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell AM, Vignola AM, Chanez P, et al. Low-affinity receptor for IgE on human bronchial epithelial cells in asthma. Immunology 1994; 82: 506–508. [PMC free article] [PubMed] [Google Scholar]
- 33. Lantz CS, Yamaguchi M, Oettgen HC, et al. IgE regulates mouse basophil FcεRI expression in vivo. J Immunol 1997; 158: 2517–2521. [PubMed] [Google Scholar]
- 34. Yamaguchi M, Lantz CS, Oettgen HC, et al. IgE enhances mouse mast cell FcεRI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med 1997; 185:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kisselgof AB, Oettgen HC. The expression of murine B cell CD23, in vivo, is regulated by its ligand, IgE. Int Immunol 1998; 10: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 36. Saini SS, Klion AD, Holland SM, et al. The relationship between serum IgE and surface levels of FcεR on human leukocytes in various diseases: correlation of expression with FcεRI on basophils but not on monocytes or eosinophils. J Allergy Clin Immunol 2000; 106: 514–520. [DOI] [PubMed] [Google Scholar]
- 37. Borkowski TA, Jouvin MH, Lin SY, et al. Minimal requirements for IgE-mediated regulation of surface FceRI. J Immunol 2001; 167: 1290–1296. [DOI] [PubMed] [Google Scholar]
- 38. Kalesnikoff J, Huber M, Lam V, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity 2001; 14: 801–811. [DOI] [PubMed] [Google Scholar]
- 39. Sihra BS, Kon OM, Grant JA, et al. Expression of high-affinity IgE receptors (FcεRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol 1997; 99: 699–706. [DOI] [PubMed] [Google Scholar]
- 40. Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcεRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol 2003; 112: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 41. MacGlashan D., Jr. IgE receptor and signal transduction in mast cells and basophils. Curr Opin Immunol 2008; 20: 717–723. [DOI] [PubMed] [Google Scholar]
- 42. Weskamp G, Ford JW, Sturgill J, et al. ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat Immunol 2006; 7: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 43. Maurer D, Fiebiger S, Ebner C, et al. Peripheral blood dendritic cells express FcεRI as a complex composed of FcεRI α- FcεRI γ-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol 1996; 157: 607–616. [PubMed] [Google Scholar]
- 44. Maurer D, Fiebiger E, Reininger B, et al. FcεRI on dendritic cells delivers IgE-bound multivalent antigens into a cathepsin S-dependent pathway of MHC class II presentation. J Immunol 1998; 161: 2731–2739. [PubMed] [Google Scholar]
- 45. Khan SH, Grayson MH. Cross-linking IgE augments human conventional dendritic cell production of CC chemokine ligand 28. J Allergy Clin Immunol 2010; 125: 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lynch JP, Mazzone SB, Rogers MJ, et al. The plasmacytoid dendritic cell: at the cross-roads in asthma. Eur Respir J 2014; 43: 264–275. [DOI] [PubMed] [Google Scholar]
- 47. Gounni AS, Lamkhioued B, Ochiai K, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 1994; 367: 183–186. [DOI] [PubMed] [Google Scholar]
- 48. Rajakulasingam K, Durham SR, O’Brien F, et al. Enhanced expression of high-affinity IgE receptor (FcεRI) α chain in human allergen-induced rhinitis with co-localization to mast cells, macrophages, eosinophils, and dendritic cells. J Allergy Clin Immunol 1997; 100: 78–86. [DOI] [PubMed] [Google Scholar]
- 49. Smith SJ, Ying S, Meng Q, et al. Blood eosinophils from atopic donors express messenger RNA for the α, β, and γ subunits of the high-affinity IgE receptor (FcεRI) and intracellular, but not cell surface, subunit protein. J Allergy Clin Immunol 2000; 105: 309–317. [DOI] [PubMed] [Google Scholar]
- 50. Kim IS, Kim MJ, Kim DH, et al. Different anti-apoptotic effects of normal and asthmatic serum on normal eosinophil apoptosis depending on house dust mite-specific IgE. Mol Biol Rep 2013; 40: 5875–5881. [DOI] [PubMed] [Google Scholar]
- 51. Palaniyandi S, Tomei E, Li Z, et al. CD23-dependent transcytosis of IgE and immune complex across the polarized human respiratory epithelial cells. J Immunol 2011; 186: 3484–3496. [DOI] [PubMed] [Google Scholar]
- 52. Palaniyandi S, Liu X, Periasamy S, et al. Inhibition of CD23-mediated IgE transcytosis suppresses the initiation and development of allergic airway inflammation. Mucosal Immunol 2015; 8: 1262–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Redhu NS, Gounni AS. The high affinity IgE receptor (FcεRI) expression and function in airway smooth muscle. Pulm Pharmacol Ther 2013; 26: 86–94. [DOI] [PubMed] [Google Scholar]
- 54. Roth M, Zhong J, Zumkeller C, et al. The role of IgE-receptors in IgE-dependent airway smooth muscle cell remodelling. PLoS One 2013; 8: e56015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Presta LG, Lahr SJ, Shields RL, et al. Humanization of an antibody directed against IgE. J Immunol 1993; 151: 2623–2632. [PubMed] [Google Scholar]
- 56. Spector S. Omalizumab efficacy in allergic disease. Panminerva Med 2004; 46: 141–148. [PubMed] [Google Scholar]
- 57. Pelaia G, Gallelli L, Renda T, et al. Update on optimal use of omalizumab in management of asthma. J Asthma Allergy 2011; 4: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hochhaus G, Brookman L, Fox H, et al. Pharmacodynamics of omalizumab: implications for optimised dosing strategy and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin 2003; 19: 491–498. [DOI] [PubMed] [Google Scholar]
- 59. Fox JA, Hotaling TE, Struble C, et al. Tissue distribution and complex generation with IgE of an anti-IgE antibody after intravenous administration in cynomolgus monkeys. J Pharmacol Exp Ther 1996; 279: 1000–1008. [PubMed] [Google Scholar]
- 60. Chang TW, Wu PC, Hsu CL, et al. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol 2007; 93: 63–119. [DOI] [PubMed] [Google Scholar]
- 61. Presta L, Shields R, O’Connell L, et al. The binding site of a human immunoglobulin E for its high affinity receptor. J Biol Chem 1994; 269: 26368–26373. [PubMed] [Google Scholar]
- 62. Domingo C. Omalizumab for severe asthma: efficacy beyond the atopic patient? Drugs 2014; 74: 521–533. [DOI] [PubMed] [Google Scholar]
- 63. Noga O, Hanf G, Brachmann I, et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J Allergy Clin Immunol 2006; 117: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 64. Holgate S, Casale T, Wenzel S, et al. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol 2005; 115: 459–465. [DOI] [PubMed] [Google Scholar]
- 65. Marcus P. Incorporating anti-IgE (omalizumab) therapy in clinical practice: practice management implications. Chest 2006; 129: 466–474. [DOI] [PubMed] [Google Scholar]
- 66. Miller CWT, Krishnaswamy N, Johnston C, et al. Severe asthma and the omalizumab option. Clin Mol Allergy 2008; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med 1997; 155: 1828–1834. [DOI] [PubMed] [Google Scholar]
- 68. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001; 108: 184–190. [DOI] [PubMed] [Google Scholar]
- 69. Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18: 254–261. [DOI] [PubMed] [Google Scholar]
- 70. Holgate ST, Chuchalin AG, Hebert J, et al. Efficacy and tolerability of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004; 34: 632–638. [DOI] [PubMed] [Google Scholar]
- 71. Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004; 59: 709–717. [DOI] [PubMed] [Google Scholar]
- 72. Ayres JG, Higgins B, Chilvers ER, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy 2004; 59: 701–708. [DOI] [PubMed] [Google Scholar]
- 73. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60: 309–316. [DOI] [PubMed] [Google Scholar]
- 74. Price D. The use of omalizumab in asthma. Prim Care Respir J 2008; 17: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsabouri S, Tseretopoulou X, Priftis K, et al. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract 2014; 2: 332–340. [DOI] [PubMed] [Google Scholar]
- 76. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154: 573–582. [DOI] [PubMed] [Google Scholar]
- 77. Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. New Engl J Med 2011; 364: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 2011; 139: 28–35. [DOI] [PubMed] [Google Scholar]
- 79. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol 2017; 140: 162–169. [DOI] [PubMed] [Google Scholar]
- 80. Molimard M, de Blay F, Didier A, et al. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med 2008; 102: 71–76. [DOI] [PubMed] [Google Scholar]
- 81. Korn S, Thielen A, Seyfried S, et al. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med 2009; 103: 1725–1731. [DOI] [PubMed] [Google Scholar]
- 82. Brusselle G, Michils A, Louis R, et al. Real-life effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med 2009; 103: 1633–1642. [DOI] [PubMed] [Google Scholar]
- 83. Cazzola M, Camiciottoli G, Bonavia M, et al. Italian real-life experience of omalizumab. Respir Med 2010; 104: 1410–1416. [DOI] [PubMed] [Google Scholar]
- 84. Molimard M, Buhl R, Niven R, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma; real-life data. Respir Med 2010; 104: 1381–1385. [DOI] [PubMed] [Google Scholar]
- 85. Pelaia G, Gallelli L, Romeo P, et al. Omalizumab decreases exacerbation frequency, oral intake of corticosteroids and peripheral blood eosinophils in atopic patients with uncontrolled asthma. Int J Clin Pharmacol Ther 2011; 49: 713–721. [DOI] [PubMed] [Google Scholar]
- 86. Storms W, Bowdish MS, Farrar JR. Omalizumab and asthma control in patients with moderate-to-severe allergic asthma: a 6-year pragmatic data review. Allergy Asthma Proc 2012; 33: 172–177. [DOI] [PubMed] [Google Scholar]
- 87. Tzortzaki EG, Georgiou A, Kampas D, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther 2012; 25: 77–82. [DOI] [PubMed] [Google Scholar]
- 88. Grimaldi-Bensouda L, Zureik M, Aubier M, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest 2013; 143: 398–405. [DOI] [PubMed] [Google Scholar]
- 89. Lopez Tiro JJ, Contreras EA, Del Pozo ME, et al. Real life study of three years omalizumab in patients with difficult-to-control asthma. Allergol Immunopathol 2015; 43: 120–126. [DOI] [PubMed] [Google Scholar]
- 90. Novelli F, Latorre M, Vergura L, et al. Asthma control in severe asthmatics under treatment with omalizumab: a cross-sectional observational study in Italy. Pulm Pharmacol Ther 2015; 31: 123–129. [DOI] [PubMed] [Google Scholar]
- 91. Sposato B, Scalese M, Latorre M, et al. Effects of omalizumab in severe asthmatics across ages: a real-life Italian experience. Respir Med 2016; 119: 141–149. [DOI] [PubMed] [Google Scholar]
- 92. Lee JH, Lee HY, Jung CG, et al. Therapeutic effect of omalizumab in severe asthma: a real-world study in Korea. Allergy Asthma Immunol Res 2018; 10: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Al-Ahmad M, Arifhodzic N, Nunkic J, et al. “Real life” efficacy and safety aspects of four-year omalizumab treatment for asthma. Med Princ Pract 2018; 27: 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Abraham I, Alhossan A, Lee CS, et al. ‘Real-life’ effectiveness studies of omalizumab in severe patients with severe allergic asthma: systematic review. Allergy 2016; 71: 593–610. [DOI] [PubMed] [Google Scholar]
- 95. Alhossan A, Lee CS, MacDonald K, et al. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract 2017; 5: 1362–1370. [DOI] [PubMed] [Google Scholar]
- 96. Licari A, Castagnoli R, Denicolò C, et al. Omalizumab in children with severe allergic asthma: the Italian real-life experience. Curr Respir Med Rev 2017; 13: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pitrez PM, de Souza RG, Roncada C, et al. Impact of omalizumab in children from a middle-income country with severe therapy-resistant asthma. Pediatr Pulmonol 2017; 52: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 98. Pace E, Ferraro M, Bruno A, et al. Clinical benefits of 7 years of treatment with omalizumab in severe uncontrolled asthmatics. J Asthma 2011; 48: 387–392. [DOI] [PubMed] [Google Scholar]
- 99. Menzella F, Galeone C, Formisano D, et al. Real life efficacy of omalizumab after 9 years of follow-up. Allergy Asthma Immunol Res 2017; 9: 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Di Bona D, Fiorino I, Taurino M, et al. Long-term ‘real life’ safety of omalizumab in patients with severe uncontrolled asthma: a nine-year study. Respir Med 2017; 130: 55–60. [DOI] [PubMed] [Google Scholar]
- 101. Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–811. [DOI] [PubMed] [Google Scholar]
- 102. Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy 2018; 11: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Humbert M, Taillé C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J 2018; 51: 1702523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kopp MV, Hamelmann E, Bendiks M, et al. Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatr Allergy Immunol 2013; 24: 427–433. [DOI] [PubMed] [Google Scholar]
- 105. Braido F, Corsico A, Rogkakou A, et al. The relationship between allergen immunotherapy and omalizumab for treating asthma. Expert Rev Respir Med 2015; 9: 129–134. [DOI] [PubMed] [Google Scholar]
- 106. Dantzer JA, Wood RA. The use of omalizumab in allergen immunotherapy. Clin Exp Allergy 2018; 48: 232–240. [DOI] [PubMed] [Google Scholar]
- 107. Pelaia C, Vatrella A, Lombardo N, et al. Biological mechanisms underlying the clinical effects of allergen-specific immunotherapy in asthmatic children. Exp Opin Biol Ther 2018; 18: 197–204. [DOI] [PubMed] [Google Scholar]
- 108. Huang YC, Leyko B, Frier M. Effects of omalizumab and budesonide on markers of inflammation in human bronchial epithelial cells. Ann Allergy Asthma Immunol 2005; 95: 443–451. [DOI] [PubMed] [Google Scholar]
- 109. Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, et al. Anti-IgE therapy with omalizumab decreases endothelin-1 in exhaled breath condensate of patients with severe persistent allergic asthma. Respiration 2010; 80: 534–542. [DOI] [PubMed] [Google Scholar]
- 110. Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration 2012; 83: 520–528. [DOI] [PubMed] [Google Scholar]
- 111. Tajiri T, Niimi A, Matsumoto H, et al. Comprehensive efficacy of omalizumab for severe refractory asthma: a time-series observational study. Ann Allergy Asthma Immunol 2014; 113: 470–475. [DOI] [PubMed] [Google Scholar]
- 112. Riccio AM, Dal Negro RW, Micheletto C, et al. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int J Immunopathol Pharmacol 2012; 25: 475–484. [DOI] [PubMed] [Google Scholar]
- 113. Mauri P, Riccio AM, Rossi R, et al. Proteomics of bronchial biopsies: galectin-3 as a predictive biomarker of airway remodelling modulation in omalizumab-treated severe asthma patients. Immunol Lett 2014; 162: 2–10. [DOI] [PubMed] [Google Scholar]
- 114. de Llano LP, Vennera Mdel C, Alvarez FJ, et al. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. J Asthma 2013; 50: 296–301. [DOI] [PubMed] [Google Scholar]
- 115. Garcia G, Magnan A, Chiron R, et al. A proof of concept randomized-controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma. Chest 2013; 144: 411–419. [DOI] [PubMed] [Google Scholar]
- 116. Lommatzsch M, Korn S, Buhl R, et al. Against all odds: anti-IgE for intrinsic asthma? Thorax 2014; 69: 94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015; 136: 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Corren J, Shapiro G, Reimann J, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol 2008; 121: 506–511. [DOI] [PubMed] [Google Scholar]
- 119. Nopp A, Johansson SG, Adédoyin J, et al. After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy 2010; 65: 56–60. [DOI] [PubMed] [Google Scholar]
- 120. Baena-Cagnani CE, Teijeiro A, Canonica GW. Four-year follow-up in children with moderate/severe uncontrolled asthma after withdrawal of a 1-year omalizumab treatment. Curr Opin Allergy Clin Immunol 2015; 15: 267–271. [DOI] [PubMed] [Google Scholar]
- 121. Kuprys-Lipinska I, Kuna P. Loss of asthma control after cessation of omalizumab treatment: real life data. Postepy Derm Alergol 2014; 31: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Solèr M. Omalizumab for severe allergic asthma: 7 years and open questions. Respiration 2014; 88: 158–161. [DOI] [PubMed] [Google Scholar]
- 123. Holgate ST, Djukanovich R, Casale T, et al. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy 2005; 35: 408–416. [DOI] [PubMed] [Google Scholar]
- 124. Cox L, Platts-Mills TAE, Finegold I, et al. American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol 2007; 120: 1373–1377. [DOI] [PubMed] [Google Scholar]
- 125. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol 2012; 129: 983–989. [DOI] [PubMed] [Google Scholar]
- 126. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol 2014; 134: 560–567. [DOI] [PubMed] [Google Scholar]
- 127. Winchester DE, Jacob A, Murphy T. Omalizumab for asthma. N Engl J Med 2006; 355: 1281–1282. [DOI] [PubMed] [Google Scholar]
- 128. Puéchal X, Rivereau P, Vinchon F. Churg-Strauss syndrome associated with omalizumab. Eur J Intern Med 2008; 19: 364–366. [DOI] [PubMed] [Google Scholar]
- 129. Bargagli E, Madioni C, Olivieri C, et al. Churg-Strauss vasculitis in a patient treated with omalizumab. J Asthma 2008; 45: 115–116. [DOI] [PubMed] [Google Scholar]
- 130. Vaglio A, Moosig F, Zwerina J. Churg-Strauss syndrome: update on pathophysiology and treatment. Curr Opin Rheumatol 2012; 24: 24–30. [DOI] [PubMed] [Google Scholar]
- 131. Cruz AA, Lima F, Sarinho E, et al. Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infections. Clin Exp Allergy 2007; 37: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Iribarren C, Rahmaoui A, Long AA, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol 2017; 139: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 133. Iribarren C, Rothman KJ, Bradley MS, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: pooled analysis of patient-level data from 25 randomized, double-blind, placebo-controlled clinical trials. J Allergy Clin Immunol 2017; 139: 1678–1680. [DOI] [PubMed] [Google Scholar]
- 134. Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy 2009; 39: 788–797. [DOI] [PubMed] [Google Scholar]
- 135. Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135: 407–412. [DOI] [PubMed] [Google Scholar]
- 136. Bousquet J, Brusselle G, Buhl R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J 2017; 50: 1701782. [DOI] [PubMed] [Google Scholar]
- 137. Pelaia C, Vatrella A, Busceti MT, et al. Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des Devel Ther 2017; 11: 3137–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pelaia C, Busceti MT, Solinas S, et al. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: results of a single-centre observational study. Pulm Pharmacol Ther. Epub ahead of print 11 September 2018. DOI: 10.1016/j.pupt.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 139. Pelaia G, Vatrella A, Busceti MT, et al. Role of biologics in severe eosinophilic asthma: focus on reslizumab, Ther Clin Risk Manag 2016; 12: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pelaia C, Vatrella A, Bruni A, et al. Benralizumab in the treatment of severe asthma: design, development and potential place in therapy. Drug Des Devel Ther 2018; 12: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pelaia C, Calabrese C, Vatrella A, et al. Benralizumab: from the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. Biomed Res Int 2018; 2018: 4839230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Drazen JM, Harrington D. New biologics for asthma. N Engl J Med 2018; 378: 2031–2032. [DOI] [PubMed] [Google Scholar]